Introduction
Acute kidney injury (AKI), a syndrome associated
with high post-operative mortality in humans, is one of the most
serious complications of cardiac surgery (1,2). The
incidence of AKI due to coronary revascularization or shock has
been increasing worldwide. Clinical and experimental studies have
demonstrated that myocardial ischemia-reperfusion (MIR) causes AKI
(2,3). The mechanism underlying the decline in
renal function during MIR was thought to be multifactorial in
origin, and associated with hypoperfusion, loss of pulsatile
perfusion, hemolysis and a systemic inflammatory response. These
factors may increase reactive oxygen species (ROS) (4). In endogenous or exogenous renal injury
such as MIR, excessively produced ROS may cause AKI (5). However, the mechanism underlying this
damage in the kidney during MIR has remained to be fully
elucidated.
Diabetes is one of the most frequent co-morbidities
in patients undergoing cardiac surgery and is an independent
predictor of AKI in patients with normal pre-operative kidney
function (6). Furthermore,
insulin-requiring diabetes is also an independent predictor of AKI
after cardiac transplantation (7).
Clinical studies have demonstrated that hyperglycemia is associated
with a significantly higher incidence of AKI. Insulin should be
administered if glucose levels are increased (8,9).
Intraoperative hyperglycemia should be avoided, as it may lead to
excessive production of ROS, which prominently occurs in the kidney
and causes acute or progressive renal damage (10).
Attenuation of oxidative stress is known to have an
important role in the renoprotective effect mediated by a variety
of treatment interventions (11).
Previous studies by our group have indicated that DJ-1, which is a
multifunctional protein that has potent anti-oxidant properties,
protected against oxidative injury in diabetic kidneys and in MIR
(12,13). Another study demonstrated that DJ-1
protein was required for the stabilization of nuclear factor
erythroid 2-related factor-2 (Nrf2), which has a central role in
the protective effect against oxidative injury, and subsequent
production of Nrf2-regulated enzymes (14). Hence, it was of interest to
investigate the role of the DJ-1/Nrf2 pathway in AKI induced by MIR
in diabetic rats.
Materials and methods
Materials
A total of 40 male Sprague Dawley rats (weight,
250–300 g; age, 9–12 weeks) were purchased from Wuhan University,
Animal Biosafety Level 3 Laboratory (Wuhan, China). All rats were
housed under standard laboratory conditions at 22–24°C, a relative
humidity of 50±15%, and a 12 h light/dark cycle. All animals had
free access to standard rat chow and water. The experimental
protocol of the present study was reviewed and approved by the
Animal Care and Use Committee of Wuhan University (Wuhan, China),
and was in accordance with the Guide for the Care and Use of
Laboratory Animals by the National Institutes of Health.
Streptozotocin (STZ) was purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). Antibodies against DJ-1 were purchased from
Cell Signaling Technology, Inc. (cat no. 5933; Beverly, MA, USA).
Antibodies to Nrf2 were purchased from Santa Cruz Biotechnology,
Inc. (cat no. sc722; Dallas, TX, USA). GAPDH and Lamin B were
purchased from Cell Signaling Technology, Inc. (cat nos. 5174 and
13435), and secondary antibodies were purchased from Santa Cruz
Biotechnology, Inc. (cat no. sc2357). All other chemicals were
obtained from commercial sources and were of the highest grade
available.
Induction of diabetes
Experimental diabetes was induced in the animals by
a single intraperitoneal administration of STZ dissolved in 0.1
mol/l citrate buffer (pH 4.5) at a dose of 65 mg/kg. Normal rats
received an equal volume of citrate buffer. Three days post-STZ
injection, tail vein blood samples were collected and glucose
levels were measured with a Onetouch glucometer (Johnson &
Johnson, New Brunswick, NJ, USA). The rats were considered diabetic
and used for the study only if their glucose levels were >15
mmol/l (15). Rats were maintained
for 8 weeks after vehicle or STZ injection.
Surgical preparation
Animals were intraperitoneally anesthetized with
pentobarbital sodium (50 mg/kg) (16) followed by a tracheotomy and
artificial ventilation. Blood pressure was recorded from the left
femoral artery using a pressure transducer with the heart rate
monitored by an electrocardiogram throughout the procedure. The
left femoral vein was cannulated for administration of drugs. A
fourth-intercostal space thoracotomy was performed and the
pericardium was excised to expose the heart. The left anterior
descending coronary artery (LAD) was ligated 2 mm above the left
auricle by a 6–0 silk suture. A small polypropylene tube was placed
between the ligature and the LAD. The artery was occluded for 30
min by tightening the ligature. After ischemia for 30 min, the
ligature was loosened to allow reperfusion for 2 h. The sham group
underwent the same surgical procedures, apart from tying the 6–0
silk suture. After 2 h of reperfusion, the rats were sacrificed,
and renal tissues and blood samples were obtained for further
analysis.
Experimental protocol
Rats were randomly assigned into one of four
experimental groups (n=8 per group) as follows: i) Sham-operated
group (sham group), non-diabetic rats that underwent isolation of
the LAD without occlusion as a control; ii) MIR group, non-diabetic
rats subjected to 30 min of myocardial ischemia and 2 h of
reperfusion after the LAD had been isolated and occluded (sham +
MIR group); iii) diabetic rats that underwent isolation of the LAD
without occlusion (diabetic group); iv) diabetic rats subjected to
30 min of myocardial ischemia and 2 h of reperfusion after the LAD
had been isolated and occluded (diabetic + MIR group).
Measurement of serum glycosylated
hemoglobin (HbA1c), creatine kinase isoenzyme MB (CK-MB) and
lactate dehydrogenase (LDH) levels
Blood samples were collected at the end of
reperfusion and centrifuged at 1,000 × g for 10 min at 4°C. Serum
was separated and stored at −20°C. HbA1c was measured using an
Olympus automatic analyzer (cat no. AU5400; Olympus Corporation,
Tokyo, Japan). CK-MB and LDH levels were measured using commercial
kits (cat nos. 01010605082 and 01010113477; Beijing Kemeidongya
Biotechnology Ltd., Beijing, China) according to the manufacturer's
instructions.
Renal histopathological
assessment
The left kidney was cut into sections and fixed in
4% formaldehyde. After embedding in paraffin, 4-µm sections were
stained with hematoxylin at 37°C for 3 min and eosin at 37°C for 10
sec for light microscopy (original magnification, ×200; Olympus
BX50; Olympus Corporation).
Histological assessment of tubular necrosis was
semi-quantitatively determined using a method modified from
McWhinnie et al (17) using
the following scoring system: 0, normal histology; 1, tubular cell
swelling, brush border loss and nuclear condensation, with up to
one-third of the tubular profile exhibiting nuclear loss; 2, same
as for score 1, but more than one-third and less than two-thirds of
the tubular profile displaying nuclear loss; and 3, same as for
score 1, but more than two-thirds of the tubular profile exhibiting
nuclear loss.
Measurement of serum cystatin C (Cys
C) and β2-microglobulin (β2-MG) levels
Blood samples were collected at the end of
reperfusion and centrifuged at 3,000 rpm, for 10 min at 4°C. Serum
was separated and stored at −20°C. Cys C and β2-MG levels were
measured using ELISA assay kits (cat nos. E-EL-R783 and
E-EL-R1085c; Elabscience Biotechnology Co., Ltd., Wuhan, China)
according to the manufacturer's instructions.
Assessment of total antioxidative
capacity (T-AOC) and malondialdehyde (MDA) in renal tissues
The renal tissues were harvested and immediately
homogenized on ice in 5 volumes of normal saline. The homogenates
were centrifuged at 1,200 × g for 10 min. The T-AOC and MDA levels
in the supernatant were measured using T-AOC and MDA assay kits
(cat nos. A015-2 and a003-2; Nanjing Jiancheng Bioengineering
Institute, Nanjing, China) according to the manufacturer's
instructions.
Immunohistochemical assessment of
renal DJ-1 and Nrf2
Paraffin-embedded renal sections were stained using
the streptavidin-biotin complex (streptavidin peroxidase, SP)
immunohistochemistry technique for DJ-1 and Nrf2 detection using SP
immunohistochemistry kits (cat no. I003-2; Nanjing Jiancheng
Bioengineering Institute) according to the manufacturer's protocol.
Brown staining in the cytoplasm and/or nucleus was considered an
indicator of positive expression. The results were
semi-quantitatively evaluated with Image-Pro® Plus
version 6.0 software (Media Cybernetics, Rockville, MD, USA)
according to optical density values of positive staining.
Western blot analysis
Cytoplasmic and nuclear proteins were extracted from
frozen renal tissues with a nuclear extraction kit (cat no. P0027;
Beyotime Institute of Biotechnology, Haimen, China) according to
the manufacturer's instructions. GAPDH was used as internal control
of cytoplasmic protein and Lamin B was used as an internal control
of nuclear protein. A BCA protein assay kit was used to determine
the protein concentration (cat no. P0012S; Beyotime Institute of
Biotechnology). Equal amounts of protein (30 µg) were separated by
12% SDS-PAGE at 100 V for 3 h. After electrophoresis, proteins were
transferred onto polyvinylidene difluoride membranes at 200 mA for
2 h. The transferred membranes were incubated overnight at 4°C with
rabbit anti-mouse polyclonal antibodies for DJ-1 and Nrf2 (each at
1:800 dilution) in Tris-buffered saline containing Tween-20 (TBS-T)
containing 5% skimmed milk. After washing three times in TBS-T,
membranes were incubated with anti-rabbit immunoglobulin G
conjugated to horseradish peroxidase at a dilution of 1:2,000 in
TBS-T containing 5% skimmed milk for 2 h at room temperature. The
immunoreactive bands were visualized by enhanced chemiluminescence
(PerkinElmer, Inc., Waltham, MA, USA) and captured on X-ray film.
The optical density of the bands was measured with a BandScan V4.0
imaging analysis system (http://bandscan.software.informer.com/).
Statistical analysis
Mean ± standard error of the mean values were
calculated to summarize all outcome measurements. Statistical
analysis was performed using GraphPad Prism software version 5.0
(GraphPad Software, Inc., La Jolla, CA, USA). The statistical
significance of the differences between groups was evaluated using
unpaired Student's t-tests for pair-wise comparisons, or a two-way
analysis of variance followed by the Tukey post hoc test for
multiple comparisons. The level of significance was set at
P<0.05 for all statistical tests.
Results
Body weight, kidney weight, fasting
blood glucose and glycosylated hemoglobin (HbA1c) content
At the end of the study, the body weight of diabetic
rats was significantly decreased as compared with that of normal
rats. The change of kidney weight was not obvious in diabetic rats
during the experimental period. The ratio of kidney weight to body
weight in diabetic rats was significantly increased as compared to
that in the normal rats. A significant increase in fasting blood
glucose in diabetic rats was observed when compared with that in
the control group. HbA1c was markedly higher in diabetic rats than
that in normal rats (Table I).
 | Table I.Effects of different time on body
weight, kidney weight, fasting blood glucose and HbA1c levels in
rats. |
Table I.
Effects of different time on body
weight, kidney weight, fasting blood glucose and HbA1c levels in
rats.
| Parameter | Sham | Sham + MIR | Diabetic | Diabetes + MIR |
|---|
| Body weight (g) |
275.77±12.02 |
262.83±14.82 |
179.78±14.52a,b |
176.93±12.77a,b |
| Kidney weight
(g) |
1.03±0.05 |
0.95±0.13 |
1.01±0.12 |
1.03±0.87 |
| Kidney/body |
|
|
|
|
| weight ratio
(×10−3) |
3.72±0.13 |
3.78±0.82 |
5.64±0.82a,b |
5.32±0.64a,b |
| Glucose (mg/dl) |
7.69±0.84 |
8.65±1.59 |
25.88±2.52a |
26.15±1.52a |
| HbA1c (%) |
4.67±0.72 |
4.66±0.26 |
13.68±2.98a |
12.74±1.58a |
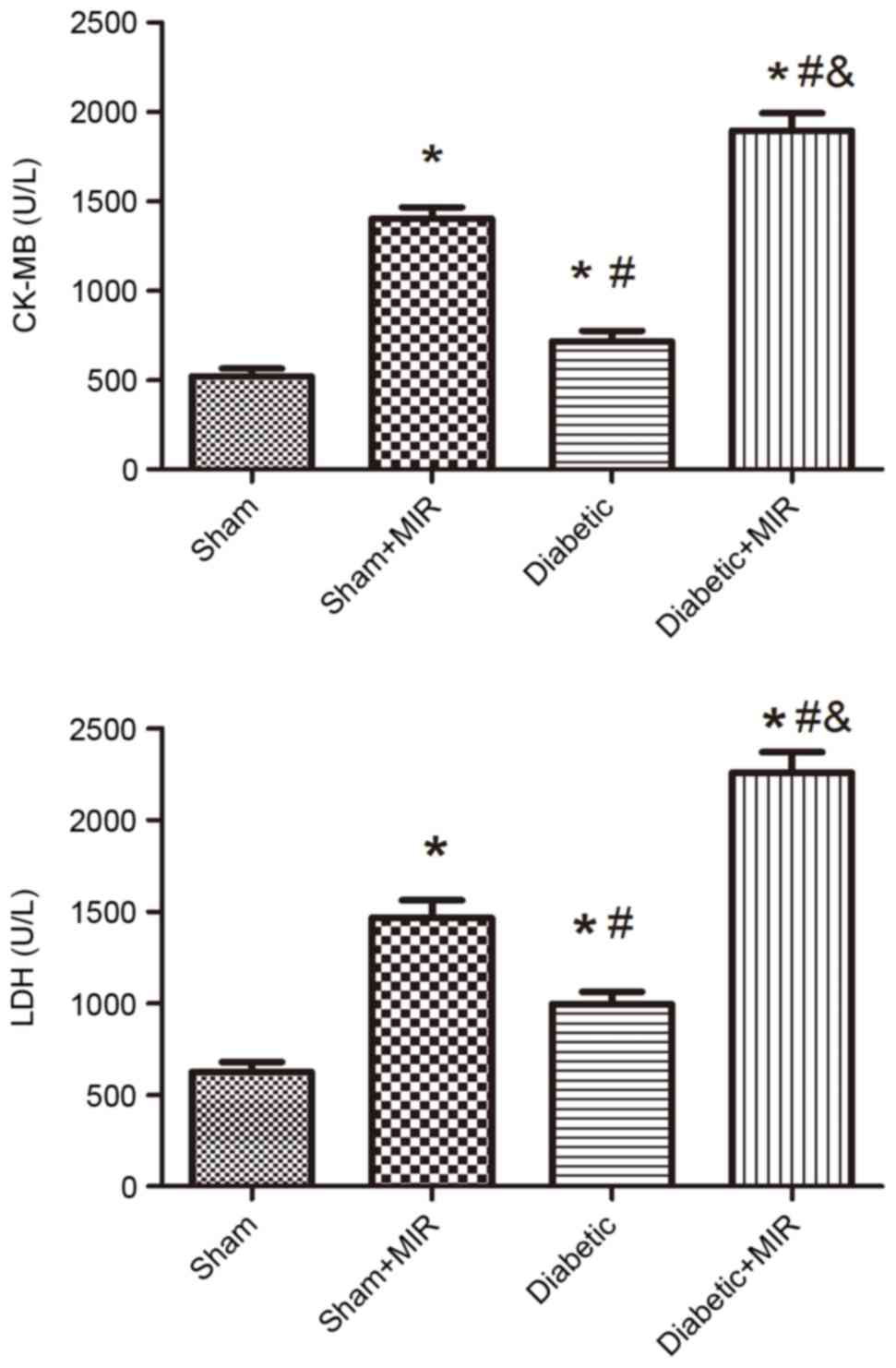
Serum CK-MB and LDH levels
To examine damage of cardiomyocytes induced by MIR,
the activities of the cardiac enzyme CK-MB and LDH as indices of
myocardial cellular injury were measured at the end of reperfusion
in the different groups. Compared with the sham group, MIR caused a
significant increase in LDH and CK-MB in the sham + MIR group, as
did the diabetic condition, but to a significantly lesser extent
(P<0.05). The damage evoked by MIR was further enhanced in the
diabetes + MIR group (P<0.05 vs. all other groups; Fig. 1).
Renal histopathology
As presented in Fig.
2, the renal tubules exhibited pathological changes, including
edema, necrosis and vacuolization in the sham + MIR and diabetic
groups. A significant aggravation of histological edema, necrosis
and vacuolization was seen in the diabetes + MIR group.
When compared with the renal histological evaluation
score of kidneys obtained from normal rats, the sham + MIR and the
diabetic groups exhibited in a significant increase in renal
histologic evaluation score individually (P<0.05). This increase
was significantly enhanced in the diabetes + MIR group (P<0.05
vs. all other groups).
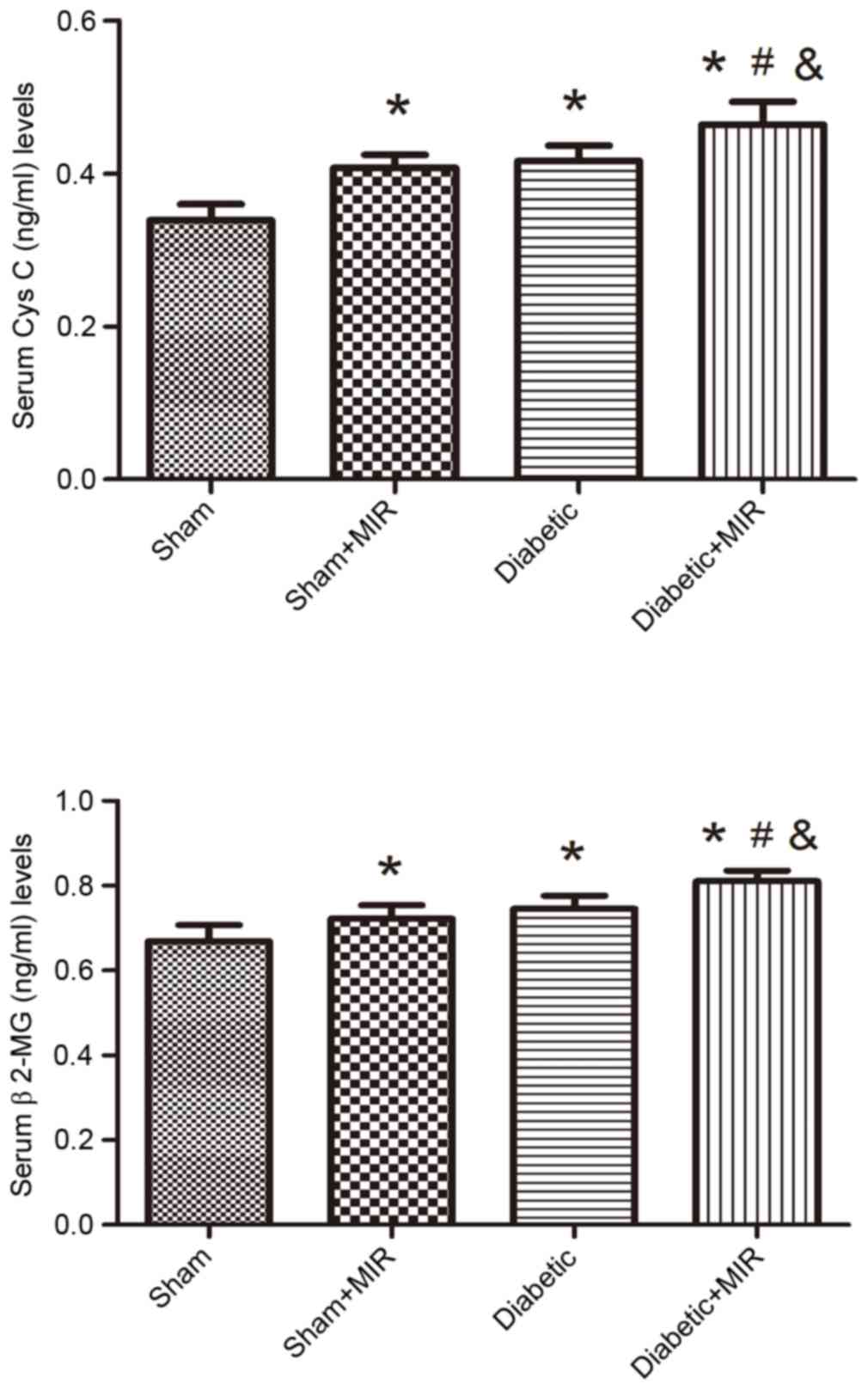
Serum Cys C and β2-MG levels
Serum Cys C and β2-MG levels were measured to
evaluate the renal injury induced by MIR in diabetic rats. MIR and
diabetes individually significantly increased serum Cys C and β2-MG
levels in comparison with those in the sham group, which was
further aggravated in the diabetes + MIR group (P<0.05; Fig. 3).
T-AOC and MDA levels in renal
tissues
As presented in Fig.
4, MIR and the diabetic condition significantly decreased the
T-AOC and significantly increased MDA levels in rats, compared with
those in the sham group, and these changes were significantly
aggravated in the diabetes + MIR group (P<0.05).
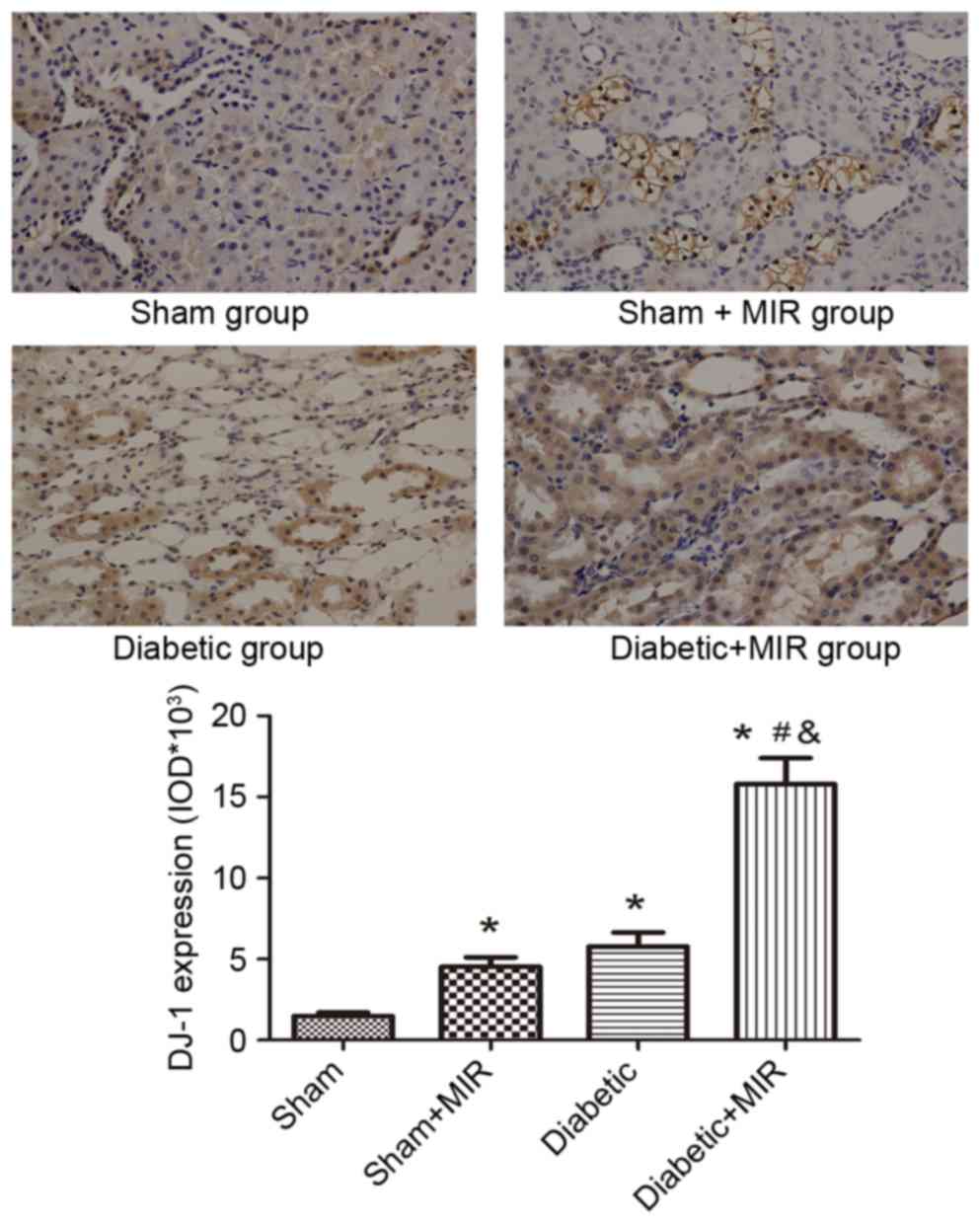
DJ-1 and Nrf2 expression in renal
tissues
Immunohistochemical analysis of the expression of
DJ-1 in the sham group revealed small brown granules in the
cytoplasm, while significant expression of DJ-1, as indicated by
brown staining in the cytoplasm, was observed in the diabetes + MIR
group (P<0.05 vs. sham group; Fig.
5). No significant difference was observed between Sham + MIR
and Diabetic groups (Fig. 5).
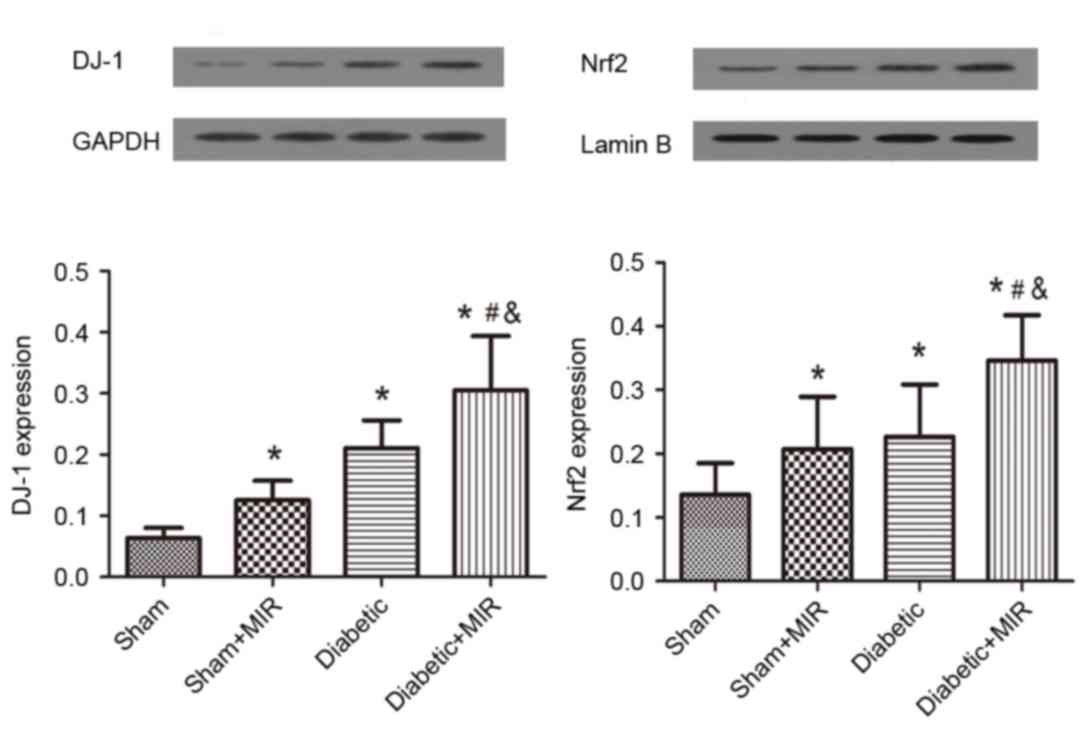
The expression of Nrf2 in the sham group displayed
as light brown immunostaining in the cytoplasm and no staining in
the nuclei. Significant expression of Nrf2, as indicated by strong
brown staining in the cytoplasm and nuclei, was observed in the
diabetes + MIR group (P<0.05; Fig.
6). No significant difference was observed between Sham + MIR
and Diabetic groups (Fig. 6).
In line with these results, western blot analysis
revealed weak expression of DJ-1 and Nrf2 in the kidneys of animals
of the sham group. By contrast, significant increases in the
protein expression of Nrf2 and DJ-1 were found in the diabetes +
MIR group (P<0.05; Fig. 7). No
significant difference was observed in Nrf2 expression between Sham
+ MIR and Diabetic groups (Fig. 7);
however, the expression of DJ-1 was significantly higher in the
diabetic group compared with the Sham + MIR group (P<0.05;
Fig. 7).
Discussion
Previous studies have revealed that acute myocardial
infarction with AKI was strongly associated with long-term
mortality, which has been increasing worldwide. However, at
present, no effective strategy is available to halt the acute
worsening of renal function in patients with decompensated heart
failure or those undergoing cardiac surgery, where even a small
change in renal function was associated with increased mortality
(18). Furthermore, diabetes
mellitus, one of the most common complications of patients
undergoing cardiac surgery and one of the major causes of
nephropathy, was found to be an independent predictor of AKI
(19). Great progress has been made
through the identification of Cys C and β2-MG as diagnostic markers
for kidney injury, even in the absence of a subsequent
manifestation of kidney dysfunction (20). In the present study, the damage of
cardiomyocytes induced by MIR was further enhanced in the diabetes
+ MIR group, as indicated by the increase of the cardiac enzyme
CK-MB and LDH as indices of myocardial cell injury. In line with
this, the increase in Cys C and β2-MG levels induced by MIR was
more pronounced in diabetic rats. In addition, the kidneys of
diabetic MIR rats exhibited glomerular hypertrophy and renal
tubular edema on histological analysis, and the renal histological
evaluation score was also increased. All of these results
demonstrated that the MIR-induced renal injury model presenting
with renal hypertrophy, renal tubular and glomerular damage was
successfully created, and that these pathologies were aggravated in
diabetic rats.
The mechanism underlying the renal injury during MIR
is thought to be multifactorial in origin, and associated with
hypoperfusion, loss of pulsatile perfusion, hemolysis and a
systemic inflammatory response. These factors may increase ROS
(21). Oxidative stress is becoming
increasingly recognized as an important causative factor of renal
damage induced by MIR (3,4). Diabetes may decrease the AOC and
increase oxidative stress, which induces the generation of ROS and
aggravates renal injury after cardiac surgery (22). T-AOC is an important marker of
oxidation, which mainly reflects non-enzymatic but includes the
activity of a minority of small molecular enzymatic systems
(23). MDA is a naturally occurring
product of lipid peroxidation and prostaglandin biosynthesis that
is mutagenic and carcinogenic (24).
The results of the present study demonstrated an increase of MDA
levels and a decrease of T-AOC levels in renal tissues following
MIR. Oxidative stress was aggravated in diabetic rats. These
findings demonstrated that a decreased anti-oxidant activity
already existed in renal injury induced by MIR and that the
anti-oxidant capacity was further weakened in diabetic rats.
Activation of DJ-1/Nrf2 has been established to have
an important protective role in oxidative stress (25,26). In
addition, a previous study by our group has found that the
renoprotective effects in diabetic rats may be enhanced by
activation of Nrf2 (12). Disruption
of DJ-1 decreased Nrf2 protein stability, whereas overexpression of
DJ-1 restored protein stability by decreasing ubiquitination of
Nrf2. DJ-1 protein was found to be required for the stabilization
of Nrf2 and cell survival by a variety of substrates (26). The results of the present study
demonstrated a significant increase in the expression of DJ-1 and
Nrf2 in the kidneys of rats following MIR. In diabetic rats, the
expression of DJ-1 and Nrf2 was further increased. All of the above
indicated that the DJ-1/Nrf2 signaling pathway has an important
role in AKI induced by MIR in diabetic rats.
In conclusion, the present study indicated that
MIR-induced renal dysfunction was exacerbated under diabetic
conditions and involved upregulation of the DJ-1/Nrf2 signaling
pathway. This may provide a novel therapeutic strategy for the
treatment of complications of cardiac surgery in diabetic
patients.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81501648).
References
|
1
|
Gallagher SM, Jones DA, Kapur A, Wragg A,
Harwood SM, Mathur R, Archbold RA, Uppal R and Yaqoob MM: Remote
ischemic preconditioning has a neutral effect on the incidence of
kidney injury after coronary artery bypass graft surgery. Kidney
Int. 87:473–481. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Choi YS, Shim JK, Kim JC, Kang KS, Seo YH,
Ahn KR and Kwak YL: Effect of remote ischemic preconditioning on
renal dysfunction after complex valvular heart surgery: A
randomized controlled trial. J Thorac Cardiovasc Surg. 142:148–154.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parlakpinar H, Ozer MK, Cicek E, Cigremis
Y, Vardi N and Acet A: Renal damage in rats induced by myocardial
ischemia/reperfusion: Role of nitric oxide. Int J Urol.
13:1327–1332. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ozer MK, Parlakpinar H, Vardi N, Cigremis
Y, Ucar M and Acet A: Myocardial ischemia/reperfusion-induced
oxidative renal damage in rats: Protection by caffeic acid
phenethyl ester (CAPE). Shock. 24:97–100. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen TH, Liao FT, Yang YC and Wang JJ:
Inhibition of inducible nitric oxide synthase ameliorates
myocardial ischemia/reperfusion injury-induced acute renal injury.
Transplant Proc. 46:1123–1126. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yi Q, Li K, Jian Z, Xiao YB, Chen L, Zhang
Y and Ma RY: Risk factors for acute kidney injury after
cardiovascular surgery: Evidence from 2,157 cases and 49,777
controls-a meta-analysis. Cardiorenal Med. 6:237–250. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hertzberg D, Sartipy U and Holzmann MJ:
Type 1 and type 2 diabetes mellitus and risk of acute kidney injury
after coronary artery bypass grafting. Am Heart J. 170:895–902.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mendez CE, Der Mesropian PJ, Mathew RO and
Slawski B: Hyperglycemia and acute kidney injury during the
perioperative period. Curr Diab Rep. 16:102016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fiaccadori E, Sabatino A, Morabito S,
Bozzoli L, Donadio C, Maggiore U and Regolisti G:
Hyper/hypoglycemia and acute kidney injury in critically ill
patients. Clin Nutr. 35:317–321. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cao A, Wang L, Chen X, Guo H, Chu S, Zhang
X and Peng W: Ursodeoxycholic acid ameliorated diabetic nephropathy
by attenuating hyperglycemia-mediated oxidative stress. Biol Pharm
Bull. 39:1300–1308. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang JY, Yang JH, Xu J, Jia JY, Zhang XR,
Yue XD, Chen LM, Shan CY, Zheng MY, Han F, et al: Renal tubular
damage may contribute more to acute hyperglycemia induced kidney
injury in non-diabetic conscious rats. J Diabetes Complications.
29:621–628. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun Q, Shen ZY, Meng QT, Liu HZ, Duan WN
and Xia ZY: The role of DJ-1/Nrf2 pathway in the pathogenesis of
diabetic nephropathy in rats. Ren Fail. 38:294–304. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu M, Zhou B, Xia ZY, Zhao B, Lei SQ,
Yang QJ, Xue R, Leng Y, Xu JJ and Xia Z: Hyperglycemia-induced
inhibition of DJ-1 expression compromised the effectiveness of
ischemic postconditioning cardioprotection in rats. Oxid Med Cell
Longev. 2013:5649022013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Milani P, Ambrosi G, Gammoh O, Blandini F
and Cereda C: SOD1 and DJ-1 converge at Nrf2 pathway: A clue for
antioxidant therapeutic potential in neurodegeneration. Oxid Med
Cell Longev. 2013:8367602013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xia Z, Kuo KH, Nagareddy PR, Wang F, Guo
Z, Guo T, Jiang J and McNeill JH: N-acetylcysteine attenuates
PKCbeta2 overexpression and myocardial hypertrophy in
streptozotocin-induced diabetic rats. Cardiovasc Res. 73:770–782.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang J, Yang J, Ding JW, Chen LH, Wang YL,
Li S and Wu H: Sequential expression of TLR4 and its effects on the
myocardium of rats with myocardial ischemia-reperfusion injury.
Inflammation. 31:304–312. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
McWhinnie DL, Thompson JF, Taylor HM,
Chapman JR, Bolton EM, Carter NP, Wood RF and Morris PJ:
Morphometric analysis of cellular infiltration assessed by
monoclonal antibody labeling in sequential human renal allograft
biopsies. Transplantation. 42:352–358. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Duan SY, Xing CY, Zhang B and Chen Y:
Detection and evaluation of renal biomarkers in a swine model of
acute myocardial infarction and reperfusion. Int J Clin Exp Pathol.
8:8336–8347. 2015.PubMed/NCBI
|
|
19
|
Song Y, Yu Q, Zhang J, Huang W, Liu Y, Pei
H, Liu J, Sun L, Yang L, Li C, et al: Increased myocardial
ischemia-reperfusion injury in renal failure involves cardiac
adiponectin signal deficiency. Am J Physiol Endocrinol Metab.
306:E1055–E1064. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
O'Neal JB, Shaw AD and Billings FT IV:
Acute kidney injury following cardiac surgery: Current
understanding and future directions. Crit Care. 20:1872016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zimmerman RF, Ezeanuna PU, Kane JC,
Cleland CD, Kempananjappa TJ, Lucas FL and Kramer RS: Ischemic
preconditioning at a remote site prevents acute kidney injury in
patients following cardiac surgery. Kidney Int. 80:861–867. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bellomo R, Auriemma S, Fabbri A, D'Onofrio
A, Katz N, McCullough PA, Ricci Z, Shaw A and Ronco C: The
pathophysiology of cardiac surgery-associated acute kidney injury
(CSA-AKI). Int J Artif Organs. 31:166–178. 2008.PubMed/NCBI
|
|
23
|
Chen Z, Wang D, Liu X, Pei W, Li J, Cao Y,
Zhang J, An Y, Nie J and Tong J: Oxidative DNA damage is involved
in cigarette smoke-induced lung injury in rats. Environ Health Prev
Med. 20:318–324. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Czerska M, Mikołajewska K, Zieliński M,
Gromadzińska J and Wasowicz W: Today's oxidative stress markers.
Med Pr. 66:393–405. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chan JY and Chan SH: Activation of
endogenous antioxidants as a common therapeutic strategy against
cancer, neurodegeneration and cardiovascular diseases: A lesson
learnt from DJ-1. Pharmacol Ther. 156:69–74. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yan YF, Yang WJ, Xu Q, Chen HP, Huang XS,
Qiu LY, Liao ZP and Huang QR: DJ-1 upregulates anti-oxidant enzymes
and attenuates hypoxia/re-oxygenation-induced oxidative stress by
activation of the nuclear factor erythroid 2-like 2 signaling
pathway. Mol Med Rep. 12:4734–4742. 2015. View Article : Google Scholar : PubMed/NCBI
|