Introduction
Mandibular tissue defects are mainly caused by
developmental deformity, trauma, and tumor resection (1,2). Trypsin
digestion and cell inoculation are the traditional methods to
repair mandibular tissue defects. This method is prone to reduce
cell activity, which leads to mass cell death, lower utilization
rate of cells, difficulty in formation of dense bone tissue and
other deficiencies. These complications hinder the recovery
expected following the clinical treatment (3–5). To
improve the utilization rate of the transplanted cells, the harvest
and inoculation of bioactive seed cells need to be optimized using
bone tissue engineering (6). The
development of bone tissue engineering provides a new avenue for
the repair of jaw injuries. Cellular secretions and maintenance of
tissue structures can be effectively retained by cell sheet
technology, which reduces the loss of seed cells (7,8). In this
study, we applied cell sheet technology to bone tissue engineering.
The scaffold surface of polylactic-co-glycolic acid (PLGA) is
covered with a cell sheet and is implanted in the region of
mandibular injury in dogs to establish normal functional bone and
bone structure.
Materials and methods
Experimental animals
We obtained 12 healthy mongrel dogs from the
Laboratory Animal Center of The Fifth People's Hospital of Jinan,
regardless of their sex, weighing 21–32 kg and ages 14–23
months.
Main instruments and reagents
Temperature-responsive culture dish (Shanghai Qifu
Biotechnology Co., Ltd., Shanghai, China), Dulbecco's modified
Eagle's medium nutrient solution (Shanghai Genmed Gene
Pharmaceutical Technology Co., Ltd., Shanghai, China), PLGA
scaffold (Changzhou New District Jiasen Medical Bracket
Instrument), inverted phase contrast microscope (Shanghai Pooher
Optoelectronics Technology Co., Ltd., Shanghai, China), scanning
electron microscope (Star Joy Co., Ltd., Guangzhou, China), cell
incubator (Precision Instrument, Shanghai, China), trypsin (Peptone
Biological Products, Shandong, China) and osteogenic inducing fluid
(Han Heng Biotechnology, Shanghai, China).
Stem cell isolation and culture
A total of 15 ml of bone marrow blood was extracted
from experimental dogs under anesthesia. Stem cells were isolated
by density gradient centrifugation and the density of stem cells
was diluted to 107 cells/ml in nutrient solution. The
cells were inoculated in 45 ml culture flasks and cultured under
saturated humidity (36°C, 6% CO2), followed by
observation under the inverted phase contrast microscope. When
cells reached 70% of confluence, they were digested with trypsin
containing 0.03% elhylene diamine tetraacetic acid and subcultured
at a 1:2 ratio.
Osteogenic induction of stem
cells
To the subcultured cells we added 5 ml high-glucose
medium containing 11% fetal calf serum and osteogenic inducing
media and cultured under saturated humidity (36°C, 6%
CO2) to promote the induction of stem cells into
osteoblasts.
Stem cell sheet preparation
The stem cells after osteogenic induction were
digested with trypsin, inoculated at a density of 107
cells/ml in temperature-responsive culture dish after, and then
placed under saturated humidity (36°C, 6% CO2). The stem
cells were laid at the bottom of culture dish 12 days later.
Subsequently, the culture dish was placed in the calorstat for 25
min and stem cells and the bottom of culture dish were separated to
form the cell sheet (3).
Stem cell inoculation and scaffold
surface wrapped with cell sheet
Scaffolds were soaked and divided in two groups. In
group A, the scaffold surface was wrapped with cell sheets
(experimental group). The scaffold surface in group B was not
wrapped with cell sheets (control group). The cells were cultured
under saturated humidity (36°C, 6% CO2) for 5 days,
followed by scanning and observing under the electron microscope.
The study was approved by the Ethics Committee of the Fifth
People's Hospital of Jinan.
Canine mandibular defect
implantation
A total of 12 dogs were divided into experimental
and control groups, with 6 animals in each group. After intravenous
anesthesia, a 6 cm-long incision was made in the lower edge of the
mandible on both sides and the skin, muscle and fascia were cut
open to expose the mandible body. A trapezoid (up broad and down
narrow) injury with shape similar to the PLGA scaffold was created
in both mandibles. We retained the inferior alveolar nerves and
vessels to prevent rejection of the implant. Scaffolds covered and
not covered with cell sheets were implanted in the experimental and
control groups, respectively. The groove of the scaffold was
embedded in the mandibular nerve vessel, and soft tissue was
carefully sutured, followed by fixation of the scaffold;
3×106 U of penicillin were administered every day for
one week following surgery.
Observation indexes
i) Gross observation: two experimental animals were
sacrificed at postoperative 3, 9 and 12 weeks, and bilateral
mandibles were removed for gross observation.
ii) Imaging: X-ray imaging of mandibles were
obtained under the same projection conditions. Optical density was
analyzed and measured by Image-Pro Plus 6.0 (Media Cybernetics
Inc., Rockville, MD, USA) software.
iii) Histological examination: Partial specimen
tissues of the same region were removed and processed by
conventional demineralization, fixation, staining, sliced, and
followed by observation under inverted phase contrast
microscope.
iv) The ratio of bone tissue surface on each slice
was calculated by Adobe Photoshop 7.0 (Adobe Systems, Inc., San
Jose, CA, USA) and Image-Pro Plus 6.0 (Media Cybernetics Inc.,
Rockville, MD, USA) software. To obtain accurate results, the
percentage of bone area was calculated after extracting the serial
slices of the same part from all the specimens.
Statistical analysis
SPSS 20.0 (IBM Corp., Armonk, NY, USA) statistical
software was used for analysis. The differences of optical density,
bone mass and bone area between two groups were compared by
analysis of variance (ANOVA). A P<0.05 was considered to
indicate a statistically significant difference.
Results
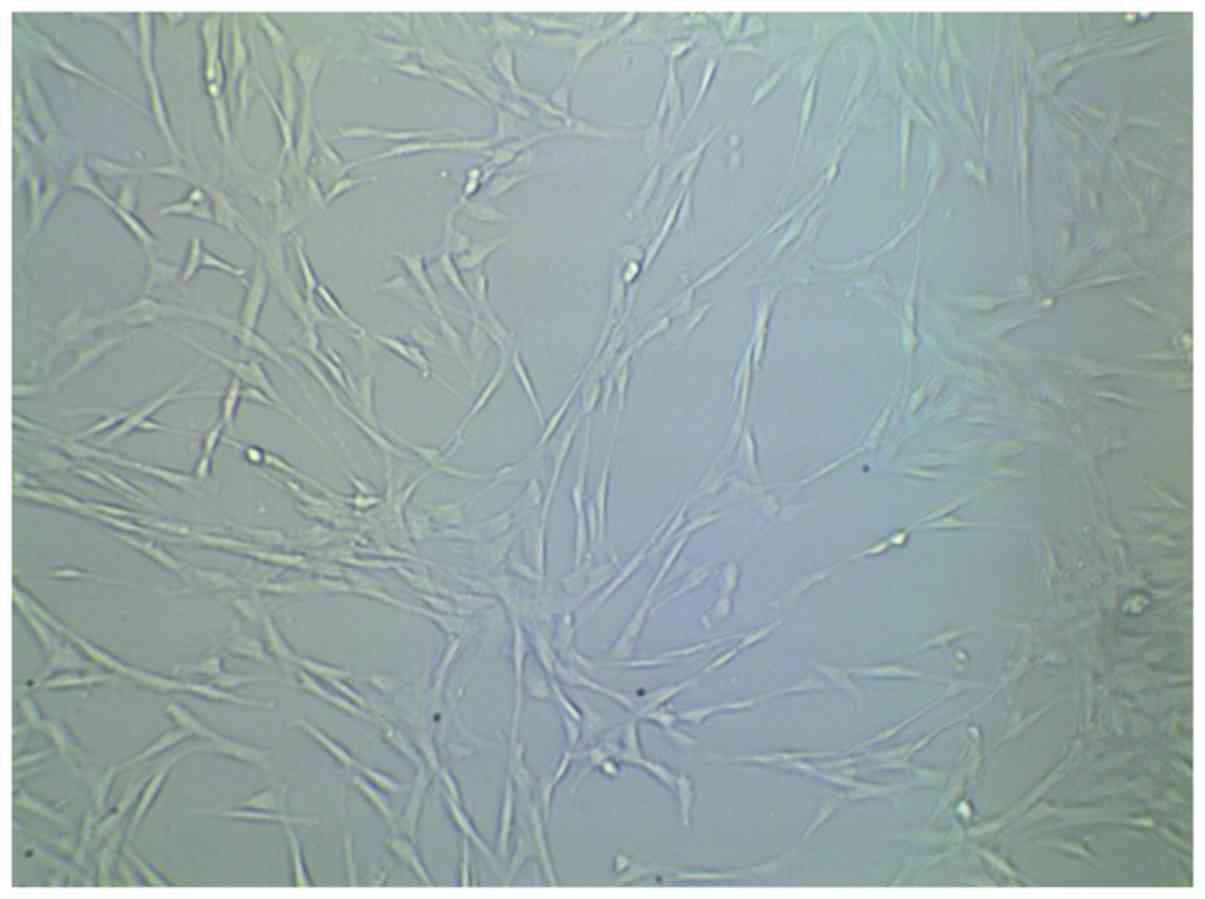
Examination of cultured stem cells
prior to implantation
Following culture of cells with and without
scaffold, we proceeded to examine them by scanning electron
microscopy. Cell sheets in the experimental group were widely
distributed on the surface on the small openings of the scaffold
material 7 days after cell inoculation (Fig. 1). Cells were tightly adhered and
fully extended, they were connected with each other and a large
amount of extracellular matrix components could be observed.
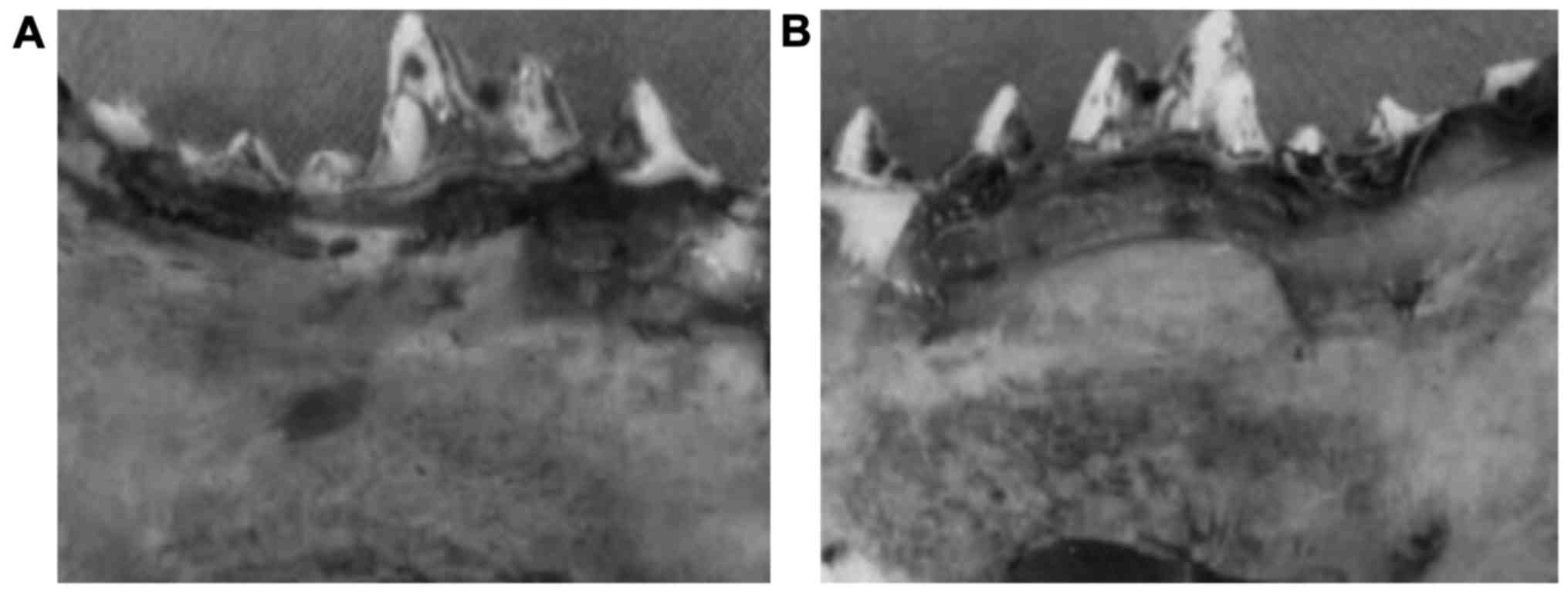
Gross observation of mandibular
implants
All the animals survived the surgery and
implantation. The gross observation of the 12 dogs showed that the
scaffold material was wrapped with soft tissue at postoperative 3
weeks, but the scaffold structure could still be seen. The broken
end of bone around the scaffold was distinct and it was relatively
soft to the touch. We found no differences between the experimental
and control groups. At postoperative 9 weeks, partial scaffold
structures could be seen on both sides. The combination of broken
end with scaffold was relatively compact, with unclear boundaries,
showing less absorption of the lower part of scaffold. The scaffold
was significantly less absorbed in the experimental group compared
to the control group. At postoperative 12 weeks, the lateral bone
injury was replaced by new bone tissue repair in the experimental
group. The compact bone at lingual position was similar to normal
bone and the broken end of the bone was healed, showing the similar
hardness to the surrounding bone tissues (Fig. 2A). The bone mass in the control group
was 2.5, which was significantly lower than 4.5 in the experimental
group (P<0.05). In contrast, the mandibular bone injury could
still be seen in the control group (Fig.
2B).
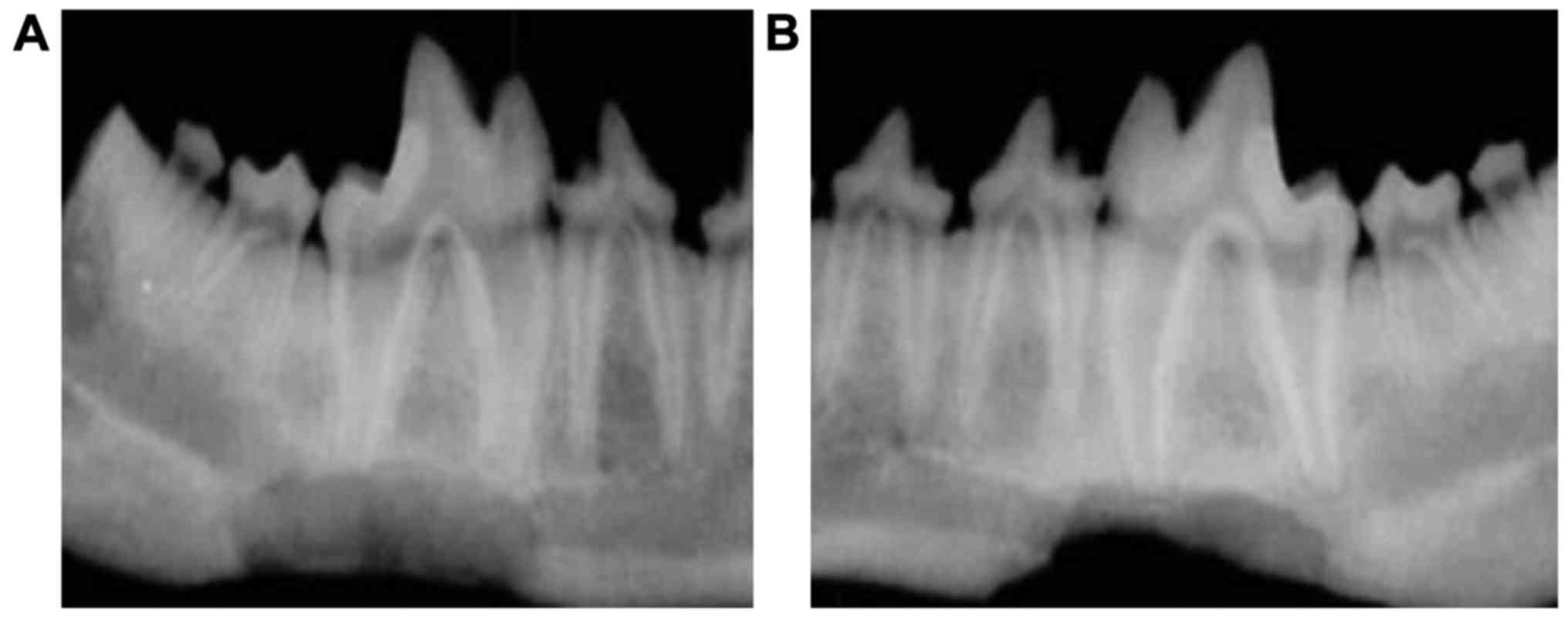
Bone imaging
Next, we conducted X-ray imaging of the injured
mandibles to examine the recovery in more detail. The optical
density of mandibles in the experimental and control groups
increased gradually over time from postoperative 3–12 weeks and the
differences were statistically significant at different time points
(P<0.05). The optical density was significantly higher in the
experimental than that in control group at the same postoperative
time point (Table I and Fig. 3). At postoperative 12 weeks, the
optical density for the experimental group reached the highest
value, but the optical density was still lower than that of normal
bone tissue. The irregular bone trabecular shadow could still be
seen within 3–12 weeks after material implantation. Over time, the
amount of bone trabecular increased and a high-density fracture
line could be observed at the broken end of the bone (Fig. 3).
 | Table I.Postoperative optical absorbance over
time. |
Table I.
Postoperative optical absorbance over
time.
|
| Postoperative
weeks |
|---|
|
|
|
|---|
| Groups | 3 | 9 | 12 |
|---|
| Control | 0.545±0.017 | 0.683±0.043 | 0.711±0.012 |
| Experimental | 0.621±0.023 | 0.802±0.065 | 0.945±0.033 |
| t-test | 7.031 | 8.004 | 9.562 |
| P-value | <0.05 | <0.05 | <0.05 |
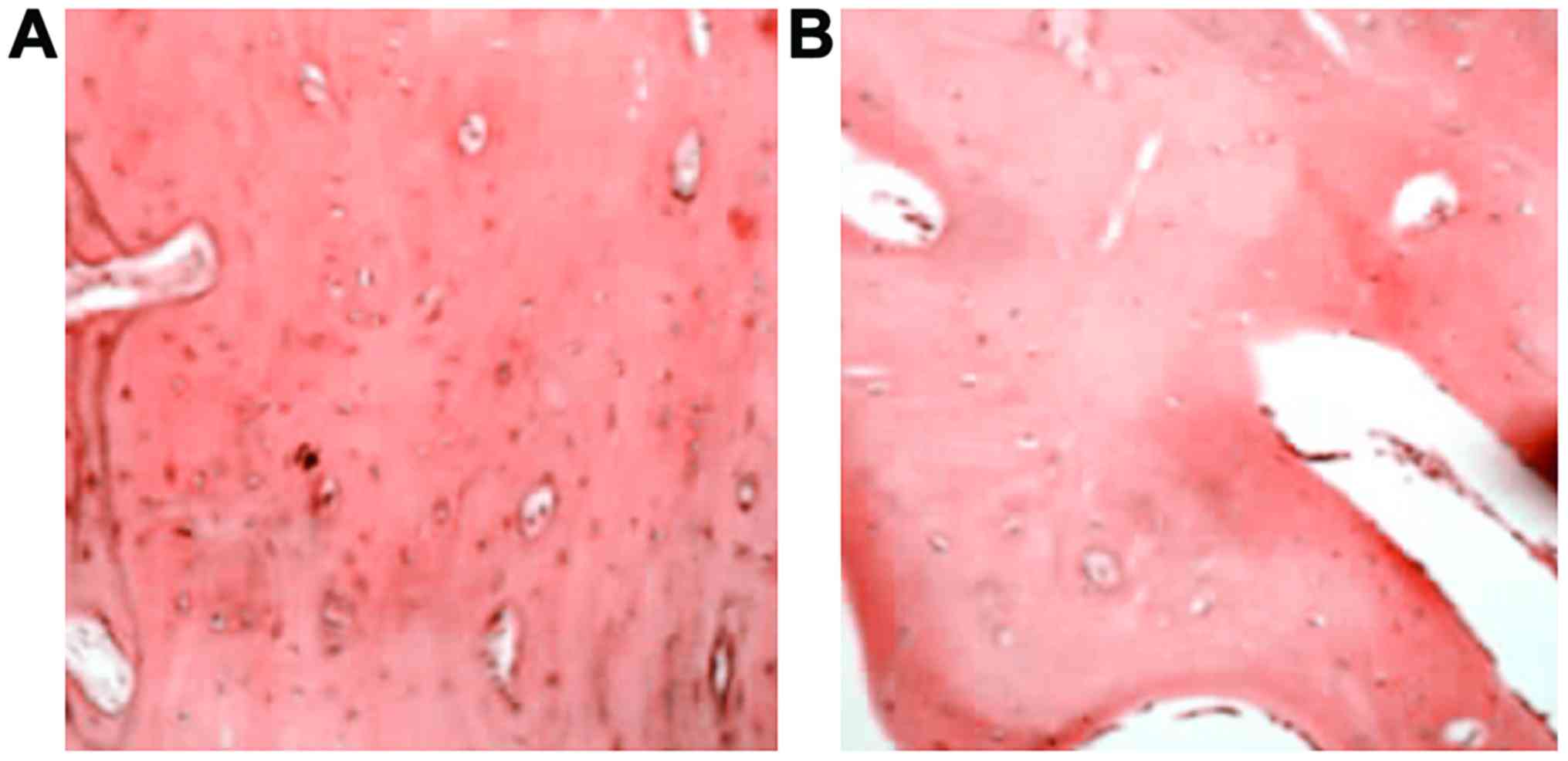
Histological observation of
osteoblast
At postoperative 12 weeks, the bone trabecula was
thick and large at bilateral positions in the experimental group
(Fig. 4A). The haversian canal was
abundant and concentric lamellar bone could be seen around it, with
red bone marrow and a large number of bone cells in good condition.
The vessels in the bone marrow were abundant containing much
calcium salt deposition. The bone trabecula and the haversian canal
were smaller in control group (Fig.
4B).
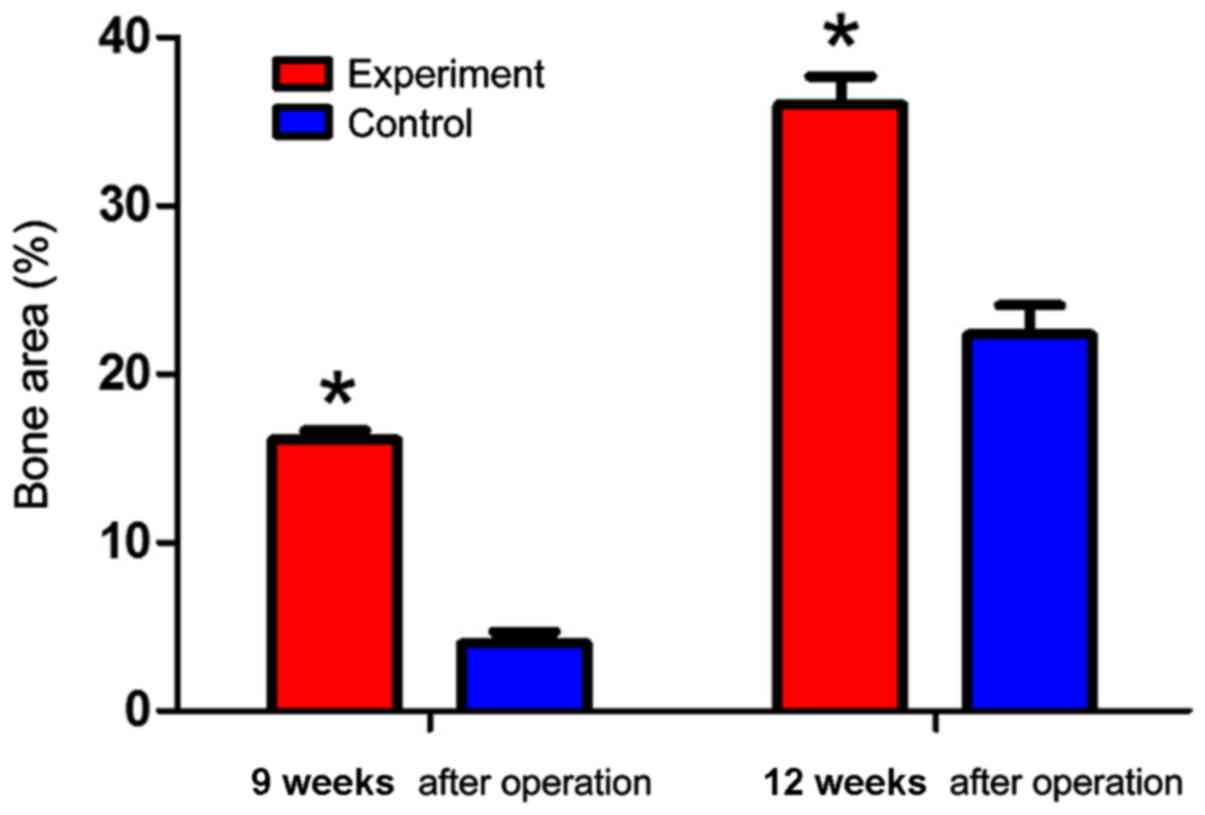
Tissue morphology
Finally, we examined the bone area recovered after
implantation. Bone areas in the experimental group were
significantly larger than those in the control group at
postoperative 9 and 12 weeks (P<0.05) (Fig. 5).
Discussion
The repair of jaw injury is still a difficult
clinical problem that needs an urgent solution (9). In previous years, the development of
bone tissue engineering provides new approaches to repair jaw
injuries. Good scaffold materials and seed cells are critical to
promote the development of bone tissue (10–12).
Cell sheets, a method of harvesting seed cells, obtains seed cells
via temperature induction (13,14).
Cells are laid at the bottom of a temperature-responsive culture
dish and cultured for 12 days at 25°C, until the formation of a
hydrated film between the cells and the material at the bottom of
the dish (15). Cells are completely
separated from the culture dish, thereby forming cell sheets. Cell
sheets have multiple advantages and have been applied in many
research fields (16–18). For instance, tubular myocardial
structure established by cell sheet technology demonstrate a degree
of functionality (19). Corneal
tissue can also be rebuilt on the oral mucosa via cell sheet
technology (20). New bone tissue
with similar structure to the normal bone can be established and
grafted.
Here, we established tissue engineering bone cells
by cell sheet technology and plastic scaffold. Canine stem cells
were induced into osteoblasts to form cell sheets and scaffold
material covered with the cell sheets were implanted in canine
mandibular injuries. The porous scaffold material, which provides
an adhesion surface for the stem cells, contributes to the smooth
growth of cells in the injury site, showing a higher degree of
plasticity and strength (21). The
PLGA scaffold used in this experiment demonstrates better
biocompatibility with biological cells. The PLGA scaffold is a
three-dimensional net-like structure and its gap size is conducive
to bone cell and vessel growth, thus ensuring the consistency of
growth rate of new bone with scaffold degradation rate (22–24). The
PLGA scaffold used in this experiment adopts a trapezoidal shape. A
groove is maintained on the dorsal side of the scaffold to
accommodate the mandibular nerve vessels, allowing the inferior
alveolar artery to penetrate into the inner part of scaffold,
thereby creating conditions to provide blood supply for the growing
bone tissue.
In conclusion, the satisfactory tissue engineering
of bone containing lamellar bone can be established by cell sheet
technology, making this technology an ideal method to repair
mandibular injuries. However, there are still multiple problems in
establishing engineered bone tissue, which require further
research. The most critical problems are the difficulty of
producing cell sheets and achieving highly functional scaffolds
wrapped with cell sheets.
References
|
1
|
Li H, Sun S and Liu H, Chen H, Rong X, Lou
J, Yang Y, Yang Y and Liu H: Use of a biological reactor and
platelet-rich plasma for the construction of tissue-engineered bone
to repair articular cartilage defects. Exp Ther Med. 12:711–719.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McDaniel JS, Pilia M, Raut V, Ledford J,
Shiels SM, Wenke JC, Barnes B and Rathbone CR: Alternatives to
autograft evaluated in a rabbit segmental bone defect. Int Orthop.
40:197–203. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang L, Zou D, Zhang S, Zhao J, Pan K and
Huang Y: Repair of bone defects around dental implants with bone
morphogenetic protein/fibroblast growth factor-loaded porous
calcium phosphate cement: A pilot study in a canine model. Clin
Oral Implants Res. 22:173–181. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kang BJ, Ryu HH, Park SS, Koyama Y,
Kikuchi M, Woo HM, Kim WH and Kweon OK: Comparing the osteogenic
potential of canine mesenchymal stem cells derived from adipose
tissues, bone marrow, umbilical cord blood, and Wharton's jelly for
treating bone defects. J Vet Sci. 13:299–310. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Heydarkhan-Hagvall S1, Schenke-Layland K,
Yang JQ, Heydarkhan S, Xu Y, Zuk PA, MacLellan WR and Beygui RE:
Human adipose stem cells: A potential cell source for
cardiovascular tissue engineering. Cells Tissues Organs.
187:263–274. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yao C, Bu L, Wang K, Li N, Wang L and Yu
Y: A study of repairing mandibular defect using tissue engineering
bone with bone marrow stem cells cell sheets in dog. Hua Xi Kou
Qiang Yi Xue Za Zhi. 30:229–233. 2012.(In Chinese). PubMed/NCBI
|
|
7
|
Udehiya RK, Amarpal, Aithal HP,
Kinjavdekar P, Pawde AM, Singh R and Sharma Taru G: Comparison of
autogenic and allogenic bone marrow derived mesenchymal stem cells
for repair of segmental bone defects in rabbits. Res Vet Sci.
94:743–752. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li H, Dai K, Tang T, Zhang X, Yan M and
Lou J: Bone regeneration by implantation of adipose-derived stromal
cells expressing BMP-2. Biochem Biophys Res Commun. 356:836–842.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sumide T, Nishida K, Yamato M, Ide T,
Hayashida Y, Watanabe K, Yang J, Kohno C, Kikuchi A, Maeda N, et
al: Functional human corneal endothelial cell sheets harvested from
temperature-responsive culture surfaces. FASEB J. 20:392–394.
2006.PubMed/NCBI
|
|
10
|
Tsai RJ and Tsai RY: From stem cell niche
environments to engineering of corneal epithelium tissue. Jpn J
Ophthalmol. 58:111–119. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yuan J, Zhang WJ, Liu G, Wei M, Qi ZL, Liu
W, Cui L and Cao YL: Repair of canine mandibular bone defects with
bone marrow stromal cells and coral. Tissue Eng Part A.
16:1385–1394. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Girolamo ND: Adult human corneal
epithelial stem cellsAdult Stem Cells. Turksen K: Springer; New
York, NY: pp. 163–197. 2014, View Article : Google Scholar
|
|
13
|
Kumashiro Y, Yamato M and Okano T: Cell
attachment-detachment control on temperature-responsive thin
surfaces for novel tissue engineering. Ann Biomed Eng.
38:1977–1988. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Raffaghello L, Bianchi G, Bertolotto M,
Montecucco F, Busca A, Dallegri F, Ottonello L and Pistoia V: Human
mesenchymal stem cells inhibit neutrophil apoptosis: A model for
neutrophil preservation in the bone marrow niche. Stem Cells.
26:151–162. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kaneshiro N, Sato M, Ishihara M, Mitani G,
Sakai H and Mochida J: Bioengineered chondrocyte sheets may be
potentially useful for the treatment of partial thickness defects
of articular cartilage. Biochem Biophys Res Commun. 349:723–731.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gao Z, Chen F, Zhang J, He L, Cheng X, Ma
Q and Mao T: Vitalisation of tubular coral scaffolds with cell
sheets for regeneration of long bones: A preliminary study in nude
mice. Br J Oral Maxillofac Surg. 47:116–122. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ueyama Y, Yagyuu T, Maeda M, Imada M,
Akahane M, Kawate K, Tanaka Y and Kirita T: Maxillofacial bone
regeneration with osteogenic matrix cell sheets: An experimental
study in rats. Arch Oral Biol. 72:138–145. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cui L, Liu B, Liu G, Zhang W, Cen L, Sun
J, Yin S, Liu W and Cao Y: Repair of cranial bone defects with
adipose derived stem cells and coral scaffold in a canine model.
Biomaterials. 28:5477–5486. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vilquin JT and Rosset P: Mesenchymal stem
cells in bone and cartilage repair: Current status. Regen Med.
1:589–604. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Binnebösel M, Ricken C, Klink CD, Junge K,
Jansen M and Schumpelick V: Safe rebuilding of the periodontal loss
an experimental study. Bull Pol Acad Sci Tech Sci. 63:527–532.
2016.
|
|
21
|
Li Y, Zhao S, Nan X, Wei H, Shi J, Li A
and Gou J: Repair of human periodontal bone defects by autologous
grafting stem cells derived from inflammatory dental pulp tissues.
Stem Cell Res Ther. 7:141–148. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jose MV, Thomas V, Johnson KT, Dean DR and
Nyairo E: Aligned PLGA/HA nanofibrous nanocomposite scaffolds for
bone tissue engineering. Acta Biomater. 5:305–315. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yan H and Tsujii K: Thermo-responsive
poly(N-isopropylacrylamide) gel containing polymeric surfactant
poly[2-(methacryloyloxyl)decylphosphate]: Correlation between rapid
collapsing characters and micelles of polymeric surfactant. J Oleo
Sci. 57:401–405. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen T, Wang Y, Bu L and Li N:
Construction of functional tissue-engineered bone using cell sheet
technology in a canine model. Exp Ther Med. 7:958–962. 2014.
View Article : Google Scholar : PubMed/NCBI
|