Introduction
Oxidized low-density lipoprotein (oxLDL) reportedly
contributes to the development and progression of atherosclerosis
(1) and is used as a biomarker of
atherosclerosis and cardiovascular risk in circulation (2). High circulating oxLDL directly exerts
oxidative stress (3), or even
induces apoptosis (4), in smooth
muscle cells and endothelial cells. oxLDL also promotes the
production or circulating long-term proinflammatory cytokines
(5) and indirectly impairs the
function of vascularity. To date, multiple markers, such as
caspases (6) and lectin-like
oxidized-low density lipoprotein receptor-1 (4), have been demonstrated to mediate the
oxLDL-induced apoptotic cascade in endothelial cells. Therefore,
oxLDL is a key contributor to the endothelial cell damage that
initiates atherosclerosis.
During the migration of smooth muscle cells (SMCs)
and the plaque rupture in atherosclerosis, matrix
metalloproteinases (MMPs) have been recognized to catalyze the
degradation of fibrous cap components such as collagens, elastin,
fibronectin and proteoglycans (7–9), and
thus contribute to the vulnerability of atherosclerotic plaques.
Among the more than 20 types of MMPs (10), MMP-1, −2, −3, −7, −8, −9, −13, and
−14 have been reported to be increased at atherosclerotic lesions
in human and animal models (9,11–14).
MMP-1 and MMP-14 predominantly localize in SMCs (15,16) and
macrophages (13), whereas MMP-8 and
−13 are produced from neutrophils (14) and macrophages (13), respectively. MMP-9 levels are
upregulated in human monocyte-derived macrophages (17); however, little is known about the
contribution of oxLDL to the production of MMP-9 in endothelial
cells.
With the exception of oxLDL, infection also
contributes to the formation of atherosclerosis. In particular,
infection with viruses, with such agents as human cytomegalovirus
(HCMV) (18,19), herpes simplex viruses (HSV) (20) and influenza virus (21,22) have
been identified to accelerate atherosclerosis. The promoted
vascular inflammation (23,24) impairs the vascularity and causes
endothelial cell (EC) dysfunction (25,26)
during viral infection. Serological studies support the association
between infection with HCMV, human immunodeficiency virus, HSV
(27,28) and influenza virus (22) with atherosclerosis. In particular,
animal and human studies have confirmed that prothrombotic and
pro-inflammatory effects are caused by influenza infection
(29). However, there are minimal
reports on the orchestrated molecular signals that are promoted by
influenza virus infection during atherosclerosis, particularly,
about the promotion of MMP by the viral infection in the background
of atherosclerosis.
In the present study, we aimed to determine whether
MMP-9 was promoted by infection with H1N1 pdm2009 influenza virus
and by treatment with oxLDL in human umbilical vein endothelial
cells (HUVECs). Subsequently, we investigated the influence of
viral infection and oxLDL treatment on the production of
proinflammatory cytokines and cellular viability in HUVECs. The
present study confirmed the synergistic enhancement of MMP-9
expression and cellular viability reduction by influenza virus
infection and oxidized-LDL treatment in human endothelial cells,
implying the contribution of influenza virus infection to the
oxLDL-induced impairment to endothelial cells.
Materials and methods
Reagents and cell culture. OxLDL was purchased from
Biomedical Technologies Inc., (Stoughton, MA, USA) and resolved in
F-12K medium with a concentration of 1 mg/ml. The human umbilical
vein endothelial HUVEC-C cell line (passage 3) was purchased from
American Type Culture Collection (Manassas, VA, USA) and limitedly
propagated (less than passage 14) in F-12K medium (Kaighn's
Modification of Ham's F-12 Medium; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS;
Invitrogen; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) at 37°C under 5% CO2. F-12 K medium
supplemented with 0.3% bovine serum albumin (BSA), 1 mg/ml tosyl
phenylalanyl chloromethyl ketone (TPCK)-treated trypsin (both from
Sigma-Aldrich; Merck KGaA) and 1% penicillin/streptomycin were used
for H1N1 pdm2009 influenza virus infection (Beijing Wantai
Biological Pharmacy Enterprise Co., Ltd., Beijing, China). For the
oxLDL treatment, ~90% confluent HUVEC-C cells were treated with 0,
10, 20 or 50 µg/ml oxLDL for 0–48 h. Following oxLDL incubation,
cells were lysed for the mRNA expression analysis or for the
western blotting analysis.
Virus infection and plaque forming assay. For viral
infection, 90% confluent HUVEC-C cells were infected with
serially-diluted H1N1 pdm2009 influenza virus of 0.001, 0.01, 1 or
5 multiplicity of infection (MOI) for 45 min at 35°C. Subsequently,
the viral supernatant was removed and cells were replenished with
F-12K medium supplemented with 0.3% BSA, 1 mg/ml TPCK-treated
trypsin and 1% penicillin/streptomycin. For the virus replication
assay, cells were inoculated for another 12, 24 or 48 h, and the
supernatant was tittered with plaque forming assay. For the cell
viability or apoptosis assay, cells were inoculated for another 12
or 24 h, and were subjected to methyl thiazolyl tetrazoliym assay
(MTT assay). For the plaque formation assay, confluent monolayer
HUVEC-C cells were inoculated with 0, 0.001, 0.01, or 0.1 MOI H1N1
pdm2009 influenza virus at 35°C for 45 min, and were overlaid with
1% hypo-temperature-solved agarose containing 0.3% BSA, 1 mg/ml
TPCK-treated trypsin and 1% penicillin/streptomycin. After 3 days
of inoculation at 35°C, cells were fixed with 4% formaldehyde for
20 min at 35°C and stained with 1% crystal violet solution at 35°C
overnight.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total cellular RNA from HUVEC-C cells was isolated
using TRIzol reagent (Thermo Fisher Scientific, Inc.) and
supplemented with RNasin Plus RNase Inhibitor (Promega Corp.,
Madison, WI, USA). The cDNA was synthesized using 1 µg of total RNA
using high capacity cDNA reverse transcription kit (Applied
Biosystems, Thermo Fisher Scientific, Inc.). RT-qPCR was performed
using a Takara One Step RT-PCR kit (Takara Biotechnology, Tokyo,
Japan). The cDNA template (50 ng) was amplified using Inventoried
TaqMan Gene Expression Assay products. The primers used were as
follows: MMP-9 forward, 5′-AACCCTGGTCACCGGACTTC-3′ and reverse,
5′-CACCCGGTTGTGGAAACTCAC-3′; TNF-α forward,
5′-AGAACTCCAGGCGGTGTCT-3′ and reverse, 5′-A-GAA-CTC CAG GCG GTG
TCT-3′; IL-1β forward, 5′TCC AGC TAC GAA TCT CCG AC3′ and reverse,
5′TCC AGC TAC GAA TCT CCG AC3′; β-actin forward,
5′-ATATCGCTGCGCTCGTCGTC-3′ and reverse, 5′-GCATCGGAACCGCTCATTGC-3′;
IL-6 forward, 5′AGT CCT GAT CCA GTT CCT GC3′ and reverse, 5′CAT TTG
TGG TTG GGT CAG GG3′. PCR was performed under the following
conditions: 95°C for 30 sec, 95°C for 15 sec, 60°C for 30 sec, and
68°C for 30 sec for 40 cycles. Relative quantification was
determined using the 2−∆∆Cq method using β-actin as
reference genes (30).
Cell viability assay
Cell viability was evaluated by MTT assay
(Invitrogen; Thermo Fisher Scientific, Inc.). Briefly, 90%
confluent HUVEC-C cells following oxLDL treatment, H1N1 PDM2009
virus infection or both were incubated with 50 µl MTT solution at
37°C for 2 h, and were dissolved completely by 150 µl DMSO at room
temperature. Optical density was subsequently measured at 570 nm
using a spectrophotometer (Bio-Rad Laboratories, Inc., Hercules,
CA, USA).
Western blot assay
Following treatment, HUVEC-C cells were lysed with a
NE-PER Nuclear and Cytoplasmic Extraction Reagents kit (Pierce;
Thermo Fisher Scientific, Inc.), and the cellular lysate was
supplemented with a protease inhibitor cocktail (Roche Diagnostics
GmbH, Wetzlar, Germany), following centrifugation at 13,400 × g for
15 min. Proteins (25 µl) were separated by 12% SDS-PAGE and
transferred to a nitrocellulose membrane (Millipore, Bedford, MA,
USA). Following blocking with 2% BSA at 4°C overnight, the membrane
was incubated with rabbit polyclone antibodies against MMP-9 (cat.
no. 444278-500UG; 1:400; Merck KGaA) or β-actin (cat. no. bs-0061R;
1:2,000; Beijing Biosynthesis Biotechnology Co., Ltd., Beijing,
China) for 1 h at 37°C. The membrane was washed with TBST for 3 min
and incubated with goat anti-rabbit IgG secondary antibody
conjugated to horseradish peroxidase (cat. no. 1662408ED; 1:500;
Bio-Rad Laboratories, Inc.) for 40 min at room temperature and an
enhanced chemiluminescence detection system (GE Healthcare Life
Sciences, Little Chalfont, UK) was used for target protein band
detection.
Results
oxLDL promotes the expression of MMP-9
in HUVEC-C cells
To investigate the regulation of the induction of
MMP-9 by oxLDL treatment in HUVEC-C cells, HUVEC-C cells were
treated with 0, 10, 20 or 50 µg/ml oxLDL for 0, 12, 24 or 48 h.
Cellular viability and the expression of MMP-9 in the oxLDL-treated
HUVEC-C cells were examined. As indicated in Fig. 1A, oxLDL treatment with 0, 10, 20 or
50 µg/ml did not significantly regulate the cellular viability of
HUVEC-C cells. However, MMP-9 expression was markedly promoted by
oxLDL treatment. MMP-9 mRNA levels were significantly higher in
HUVEC-C cells treated with 10 (P<0.05), 20 (P<0.01) or 50
(P<0.001) µg/ml oxLDL for 12 h, with a dose-dependent increase
noted between 20 and 50 µg/ml oxLDL (P<0.05; Fig. 1B). Western blotting results confirmed
the promotion of MMP-9 at a protein level (P<0.05, P<0.01 and
P<0.001 for 10, 20 and 50 µg/ml, respectively; Fig. 1C), with a dose-dependent increase
noted between 10 and 20 µg/ml oxLDL (P<0.05). MMP-9 promotion
was also time-dependent, the MMP-9 in protein level was
significantly different between 12 and 48 h post-treatment with 50
µg/ml oxLDL (P<0.05 and P<0.01, respectively; Fig. 1D). In addition, MMP-9 activity was
also examined in the oxLDL-treated HUVEC-C cells. As shown in
Fig. 1E, MMP-9 activity was markedly
promoted by the oxLDL treatment with 20 or 50 µg/ml. These findings
suggest that treatment with oxLDL promoted the expression of MMP-9
in human endothelial HUVEC-C cells.
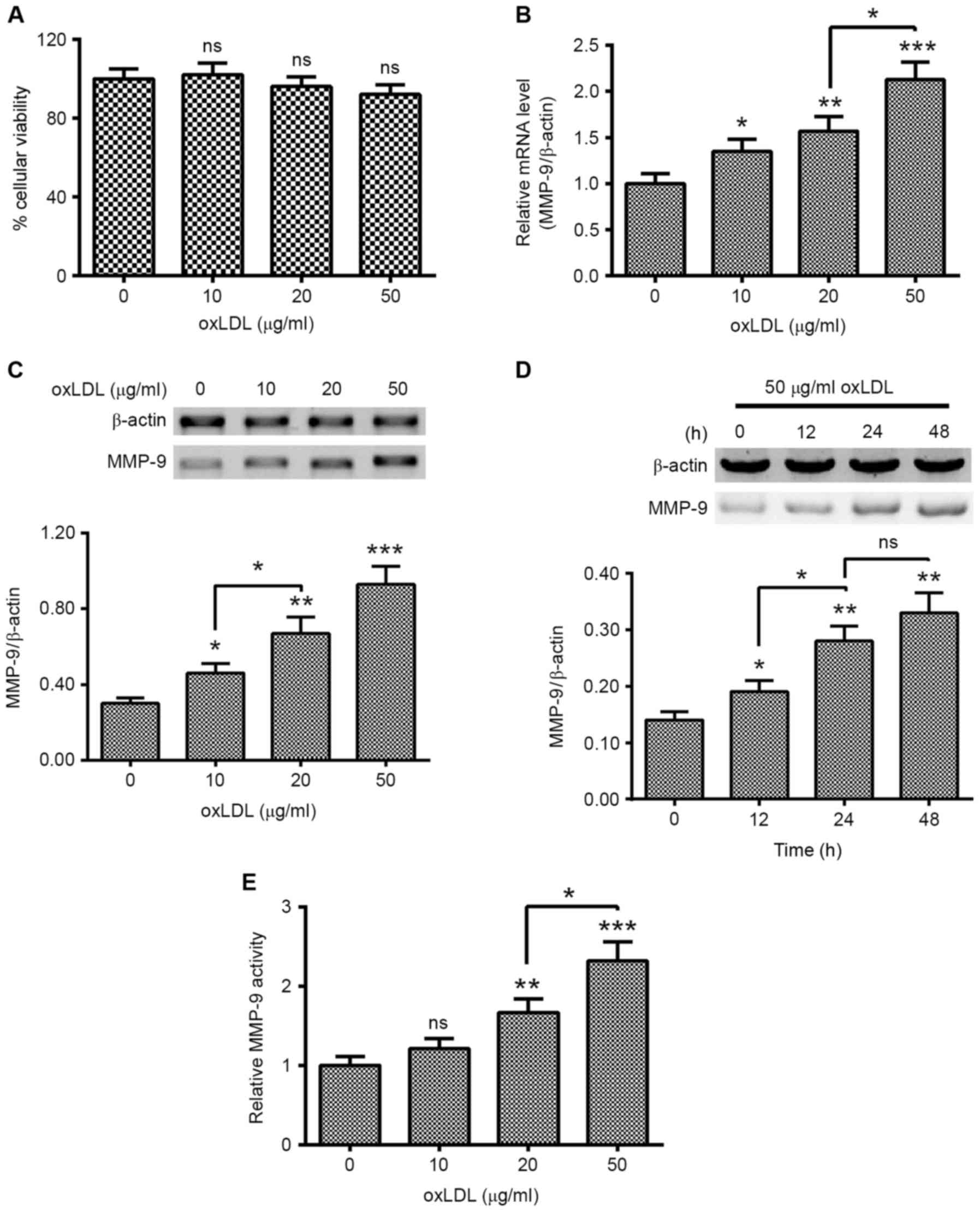 | Figure 1.oxLDL treatment promotes MMP-9
expression in human endothelial HUVEC-C cells. (A) MTT assay of
HUVEC-C cells following treatment with 0, 10, 20 or 50 µg/ml oxLDL
for 24 h. (B) mRNA levels of MMP-9 in HUVEC-C cells treated with 0,
10, 20 or 50 µg/ml oxLDL for 12 h. (C) Western blot assay of
cytosolic MMP-9 in HUVEC-C cells treated with 0, 10, 20 or 50 µg/ml
oxLDL for 24 h. (D) Western blot assay of cytosolic MMP-9 in
HUVEC-C cells treated with 50 µg/ml oxLDL for 0, 12, 24 or 48 h.
(E) MMP-9 activity in HUVEC-C cells treated with 0, 10, 20 or 50
µg/ml oxLDL for 24 h. Data are presented as the average of
triplicate results. *P<0.05, **P<0.01 and ***P<0.001 vs. 0
µg/ml oxLDL or 0 h. NS, not significant; HUVEC, human umbilical
vein endothelial cells; oxLDL, oxidized low density lipoprotein;
MMP, matrix metalloproteinase. |
H1N1 PDM2009 influenza virus infects
and replicates in HUVEC-C cells
To investigate the influence of influenza virus
infection on OxLDL-promoted MMP-9 expression in endothelial cells,
we determined the infection efficiency of H1N1 PDM2009 influenza
virus in HUVEC-C cells. First, we determined the plaque forming
capacity of H1N1 PDM2009 virus in HUVEC-C cells. Fig. 2A demonstrated that inoculation with
0.001, 0.01 or 0.1 MOI H1N1 PDM2009 promoted plaque forming in
HUVEC-C cells; and the plaque number positively correlated with the
MOI of H1N1 PDM2009. The growth curve of H1N1 PDM2009 influenza
virus was also determined, which indicated that either a MOI of
0.001 or 0.01 H1N1 PDM2009 influenza virus replicated efficiently
in HUVEC-C cells, or the replication continued from 48 h
post-infection (Fig. 2B). In
addition, the viability of HUVEC-C cells following infection with 5
MOI H1N1 PDM2009 was significantly reduced at 12 or 24 h
post-infection, as compared with 0 h (P<0.05 and P<0.01,
respectively; Fig. 2C). Similarly,
the viability of HUVEC-C cells following infection with 1 MOI H1N1
PDM2009 was significantly reduced at 24 h post-infection, as
compared with 0 h (P<0.05). Therefore, the H1N1 PDM2009
influenza viruses infected and replicated efficiently in the human
endothelial HUVEC-C cells.
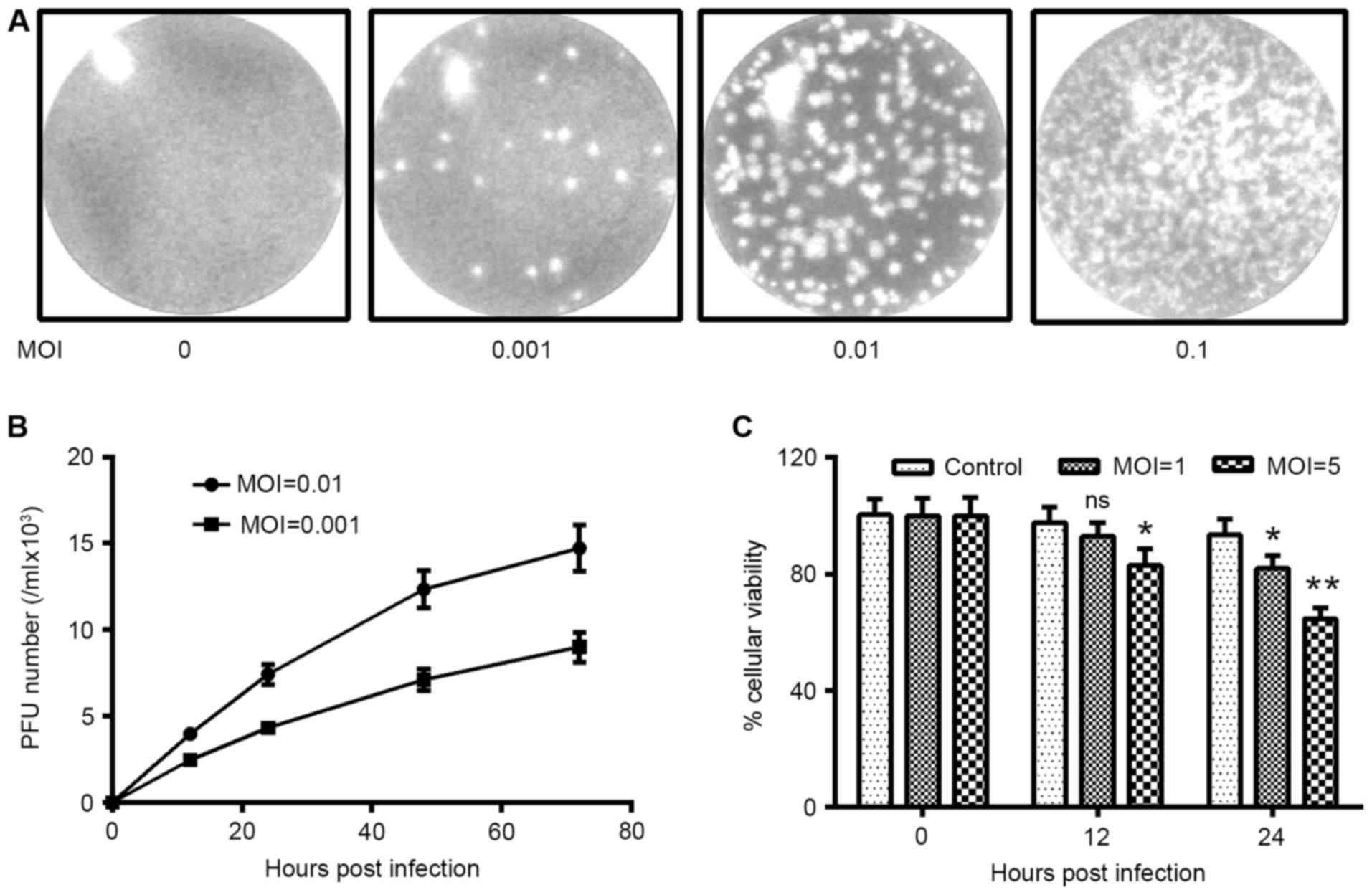 | Figure 2.H1N1 PDM2009 virus infects and forms
plaques in human endothelial HUVEC-C cells. (A) Plaque forming
assay in HUVEC-C cells, which were infected with 0, 0.001, 0.01, or
0.1 MOI H1N1 PDM2009 virus. (B) Growth curve of H1N1 PDM2009 virus
in HUVEC-C cells, with a MOI of 0.001 or 0.01. (C) MTT assay for
the viability of HUVEC-C cells, following infection with 1 or 5 MOI
H1N1 PDM2009 virus for 0, 12 or 24 h. Experiments were
independently repeated in triplicate. *P<0.05 and **P<0.01
vs. 0 h post-infection. HUVEC, human umbilical vein endothelial
cells; NS, not significant; oxLDL, oxidized low density
lipoprotein; MOI, multiplicity of infection. |
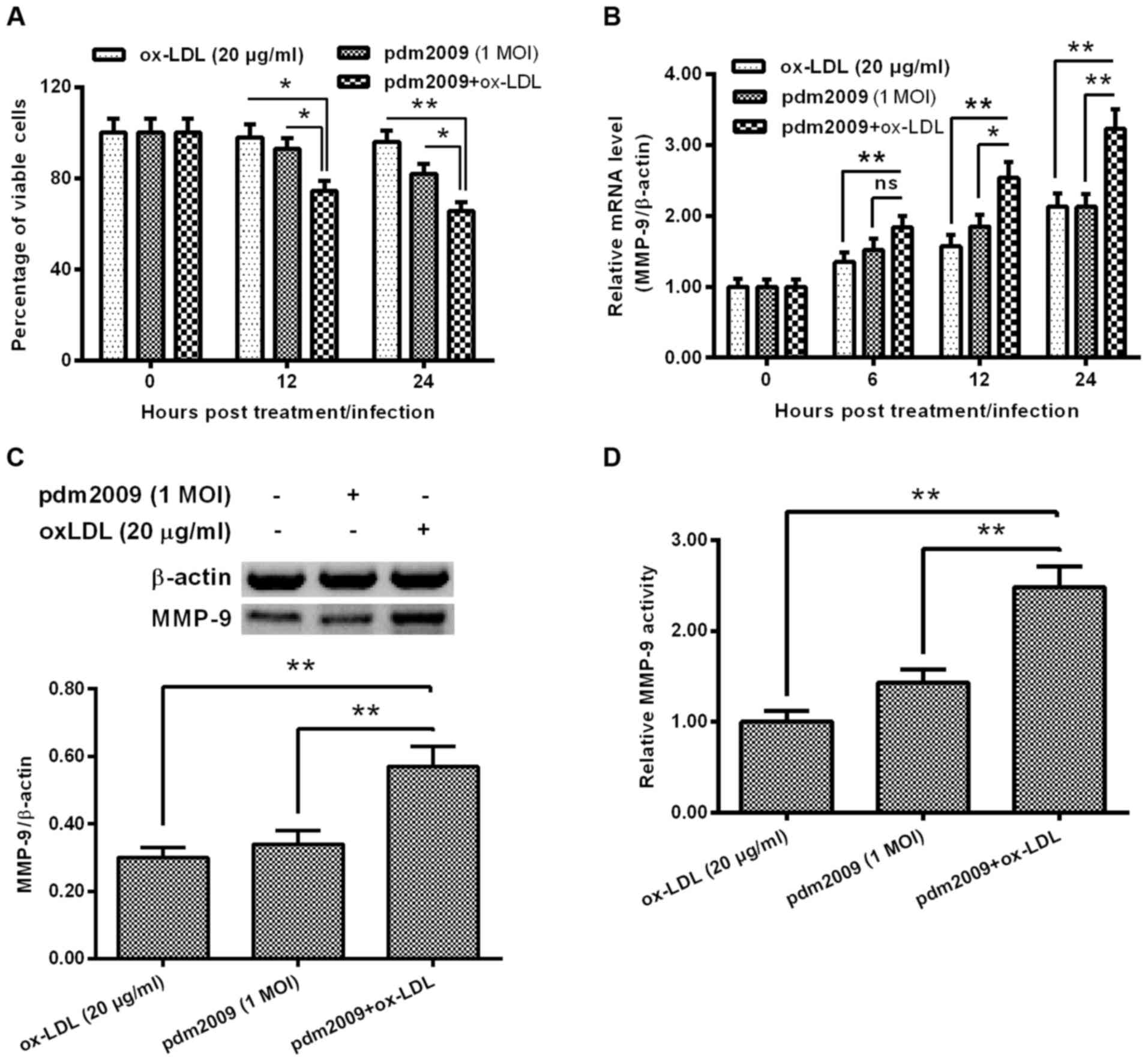
H1N1 PDM2009 virus infection
synergistically enhances oxLDL-promoted MMP-9 in HUVEC-C cells
In order to explore the influence of H1N1 PDM2009
virus infection on oxLDL-induced MMP-9 expression in HUVEC-C cells,
we examined the influence of H1N1 PDM2009 virus infection and oxLDL
treatment on the viability of HUVEC-C cells. As indicated in
Fig. 3A, the cellular viability
reduction was more significant in the oxLDL-treated (20 µg/ml)
HUVEC-C cells, which were also infected with 1 MOI H1N1 PDM2009
virus at 12 or 24 h post-treatment/infection (P<0.05 and
P<0.01, respectively; Fig. 3A).
MMP-9 expression was markedly higher in the oxLDL-treated and
virus-infected HUVEC-C cells than in the either oxLDL-treated or
virus-infected HUVEC-C cells (P<0.05 and P<0.01,
respectively; Fig. 3B). This
synergistic effect on MMP-9 promotion was confirmed at the protein
level via western blotting in the HUVEC-C cells (P<0.01;
Fig. 3C). In addition, MMP-9
activity was also synergistically upregulated by oxLDL treatment
and H1N1 PDM2009 virus infection (P<0.01; Fig. 3D). These results indicate that the
H1N1 PDM2009 virus infection synergistically enhanced oxLDL-induced
MMP-9 expression in HUVEC-C cells.
H1N1 PDM2009 virus and oxLDL
synergistically promote pro-inflammatory cytokines in HUVEC-C
cells
To investigate whether oxLDL treatment and H1N1
PDM2009 virus infection synergistically induced pro-inflammatory
cytokines in the endothelial HUVEC-C cells, we examined the
expression levels of TNF-α, IL-1β and IL-6 in HUVEC-C cells,
following treatment with 20 µg/ml oxLDL, infection with 1 MOI H1N1
PDM2009 virus or with both treatments. mRNA levels of TNF-α were
markedly promoted by either treatment with 20 µg/ml oxLDL or by
infection with 1 MOI H1N1 PDM2009 virus (P<0.05 and P<0.01,
respectively; Fig. 4A). Such
promotion of TNF-α was more notable in the HUVEC-C cells subjected
to both 20 µg/ml oxLDL treatment and infection with 1 MOI H1N1
PDM2009 virus (P<0.05). Synergistic promotion by oxLDL treatment
and virus infection was also recognized in IL-1β and IL-6. mRNA
levels of both cytokines were also significantly higher in the
HUVEC-C cells subjected to both 20 µg/ml oxLDL treatment and
infection with 1 MOI H1N1 PDM2009 virus (P<0.05 and P<0.01,
respectively; Fig. 4B and C).
Therefore, these findings demonstrated the synergistic promotion of
pro-inflammatory cytokines in HUVEC-C cells by oxLDL treatment and
influenza virus infection.
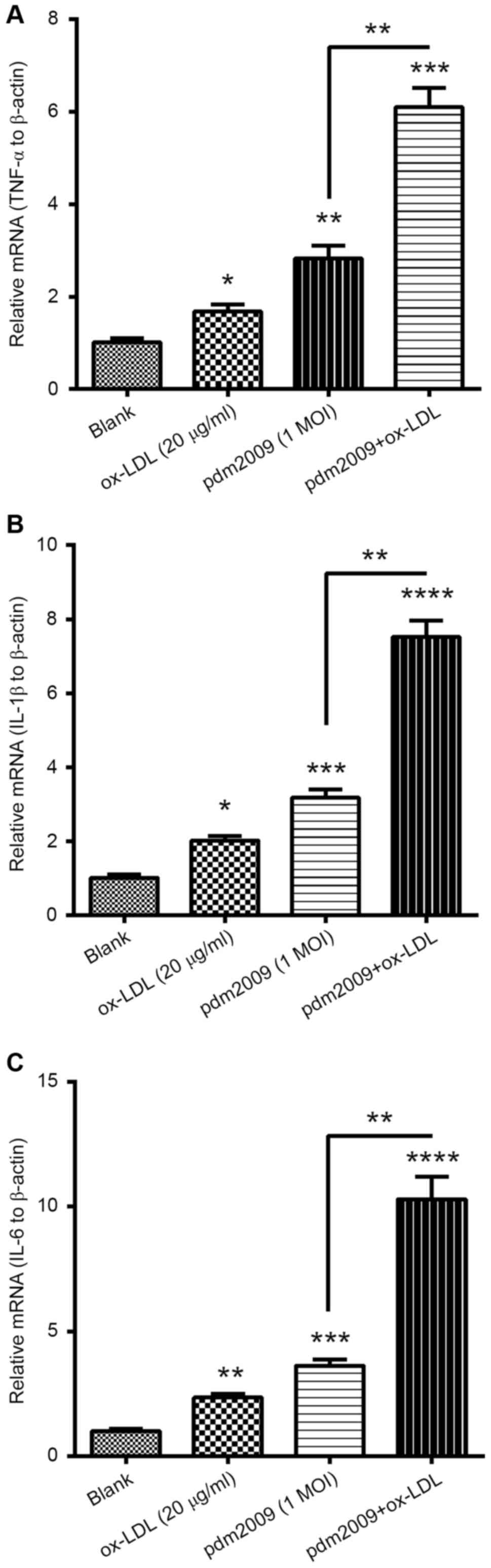 | Figure 4.RT-qPCR analysis of pro-inflammatory
cytokines in H1N1 PDM2009 virus-infected and oxLDL-treated human
endothelial HUVEC-C cells. HUVEC-C cells were treated with 20 µg/ml
oxLDL and/or infected with 1 MOI H1N1 PDM2009 virus for 12 h.
Subsequently, RT-qPCR was performed to quantify the mRNA levels of
(A) TNF-α, (B) IL-1β and (C) IL-6. Data are expressed as the mean ±
standard deviation of experiments independently repeated in
triplicate. *P<0.05, **P<0.01, ***P<0.001 and
****P<0.0001 vs. blank. RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; HUVEC, human
umbilical vein endothelial cells; oxLDL, oxidized low density
lipoprotein; MOI, multiplicity of infection; TNF, tumor necrosis
factor; IL, interleukin. |
Discussion
The mechanism underlining the development of
atherosclerosis, which is mediated by oxLDL and infection, is not
well-documented. oxLDL-, free radical-, or infection-induced
inflammatory responses have been demonstrated to lead to
endothelial dysfunction (30), and
thus may contribute to atherosclerosis. In the present study, we
demonstrated the promotion to MMP-9 expression by ox-LDL treatment
in HUVEC-C cells. oxLDL treatment significantly promoted mRNA and
protein levels of MMP-9 in the in vitro-cultured HUVEC-C
cells dose-dependently and time-dependently. MMP-9 activity was
also markedly promoted by oxLDL treatment in HUVEC-C cells.
Previous in vivo results also demonstrated MMP-9
overexpression in human progressive atherosclerotic plaques
(9); and high levels of MMP-9 have
been associated with an increased risk of severe atherosclerosis
and unstable plaques in atherosclerotic patients (31). The present study also demonstrated
that H1N1 PDM2009 influenza virus infected and replicated
efficiently in HUVEC-C cells and the H1N1 PDM2009 virus infection
synergistically enhanced the oxLDL-promoted MMP-9 levels in HUVEC-C
cells. Therefore, we propose that the promotion of MMP-9 by oxLDL
underlines oxLDL-induced atherosclerosis.
Inflammation involves the development of
atherosclerosis both via mediating the effects of above-mentioned
risk factors (ox-LDL and infection) and by directly affecting the
vessel wall (32). The vascular
inflammation (23,24) impairs the vascularity and causes
endothelial cell dysfunction. A previous mice model study indicated
that influenza virus directly infects, and resides in
atherosclerotic arteries, in association with systemic and
arterial-level pro-inflammatory changes (33). Influenza virus infection-induced
autoimmune mechanisms have also been shown to participate in
athermanous lesions (34). Our study
findings indicated that H1N1 PDM2009 virus infection
synergistically promotes pro-inflammatory cytokines with oxLDL in
HUVEC-C cells. mRNA levels of TNF-α, IL-1β and IL-6 were markedly
and synergistically promoted by treatment with 20 µg/ml oxLDL and
infection with 1 MOI H1N1 PDM2009 virus.
A previous study demonstrated that influenza virus
aggravates the ox-LDL-induced apoptosis of human endothelial cells
by promoting p53 signaling (35).
Infection with A/Porto Rico/8/1934 (H1N1) (PR8) influenza virus in
human endothelial EA.hy926 cells induced apoptosis, which was
aggravated by ox-LDL treatment. p53 signaling was also
synergistically activated by both influenza virus infection and
oxLDL treatment. Our study expanded the current understanding of
the synergistical regulation by both oxLDL treatment and influenza
virus infection in human endothelial cells.
In conclusion, the present study is the first to
demonstrate the synergistical promotion of the expression of MMP-9
and pro-inflammatory cytokines in human endothelial HUVEC-C cells.
Such synergistical promotion may contribute to influenza virus
infection and oxLDL-mediated endothelial dysfunction.
References
|
1
|
Ehara S, Ueda M, Naruko T, Haze K, Itoh A,
Otsuka M, Komatsu R, Matsuo T, Itabe H and Takano T: Elevated
levels of oxidized low density lipoprotein show a positive
relationship with the severity of acute coronary syndromes.
Circulation. 103:1955–1960. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Verhoye E and Langlois: Asklepios
Investigators: Circulating oxidized low-density lipoprotein: A
biomarker of atherosclerosis and cardiovascular risk? Clin Chem Lab
Med. 47:128–137. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Itabe H: Oxidized low-density lipoprotein
as a biomarker of in vivo oxidative stress: From atherosclerosis to
periodontitis. J Clin Biochem Nutr. 51:1–8. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Imanishi T, Hano T, Sawamura T, Takarada S
and Nishio I: Oxidized low density lipoprotein potentiation of
Fas-induced apoptosis through lectin-like oxidized-low density
lipoprotein receptor-1 in human umbilical vascular endothelial
cells. Circ J. 66:1060–1064. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bekkering S, Quintin J, Joosten LA, van
der Meer JW, Netea MG and Riksen NP: Oxidized low-density
lipoprotein induces long-term proinflammatory cytokine production
and foam cell formation via epigenetic reprogramming of monocytes.
Arterioscler Thromb Vasc Biol. 34:1731–1738. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen J, Mehta JL, Haider N, Zhang X,
Narula J and Li D: Role of caspases in Ox-LDL-induced apoptotic
cascade in human coronary artery endothelial cells. Circ Res.
94:370–376. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Galis ZS, Sukhova GK, Lark MW and Libby P:
Increased expression of matrix metalloproteinases and matrix
degrading activity in vulnerable regions of human atherosclerotic
plaques. J Clin Invest. 94:2493–2503. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Falk E, Shah PK and Fuster V: Coronary
plaque disruption. Circulation. 92:657–671. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Galis ZS and Khatri JJ: Matrix
metalloproteinases in vascular remodeling and atherogenesis: The
good, the bad and the ugly. Circ Res. 90:251–262. 2002.PubMed/NCBI
|
|
10
|
Visse R and Nagase H: Matrix
metalloproteinases and tissue inhibitors of metalloproteinases:
Structure, function and biochemistry. Circ Res. 92:827–839. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kuzuya M and Iguchi A: Role of matrix
metalloproteinases in vascular remodeling. J Atheroscler Thromb.
10:275–282. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Flamant M, Placier S, Dubroca C, Esposito
B, Lopes I, Chatziantoniou C, Tedgui A, Dussaule JC and Lehoux S:
Role of matrix metalloproteinases in early hypertensive vascular
remodeling. Hypertension. 50:212–218. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sukhova GK, Schonbeck U, Rabkin E, Schoen
FJ, Poole AR, Billinghurst RC and Libby P: Evidence for increased
collagenolysis by interstitial collagenases-1 and −3 in vulnerable
human atheromatous plaques. Circulation. 99:2503–2509. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Herman MP, Sukhova GK, Libby P, Gerdes N,
Tang N, Horton DB, Kilbride M, Breitbart RE, Chun M and Schönbeck
U: Expression of neutrophil collagenase (matrix
metalloproteinase-8) in human atheroma: A novel collagenolytic
pathway suggested by transcriptional profiling. Circulation.
104:1899–1904. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nikkari ST, O'Brien KD, Ferguson M,
Hatsukami T, Welgus HG, Alpers CE and Clowes AW: Interstitial
collagenase (MMP-1) expression in human carotid atherosclerosis.
Circulation. 92:1393–1398. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rajavashisth TB, Xu XP, Jovinge S, Meisel
S, Xu XO, Chai NN, Fishbein MC, Kaul S, Cercek B, Sharifi B and
Shah PK: Membrane type 1 matrix metalloproteinase expression in
human atherosclerotic plaques: Evidence for activation by
proinflammatory mediators. Circulation. 99:3103–3109. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu XP, Meisel SR, Ong JM, Kaul S, Cercek
B, Rajavashisth TB, Sharifi B and Shah PK: Oxidized low-density
lipoprotein regulates matrix metalloproteinase-9 and its tissue
inhibitor in human monocyte-derived macrophages. Circulation.
99:993–998. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Roivainen M, Viik-Kajander M, Palosuo T,
Toivanen P, Leinonen M, Saikku P, Tenkanen L, Manninen V, Hovi T
and Mänttäri M: Infections, inflammation and the risk of coronary
heart disease. Circulation. 101:252–257. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ravnskov U and McCully KS: Infections may
be causal in the pathogenesis of atherosclerosis. Am J Med Sci.
344:391–394. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hechter RC, Budoff M, Hodis HN, Rinaldo
CR, Jenkins FJ, Jacobson LP, Kingsley LA, Taiwo B, Post WS,
Margolick JB and Detels R: Herpes simplex virus type 2 (HSV-2) as a
coronary atherosclerosis risk factor in HIV-infected men:
Multicenter AIDS cohort study. Atherosclerosis. 223:433–436. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gurevich VS, Pleskov VM, Levaia MV,
Bannikov AI, Mitrofanova LB and Urazgil'Deeva SA: Influenza virus
infection in progressing atherosclerosis. Kardiologiia. 42:21–24.
2002.PubMed/NCBI
|
|
22
|
Auer J, Berent R, Weber T and Eber B:
Influenza virus infection, infectious burden and atherosclerosis.
Stroke. 33:1454–1455. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Streblow DN, Orloff SL and Nelson JA: Do
pathogens accelerate atherosclerosis? J Nutr. 131:2798S–2804S.
2001.PubMed/NCBI
|
|
24
|
Söderberg-Nauclér C: Does cytomegalovirus
play a causative role in the development of various inflammatory
diseases and cancer? J Intern Med. 259:219–246. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Epstein SE, Zhou YF and Zhu J: Infection
and atherosclerosis: Emerging mechanistic paradigms. Circulation.
100:e20–e28. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Libby P, Ridker PM and Maseri A:
Inflammation and atherosclerosis. Circulation. 105:1135–1143. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nieto FJ, Adam E, Sorlie P, Farzadegan H,
Melnick JL, Comstock GW and Szklo M: Cohort study of
cytomegalovirus infection as a risk factor for carotid
intimal-medial thickening, a measure of subclinical
atherosclerosis. Circulation. 94:922–927. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Smieja M, Gnarpe J, Lonn E, Gnarpe H,
Olsson G, Yi Q, Dzavik V, McQueen M and Yusuf S: Heart Outcomes
Prevention Evaluation (HOPE) Study Investigators: Multiple
infections and subsequent cardiovascular events in the heart
outcomes prevention evaluation (HOPE) study. Circulation.
107:251–257. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Celik S, Shankar V, Richter A, Hippe HJ,
Akhavanpoor M, Bea F, Erbel C, Urban S, Blank N, Wambsganss N and
Katus HA: Proinflammatory and prothrombotic effects on human
vascular endothelial cells of immune-cell-derived light. Eur J Med
Res. 14:147–156. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Halade GV, Jin YF and Lindsey ML: Matrix
metalloproteinase (MMP)-9: A proximal biomarker for cardiac
remodeling and a distal biomarker for inflammation. Pharmacol Ther.
139:32–40. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gao X, Xu X, Belmadani S, Park Y, Tang Z,
Feldman AM, Chilian WM and Zhang C: TNF-alpha contributes to
endothelial dysfunction by upregulating arginase in
ischemia/reperfusion injury. Arterioscler Thromb Vasc Biol.
27:1269–1275. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Haidari M, Wyde PR, Litovsky S, Vela D,
Ali M, Casscells SW and Madjid M: Influenza virus directly infects,
inflames and resides in the arteries of atherosclerotic and normal
mice. Atherosclerosis. 208:90–96. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gurevich VS: Influenza, autoimmunity and
atherogenesis. Autoimmun Rev. 4:101–105. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Suo J, Zhao L, Wang J, Zhu Z, Zhang H and
Gao R: Influenza virus aggravates the ox-LDL-induced apoptosis of
human endothelial cells via promoting p53 signaling. J Med Virol.
87:1113–1123. 2015. View Article : Google Scholar : PubMed/NCBI
|