Introduction
In 2011, more than 10,000 women in Japan were
diagnosed with uterine cervical carcinoma and almost 2,900
succumbed to this disease (1). The
major histopathological type of cervical cancer is squamous cell
carcinoma, and the second most frequent type is adenocarcinoma,
accounting for 15–20% of cases (2).
The incidence of cervical adenocarcinoma is increasing, and its
prognosis is poor compared with that of squamous cell carcinoma due
to its low response to chemotherapy and radiotherapy (1,3).
According to the National Comprehensive Cancer Network guidelines,
treatment of cervical cancer is stratified by stage (3). However, the same treatment is used for
adenocarcinoma and squamous cell carcinoma of the cervix (4). Therefore, a novel therapy for cervical
adenocarcinoma is required.
Src is a non-receptor tyrosine kinase protein that
interacts with receptor tyrosine kinases. Src binds its own Src
homology 2 (SH2) domain with a short peptide sequence containing
phosphorylated tyrosine (Tyr 530) at the C-terminal tail, and SH3
is coupled with the proline-rich linker domain. SH2 and SH3
inactivate the structure of the metabolic region. When Tyr 530 is
dephosphorylated, intramolecular interaction is destabilized,
leading to autophosphorylation of Tyr 419 and activation of Src
(5). Src is an important factor in
cell survival, proliferation, angiogenesis and metastasis (5,6). Src
family kinases are frequently overexpressed and activated in a
variety of epithelial and non-epithelial cancers and contribute to
resistance to chemotherapy (7).
Thus, Src is being investigated for molecularly targeted therapy in
cervical adenocarcinoma.
Dasatinib is an inhibitor of the Src family of
tyrosine kinases and of the Bcr-Abl tyrosine kinase (4,5). It was
originally approved as a drug for the treatment of chronic myeloid
leukemia and Philadelphia chromosome-positive acute lymphoblastic
leukemia (4,5). Clinical trials have been conducted
using dasatinib in combination with anticancer drugs for the
treatment of breast, colon, prostate and other cancers (8–17).
Single-agent dasatinib has not exhibited any clinical activity in
breast, pancreatic, colorectal and ovarian carcinoma (8,12,13,15).
However, combination therapy with dasatinib and anticancer drugs
has been demonstrated to be clinically effective in the treatment
of breast, prostate, ovarian and pancreatic cancers (9–11,14,16,17).
A number of studies have reported Src expression in
cervical cancer (18–22). However, data are lacking on its
expression in cervical adenocarcinoma. The present study aimed to
confirm Src expression in human cervical adenocarcinoma cell lines
and to determine the mechanism of inhibition of Src signaling by
dasatinib in vitro.
Materials and methods
Cell lines and cultures
HeLa (RCB0007) and TCO-2 (RCB0689) cells were
cultured in Eagle's minimum essential medium (Wako Pure Chemical
Industries, Ltd., Osaka, Japan), supplemented with 10% fetal bovine
serum (Thermo Fisher Scientific, Inc., Waltham, MA, USA) and 1%
penicillin-streptomycin solution in 5% CO2 at 37°C.
These cell lines were derived from women with uterine cervical
adenocarcinoma, and were provided by the RIKEN BioResource Center
(Tsukuba, Japan) through the National Bio-Resource Project of the
Japanese Ministry of Education, Culture, Sports, Science and
Technology (MEXT). In vitro experiments were conducted with
subconfluent cells.
Drugs and reagents
Anti-Src (cat. no. 2109), anti-phospho (p)-Akt (cat.
no. 9271S), anti-p-p44/42 MAPK (Erk1/2) (cat. no. 9101) and
anti-β-actin (cat. no. 4967) antibodies for western blotting were
all purchased from Cell Signaling Technology, Inc. (Danvers, MA,
USA). Anti-p-Src Y419 (cat. no. AF2685) was purchased from R&D
Systems, Inc. (Minneapolis, MN, USA). Dasatinib (Focus
Biomolecules, Plymouth Meeting, PA, USA) was prepared as a
10-mmol/l stock solution in dimethyl sulfoxide (DMSO). Paclitaxel
was purchased from Bristol-Myers Squibb (New York, NY, USA) and
oxaliplatin was purchased from AdooQ Bioscience (Irvine, CA,
USA).
Cytotoxicity assays
The cytotoxicity of paclitaxel, oxaliplatin and the
combination of each of these drugs with dasatinib in HeLa and TCO-2
cells was examined using the Cell Proliferation reagent WST-1
(Roche Applied Science, Penzberg, Germany) according to the
manufacturer's instructions. The cells were seeded in 96-well
plates (5,000 cells/well) with serum-containing medium and
incubated for 24 h. The medium was then replaced with 100 µl fresh
serum free medium containing various concentrations of drug:
Paclitaxel (0, 0.0005, 0.001, 0.005, 0.01, 0.05, 1 and 10 µg/ml),
oxaliplatin (0, 0.1, 0.5, 1, 5, 10, 50, 100 and 500 µg/ml), and
dasatinib (0, 0.0001, 0.001, 0.01, 0.1, 1, 10 and 100 µM).
Treatment was stopped at 48 h, and the WST-1 reagent was added to
each well. Following a 2-h incubation period at 37°C and 5%
CO2, the absorbance at 450 nm of the samples against a
background control as blank was measured using a microplate reader
(Thermo Fisher Varioskan Flash; Thermo Fisher Scientific, Inc.).
Each sample was analyzed in triplicate.
Apoptosis assay
For evaluation of apoptosis, caspase-3/7 activity
was measured. The cells were plated at 5,000 cells/well in black
96-well plates and incubated for 24 h. The cells were then treated
with either paclitaxel (0.005 µg/ml) or oxaliplatin (5 µg/ml) in
the presence or absence of dasatinib (10 µM) for 48 h. Following
the treatment, 100 µl reagent from the Apo-ONE Homogeneous
Caspase-3/7 assay kit (Promega Corporation, Fitchburg, WI, USA) was
added to each well, which contained 100 µl blank, control or
treated cells in culture. The plates were gently mixed with a plate
shaker at 500 rpm for 30 sec. The fluorescence of each well was
measured using a microplate reader (Thermo Fisher Varioskan Flash)
with a 480/520 excitation/emission filter and a gain setting of
25.
Western blotting
Cells were harvested to 80% confluence, and
dasatinib and anticancer agents were added as indicated. Cells were
lysed in M-PER Mammalian Protein Extraction reagent (Thermo Fisher
Scientific, Inc.). Protein concentrations were measured with a
Pierce BCA Protein Assay kit (Thermo Fisher Scientific, Inc.). The
total protein (10 µg) was separated by 7.5% SDS-PAGE and
transferred electrophoretically onto a polyvinylidene difluoride
membrane. The blots were blocked for 3 h at room temperature with
3% bovine serum albumin (Wako Pure Chemical Industries, Ltd.) and
then probed with the aforementioned primary antibodies in TBST
(1:1,000) at 4°C overnight. Secondary antibody binding was
conducted by incubating the blots with peroxidase-conjugated
AffiniPure Goat Anti-Rabbit IgG (H+L) from Jackson ImmunoResearch
Laboratories, Inc. (West Grove, PA, USA) at a dilution of 1:10,000
for 1 h at room temperature. Proteins were visualized by incubation
with ECL Prime Western Blotting Detection reagent (GE Healthcare,
Little Chalfont, UK) and detected with an image analyzer (LAS
Amersham Imager 600; GE Healthcare). Anti-β-actin antibody was used
as a positive control. Densitometry was performed to interpret
differences in the results using ImageJ software version 1.51
(National Institutes of Health; Bethesda, MD, USA).
Statistical analysis
Statistical analyses were performed using SPSS
software, version 20 (IBM Corp., Armonk, NY, USA). Inhibition of
cell growth by anticancer agents and/or dasatinib was evaluated
using Dunnett's test. One-way analysis of variance with
Bonferroni's adjustment was used for the caspase-3/7 assay. DMSO
was used for comparison as a negative control. All experiments were
performed in triplicate. All P-values were two-sided, and P<0.05
was considered to indicate a statistically significant difference
in all statistical analyses.
Results
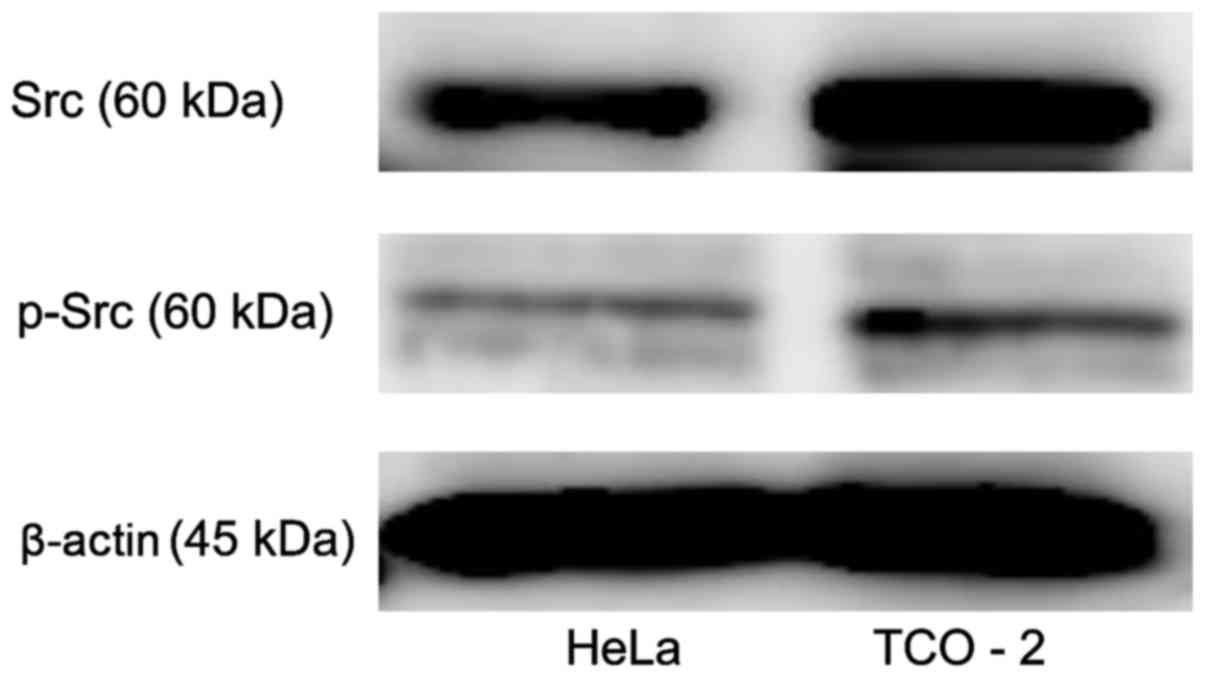
Src and p-Src protein expression in
cervical adenocarcinoma cell lines
Src kinase protein expression was evaluated in two
cervical adenocarcinoma cell lines using western blot analysis
(Fig. 1). The two cell lines clearly
exhibited Src and p-Src expression. The TCO-2 cell line exhibited
higher expression levels of Src and p-Src than did the HeLa cell
line.
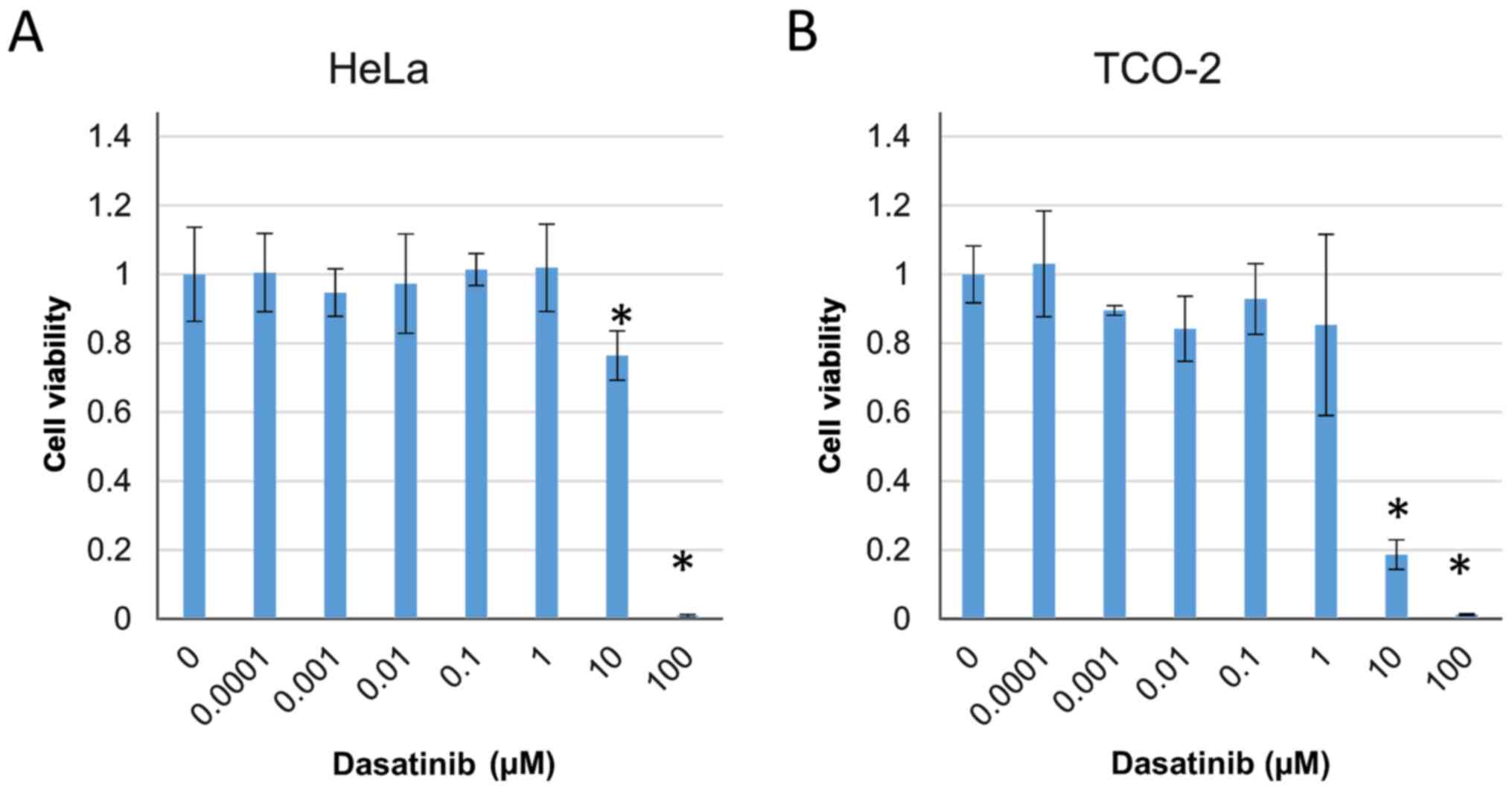
Combinations of dasatinib and
anticancer drugs significantly suppress the proliferation of
cervical adenocarcinoma cells
The cytotoxic effect of dasatinib was investigated
in HeLa and TCO-2 cells using a WST-1 assay. The effect of
treatment with various concentrations of dasatinib (0, 0.0001,
0.001, 0.01, 0.1, 1, 10 and 100 µM) on cell survival in the two
cell lines was evaluated. Cell proliferation was significantly
suppressed by treatment with ≥10 µM dasatinib compared with that in
the control group treated with DMSO only (Fig. 2).
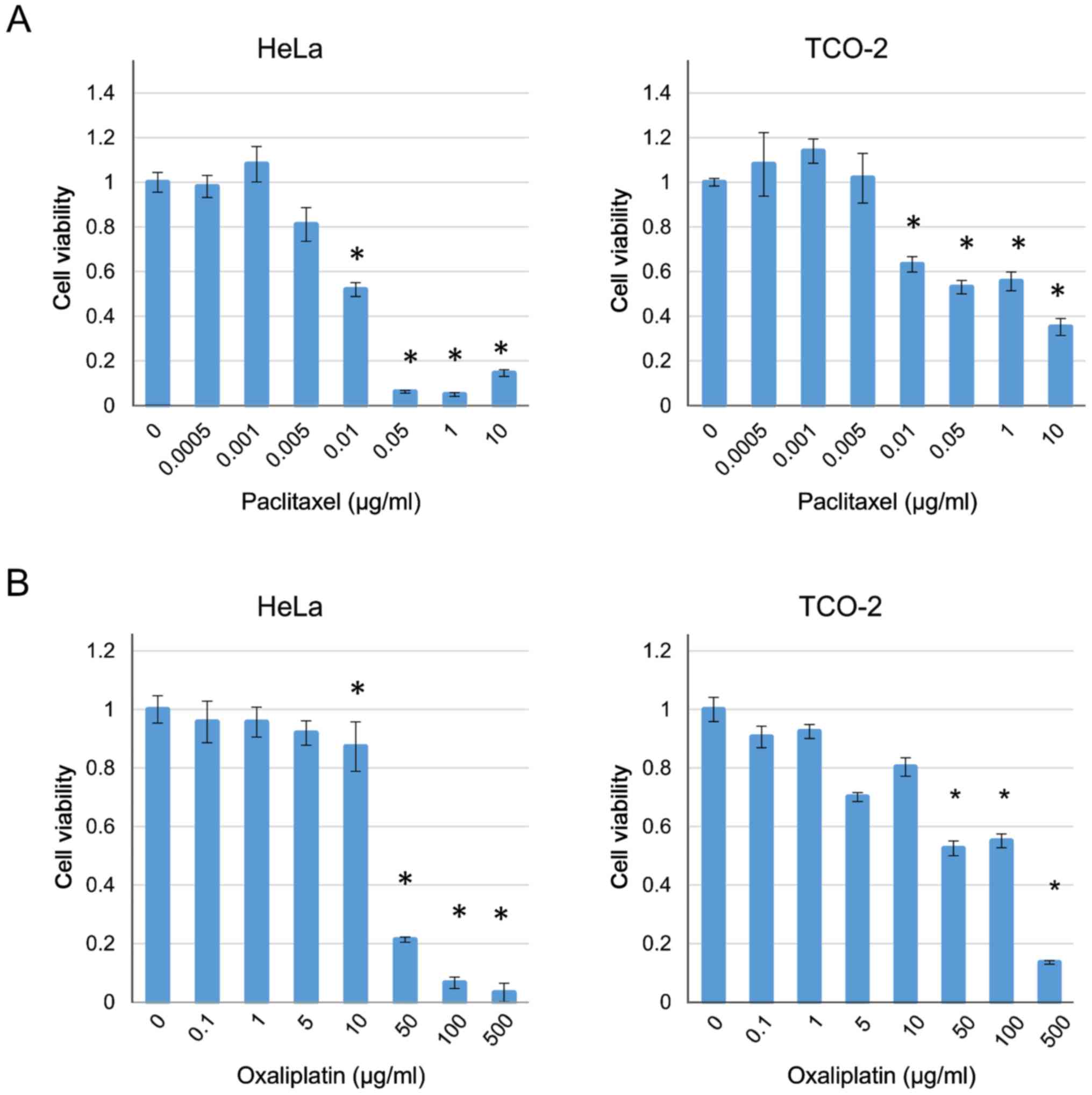
The cytotoxicity of paclitaxel and oxaliplatin was
also investigated in the HeLa and TCO-2 cell lines. Treatment with
each drug suppressed cell growth in a dose-dependent manner
(Fig. 3). Based on these results,
the concentrations of paclitaxel and oxaliplatin were fixed
(paclitaxel 0.005 µg/ml, oxaliplatin 5 µg/ml) as they were the
highest concentrations that did not have a cytotoxic effect, and
various concentrations of dasatinib were used with each of the two
anticancer drugs. In all cases, when compared with the group
receiving the anticancer drug alone (control), a significant
difference was observed with the use of ≥10 µM dasatinib (Fig. 4). Therefore, a decision was made to
culture the cells with 10 µM dasatinib and collect the protein for
analysis.
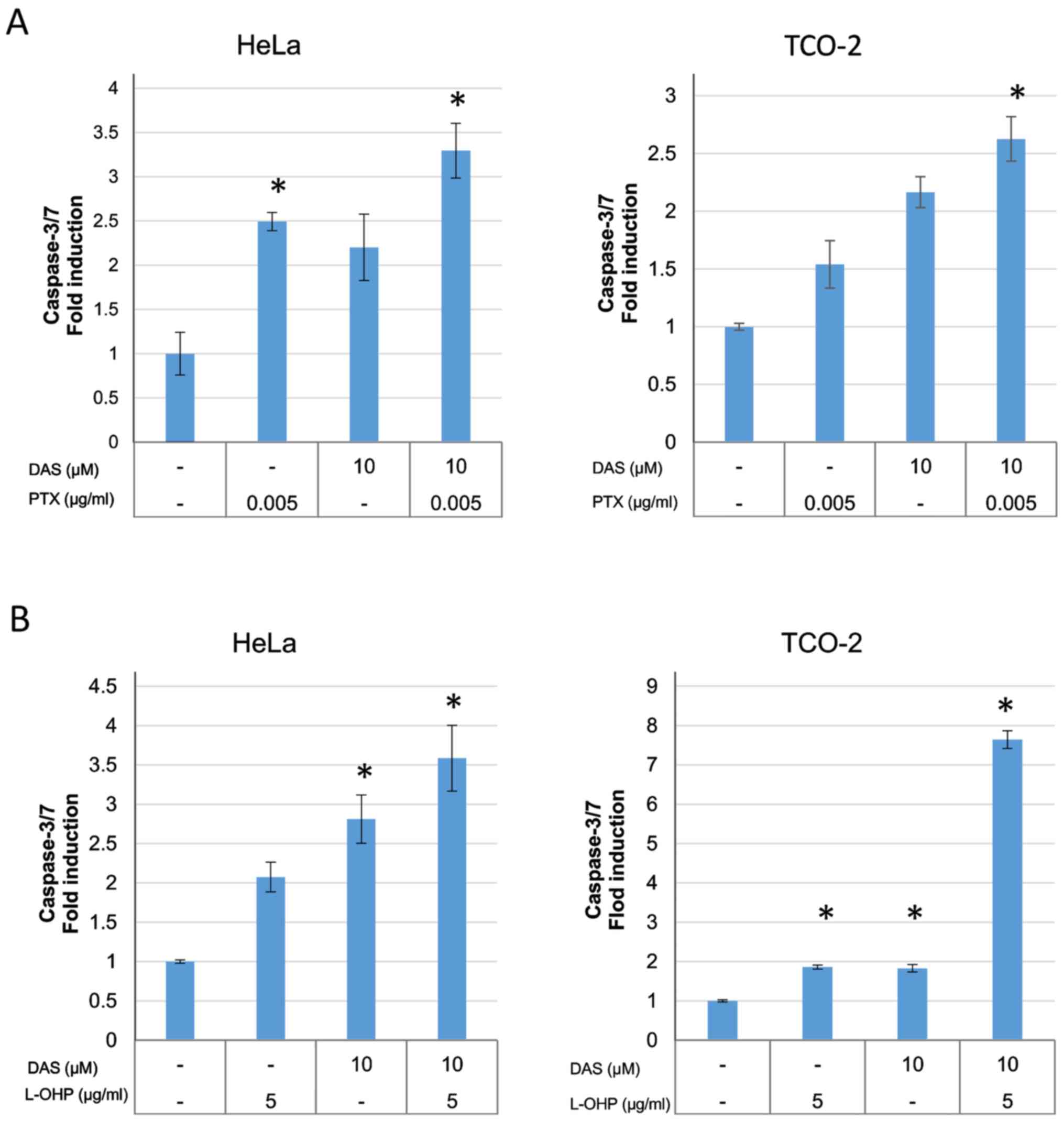
Combination of dasatinib with
anticancer agents causes apoptosis in HeLa and TCO-2 cells
The activity of caspase-3/7 was measured using a
caspase-3/7 assay kit in order to investigate the apoptosis
associated with combination treatment (Fig. 5). The anticancer drug alone,
dasatinib alone, and the anticancer drug plus dasatinib combination
groups were compared to determine whether there were differences
between the groups. The induction of caspase-3/7 was significantly
increased in the combination group when compared with control group
(DMSO only), suggesting the induction of cell death by
apoptosis.
Combination of dasatinib with
anticancer agents inhibits the level of p-Src expression and
downstream signal transduction
Src signaling was investigated, with the use of
western blot analysis to evaluate p-Src, p-Akt, and p-Erk
expression (Fig. 6). When dasatinib
was used in combination with an anticancer agent, the Src
phosphorylation was suppressed, and phosphorylation in the
downstream Akt and MAPK pathways was also inhibited (4).
Discussion
The purpose of this study was to confirm Src
expression in human cervical adenocarcinoma cell lines and to
determine the mechanism underlying the inhibition of Src signaling
by dasatinib at the cellular and protein levels. The present
authors performed an immunohistochemical analysis to reveal Src
expression in human adenocarcinoma cells. A total of 49 specimens
were obtained from patients who underwent surgery between 2001 and
2011 at the Tokushima University Hospital (Tokushima, Japan). In
the normal cervical gland tissue, Src expression was not observed
(0/12). In the invasive cervical adenocarcinoma tissue, the
expression rate of Src was 44% (16/36), which was significantly
higher than that in the normal cervical gland tissue (data not
shown). This demonstrated that Src was expressed in human
adenocarcinoma cell lines. In HeLa and TCO-2 cell lines, cell
proliferation was significantly suppressed and apoptosis was
significantly induced by dasatinib in combination with paclitaxel
or oxaliplatin in the present study. Dasatinib in combination with
paclitaxel or oxaliplatin suppressed Src phosphorylation and also
suppressed downstream signaling via the Akt and MAPK pathways, as
determined by western blot analysis. These results support the
hypothesis that Src is expressed in cervical adenocarcinoma and
that antitumor drugs become more effective when Src signaling is
inhibited.
Uterine cervical carcinoma is one of the most
serious diseases worldwide, and surgery, chemotherapy and
radiotherapy have been used to treat it (2,4).
Patients in the advanced stage in various solid tumors show
resistance to treatment, and a number of studies have investigated
novel antitumor agents, with a particular focus on molecularly
targeted drugs. In recent years, Src inhibitors have been studied
extensively (8–17).
Clinical trials have revealed no effect of dasatinib
alone on breast, pancreatic, colorectal and ovarian tumor
progression, overall survival or progression-free survival
(8,12,13,15).
This may be due to the activation of other signaling molecules,
such as the Src family kinases Janus kinase and FGR (14). However, combination therapy with
dasatinib and anticancer drugs has been found to be effective for
inhibiting tumor growth (9–11,14,16,17).
These results are consistent with a number of in vitro
experiments (18–22) and the present study.
Mucinous ovarian carcinoma is resistant to
oxaliplatin; however, this resistance was diminished when dasatinib
was used in combination with oxaliplatin (17). Combination treatment with dasatinib
and paclitaxel has a synergistic effect against cancer through Src
signaling (19). The Src inhibitor
PP2 has been shown to inhibit cervical cancer cell proliferation
via the downregulation of p-Src (23), and p-Src is a predictor of relapse of
squamous cell carcinoma (24).
However, to the best of our knowledge, the effect of Src inhibitors
on adenocarcinoma of the uterine cervix has not been reported, and
the present study is the first of its kind to delineate Src
expression in cervical adenocarcinoma.
In HeLa and TCO-2 cell lines, cell proliferation was
significantly suppressed by ≥10 µM dasatinib alone. When dasatinib
was combined with a non-cytotoxic concentration of paclitaxel or
oxaliplatin, ≥1 µM dasatinib significant suppressed cell
proliferation in the majority of cases. This indicates that
combining dasatinib with an anticancer drug enhances the
suppression of cell growth.
In the present study, western blotting results
indicated that not all the phosphorylated proteins tested (p-Src,
p-Akt and p-Erk) were activated by paclitaxel or oxaliplatin alone
compared with controls. However, the anticancer agents alone also
did not reliably suppress the activation of phosphorylated
proteins, suggesting that they alone do not necessarily increase
resistance. Cervical adenocarcinoma cells may respond in a
different manner than do mucinous ovarian cancer cells. Although
the concentrations of dasatinib used in the experiments in the
present study were not sufficient to suppress the expression of the
phosphorylated proteins, suppression was observed when dasatinib
was used in combination with an anticancer agent compared with the
control. The results indicate that Src signaling may be suppressed
by combining an anticancer agent with dasatinib. However, the
effect of the suppression of Src in vivo is unclear. Further
studies are required to identify the mechanism underlying the
effects of dasatinib in animal models.
In conclusion, Src is expressed in cervical
adenocarcinoma cell lines. Dasatinib inhibits intracellular Src
signaling and causes apoptosis. The results of the present study
suggest that anti-Src molecularly targeted therapy may be used as a
novel treatment strategy for cervical adenocarcinoma.
Acknowledgements
The authors thank Dr. Takeshi Iwasa for useful
discussions and advice. The present study was supported by the
Support Center for Advanced Medical Sciences, Institute of
Biomedical Sciences, Tokushima University Graduate School.
References
|
1
|
The Editorial Board of the Cancer
Statistics in Japan: Cancer Statistics in Japan. 2014.
|
|
2
|
Fujiwara K, Monk B and
Devouassoux-Shisheboran M: Adenocarcinoma of the uterine cervix:
Why is it different? Curr Oncol Rep. 16:4162014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
NCNN Guideline: NCCN Clinical Practice
Guidelines in Oncology, cervical cancer. 2016.
|
|
4
|
Gien LT, Beauchemin MC and Thomas G:
Adenocarcinoma: A unique cervical cancer. Gynecol Oncol.
116:140–146. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wheeler DL, Iida M and Dunn EF: The role
of Src in solid tumors. Oncologist. 14:667–678. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Summy JM and Gallick GE: Treatment for
advanced tumors: SRC reclaims center stage. Clin Cancer Res.
12:1398–1401. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Le XF and Bast RC Jr: Src family kinases
and paclitaxel sensitivity. Cancer Biol Ther. 12:260–269. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schott AF, Barlow WE, Van Poznak CH, Hayes
DF, Moinpour CM, Lew DL, Dy PA, Keller ET, Keller JM and Hortobagyi
GN: Phase II studies of two different schedules of dasatinib in
bone metastasis predominant metastatic breast cancer: SWOG S0622.
Breast Cancer Res Treat. 159:87–95. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fornier MN, Morris PG, Abbruzzi A,
D'Andrea G, Gilewski T, Bromberg J, Dang C, Dickler M, Modi S,
Seidman AG, et al: A phase I study of dasatinib and weekly
paclitaxel for metastatic breast cancer. Ann Oncol. 22:2575–2581.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Araujo JC, Mathew P, Armstrong AJ, Braud
EL, Posadas E, Lonberg M, Gallick GE, Trudel GC, Paliwal P, Agrawal
S and Logothetis CJ: Dasatinib combined with docetaxel for
castration-resistant prostate cancer: Results from a phase 1–2
study. Cancer. 118:63–71. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Araujo JC, Trudel GC, Saad F, Armstrong
AJ, Yu EY, Bellmunt J, Wilding G, McCaffrey J, Serrano SV, Matveev
VB, et al: Docetaxel and dasatinib or placebo in men with
metastatic castration-resistant prostate cancer (READY): A
randomised, double-blind phase 3 trial. Lancet Oncol. 14:1307–1316.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chee CE, Krishnamurthi S, Nock CJ, Meropol
NJ, Gibbons J, Fu P, Bokar J, Teston L, O'Brien T, Gudena J, et al:
Phase II study of dasatinib (BMS-354825) in patients with
metastatic adenocarcinoma of the pancreas. Oncologist.
18:1091–1092. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sharma MR, Wroblewski K, Polite BN, Knost
JA, Wallace JA, Modi S, Sleckman BG, Taber D, Vokes EE, Stadler WM
and Kindler HL: Dasatinib in previously treated metastatic
colorectal cancer: A phase II trial of the University of Chicago
Phase II Consortium. Invest New Drugs. 30:1211–1215. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gold K, Lee J, Harun N, Tang X, Price J,
Kawedia JD, Tran HT, Erasmus JJ, Blumenschein GR and William WN: A
phase I/II study combining erlotinib and dasatinib for non-small
cell lung cancer. Oncologist. 19:1040–1041. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schilder RJ, Brady WE, Lankes HA, Fiorica
JV, Shahin MS, Zhou XC, Mannel R, Pathak HB, Hu W, Alpaugh K, et
al: Phase II evaluation of dasatinib in the treatment of recurrent
or persistent epithelial ovarian or primary peritoneal carcinoma: A
gynecologic oncology group study. Gynecol Oncol. 127:70–74. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Secord AA, Teoh DK, Barry WT, Yu M,
Broadwater G, Havrilesky LJ, Lee PS, Berchuck A, Lancaster J and
Wenham RM: A phase I trial of dasatinib, a Src-family kinase
inhibitor, in combination with paclitaxel and carboplatin in
patients with advanced or recurrent ovarian cancer. Clin Cancer
Res. 18:5489–5498. 2013. View Article : Google Scholar
|
|
17
|
Hong DS, Choe DH, Naing A, Wheler JJ,
Falchook GS, Piha-Paul S, Moulder SL, George GC, Choe JM, Strauss
LC, et al: A phase 1 study of gemcitabine combined with dasatinib
in patients with advanced solid tumors. Invest New Drugs.
31:918–926. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matsuo K, Nishimura M, Bottsford-Miller
JN, Huang J, Komurov K, Armaiz-Pena GN, Shahzad MM, Stone RL, Roh
JW, Sanguino AM, et al: Targeting Src in mucinous ovarian
carcinoma. Clin Cancer Res. 17:5367–5378. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pengetnze Y, Steed M, Roby KF, Terranova
PF and Taylor CC: Src tyrosine kinase promotes survival and
resistance to chemotherapeutics in a mouse ovarian cancer cell
line. Biochem Biophys Res Commun. 309:377–383. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xiao J, Xu M, Hou T, Huang Y, Yang C and
Li J: Dasatinib enhances antitumor activity of paclitaxel in
ovarian cancer through Src signaling. Mol Med Rep. 12:3249–3256.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Teoh D, Ayeni TA, Rubatt JM, Adams DJ,
Grace L, Starr MD, Barry WT, Berchuck A, Murphy SK and Secord AA:
Dasatinib (BMS-35482) has synergistic activity with paclitaxel and
carboplatin in ovarian cancer cells. Gynecol Oncol. 121:187–192.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu T, Hu W, Dalton HJ, Choi HJ, Huang J,
Kang Y, Pradeep S, Miyake T, Song JH, Wen Y, et al: Targeting Src
and tubulin in mucinous ovarian carcinoma. Clin Cancer Res.
19:6532–6543. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kong L, Deng Z, Zhao Y, Wang Y, Sarkar FH
and Zhan Y: Down-regulation of phospho-non-receptor Src tyrosine
kinases contributes to growth inhibition of cervical cancer cells.
Med Oncol. 28:1495–1506. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kong L, Deng Z, Shen H and Zhang Y: Src
family kinase inhibitor PP2 efficiently inhibits cervical cancer
cell proliferation through down-regulating phospho-Src-Y416 and
phospho-EGFR-Y1173. Mol Cell Biochem. 348:11–19. 2011. View Article : Google Scholar : PubMed/NCBI
|