Introduction
Pulmonary hypertension (PH) in neonates is a common
diagnosis, and is often accompanied by an increase in pulmonary
vascular resistance. PH is considered to be the most frequent
complication of chronic neonatal lung disease, particularly in
bronchopulmonary dysplasia (BPD) (1,2). Hypoxia
is a primary cause of neonatal PH, and hypoxia may induce PH in
adult rats (3,4). In the present study, hypoxic conditions
were used to induce PH in newborn rats. Hypoxia-induced PH (HPH) in
neonates is characterized by an increase in pulmonary vascular
resistance and irreversible pulmonary vascular remodeling involving
the proliferation of pulmonary artery smooth muscle cells (PASMCs)
(5). The pulmonary vascular changes,
which mimic those seen in infants with severe BPD, are an important
cause of high morbidity and mortality (2). However, the mechanisms that contribute
to PASMC proliferation and pulmonary vascular remodeling are not
well understood.
Four and a half LIM domains 1 (FHL1) belongs to the
Lin-1, Isl-1 and Mec-3 (LIM)-only protein family, and serves vital
roles in gene transcription and cell proliferation, differentiation
and apoptosis (6). Previous studies
have suggested that FHL1 is associated with the development of
abnormalities in skeletal muscle and cardiac muscle, including
reduced body myopathy (7) and
hypertrophic cardiomyopathy (8).
Kwapiszewska et al (9)
documented that FHL1 was an important factor in pulmonary vascular
remodeling in patients with idiopathic pulmonary arterial
hypertension, as well as in adult rat models of PH, and that
altered expression of FHL1 affected the proliferation and migration
of human PASMCs. However, to the best of our knowledge, no study
has investigated whether FHL1 is related to PASMC proliferation and
pulmonary vascular remodeling in newborn rats with HPH.
Cellular proliferation is regulated by a series of
cell cycle modulators, including cyclin-dependent kinase inhibitors
(CDKIs). P21Cip1 is one of a few major CDKIs (10). Brock et al (11) reported that, in an HPH mouse model,
microRNA-130a controlled the proliferation of pulmonary smooth
muscle cells by directly downregulating P21. Furthermore, other
studies have demonstrated that rosiglitazone inhibited rat PASMC
proliferation by altering the expression levels of heme oxygenase-1
and P21, and that rosiglitazone ameliorated rat pulmonary
hypertension by inducing heme oxygenase-1 and P21 expression
(12,13). However, whether P21 serves a role in
HPH in newborn rats is unknown. Thus, the aim of the current study
was to investigate the expressions of FHL1 and P21 in the pulmonary
vessels of newborn rats with HPH, as well as the effects of these
proteins on pulmonary vascular remodeling.
Materials and methods
Animal models
All animal experimental procedures and protocols
were approved by the Ethics Committee of China Medical University
(Shenyang, China). Animals were provided by the Animal Department
Experiment Center, Shengjing Hospital of China Medical University
(Shenyang, China). A total of 32 newborn Sprague-Dawley (SD) rats
(male:female, 1:1; weight, 5–7 g) were randomly assigned to an HPH
model group or a control group (n=16). All rats were housed with
maternal rats (n=4; weight, 280–320 g; age, 10–12 weeks). Newborn
rats in the HPH model group were housed in a hypoxic atmosphere
(FiO2, 0.10; temperature, 25–27°C; humidity, 50–70%; 12
h light/dark cycle), and control rats were housed in a normal air
atmosphere (FiO2, 0.21; temperature, 25–27°C; humidity,
50–70%; 12 h light/dark cycle) as previously described (14,15).
Maternal rats had free access to food and water and were switched
between the groups every 24 h to avoid decreases in feeding ability
caused by hypoxia.
Hemodynamic studies and measurement of
right ventricular (RV) hypertrophy index
On day 14 of exposure to hypoxic conditions or room
air, pups were anesthetized with an intraperitoneal injection of
sodium pentobarbital (50 mg/kg). Tracheal intubation was performed
following tracheotomy, and the animals were connected to an animal
ventilator (ALC-V8D; Shanghai Alcott Biotech Co., Shanghai, China)
to provide breathing support with the following respiratory
parameters: Respiratory rate, 100 breaths/min; tidal volume, 0.2
ml. Blunt dissection of the left third and fourth intercostal
spaces was performed until the heart was fully exposed. The tip of
an intravenous trocar was punched into the right ventricle and the
opposite end of the trocar was connected to a pressure sensor
(BL-420F; Biological Signal Acquisition and Analysis System,
Chengdu Taimeng Software Co., Ltd., Chengdu, China) to determine
the RV mean pressure and RV systolic pressure (RVSP). The RV free
wall was subsequently removed from the left ventricle (LV) and
septum (S), and the weights of the RV and the LV plus S were
measured. The RV hypertrophy index (RVHI) was calculated to
evaluate the HPH model, and was determined as the ratio of the
weight of the RV to that of the LV plus S: RV/LV+S. In addition,
RVHI was calculated on day 7 and day 14 of exposure to hypoxia or
room air in 8 pups from each subgroup.
Sample preparation and determination
of pulmonary vascular remodeling
On days 7 and 14 of exposure to hypoxia or room air,
8 pups from each subgroup were anesthetized with an intraperitoneal
injection of sodium pentobarbital (50 mg/kg), and whole lungs were
collected. The inferior lobes of the right lungs were fixed in 4%
paraformaldehyde at 4°C for 48 h for hematoxylin and eosin
(H&E) or immunohistochemical staining, while the remaining
lungs were stored at −80°C for polymerase chain reaction (PCR) and
western blot analyses. The inferior lobes of the right lungs were
embedded with paraffin and 4–5-µm-thick sections were
deparaffinized in graded alcohol solutions and xylene, and stained
with hematoxylin (at room temperature for 3 min) and eosin (at room
temperature for 1 min) using a staining kit (cat. no. G1120;
Beijing Solarbio Science and Technology Co., Ltd., Beijing, China).
A total of 3 paraffin-embedded sections were randomly selected from
8 rats/subgroup, and 5 pulmonary arterioles with a diameter of 50
to 100 µm from each rat were then selected and images were captured
using a light microscope. The pulmonary artery medical wall
thickness/external diameter ratio (WT%) and pulmonary artery
medical wall cross-sectional area/total vessel area (WA%) were
analyzed in collected images using Image-Pro Plus 6.0 software
(Media Cybernetics, Inc., Rockville, MD, USA) to assess medial wall
hypertrophy, as previously described (15).
Immunohistochemistry
Briefly, sections of lung (4-5-µm thick) were
deparaffinized in a graded series of alcohol and xylene. Following
the manufacturer's instructions (immunohistochemistry kit; cat. no.
kit-9710; Fuzhou Maixin Biotech Co., Ltd., Fuzhou, China), all
sections were subsequently blocked with 3%
H2O2 (37°C for 20 min) and goat serum
(provided in kit-9710; 40 min at room temperature). Sections were
subsequently incubated overnight at 4°C with FHL1 primary antibody
(rabbit anti-rat FHL1, 1:200, cat. no. ab133661), P21 primary
antibody (rabbit anti-rat p21, 1:400, cat. no. ab18209) or
proliferating cell nuclear antigen (PCNA) primary antibody (rabbit
anti-rat PCNA, 1:400, cat. no. ab2426) (all from Abcam, Cambridge,
UK). The next day, sections were incubated with biotin-labeled goat
anti-rabbit IgG secondary antibody (provided in kit-9710; 20 min at
37°C) and then incubated with a horseradish peroxidase marker
(provided in kit-9710; 20 min at 37°C). The paraffin sections were
developed with DAB (30 sec at room temperature, cat. no. ZLI-9017
DAB kit; OriGene Technologies, Inc., Rockville, MD, USA) and
counterstained with 10% hematoxylin for 3 min at room temperature.
Sections were subsequently dehydrated in a graded series of
alcohol, treated with xylene and mounted using neutral balsam. A
light microscope was used for image acquisition, and the deposition
of brown particles indicated a positive result.
Total RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
A total of 8 samples (60 mg) from each group were
obtained and total RNA was extracted using TRIzol reagent (Thermo
Fisher Scientific, Inc., Waltham, MA, USA), according to the
manufacturer's instructions. RNA purity and concentration were
determined according to the OD 260/280 nm ratio. mRNA was reverse
transcribed into cDNA using the PrimeScript RT Reagent kit (Takara
Biotechnology Co., Ltd., Dalian, China). PCR was performed with a
20 µl final volume reaction mixture using a SYBR-Green PCR reagent
kit (Takara Biotechnology Co., Ltd.) according to the
manufacturer's instructions on the Applied Biosystems 7500
Real-Time PCR system (Applied Biosystems 7500; Thermo Fisher
Scientific, Inc.). The cDNA PCR conditions were as follows: 1 cycle
of 95°C for 30 sec, then 40 cycles of 95°C for 5 sec and 60°C for
34 sec. The gene expression levels were calculated with the
2−∆∆Cq method (16).
Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) served as an
internal control. The primer sequences are listed in Table I.
 | Table I.Reverse transcription-quantitative
polymerase chain reaction primers. |
Table I.
Reverse transcription-quantitative
polymerase chain reaction primers.
| Genes | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| FHL1 |
CGTGCCAGTAGCGATTCTTAT |
GCTGCCTGAAGTGCTTTGAC |
| P21 |
CATGTCCGATCCTGGTGATG |
CGAACAGACGACGGCATACTT |
| GAPDH |
AGACAGCCGCATCTTCTTGT |
CTTGCCGTGGGTAGAGTCAT |
Western blotting
The total protein extracted
(radioimmunoprecipitation assay lysis buffer, cat. no. P0013B;
Beyotime Institute of Biotechnology, Shanghai, China) from lung
tissue was quantified using a bicinchoninic acid protein assay kit.
A total of 50 µg of each protein extract loaded per lane was
separated by 12% SDS-PAGE and transferred to polyvinylidene
difluoride membranes (EMD Millipore, Billerica, MA, USA). The
membranes were incubated at room temperature for 2 h in 5% bovine
serum albumin (Beijing Solarbio Science and Technology Co., Ltd.,
Beijing, China) to block non-specific binding. The membranes were
then incubated with primary antibodies against FHL1 (rabbit
anti-rat FHL1, 1:400, cat. no. ab133661), P21 (rabbit anti-rat p21,
1:500, cat. no. ab18209) and GAPDH (1:5,000, mouse monoclonal, cat.
no. ab9484) (all from Abcam) diluted in Tris-buffered saline with
Tween-20 (TBST) at 4°C overnight. Following three washes in TBST,
the membranes were incubated with a peroxidase-conjugated secondary
antibody [goat anti-rabbit immunoglobulin G (IgG) or goat
anti-mouse IgG, 1:2,000; OriGene Technologies, Inc.] for 2 h at
room temperature. The membranes were subsequently imaged using
enhanced chemiluminescence reagents (Santa Cruz Biotechnology,
Inc., Dallas, TX, USA). The density of the protein bands was
analyzed using Image-Pro Plus 6.0 software (Media Cybernetics,
Inc.) and normalized to that of GAPDH.
Statistical analysis
Data are presented as the mean + standard error of
the mean. Statistical analyses were performed with SPSS 17.0
software (SPSS Inc., Chicago, IL, USA). Student's t-tests were used
for inter-group comparisons, and a Pearson's test was used for
correlation analysis. P<0.05 was considered to indicate
statistical significance.
Results
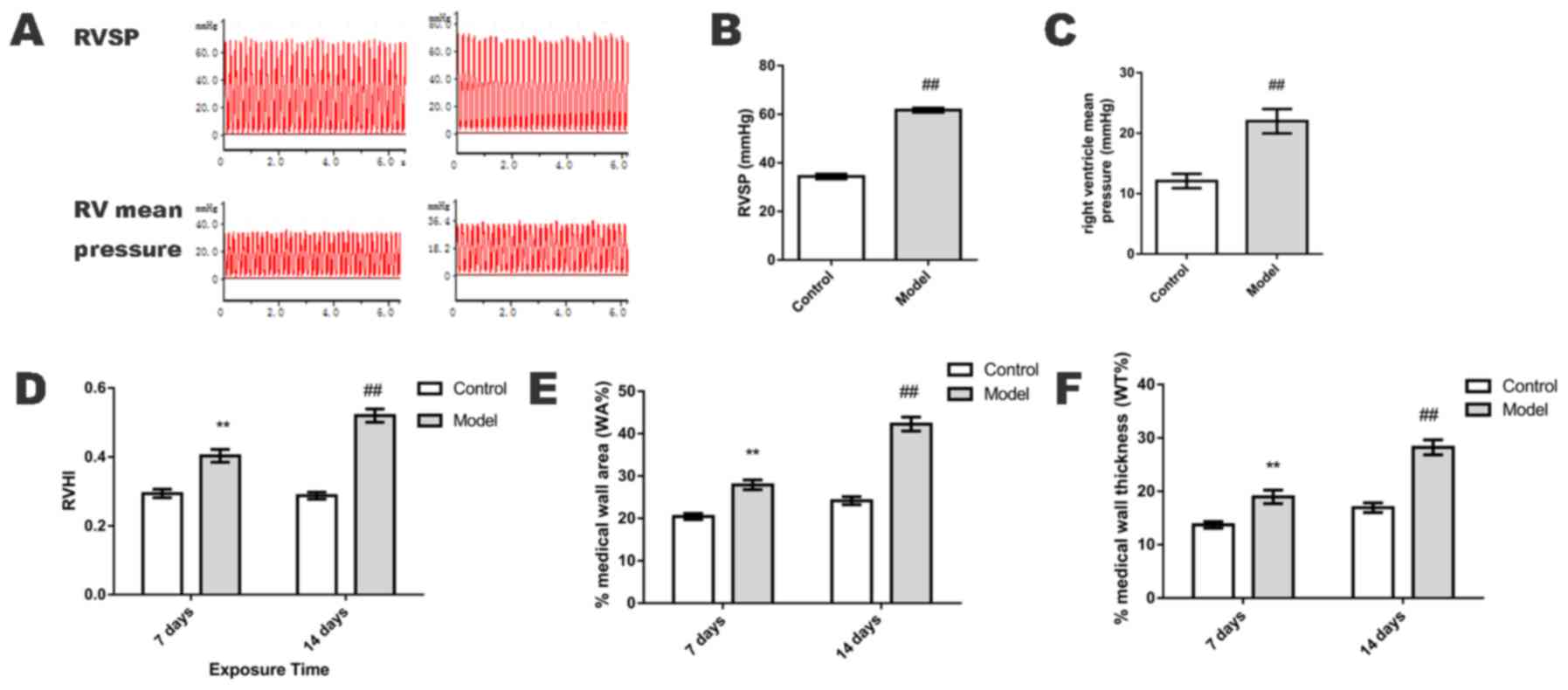
RV mean pressure, RVSP and RVHI
On day 14, RV mean pressure and RVSP were
significantly elevated in the HPH group when compared with the
control group (P<0.01; Fig.
1A-C). On days 7 and 14, RVHI was also significantly increased
in the HPH group when compared with the control group (P<0.01;
Fig. 1D).
H&E staining and pulmonary
vascular remodeling (WA% and WT%)
Upon comparison of the small pulmonary arteries
(50–100 µm in diameter), the WA% and WT% of the HPH group were
significantly greater than those in the control group on days 7 and
14 (P<0.01; Fig. 1E and F), as
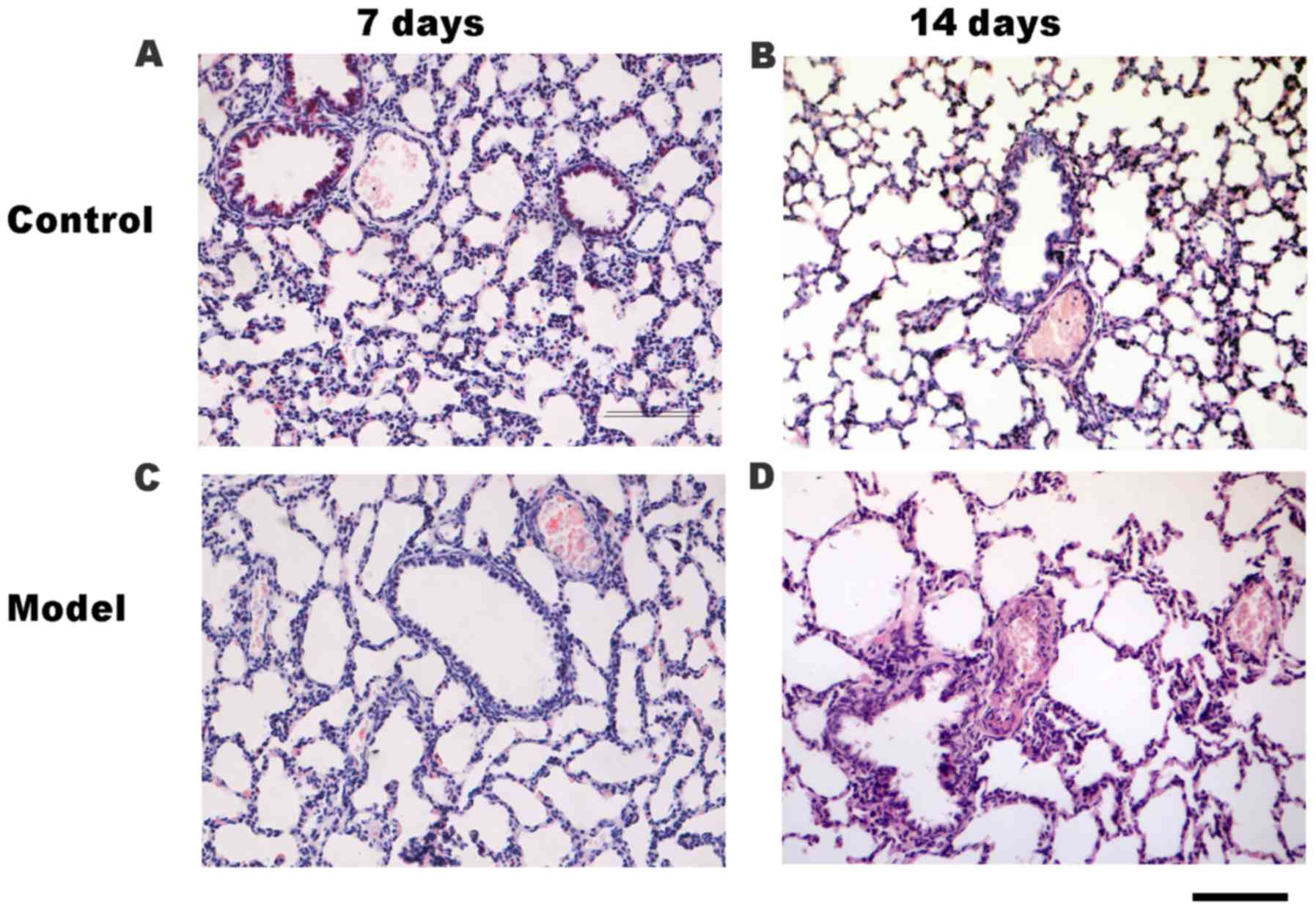
determined by H&E staining (Fig.
2). Accordingly, compared with the control group (Fig. 2A and B), the small pulmonary artery
wall was thickened and the lumen was narrowed in the HPH group on
days 7 (Fig. 2C) and 14 (Fig. 2D). Additionally, compared with the
control group on day 14 (Fig. 2B),
the lung histology of rats in the HPH group (Fig. 2D) was characterized by increased
alveolar space, a thickened alveolar wall and decreased secondary
septa.
Pulmonary artery smooth muscle cell
(PASMC) proliferation
The expression of PCNA protein may be used as an
indicator of cell proliferation (17), and thus was used to assess the
proliferation of PASMCs by immunohistochemical staining. The
expression of PCNA protein was identified in the nucleus of PASMCs
in both the HPH and control groups (Fig.
3A-D). PCNA expression in the pulmonary arterioles of the HPH
group on days 7 (Fig. 3C) and 14
(Fig. 3D) were greater than those in
the control group at the same times (Fig. 3A and B).
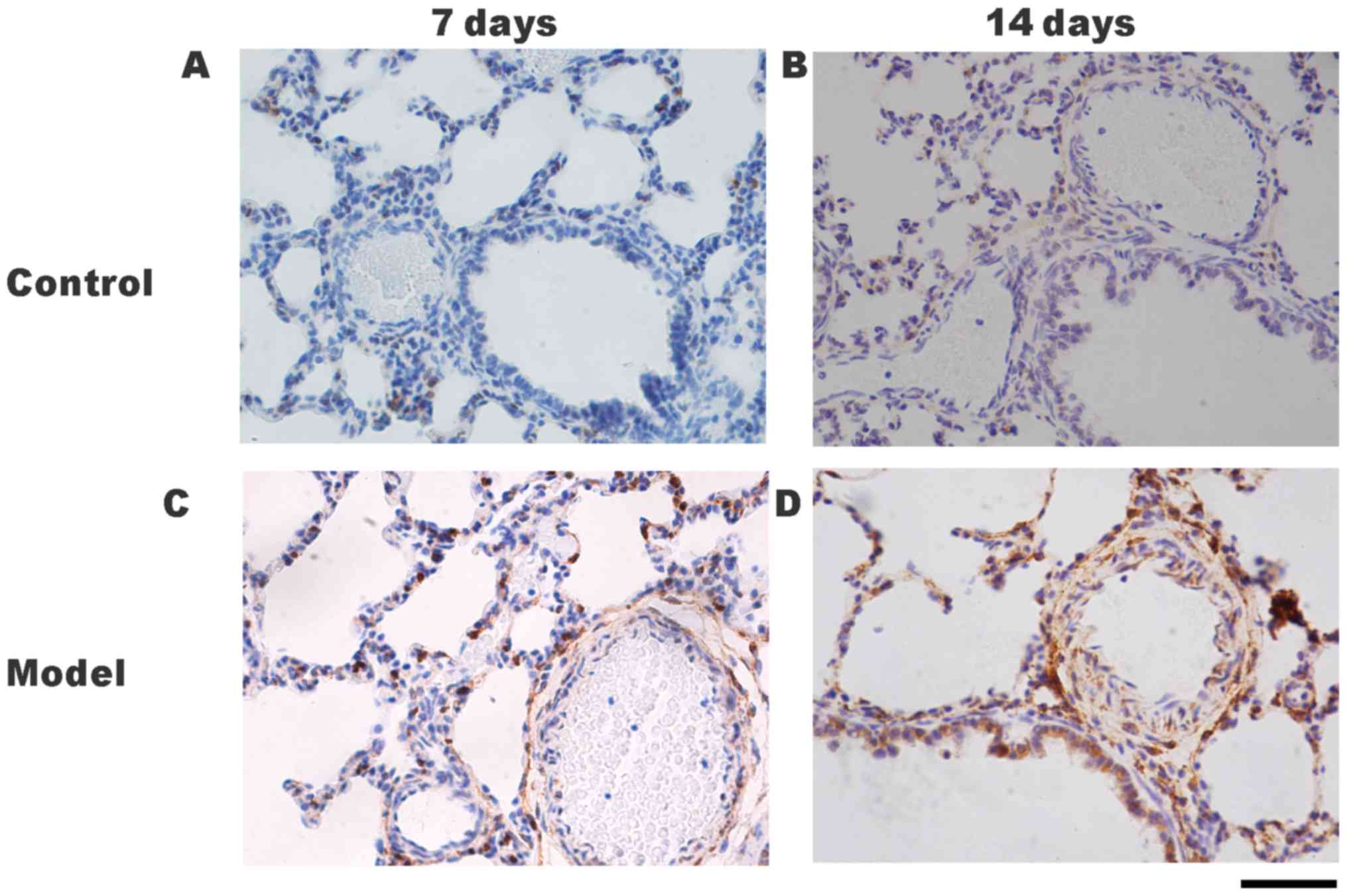
FHL1 and P21 localization in rat lung
tissues following hypoxia
The protein localizations of FHL1 and P21 in the
lung tissues were determined by immunohistochemical staining. Only
slight positive staining for FHL1 was detected in the small
pulmonary arteries of the control group on days 7 (Fig. 4A) and 14 (Fig. 4B). Positive staining for FHL1 was
detected in the small pulmonary arteries of the HPH group on days 7
(Fig. 4C) and 14 (Fig. 4D) P21 protein was detected in the
nuclei and cytoplasm of PASMCs (Fig.
5A-D). Positive staining for P21 was identified in the
cytoplasm of PASMCs in the HPH group on days 7 (Fig. 5C) and 14 (Fig. 5D). However, P21 expression in the
pulmonary arterioles of the HPH group on days 7 and 14 (Fig. 5C and D) were markedly lower than
those in the control group at the same times (Fig. 5A and B).
Protein expression levels of FHL1 and
P21
The protein expression levels of FHL1 and P21 in the
lung tissues were determined by western blotting. Compared with the
control group, the protein expression of FHL1 was significantly
increased in the HPH model group on days 7 (P<0.01) and 14
(P<0.05; Fig. 6A and B). By
contrast, compared with the control group, the protein expression
of P21 was significantly reduced in the HPH group on days 7
(P<0.01) and 14 (P<0.01; Fig. 6A
and C).
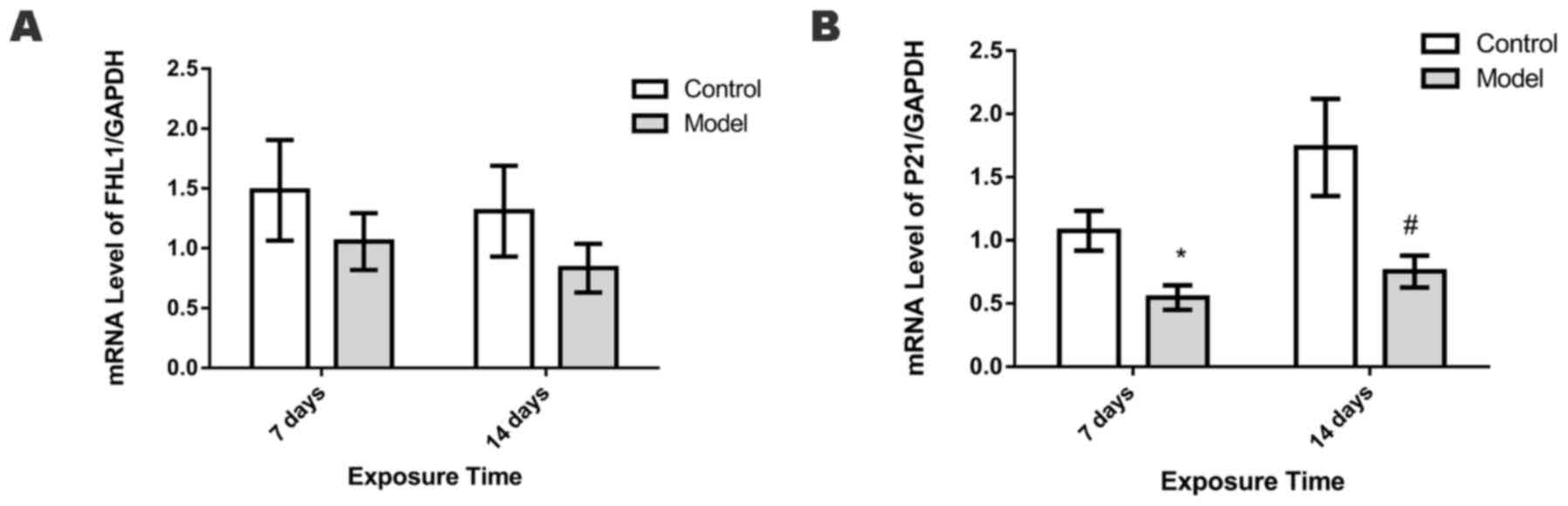
mRNA expression levels of FHL1 and
P21
The mRNA expression levels of FHL1 in the lungs did
not differ significantly between the control and HPH groups on days
7 and 14 (Fig. 7A). By contrast,
compared with the control group, the HPH group exhibited
significantly lower levels of P21 mRNA on days 7 and 14 (P<0.05;
Fig. 7B).
Associations between FHL1, P21 and
HPH
Pearson correlation analysis indicated that the
protein expression of FHL1 was negatively associated with the
protein expression of P21 (r=−0.504, P<0.01). Furthermore, WA%
and WT% were positively associated with the expression of FHL1
(r=0.627, P<0.001 and r=0.589, P<0.001; respectively).
Additionally, WA% and WT% were negatively associated with the
expression of P21 (r=−0.805, P<0.001 and r=−0.749, P<0.001;
respectively).
Discussion
PH in neonates is a critical disease with a
relatively high mortality rate of 10–20% (18). PH may be triggered by hypoxic lung
diseases, including apnea; however, hypoxia is among the main
causes of PH (1,5,19). In
clinical scenarios, HPH is common; however, if sustained hypoxia is
not controlled, hypoxia-induced pulmonary vascular contraction may
lead to irreversible pulmonary vascular remodeling, which is
associated with a poor prognosis (5).
HPH is characterized by pulmonary vasoconstriction
and irreversible pulmonary vascular remodeling, which involves the
proliferation of vascular endothelial cells and PASMCs, and
deposition of collagen in the adventitia (20–22). In
the present study, 2-day-old SD rats were exposed to hypoxia to
induce a model of HPH, and parameters including RV mean pressure,
RVHI, WT and WA% were measured to evaluate the development of HPH.
The results demonstrated that RV mean pressure, RVHI, WT and WA%
were significantly increased after 14 days of hypoxia, which was
consistent with previous studies (5,15,23).
Furthermore, after 14 days of exposure to hypoxia, the lung
histology was characterized by increased alveolar space, a
thickened alveolar wall and decreased secondary septa. These
observations were consistent with a study by Deruelle et al
(15). Additionally,
immunohistochemical staining indicated that the protein expression
of PCNA was significantly higher in the HPH model group than in the
control group, which suggested that hypoxia stimulated the
proliferation of PASMCs in vitro.
Many factors contribute to the proliferation of
PASMCs. For instance, high expression of hypoxia-inducible factor
(HIF)-1α has been related to PASMC proliferation in HPH adult rat
models and newborn lamb models of persistent PH (24–26). The
present study demonstrated that, in an HPH neonatal rat model,
FHL-1 expression was increased in the small pulmonary arteries,
which was concurrent with increased PASMC proliferation.
Kwapiszewska et al (9)
documented that FHL1 was markedly upregulated in the lungs of
patients with idiopathic pulmonary arterial hypertension, as well
as in adult mouse and rat models of HPH. They also observed that
knockdown of FHL1 decreased the migration and proliferation of
human PASMCs, while FHL1 overexpression had the opposite effects.
Furthermore, they concluded that the expression of FHL1 was
regulated by HIF-1α and −2α (9).
Therefore, in the present HPH neonatal rat model, the effect of
FHL1 on the proliferation of PASMCs may have been regulated by
HIF-1α.
Nadeau et al (27) observed that vascular endothelial
growth factor (VEGF) was upregulated in a neonatal pig model of
HPH. In human HepG2 hepatoma cells, FHL1 decreased the expression
and activity of VEGF by blocking the dimerization of HIF-1α and
HIF-1β (28). Thus, FHL1 may have
exerted similar effect in the current HPH neonatal rat model.
Additionally, the present data was consistent with previous
findings that high expression of FHL1 was correlated with PASMC
proliferation induced by cigarette smoke extract (29).
Excessive PASMC proliferation contributes to
pulmonary vascular remodeling in HPH (30,31). The
cyclin-dependent inhibitor P21 serves an important role in the
proliferation of PASMCs, and has been identified as a critical
factor in cell cycle progression following exposure to hypoxia
(11). However, previous results
contradict the potential role of P21 in PH. Yu et al
(32) observed that, in an HPH adult
mouse model, P27 expression was significantly suppressed, while the
expressions of other CDKIs, including P21, were not affected. To
clarify the role of FHL1 in cell proliferation, the expression of
P21 in the lungs was investigated. Compared with the control group,
P21 expression was significantly decreased in the HPH model group.
Similarly, a previous study documented that a loss of P21
expression contributed to PASMC proliferation in fetal lambs
acclimatized to long-term high-altitude hypoxia (33).
In the present study, the protein expression of FHL1
was significantly increased on days 7 and 14 of hypoxia, while P21
protein expression significantly decreased. Pearson correlation
analysis indicated that the protein expressions of FHL1 and P21
were correlated with WA% and WT%. Furthermore, the protein
expression of P21 was negatively correlated with that of FHL1.
These results suggest that hypoxia increased the expression of FHL1
expression, which subsequently downregulated P21 in the HPH
neonatal rat model. In turn, downregulation of P21 may have
triggered PASMC proliferation, resulting in pulmonary vascular
remodeling.
In conclusion, the present results indicated that
the expression profiles of FHL1 and P21 were altered in the PASMCs
of neonatal rats with HPH. Additionally, the protein expressions of
FHL1 and P21 were correlated with WA% and WT%, indicating that FHL1
and P21 may serve important roles in pulmonary vascular remodeling.
However, further study is required to verify the roles of FHL1 and
P21 in the proliferation of PASMCs in neonatal rats with HPH.
Acknowledgements
The present study was supported by the Natural
Science Foundation of China (grant nos. 81471489 and 81571479).
Glossary
Abbreviations
Abbreviations:
|
PH
|
pulmonary hypertension
|
|
PASMCs
|
pulmonary artery smooth muscle
cells
|
|
FHL1
|
four and a half LIM domains 1
|
|
HPH
|
hypoxia-induced pulmonary
hypertension
|
|
RV
|
right ventricle
|
|
LV
|
left ventricle
|
|
RVHI
|
right ventricular hypertrophy
index
|
|
RVSP
|
right ventricular systolic
pressure
|
|
WT
|
medial wall thickness
|
|
WA
|
medial wall area
|
|
S
|
septum
|
|
PCNA
|
proliferating cell nuclear antigen
|
|
BPD
|
bronchoplumonary dysplasia
|
|
CDKI
|
cyclin-dependent kinase inhibitor
|
|
HIF
|
hypoxia-inducible factor
|
|
VEGF
|
vascular endothelial growth factor
|
|
TBST
|
Tris-buffered saline with Tween-20
|
|
SD
|
Sprague-Dawley
|
References
|
1
|
Jain A and McNamara PJ: Persistent
pulmonary hypertension of the newborn: Advances in diagnosis and
treatment. Semin Fetal Neonatal Med. 20:262–271. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Young KC, Torres E, Hehre D, Wu S,
Suguihara C and Hare JM: Antagonism of stem cell factor/c-kit
signaling attenuates neonatal chronic hypoxia-induced pulmonary
vascular remodeling. Pediatr Res. 79:637–646. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shao J, Wang P, Liu A, Du X, Bai J and
Chen M: Punicalagin prevents hypoxic pulmonary hypertension via
anti-oxidant effects in rats. Am J Chin Med. 44:785–801. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mohsenin V: The emerging role of microRNAs
in hypoxia-induced pulmonary hypertension. Sleep Breath.
20:1059–1067. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang L, Zhou Y, Li M and Zhu Y: Expression
of hypoxia-inducible factor-1α, endothelin-1 and adrenomedullin in
newborn rats with hypoxia-induced pulmonary hypertension. Exp Ther
Med. 8:335–339. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou Z, Lu J, Dou J, Lv Z, Qin X and Lin
J: FHL1 and Smad4 synergistically inhibit vascular endothelial
growth factor expression. Mol Med Rep. 7:649–653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Malfatti E, Olive M, Taratuto AL, Richard
P, Brochier G, Bitoun M, Gueneau L, Laforêt P, Stojkovic T,
Maisonobe T, et al: Skeletal muscle biopsy analysis in reducing
body myopathy and other FHL1-related disorders. J Neuropathol Exp
Neurol. 72:833–845. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Friedrich FW, Wilding BR, Reischmann S,
Crocini C, Lang P, Charron P, Müller OJ, McGrath MJ, Vollert I,
Hansen A, et al: Evidence for FHL1 as a novel disease gene for
isolated hypertrophic cardiomyopathy. Hum Mol Genet. 21:3237–3254.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kwapiszewska G, Wygrecka M, Marsh LM,
Schmitt S, Trösser R, Wilhelm J, Helmus K, Eul B, Zakrzewicz A,
Ghofrani HA, et al: Fhl-1, a new key protein in pulmonary
hypertension. Circulation. 118:1183–1194. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vermeulen K, Van Bockstaele DR and
Berneman ZN: The cell cycle: A review of regulation, deregulation
and therapeutic targets in cancer. Cell Prolif. 36:131–149. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brock M, Haider TJ, Vogel J, Gassmann M,
Speich R, Trenkmann M, Ulrich S, Kohler M and Huber LC: The
hypoxia-induced microRNA-130a controls pulmonary smooth muscle cell
proliferation by directly targeting CDKN1A. Int J Biochem Cell
Biol. 61:129–137. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li M, Li Z, Sun X, Yang L, Fang P, Liu Y,
Li W, Xu J, Lu J, Xie M and Zhang D: Heme oxygenase-1/p21WAF1
mediates peroxisome proliferator-activated receptor-gamma signaling
inhibition of proliferation of rat pulmonary artery smooth muscle
cells. FEBS J. 277:1543–1550. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang D, Wang G, Han D, Zhang Y, Xu J, Lu
J, Li S, Xie X, Liu L, Dong L and Li M: Activation of PPAR-γ
ameliorates pulmonary arterial hypertension via inducing heme
oxygenase-1 and p21 (WAF1): An in vivo study in rats. Life Sci.
98:39–43. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu XF, Gu WZ, Wu XL, Li RY and Du LZ:
Fetal pulmonary vascular remodeling in a rat model induced by
hypoxia and indomethacin. J Matern Fetal Neonatal Med. 24:172–182.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Deruelle P, Balasubramaniam V, Kunig AM,
Seedorf GJ, Markham NE and Abman SH: BAY 41–2272, a direct
activator of soluble guanylate cyclase, reduces right ventricular
hypertrophy and prevents pulmonary vascular remodeling during
chronic hypoxia in neonatal rats. Biol Neonate. 90:135–144. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xiang H, Yuan L, Gao X, Alexander PB,
Lopez O, Lau C, Ding Y, Chong M, Sun T, Chen R, et al: UHRF1 is
required for basal stem cell proliferation in response to airway
injury. Cell Discov. 3:170192017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Perez KM and Laughon M: Sildenafil in term
and premature infants: A systematic review. Clin Ther.
37:2598–2607. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Steinhorn RH: Advances in neonatal
pulmonary hypertension. Neonatology. 109:334–344. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
He Y, Cao X, Liu X, Li X, Xu Y, Liu J and
Shi J: Quercetin reverses experimental pulmonary arterial
hypertension by modulating the TrkA pathway. Exp Cell Res.
339:122–134. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li L, Wang X, Wang L, Qu L, Zhu X, Li M,
Dang X, Li P, Gao Y, Peng Z, et al: Mammalian target of rapamycin
overexpression antagonizes chronic hypoxia-triggered pulmonary
arterial hypertension via the autophagic pathway. Int J Mol Med.
36:316–322. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Umesh A, Paudel O, Cao YN, Myers AC and
Sham JS: Alteration of pulmonary artery integrin levels in chronic
hypoxia and monocrotaline-induced pulmonary hypertension. J Vasc
Res. 48:525–537. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu YP, Zhu JJ, Cheng F, Jiang KW, Gu WZ,
Shen Z, Wu YD, Liang L and Du LZ: Ghrelin ameliorates
hypoxia-induced pulmonary hypertension via phospho-GSK3 β/β-catenin
signaling in neonatal rats. J Mol Endocrinol. 47:33–43. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yue J, Guan J, Wang X, Zhang L, Yang Z, Ao
Q, Deng Y, Zhu P and Wang G: MicroRNA-206 is involved in
hypoxia-induced pulmonary hypertension through targeting of the
HIF-1α/Fhl-1 pathway. Lab Invest. 93:748–759. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang L, Pu Z and Wang J, Zhang Z, Hu D
and Wang J: Baicalin inhibits hypoxia-induced pulmonary artery
smooth muscle cell proliferation via the AKT/HIF-1α/p27-associated
pathway. Int J Mol Sci. 15:8153–8168. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wedgwood S, Lakshminrusimha S, Schumacker
PT and Steinhorn RH: Hypoxia inducible factor signaling and
experimental persistent pulmonary hypertension of the newborn.
Front Pharmacol. 6:472015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nadeau S, Baribeau J, Janvier A and
Perreault T: Changes in expression of vascular endothelial growth
factor and its receptors in neonatal hypoxia-induced pulmonary
hypertension. Pediatr Res. 58:199–205. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin J, Qin X, Zhu Z, Mu J, Zhu L, Wu K,
Jiao H, Xu X and Ye Q: FHL family members suppress vascular
endothelial growth factor expression through blockade of
dimerization of HIF1α and HIF1β. IUBMB Life. 64:921–930. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Y, Pu G, Chen C and Yang L: Inhibition
of FHL1 inhibits cigarette smoke extract-induced proliferation in
pulmonary arterial smooth muscle cells. Mol Med Rep. 12:3801–3808.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huetsch JC, Jiang H, Larrain C and Shimoda
LA: The Na+/H+ exchanger contributes to
increased smooth muscle proliferation and migration in a rat model
of pulmonary arterial hypertension. Physiol Rep. 4:e127292016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wei C, Li HZ, Wang YH, Peng X, Shao HJ, Li
HX, Bai SZ, Lu XX, Wu LY, Wang R and Xu CQ: Exogenous spermine
inhibits the proliferation of human pulmonary artery smooth muscle
cells caused by chemically-induced hypoxia via the suppression of
the ERK1/2- and PI3K/AKT-associated pathways. Int J Mol Med.
37:39–46. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu L, Quinn DA, Garg HG and Hales CA: Gene
expression of cyclin-dependent kinase inhibitors and effect of
heparin on their expression in mice with hypoxia-induced pulmonary
hypertension. Biochem Biophys Res Commun. 345:1565–1572. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yang Q, Lu Z, Ramchandran R, Longo LD and
Raj JU: Pulmonary artery smooth muscle cell proliferation and
migration in fetal lambs acclimatized to high-altitude long-term
hypoxia: Role of histone acetylation. Am J Physiol Lung Cell Mol
Physiol. 303:L1001–L1010. 2012. View Article : Google Scholar : PubMed/NCBI
|