Introduction
Acute pancreatitis is usually mild and exhibits a
wide range of clinical manifestations. The mortality rate of
patients with infected necrotizing pancreatitis (INP) is 8–39% and
~20% of patients with acute pancreatitis succumb (1). INP is a severe condition (2) and is a major cause of mortality as its
symptoms include early organ failure and infection of pancreatic or
peripancreatic necrotic tissue, leading to sepsis and multiple
organ failure (3).
The traditional method of treating patients with INP
by laparotomy remains the ‘gold standard’. However, this surgical
approach is associated with post-operative mortality and morbidity,
as well as organ dysfunction (4–6);
therefore, laparotomy should be delayed as long as possible to
decrease mortality and morbidity rates (7). Besselink et al (8) conducted a PANTER study to compare the
efficacy of PN with the step-up approach in patients with INP. The
step-up approach reduced the rate of major morbidity in patients
with suspected INP compared with those undergoing maximal
necrosectomy via laparotomy (8). The
minimally invasive step-up approach, which involves percutaneous
catheter drainage (PCD), followed by minimally invasive PN if
necessary, has many advantages including damage control, fewer
complications, and a decreased likelihood of multiple organ failure
(9). In 2010, Van Santvoort et
al (10) demonstrated that the
minimally invasive step-up approach was the least invasive of all
techniques, and is the optimal method of treating patients with INP
and secondary infection.
PCD is the primary strategy in the step-up approach
and a method of controlling sepsis (10). It has been applied to treat patients
with acute pancreatitis and decreases the risk of morbidity and
mortality (11). Furthermore, it is
a well-regarded first minimal access technique, which is used to
treat acute pancreatitis and avoid the use of PN. Guo et al
(12) has suggested that acute
necrotic collection and computed tomography (CT) mean density of
necrotic fluid collection may affect the success rate of PCD.
Indeed, previous studies have demonstrated that a high proportion
of patients fail to improve following the use of PCD (11,13). If
PCD does not improve clinical symptoms, PN should be performed
following PCD (10). Although a
number of recent studies have investigated the predictive factors
of PCD (12,14), few studies have identified methods of
predicting whether PN is required following PCD failure. Therefore,
the current study aimed to identify predictors of PN following the
use of PCD as primary treatment in patients with INP.
Patients and methods
Patients
Patients with suspected INP and peripancreatic fluid
collection in the Department of General Surgery, Capital Medical
University (Beijing, China) were enrolled in the present study
between October 2010 and October 2015. A series of consecutive
patients were diagnosed with acute suspected INP and peripancreatic
fluid collection.
The inclusion criteria were a maximum extent of
fluid collection of ≥100 ml and fluid collections taken within 2
weeks of disease onset. Patients were excluded if they had mild
pancreatitis, did not receive PCD as primary treatment or had a
pancreatic pseudocyst.
The process of patient screening and treatment
management in the current study is illustrated in Fig 1. All experiments performed in the
current study were approved by the Ethics Committee of Xuanwu
Hospital, Capital Medical University. Written informed consent was
obtained from all participants for their clinical data to be
applied in the present study.
PCD procedure
All 74 patients (female, 27; male, 47) initially
received resuscitative measures, gastrointestinal decompression,
antibiotic prophylaxis and conservative medical care. The needle
aspiration procedure and pus culture were performed under guidance
with ultrasound or CT, and a pigtail catheter was put in place.
Percutaneous catheters ranged in size from 8–32 French gauge (Fr)
and the mean number of ultrasound or CT guidance procedures
performed per patient was 1.5 (range, 1–5 per patient). Drains were
routinely flushed with 0.9% saline solution every 6 h to avoid
tubes becoming blocked and thicker drainage tubes were used if this
could not be avoided. If patients exhibited clinical improvements
and reduced peripancreatic fluid collection was confirmed by CT
reassessment 72 h later, primary PCD was considered a success.
Under reassessed CT guidance, multiple drainage treatments were
performed in areas including the retorperitineal or transperitoneal
regions. However, if peripancreatic fluid collection was only
reduced in the drainage area, with no reduction of peripancreatic
fluid collection away from the drainage sites, multi-points
puncture and drainage should be performed under multiple CT or
ultrasound guidance to reduce peripancreatic fluid collection. If
there was continued clinical deterioration following ≥1 drainage,
the PCD procedure was considered to be a failure.
PN after PCD
Following PCD, 42/74 of the patients (57%) were
treated with further PN. Videoscopic assisted retroperitoneal
debridement (VARD) or video-assisted laparoscopic debridement were
primarily used to debride necrotic tissues of the peripancreas. The
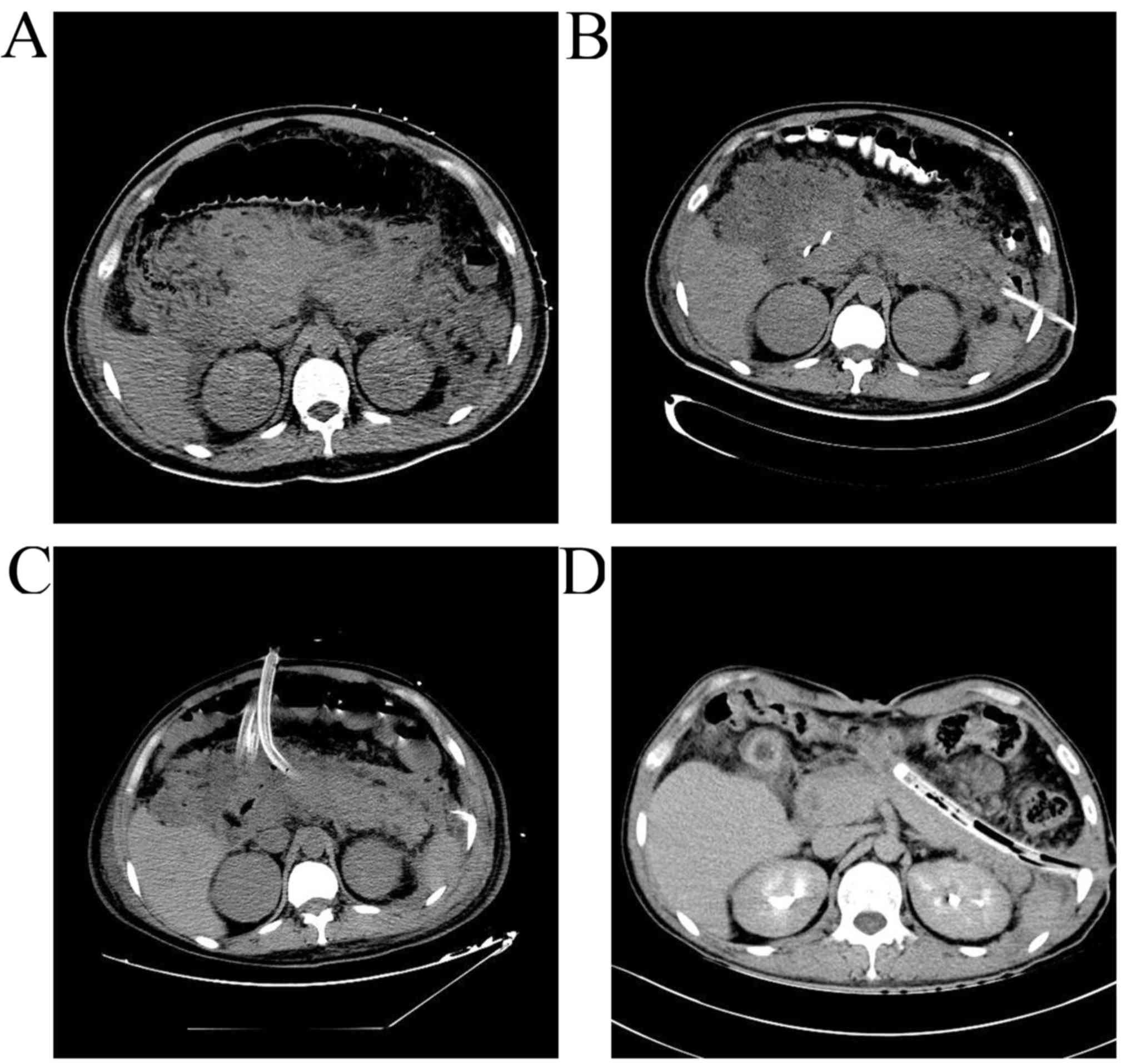
CT scans of patients who received PN following PCD are presented in
Fig 2.
Patient data recorded included age, etiology,
referral following INP onset, days spent in hospital, the span time
from onset to PCD, number of PCD catheters, catheter size, the
number of PCDs performed, duration of drainage, site of PCD,
severity score [Modified computerized tomography severity index
(MCTSI) (15) and Modified Marshall]
(16), maximum extent of necrosis,
maximum extent of fluid collection, reduction of fluid collection
following PCD, multiple organ failure, mortality rates and
laboratory parameters [C-reactive protein (CPR), procalcitonin
(PTC) and white blood cell (WBC) count].
Peripancreatic fluid collection and the extent of
peripancreatic necrosis was measured using a GE ADW4.5 workstation
(GE Healthcare, Chicago, USA) and is presented in Fig. 3. The nonnecrotic and necrotic tissues
of the pancreas were selected following the technique provided by
Quick Paint of Segment (a setting in the GE ADW4.5
workstation) and the volume of necrotic and non-necrotic tissue in
the pancreas was determined following the technique provided by
Measure Volume of Display (a setting of the GE ADW4.5
workstation; Brush Diameter was set as 1.0 mm).
Statistical analysis
Patients were divided into two groups: A PCD-alone
group (n=32) and a PCD+necrosectomy group (n=42). The clinical data
of patients in these groups are presented in Table I. Statistical analyses were performed
using SPSS Statistics 20.0 (IBM Corp, Armonk, NY, USA) and
measurement data were evaluated using a independent-sample t-test
to compare between two groups. Frequency counts and percentages
were applied to describe categorical data, which were assessed
using a χ2 test. For grade data, a Mann-Whitney test was
applied. Multivariable logistic regression analysis was applied to
assess the odds ratio and 95% confidence intervals were used to
identify the independent predictors of PN following PCD. P<0.05
was considered to indicate a statistically significant difference.
The model with the identified predictors was further confirmed by
bootstrapping, which was performed internally by a calibration plot
with bootstrap sampling (n=200). The calibration plot of an
accurate model may fall along the 45° line. A nomogram was
performed using R software version 3.13 (http://www.r-project.org). The nomogram was validated
internally in the training set and externally in the validation
set. The internal validation was performed using the calibration
method. The external validation was performed by calculating the
area under the receiver operating characteristic (ROC) curve (AUC).
The calibration plot with bootstrapping was used to illustrate the
association between the actual probability and predicted
probability. The AUC ranged from 0 to 1, with 1 indicating prefect
concordance, 0.5 indicating no better than chance, and 0 indicating
discordance.
 | Table I.Baseline Characteristics of the
patients with INP enrolled in this study. |
Table I.
Baseline Characteristics of the
patients with INP enrolled in this study.
| Characteristic | PCD-alone group | PCD+necrosectomy
group | P-values |
|---|
| Number of
patients | 32 | 42 |
|
| Demographic data
(years) |
|
|
|
| Age | 49±14.6 | 50±15.9 | 0.921 |
| Etiology |
|
| 0.755 |
|
Gallstone | 13 | 18 |
|
|
Hyperlipemia | 8 | 14 |
|
| Alcohol
abuse | 7 | 6 |
|
|
Other | 4 | 4 |
|
| Indexes of medical
economics (days) |
|
|
|
| Referral
following | 12±3.8 | 11±3.4 | 0.820 |
| INP
onset |
|
|
|
| Days in
hospital | 42.8±16.15 | 69.6±23.88 | 0.014a |
| Days in
intensive care unit | 12.4±6.56 | 22.7±4.12 | 0.019a |
Results
Clinicopathological
characteristics
There were no significant differences in age,
etiology or referral after INP onset between the two groups. The
primary etiology of INP was gallstone disease (13 in the PCD-alone
group and 18 in the PCD+necrosectomy group), followed by
hyperlipemia (8 in the PCD-alone group and 14 in the
PCD+necrosectomy group). The number of days spent in hospital or
the intensive care unit by patients in the PCD+necrosectomy group
was significantly higher than patients in the PCD-alone group (both
P<0.05; Table I).
Parameters and outcomes of the PCD
procedure
All 74 patients with INP underwent primary PCD under
ultrasound or CT guidance and if necessary this was followed by PN.
There were no significant differences in the technical details of
PCD between the two groups (Table
II). The mean interval between the onset of acute INP to PCD in
the PCD-alone group (28 days) was slightly shorter than that of the
PCD+necrosectomy group (32 days). However, this difference was not
significant. The mean number of PCD catheters was 1.5 per patient
(range, 1–5 per patient) in each group. The median catheter size
was 16 Fr (range, 8–32 Fr) in each group and the most common size
of the initial PCD catheter was 16 Fr in the two groups. The median
duration of drainage was relatively longer in the PCD-alone group
than that in the PCD+necrosectomy group (25 vs. 20 days), although
this difference was not significant. A total of 26 cases in the
PCD-alone success group underwent one PCD procedure. To avoid
further PN, an additional 6 cases underwent the PCD procedure 2–5
times and did not require PN. In the PCD+necrosectomy group, 8/42
cases (19%) underwent the PCD procedure 2–5 times, but still
underwent PN.
 | Table II.Technical details of PCD and
outcomes. |
Table II.
Technical details of PCD and
outcomes.
| Variable | PCD-alone group | PCD+necrosectomy
group | P-values |
|---|
| Number of
patients | 32 | 42 |
|
| Onset to PCD
(days) | 28.1±6.11 | 32.0±7.19 | 0.384 |
| Number of PCD
catheters | 1.5±0.77 | 1.5±0.63 | 0.948 |
| Median (range) | 2 (1–5) | 2 (1–5) |
|
| Catheter size, Fr
(range) | 16 (8–32) | 16 (8–32) | 0.980 |
| No. of PCDs
performed |
|
| 0.974 |
| 1 | 26 | 34 |
|
|
2–5 | 6 | 8 |
|
| Duration of
drainage (days) | 31.9±24.59 | 28.9±25.73 | 0.510 |
| Median (range) | 25 (3–98) | 20 (1–95) |
|
Differences in parameters between the
PCD-alone group and the PCD+necrosectomy group
Initial MCTSI and Modified Marshall scores were
significantly higher in the PCD+necrosectomy group than in the
PCD-alone group (both P<0.05; Table
III). The PCD+necrosectomy group also had a significantly
greater amount of maximum extent of necrosis, maximum extent of
fluid collection and reduction of fluid collection following PCD
compared with those in the PCD-alone group (P<0.05).
 | Table III.The laboratory and clinical related
parameters between the two groups. |
Table III.
The laboratory and clinical related
parameters between the two groups.
| Variable | PCD-alone
group | PCD+necrosectomy
group | P-values |
|---|
| Number of
patients | 32 | 42 |
|
| Severity score |
|
|
|
|
MCTSI | 6.0±1.48 | 7.3±1.87 | 0.020a |
|
Modified Marshall | 3.0±1.11 | 3.67±0.87 | 0.011a |
| Maximum extent of
necrosis |
|
| 0.011a |
|
<30% | 11 | 4 |
|
|
30–50% | 9 | 12 |
|
|
>50% | 12 | 26 |
|
| Maximum extent of
fluid collection |
|
| 0.008a |
| 100–300
ml | 4 | 0 |
|
| 300–500
ml | 23 | 25 |
|
| >500
ml | 5 | 17 |
|
| Reduction of fluid
collection following |
|
| 0.003a |
| PCD |
|
|
|
|
<50% | 10 | 28 |
|
|
>50% | 22 | 14 |
|
| Proportion of
reduction fluid collection (%) | 69.5 (30–90) | 35.0 (15–80) | 0.001a |
| Multiple organ
failure, n (%) | 6 (18.7) | 24 (57.1) | 0.001a |
| Mortality, n
(%) | 1 (3.1%) | 4 (9.5%) | 0.277 |
| Laboratory
parameters, initial |
|
|
|
| CPR
(mg/l) | 63.0±13.47 | 67.9±17.98 | 0.048a |
| PTC
(ng/ml) | 1.43±0.58 | 1.72±0.51 | 0.027a |
| WBC
(×109) | 16.4±7.48 | 13.9±5.67 | 0.103 |
The frequency of multiple organ failure was
significantly higher in the PCD+necrosectomy group than that in PCD
alone group (P<0.05; Table
III). Only 1 patient in the PCD-alone group succumbed to
multiple organ failure. By contrast, in the PCD+necrosectomy group
3 patients suffered from multiple organ failure and 1 patient
succumbed following multiple organ failure with uncontrolled sepsis
(Table III).
There were significant differences in the initial
serum CRP and PTC levels between the two groups (P<0.05),
however, there was no significant difference in the initial WBC
levels between groups (Table
III).
Multivariable logistic regression
analysis of the predictors of PN following PCD intervention
A total of nine parameters were used in the
univariate analysis (initial MCTSI scores, initial Modified
Marshall scores, maximum extent of necrosis, maximum extent of
peripancreatic fluid collection, reduction of fluid collection by
<50% following PCD, organ failure, initial serum CRP level, PTC
level and WBC count), which were assessed by multivariable logistic
regression analysis. It was identified that the reduction of fluid
collection following PCD (P=0.021), maximum extent of
peripancreatic necrosis (P=0.019) and multiple organ failure
(P=0.017) were predictors of PN following PCD (Table IV).
 | Table IV.Predictors of PN following PCD. |
Table IV.
Predictors of PN following PCD.
| Variable | 95% CI (lower
OR-upper OR) | P-values |
|---|
| Reduction of fluid
collection after PCD | 0.269
(0.088–0.818) | 0.021a |
| Maximum extent of
necrosis | 2.397
(1.158–4.962) | 0.019a |
| Multiple organ
failure | 4.256
(1.295–13.985) | 0.017a |
In addition, the number of patients experiencing a
reduction of fluid collection following PCD in the PCD+necrosectomy
group (14/42) was significantly lower (P<0.05) than that of the
PCD-alone group (22/32). More patients in the PCD+necrosectomy
group (26/42) had peripancreatic tissue necrosis of >50%
compared with those in the PCD-alone group (12/32; P<0.05).
Furthermore, significantly more patients in the PCD+necrosectomy
group (24/42) had multiple organ failure than those in the
PCD-alone group (6/32; P<0.05; Table III).
Final prediction model
The results of the bootstrap analysis of 200
resamples (Fig. 4) indicated that
final multivariable analysis confirmed three predictors of PN
following PCD as a primary treatment of patients with INP:
Reduction of fluid collection following PCD, maximum extent of
peripancreatic necrosis and multiple organ failure. The receiver
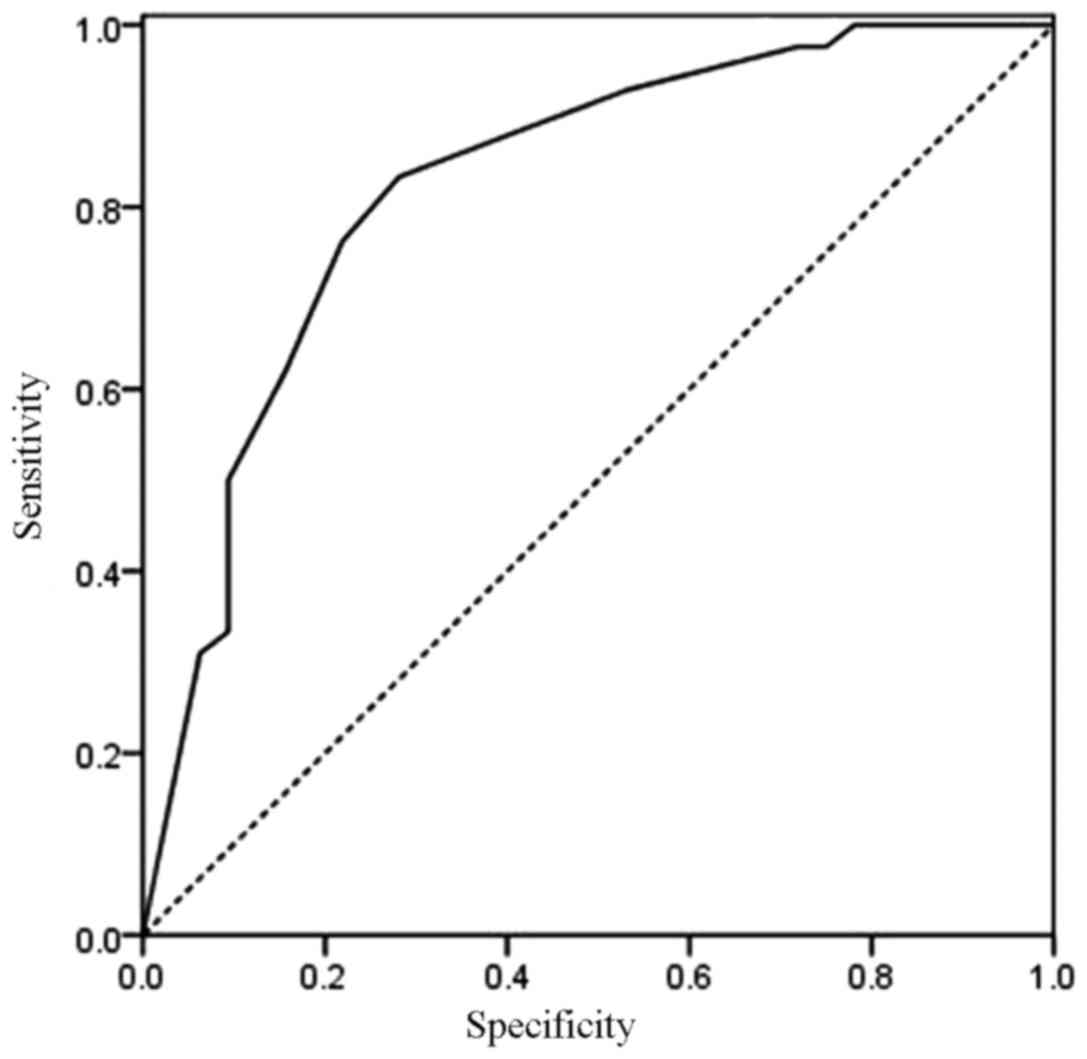
operating characteristic curve (Fig.
5) of the ROC model had an area under of 0.827 (95% CI,
0.728–0.925). Then, the nomogram was performed. The three factors
were independently associated with success of catheter drainage. A
nomogram was designed (Fig. 6) to
determine the association of these three factors with the success
of catheter drainage. This indicated that patients with a reduction
in fluid collection after PCD of <50%, a maximum extent of
peripancreatic necrosis of <30% and no multiple organ failure
had a ~93% chance of success of experiencing primary catheter
drainage. However, unfavorable scores (≥240 points) resulted in ~7%
chance of success of primary catheter drainage.
Discussion
In 1998, Freeny et al (17) primarily treated patients with INP
using imaging-guided PCD. Over the past two decades, PCD has been
applied to treat patients with uncomplicated INP and the use of PCD
to treat patients with INP has been assessed (18). PCD is able to treat patients with INP
and stabilize sepsis, thereby avoiding the use of PN. Van Baal
et al (19) reviewed the
outcomes of PCD in a mixed group of patients with PN. Out of all
the patients assessed, 55.7% recovered following PCD alone. Baudin
et al (20) suggested that
PCD was a safe and effective method of treating acute INP, although
35.4% of patients treated in this manner required further surgery.
Additionally, it was suggested that CT or ultrasound guided PCD
could be used to drain fluid collection around necrotic lesions and
avoid the use of surgical necrosectomy. However, Van Santvoort
et al (10) indicated that
PCD failed in 32.7% (17/52) patients and that 14 of these patients
subsequently required PN. This combinatorial treatment of PN
following PCD significantly increased the success rate in patients
with INP compared with patients undergoing open necrosectomy and
consequently contributed to improved long-term patient prognosis
(10).
Fluid collection may disperse following initial PCD
and the reduction of fluid collection influences the success rate
of PCD. Guo et al (12)
indicated that the CT mean density of necrotic fluid collection and
acute necrotic collection may influence the success rate of PCD.
Patients in the PCD-alone group had a lower CT mean density of
necrotic fluid collection compared with the failed PCD group (20/35
vs. 5/16, P=0.04). Following multivariate analysis of the possible
predictors of surgery, only CT mean density of necrotic fluid
collection (OR, 1.63; 95% CI 1.04–2.94; P=0.006) was identified as
a significant factor. The potential explanation for this is that a
higher CT density signifies a greater proportion of solid form in
the necrotic fluid collection, leading to obstruction of the
drainage tube. Therefore, in such cases, PCD will fail to reduce
necrotic fluid collection. This indicates that reduction of fluid
collection by PCD may influence its success rate and multiple PCD
or alteration of the drainage tube could reduce the collection of
fluid caused by obstruction. It was also demonstrated that 69%
(22/32) of patients in the PCD-alone group achieved >50%
reduction in fluid collection, which was significantly greater than
those in the PCD+necrosectomy group (14/42; 33%). Therefore,
patients with reduction of fluid collection of <50% following
PCD have a higher chance of requiring PN than those experiencing a
reduction of >50%.
Minimally invasive PN was identified as an important
intervention following the failure of PCD in the step-up approach,
which included retroperitoneal necrosectomy and endoscopic
transgastric necrosectomy (7). Van
Santvoort et al (10)
confirmed that minimally invasive PN following PCD reduced the risk
of major complications or mortality occurring in patients with INP
compared with those undergoing open necrosectomy. However, there
are no definitive criteria able to predict which subset of patients
with INP require minimally invasive PN following initial PCD among
those managed using the step-up approach. The aim of the present
study was to investigate the circumstances under which PN benefits
patients with INP that have undergone PCD and to identify
predictors for PN.
Babu et al (21) conducted a prospective study
investigating 70 patients with severe acute pancreatitis and
suggested that a maximum extent of necrosis of >50% in the
pancreas may not be a predictor of surgery in the early course of
severe acute pancreatitis. However, Liu et al (14) indicated that the maximum extent of
necrosis is an important indicator in a step-up approach, which
suggests that PN should be performed. The present study also
indicated that a maximum extent of peripancreatic necrosis of
>50% increased the likelihood of PN. Babu et al (21) identified that organ failure within 1
week of disease onset was a predictor of surgery in the early
course of severe acute pancreatitis. In the present study,
necrosectomy was performed 4 weeks after disease onset in the
majority of patients. A total of 31/74 (41.8%) patients were
treated by PCD alone and one patient in the PCD-alone group
succumbed to multiple organ failure. A necrosectomy was performed
in 42/74 (56.8%) cases. A total of 4 patients in the
PCD+necrosectomy group succumbed due to multiple organ failure and
uncontrolled sepsis. Multiple organ failure is a key factor leading
to mortality in patients with acute INP (3). It has been reported that the incidence
of organ failure in patients with acute INP is 54% (1). Patients with no organ failure have a 0%
mortality rate, those with single organ failure have a median
mortality of 3% and those with multiple organ failure have a median
mortality rate of 47% (1). Van Baal
et al (19) performed a
systematic literature search investigating the mortality rates of
patients undergoing PCD treatment for INP and determined that the
overall mortality rate was 17.4% (67/384). However, Rocha et
al (22) suggested that the
mortality rate in patients with INP and multiple organ failure
treated with PCD alone was 6/11 (55%) and Mortelé et al
(23) identified that 5/11 (45%)
patients treated with PCD alone succumbed from multiple organ
failure. Although PCD may successfully treat ~50% of patients with
INP, it does not appear to reduce mortality rates following
multiple organ failure. In the current study, multiple organ
failure occurred in 4/32 (12.5%) patients who underwent PCD-alone,
during which 1/4 patients (25%) succumbed. Multiple organ failure
occurred in 24/42 (57.1%) patients who underwent PCD+necrosectomy,
during which 4/24 patients (16.7%) succumbed. For patients
experiencing multiple organ failure, the mortality rate in the
PCD+necrosectomy group was significantly lower than that of the
PCD-alone group. PN may therefore reduce mortality rates among
patients with multiple organ failure and multiple organ failure may
also be an effective predictor of necrosectomy in patients with
INP.
There were a number of limitations in the current
study. Although it provides the evidence for the predictors of PN
following PCD, further studies with larger sample sizes are
required, including a multicenter randomized controlled trial.
Furthermore, the step-up approach requires unique expertise, thus
the treatment received by patients may have been influenced by the
variability of experience among radiologists and surgeons. This is
inherent in any retrospective study and difficult to control. These
novel predictors identified in the current study require further
investigation if they are to be developed for clinical
treatment.
In conclusion, a reduction of fluid collection by
<50% following PCD, maximum extent of peripancreatic necrosis of
>50% and multiple organ failure may be effective predictors of
necrosectomy in patients with INP following PCD failure.
Acknowledgements
The authors wish to thank the Beijing Municipal
Administration of Hospitals Clinical Medicine Development for their
support (grant no. XMLX201404).
References
|
1
|
Banks PA and Freeman ML: Practice
Parameters Committee of the American College of Gastroenterology:
Practice guidelines in acute pancreatitis. Am J Gastroenterol.
101:2379–2400. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
van Baal MC, Bollen TL, Bakker OJ, van
Goor H, Boermeester MA, Dejong CH, Gooszen HG, van der Harst E, van
Eijck CH, van Santvoort HC, et al: The role of routine fine-needle
aspiration in the diagnosis of infected necrotizing pancreatitis.
Surgery. 155:442–448. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guo Q, Li A, Xia Q, Liu X, Tian B, Mai G,
Huang Z, Chen G, Tang W, Jin X, et al: The role of organ failure
and infection in necrotizing pancreatitis: A prospective study. Ann
Surg. 259:1201–1207. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Götzinger P, Sautner T, Kriwanek S,
Beckerhinn P, Barlan M, Armbruster C, Wamser P and Függer R:
Surgical treatment for severe acute pancreatitis: Extent and
surgical control of necrosis determine outcome. World J Surg.
26:474–478. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rau B, Bothe A and Beger HG: Surgical
treatment of necrotizing pancreatitis by necrosectomy and closed
lavage: Changing patient characteristics and outcome in a 19-year,
single-center series. Surgery. 138:28–39. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gou S, Xiong J, Wu H, Zhou F, Tao J, Liu T
and Wang C: Five-year cohort study of open pancreatic necrosectomy
for necotizing pancreatitis suggests it is a safe and effective
operation. J Gastrointest Surg. 17:1634–1642. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bausch D, Wellner U, Kahl S, Kuesters S,
Richter-Schrag HJ, Utzolino S, Hopt UT, Keck T and Fischer A:
Minimally invasive operations for acute necrotizing pancreatitis:
Comparison of minimally invasive retroperitoneal necrosectomy with
endoscopic transgastric necrosectomy. Surgery. 152:S128–S134. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Besselink MG, van Santvoort HC,
Nieuwenhuijs VB, Boermeester MA, Bollen TL, Buskens E, Dejong CH,
van Eijck CH, van Goor H, Hofker SS, et al: Minimally invasive
‘step-up approach’ versus maximal necrosectomy in patients with
acute necrotising pancreatitis (PANTER trial): Design and rationale
of a randomised controlled multicenter trial [ISRCTN13975868]. BMC
Surg. 6:62006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Worhunsky DJ, Qadan M, Dua MM, Park WG,
Poultsides GA, Norton JA and Visser BC: Laparoscopic transgastric
necrosectomy for the management of pancreatic necrosis. J Am Coll
Surg. 219:735–743. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
van Santvoort HC, Besselink MG, Bakker OJ,
Hofker HS, Boermeester MA, Dejong CH, van Goor H, Schaapherder AF,
van Eijck CH, Bollen TL, et al: A step-up approach or open
necrosectomy for necrotizing pancreatitis. N Engl J Med.
362:1491–1502. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kumar N, Conwell DL and Thompson CC:
Direct endoscopic necrosectomy versus step-up approach for
walled-off pancreatic necrosis: Comparison of clinical outcome and
health care utilization. Pancreas. 43:1334–1339. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Guo Q, Li A and Hu W: Predictive factors
for successful ultrasound-guided percutaneous drainage in
necrotizing pancreatitis. Surg Endosc. 30:2929–2934. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Albers D, Toermer T, Charton JP, Neuhaus H
and Schumacher B: Endoscopic therapy for infected pancreatic
necrosis using fully covered self-expandable metal stents:
Combination of transluminal necrosectomy, transluminal and
percutaneous drainage. Z Gastroenterol. 54:26–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu WH, Wang T, Yan HT, Chen T, Xu C, Ye
P, Zhang N, Liu ZC and Tang LJ: Predictors of percutaneous catheter
drainage (PCD) after abdominal paracentesis drainage (APD) in
patients with moderately severe or severe acute pancreatitis along
with fluid collections. PLoS One. 10:e01153482015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zaheer A, Singh VK, Qureshi RO and Fishman
EK: The revised Atlanta classification for acute pancreatitis:
Updates in imaging terminology and guidelines. Abdom Imaging.
38:125–136. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Banks PA, Bollen TL, Dervenis C, Gooszen
HG, Johnson CD, Sarr MG, Tsiotos GG and Vege SS: Acute Pancreatitis
Classification Working Group: Classification of acute
pancreatitis-2012: Revision of the Atlanta classification and
definitions by international concensus. Gut. 62:102–111. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Freeny PC, Hauptmann E, Althaus SJ,
Traverso LW and Sinanan M: Percutaneous CT-guided catheter drainage
of infected acute necrotizing pancreatitis: Techniques and results.
AJR Am J Roentgenol. 170:969–975. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Terayama T, Hifumi T, Kiriu N, Kato H,
Koido Y, Ichinose Y, Morimoto K and Yasuhiro K: A minimally
invasive multiple percutaneous drainage technique for acute
necrotizing pancreatitis. World J Emerg Med. 5:310–312. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van Baal MC, van Santvoort HC, Bollen TL,
Bakker OJ, Besselink MG and Gooszen HG: Dutch Pancreatitis Study
Group: Systematic review of percutaneous catheter drainage as
primary treatment for necrotizing pancreatitis. Br J Surg.
98:18–27. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Baudin G, Chassang M, Gelsi E, Novellas S,
Bernardin G, Hébuterne X and Chevallier P: CT-guided percutaneous
catheter drainage of acute infectious necrotizing pancreatitis:
Assessment of effectiveness and safety. AJR Am J Roentgenol.
199:192–199. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Babu RY, Gupta R, Kang M, Bhasin DK, Rana
SS and Singh R: Predictors of surgery in patients with severe acute
pancreatitis managed by the step-up approach. Ann Surg.
257:737–750. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rocha FG, Benoit E, Zinner MJ, Whang EE,
Banks PA, Ashley SW and Mortele KJ: Impact of radiologic
intervention on mortality in necrotizing pancreatitis: The role of
organ failure. Arch Surg. 144:261–265. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mortelé KJ, Girshman J, Szejnfeld D,
Ashley SW, Erturk SM, Banks PA and Silverman SG: CT-guided
percutaneous catheter drainage of acute necrotizing pancreatitis:
Clinical experience and observations in patients with sterile and
infected necrosis. AJR Am J Roentgenol. 192:110–116. 2009.
View Article : Google Scholar : PubMed/NCBI
|