Introduction
Oral cancer is one of the most common cancers. Male
patients between the fifth and sixth decade are mainly affected by
oral squamous cell carcinoma (OSCC) (1). Advanced stages are often present at
primary diagnosis. Unfortunately, new insights into the
understanding and further treatment options of OSCC are still
lacking (1). Surgery has advanced
opportunities for good functional and reconstructive results
(2). Nevertheless, recurrence and
second tumours are dominating and influencing survival rates
(3). TNM classification and grading
were discussed as having a high impact on patients outcomes.
However, even recent studies indicate that this is not a sufficient
explanation system for predicting recurrence (4). Poor survival rates reflect the status
quo of OSCC and further therapy is frustrating in many cases. The
need to identify molecular markers with a therapeutical impact on
metastasis and recurrence is urgent. The correlation between tumour
biology and survival rates is becoming more relevant. Further,
tumour biology might lead to suitable therapeutic strategies. The
creation of a variable patient-specific key target therapy with
acceptable side effects is a main goal of today's research
(5). Various molecular markers have
emerged and have provided a new understanding of pathogenesis in
OSCC. Epidermal growth factor receptor (EGFR) is one well studied
target. The EGFR tyrosin kinase and its signal transduction pathway
is a key route for distinct molecular interactions. Intracellular
signalling chains, e.g., Ras/mitogen-activated protein kinase
(MAPK) and the activation of transcription and extracellular
chains, e.g., extracellular signal-regulated kinase (ERK) are
activated through the EGFR. Endpoints of the signalling chain are
supporting tumour growth, invasion, angiogenesis, metastases and
interactions with lymph nodes. Essential research was carried out
on the EGFR pathways with the emergence of EGFR antibody therapies
(6).
Recently, three EGFR antibodies were developed.
Cetuximab is a monoclonal immunglobulin G1 antibody inhibiting the
receptor, whereas Erlotinib and Afatinib are blocking proteins of
the ErbB family (7) and inhibit the
tyrosin kinase activity of the receptor. To date, Cetuximab is the
only EGFR antibody used in OSCC. EGFR overexpression was associated
with poor prognosis and decreased survival rates in several OSCC
studies. The first clinical study use of Cetuximab therapy in
combination with radiotherapy in advanced OSCC was assessed in the
often-cited study of Bonner et al (8). Effects of EGFR antibody therapy on
survival rates were reported within this trial, but with a limited
time benefit for the patients.
Despite these milestones at the beginning of the
clinical use, the treatment response to EGFR inhibitors is not
always sufficient resulting in a low or lack of impact on survival
rates and is also dependent on the amount of EGFR expression
(6). Further, up to 12 potential
ligands next to EGF exist that could interact with the receptor and
mutations of the receptor are not rare and could negatively
interact with the response (9).
Therefore improvements in therapy influencing survival rates are
necessary. One option to maximise therapeutical effects is to block
more targets than one in a single signal transduction pathway.
Dual-blocking or dual-targeting was also considered as a method to
enhance the anti-tumour response and is a topic of high clinical
impact (10). The combination of
antibodies targeting two patterns offers the chance to strengthen
effiacacy without increasing side-effects. One substrate of the
EGFR cascade is Cortactin (11).
Cortactin is located within the cytoplasm and around the nucleus.
It also co-localises with actin in the plasma membrane and at
peripheral adhesion sites (12,13).
Currently, the activity of Cortactin presents many unsolved
questions because of its complexity (14). Important actions of Cortactin include
cell spreading and adhesion (15).
Hence, Cortactin is clearly also essential in tumour progress. An
interpretation of the intensity of staining, of the proportion of
stained cells, of the score of positivity and of the use of
recommended scores is possible and has to be carried out carefully
and independently (16). Therefore,
our aim was, to use immunohistochemistry (IHC) indexing to create
subgroups with meaningful numbers of patient samples in order to
avoid overlaps, any interference of subgroups with insufficient
numbers and any lack of clarity. Our objective was further to
investigate the clinicopathological and prognostic significance of
the co-expression of EGFR and Cortactin via immunohistochemical
staining and to determine whether a collective of OSCC patients had
sufficient numbers for evaluation.
Patients and methods
Patients
In total, 222 patients were included in the current
study. They were treated between 2009 and 2011 at our maxillofacial
surgery department. Relevant data (Table
I) from patients diagnosed with OSCC for statistical evaluation
and formalin fixed and paraffin embedded tissue (FFPE) for
laboratory use were available in every single case. Regular follow
up examinations of every included patient were held at our
department according to the German guidelines of oral cancer
(17). All included patients
received regular follow up. In the first 2 years after the
diagnosis the follow up was done every 3 months, after 2 years the
follow up was done every 6 months until the fifth year. After the
fifth year our follow up was completed.
 | Table I.Clinical Parameters of the cohort-not
subdivided. |
Table I.
Clinical Parameters of the cohort-not
subdivided.
| Clinical
Parameters | Total (n=222) |
|---|
| Median age in years
(range) | 60.1
(49.2–69.7) |
| Gender |
|
Male/female | 175/47 |
| UICC stage |
|
| I | 25 |
| II | 40 |
|
III | 42 |
|
IVa | 118 |
| Tumour size |
|
| T1 | 46 |
| T2 | 92 |
| T3 | 34 |
|
T4a/b | 50 |
| N Stage |
|
| N0 | 97 |
| N1 | 37 |
| N2 | 88 |
|
Extracapsular spread | 24 |
| Grading |
|
| G1 | 12 |
| G2 | 113 |
| G3 | 97 |
The therapy regimes of the included patients were
primary surgery, with intra-operative margin control via the help
of frozen sections and with neck dissection with the intention of
curative treatment. All tumour tissues were collected at the main
tumour operation, which also included neck dissection. The tumour
was operated by excisional biopsy of the whole tumour.
Postoperative adjuvant cisplatin-based chemoradiation was performed
in cases of pN1, pN2 or tumour infiltration of the jaw or locally
infiltrating tumour growth of the oral cavity (T4a/b) and of
positive microscopic resection margins and/or extracapsular spread,
also according to the German guidelines for oral cancer as
previously described (18).
Exclusion criteria were death resulting from a cause
other than OSCC, distant metastasis at primary diagnosis and the
use of primary radiochemotherapy before operation. The methods were
approved by the ethics committee of the Technische Universität
München (no. 212108) and are in accordance with the Declaration of
Helsinki.
Tissue microarray (TMA)
construction
Two independent pathologists defined the centre of
the tumour and the invasion front of every study patient. The
tissue was formalin-fixed and paraffin-embedded in blocks. The
pathologists then marked the areas to be represented in the TMA. A
minimum of two tumour cores from the centre of the tumour, the
invasion front and the corresponding lymph nodes with a 6-mm core
size were assembled into the TMA by using a Tissue Microarrayer
(Beecher Instruments, Inc., Sun Prairie, WI, USA) as previously
described (18,19). All lymph nodes, used for the TMA,
were positive lymph nodes if the patient had positive lymph nodes.
If the patient had no positive lymph nodes, negative lymph nodes
were taken. Therefore lymph nodes of every patient were presented
in the TMA.
Immunohistochemistry
Immunohistochemical staining was performed as
described previously (20) by using
4-µm-thick sections of the TMA. The sections were incubated with
primary antibodies against EGFR (1:50; Dako, Hamburg, Germany); and
Cortactin (1:100; BD Bioscience, Heidelberg; Germany) overnight
according to the manufacturers' recommendations.
Scoring
Immunohistochemical samples were blind-scored by two
investigators and checked by one pathologist. EGFR and Cortactin
staining was evaluated under a light microscope (magnification,
×200). The immunostaining intensity and positive cell proportion
were assessed for both markers. Further, the staining was evaluated
via an immunoreactive score (IRS) (21). We also evaluated the EGFR expression
of both the cell cytoplasm and the cell membrane independently,
since EGFR has two cellular loci of expression.
The staining intensity score was adjusted on a scale
of 0-1-2-3: no staining was scored as 0; weak staining as 1;
intermediate staining as 2; and strong staining as 3. Positive cell
proportion was also assigned (0<25%; 1 if 25–50%; 2 if 50–75%;
and 3 if >75%) as previously described (22). For a combination of quality and
quantity, scores of intensity and quantity were multiplied (IRS
results: 0-1-2-4-6-9). For the evaluated markers of the current
cohort a final cut off score was determined as I (low expression:
0–4) and II (high expression: 6–9).
Statistical analysis
Data was analyzed with the SPSS for Windows, release
24.0.0, 2016 (SPSS, Inc., Chicago, IL, USA) and results were
presented as figures. Cox regression and Kaplan Meier curves were
used for survival analysis. Categories were tested for associations
by using cross tabs (Chi-square test). To compare groups, the
non-parametric Mann Whitney U-test was used. P<0.05 was
considered to indicate a statistically significant difference.
Results
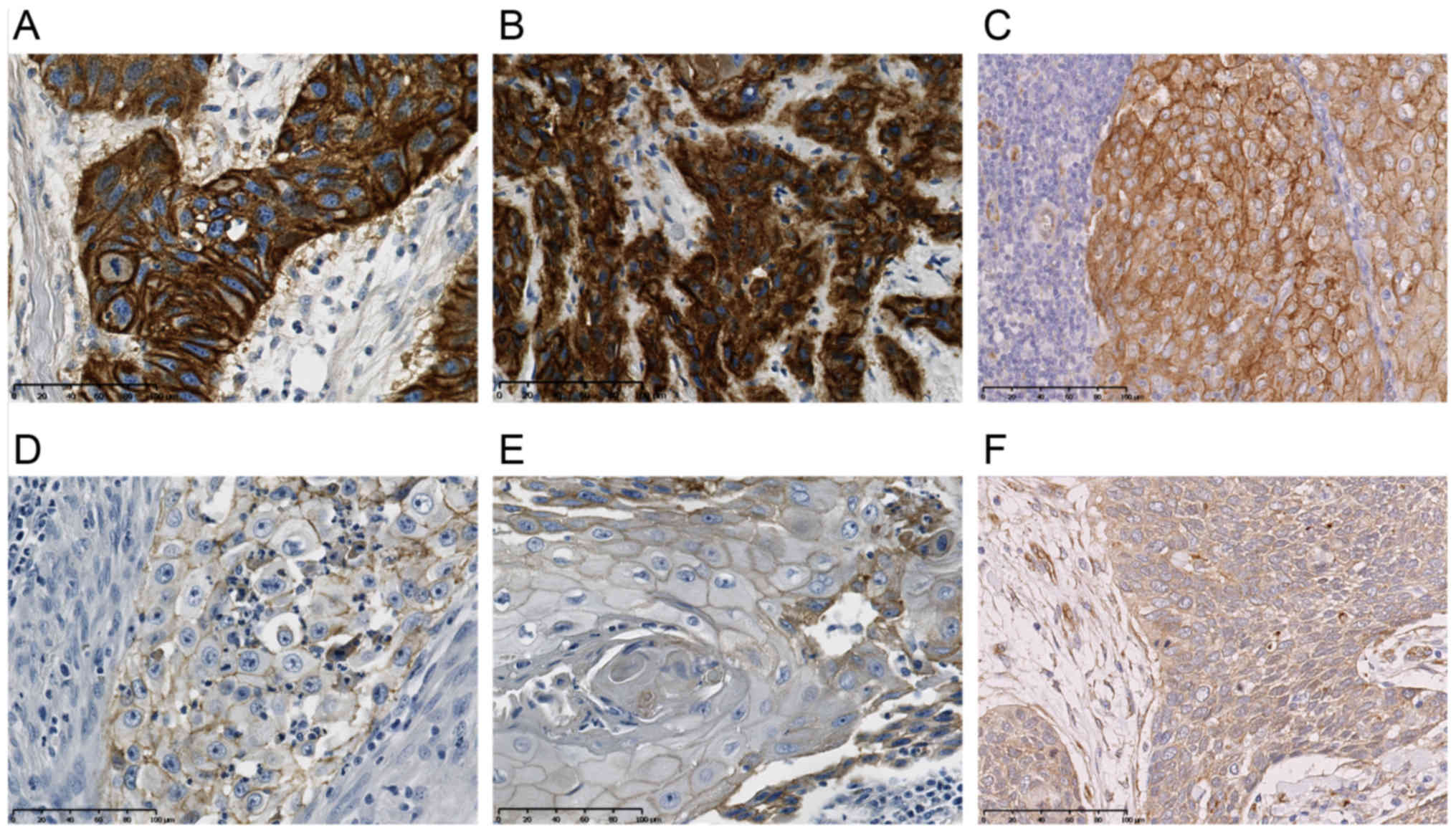
IHC scoring system of EGFR and
Cortactin
We analysed staining as described in the methods
section for cytoplasmic EGFR, membrane EGFR and Cortactin. The
staining results of the cut off value groups I and II for the
evaluated markers of the current cohort are shown in Fig. 1 (cytoplasmic EGFR, membrane EGFR and
Cortactin).
Association of Cortactin expression to
the survival rates
We used the Chi-square test to compare the
clinicopathological parameters between the Cortactin low expression
(I) and high expression (II) group. We did these tests for every
TMA localisation. All the following results are only valid for the
expression of the central tumour area. The invasion front and lymph
nodes had no impact on the evaluation of expression. The analysis
showed that overall survival was significantly poorer (P=0.037) in
the case of Cortactin II: 50.3 months [SD 3.59; 95% confidence
interval (CI): 43.28–57.36] compared with Cortactin I: 63.7 months
(SD 4.82; 95% CI: 54.22–73.13: Kaplan Meier curves of Cortactin).
Remarkably, during the analysis, Cortactin with a high expression
score had an influence on clinicopathological data (Table II). Cortactin II was significantly
associated with advanced UICC stages, especially III and IV
(P=0.032). T1 stages were rare in Cortactin II (P=0.021). The
incidence of lymphatic invasion (P=0.049) also dominated in
Cortactin II and showed significantly more N2 stages in this
cohort. Grading (P=0.057) and extracapsular spread (P=0.15) had no
influence.
 | Table II.Clinical parameters of the
cohort-subdivided to EGFR and Cortactin expression. |
Table II.
Clinical parameters of the
cohort-subdivided to EGFR and Cortactin expression.
| Clinical
parameters | EGFR | Cortactin |
|---|
| Median age in years
(range) | 57.6
(49.5–64.8) | 57.4
(44.3–65.8) |
| Gender |
|
|
| Male/female | 84/15 | 91/32 |
| UICC stage |
| I | 15 | 10 |
| II | 14 | 26 |
|
III | 19 | 23 |
|
IVa | 49 | 66 |
| Tumour size |
| T1 | 28 | 18 |
| T2 | 33 | 59 |
| T3 | 12 | 22 |
|
T4a/b | 25 | 25 |
| N Stage |
| N0 | 44 | 53 |
| N1 | 21 | 16 |
| N2 | 30 | 58 |
|
Extracapsular spread | 8 | 16 |
| Grading |
|
|
| G1 | 7 | 5 |
| G2 | 47 | 45 |
| G3 | 66 | 52 |
Association of EGFR expression to the
survival rates
The Chi-square test was also used to compare the
clinicopathological parameters between the EGFR low expression (I)
and high expression (II) group. We performed these tests for every
TMA localisation as for the Cortactin cohort. We evaluated
cytoplasmic EGFR and membrane EGFR. All the following results are
only valid for the expression of the central tumour area. The
invasion front and lymph nodes had no impact on the evaluation of
expression. The analysis showed that overall survival did not
differ in dependence on EGFR expression (cytoplasmic EGFR, P=0.636;
membrane EGFR, P=0.978). The average survival of the cytoplasmic
EGFR cohort for I was: 84.5 months (SD 7.98; 95% CI: 68.92–100.18)
and for II was 89.6 months [SD 7.49; 95% CI: 74.92–104.30, Fig. 2A (Kaplan Meier curves of cytoplasmic
EGFR)]. The membrane EGFR cohort had an average survival for I of
99.5 months (SD 13.29; 95% CI: 73.43–125.50) and for II of 99.5
months [SD 13.29; 95% CI: 73.43–125.50, Fig. 2B (Kaplan Meier curves of membrane
EGFR)]. Furthermore, cytoplasmic EGFR and membrane EGFR in an high
expression score did not have an influence on clinicopathological
data (Table II). EGFR was not
significantly associated with advanced UICC stages (cytoplasmic
EGFR, P=0.094; membrane EGFR, P=0.113) nor T stage (cytoplasmic
EGFR, P=0.670; membrane EGFR, P=0.439) or N stage (cytoplasmic
EGFR, P=0.473; membrane EGFR, P=0.113). Moreover, grading (P=0.33)
had no influence. Remarkably, extracapsular spread was
significantly associated with high cytoplasmic EGFR expression
(P=0.034).
Association of EGFR expression to
Cortactin expression and the survival rates
Interestingly, a strong co-expression in the tumour
centre of EGFR II and Cortactin II led significantly to reduced
survival rates (P=0.04) with a median of a reduction in survival by
8 months.
Clinical data
Relevant clinical data from the 222 included
patients with an OSCC diagnosis are listed in Table I. The average survival of the cohort
was 88.4 months (SD 4.8; 95% CI: 78.84–90.04). Risk factors such as
smoking and alcohol consumption were evaluated, with approximately
50% of the patients having a positive anamnesis. Expression of EGFR
II and Cortactin II in combination of smoking and regular alcohol
consumption was observed in 10% of all patients.
Lymph node recurrence played a major role in
survival (P=0.028), whereas local recurrence did not (P=0.128).
Lymph node recurrence occurred in 21 patients, which means that
recurrence of lymph nodes metastasis occured after surgical removal
and neck dissection. Local recurrence was evaluated in 35
patients.
The UICC stage (P=0.031), age of patients (P=0.012)
and lymph node metastasis (P=0.003) at the time of primary
diagnosis had a significant influence on overall survival
rates.
In contrast, patient gender, T category, extra
capsular spread and tumour grading were not significantly
associated with overall tumour-related survival (P>0.05) and
were independent of the marker expression status.
Discussion
The EGF cascade is an important pathway that is
upregulated in a high percentage of human tumours (23). EGF and its receptor have complex
influences on cell signalling and are key targets in oncology. EGFR
expression has been studied in various malignomas (24) and EGFR interaction was often
correlated with survival rates (25). Several studies emphasised that
expression level of EGFR is proportional to recurrence, therapy
failure and worse overall survival in OSCC (26). However, on the other hand, various
authors have argued that this does not reflect reality and, to
date, many trials are questioning the statement of the
proportionality of EGFR to worse survival rates (27). Therefore, one of our aims was to do
further research in the field of EGFR expression in a cohort of
OSCC with a large number of patients. Our results from the current
study do not confirm the unlimited correlation of high EGFR
expression to lower survival rates. Monoclonal antibody therapy is
linked to specific targets such as glycoproteins, vascular targets,
growth factors, stromal antigens and the cluster of differentiation
antigens (28). These therapies are
applied only in well-defined special clinical cases. Currently,
these individual target therapies are rescue therapies in OSCC and
other malignancies and are applied after the first- or second-line
therapy failed or in the case of recurrence after the first line
therapy protocol has been administered (29). Further, these antibody therapies
depend on the expression of the molecular target in order to be
started. In the case of OSCC, EGFR antibody therapy is selected if
cisplatin-based radiotherapy was unable to lead the tumour into
remission (6). Cetuximab is the
antibody of choice in the therapy of OSCC. The tyrosin kinase
inhibitors Erlotinib and Afatinib are developed e.g., for use in
lung cancer and gastric cancer (30,31). Yet
these tyrosin kinase inhibiting antibodies are not authorised for
the clinical use in OSCC. Due to our results showing the lack of
influence of EGFR expression on survival rates, as previously
suggested by other studies, we emphasise hereby the importance of
double-target blocking with additional key targets as EGFR
monotherapy might not be sufficient to eliminate EGFR-positive
tumours (32). Another important
issue is that resistance of EGFR to the antibody therapy are
emerging, and could cause an altered therapy response. In
particular, associations to the expression of multi-drug resistance
proteins are newly being discussed (33,34).
Molecular cross-talk offers options for identifying targets for
future therapies (35). Further, a
strong clinical correlation of every target is necessary. Several
co-targets are considered in the literature. Cortactin plays a
major role in cell interactions and Cortactin influences survival
in OSCC in a significant way according to our current results. We
could set a proof-of-principle in our cohort with regard to the
influence of Cortactin in OSCC. To the best of our knowledge, the
expression of both EGFR and Cortactin was not evaluated previously
in OSCC. Our results show, that these interactions should not be
disregarded. Discordance to previous published results regarding
Cortactin expression can be explained on the basis of the use od
smaller cohorts (36). Evaluation
methods such as IHC scoring must be extremely detailed and well
thought out to provide safe prognostic values of potential
biomarkers (16,37). Therefore, we conducted IRS scoring
and further divided the collective into the score cohorts I and II
to avoid any interferences of subgroups with insufficient numbers.
Moreover, we evaluated distinct localisations of the tumour, as
also heed in the TMA: the centre of the tumour, the invasion front
and the corresponding lymph nodes because of the potential
differential expression of the biomarkers (38). Hence, we evaluated the mentioned
tumour regions independently. Our results showed that significant
interactions of Cortactin occur in the central tumour area. The
area of tumour invasion and the lymph nodes play no significant
role in Cortactin expression and have no influence on clinical
features. In previous studies, Cortactin expression was reported in
advanced stages of OSCC (39).
Nevertheless, none of these few studies evaluated distinct tumour
areas separately for Cortactin (40)
as it was conducted successfully in the present study. EGFR
staining is very common in clinical routine and is the basis of
several studies. However, to our knowledge, studies having the
topic EGFR and OSCC did not differ between the two expression sites
of EGFR as we have for cytoplasmic EGFR and membrane EGFR (41). In the present study we were able to
evaluate differences in these localisations. We found a significant
correlation of high cytoplasmic EGFR expression and extracapsular
spread in the central tumour area. In the literature, this
interesting fact was not reported before. Only the general presence
of EGFR expression, rather than its detailed cellular localisation
and increased extracapsular spread were reported (42). According to the present understanding
of molecular oncology, the differential results regarding the
localisations of EGFR expressions might lead to significantly
different outcomes and should be taken into consideration. The
results of our current study based on a cohort with a large size
and the complete availability of after-care data, suggest that the
staining and evaluation of Cortactin expression have translational
clinical impact and the results of our study are of high relevance.
In particular, the majority of included patients were primarily
diagnosed with advanced UICC stages (III and IV). Curative surgical
treatment is often not possible for these stages and further
therapy strategies are all the more important for these cohorts.
Our results indicate for the first time that Cortactin is a protein
having a concomitant and not a compensatory pathway next to EGFR.
This result is essential, since cross-talk therapy is based on
molecules that are independent of each other in expression. In
summary, we showed that the dual-antibody-therapy targeting EGFR
and Cortactin is superior to EGFR targeting alone in OSCC (43). Cortactin might represent an important
molecule for the therapeutic approaches urgently needed to solve
the problems of mutations and therapy resistances (44) and of recurrence. Regarding other
malignancies, for example lymphocytic leukaemia, Cortactin plays
also an important role as a checkpoint molecule (45). In colon cancer, Cortactin promotes
cell migration and invasion (46).
These findings are showing the importance of further studies with
the subject Cortactin.
Immunohistochemical evaluations have their
limitations. Because only protein expression can be evaluated by
IHC, genetic profiles and further cellular interactions remain
unknown. Further studies are needed to answer these open molecular
questions.
Our results indicate that Cortactin could be a
prognostic marker for OSCC and also that the co-expression of EGFR
and Cortactin could have a clinical impact on survival rates. The
development of a Cortactin antibody to improve the stagnated
survival rates of OSCC patients is worthy of further studies.
Mainly in advanced UICC stages (III and IV) this cross link
antibody therapy could be the future therapy of choice, since
conventional therapies have only a limited range. The genetic
regulations of these markers should now be evaluated to
substantiate the findings of the current study.
Acknowledgements
The authors thank Daniela Hellmann for excellent
technical support. This study was supported by the German Research
Foundation (DFG) and the Technical University of Munich (TUM) in
the framework of the Open Access Publishing Program.
References
|
1
|
Scully C and Bagan JV: Recent advances in
oral oncology. Oral Oncol. 43:107–115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Breeze J, Morrison A, Dawson D, Tipper J,
Rehman K, Grew N and Pigadas N: Health-related quality of life
after treatment for neoplasia of the major salivary glands: A pilot
study. Br J Oral Maxillofac Surg. 54:806–811. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
González-García R, Naval-Gías L,
Román-Romero L, Sastre-Pérez J and Rodríguez-Campo FJ: Local
recurrences and second primary tumors from squamous cell carcinoma
of the oral cavity: A retrospective analytic study of 500 patients.
Head Neck. 31:1168–1180. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lindenblatt Rde C, Martinez GL, Silva LE,
Faria PS, Camisasca DR and Lourenço Sde Q: Oral squamous cell
carcinoma grading systems-analysis of the best survival predictor.
J Oral Pathol Med. 41:34–39. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gupta S, Khan H, Kushwaha VS, Husain N,
Negi M, Ghatak A and Bhatt M: Impact of EGFR and p53 expressions on
survival and quality of life in locally advanced oral squamous cell
carcinoma patients treated with chemoradiation. Cancer Biol Ther.
16:1269–1280. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Argiris A: EGFR inhibition for recurrent
or metastatic HNSCC. Lancet Oncol. 16:488–489. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cho HS and Leahy DJ: Structure of the
extracellular region of HER3 reveals an interdomain tether.
Science. 297:1330–1333. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bonner JA, Harari PM, Giralt J, Azarnia N,
Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, et al:
Radiotherapy plus cetuximab for squamous-cell carcinoma of the head
and neck. N Engl J Med. 354:567–578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakamura H, Koizumi H, Kimura H, Marushima
H, Saji H and Takagi M: Epidermal growth factor receptor mutations
in adenocarcinoma in situ and minimally invasive adenocarcinoma
detected using mutation-specific monoclonal antibodies. Lung
Cancer. 99:143–147. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mabry R, Gilbertson DG, Frank A, Vu T,
Ardourel D, Ostrander C, Stevens B, Julien S, Franke S, Meengs B,
et al: A dual-targeting PDGFRbeta/VEGF-A molecule assembled from
stable antibody fragments demonstrates anti-angiogenic activity in
vitro and in vivo. MAbs. 2:20–34. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Buday L and Downward J: Roles of cortactin
in tumor pathogenesis. Biochim Biophys Acta. 1775:263–273.
2007.PubMed/NCBI
|
|
12
|
Wu H, Reynolds AB, Kanner SB, Vines RR and
Parsons JT: Identification and characterization of a novel
cytoskeleton-associated pp60src substrate. Mol Cell Biol.
11:5113–5124. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Belsches AP, Haskell MD and Parsons SJ:
Role of c-Src tyrosine kinase in EGF-induced mitogenesis. Front
Biosci. 2:d501–d518. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Meiler E, Nieto-Pelegrín E and
Martinez-Quiles N: Cortactin tyrosine phosphorylation promotes its
deacetylation and inhibits cell spreading. PLoS One. 7:e336622012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kruchten AE, Krueger EW, Wang Y and
McNiven MA: Distinct phospho-forms of cortactin differentially
regulate actin polymerization and focal adhesions. Am J Physiol
Cell Physiol. 295:C1113–C1122. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Halon A, Donizy P, Biecek P,
Rudno-Rudzinska J, Kielan W and Matkowski R: HER-2 expression in
immunohistochemistry has no prognostic significance in gastric
cancer patients. ScientificWorldJournal. 2012:9412592012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wolff KD, Follmann M and Nast A: The
diagnosis and treatment of oral cavity cancer. Dtsch Arztebl Int.
109:829–835. 2012.PubMed/NCBI
|
|
18
|
Götz C, Drecoll E, Straub M, Bissinger O,
Wolff KD and Kolk A: Impact of HPV in oral squamous cell carcinoma.
Oncotarget. 7:76704–76712. 2016.PubMed/NCBI
|
|
19
|
Young RJ, Urban D, Angel C, Corry J, Lyons
B, Vallance N, Kleid S, Iseli TA, Solomon B and Rischin D:
Frequency and prognostic significance of p16 (INK4A) protein
overexpression and transcriptionally active human papillomavirus
infection in laryngeal squamous cell carcinoma. Br J Cancer.
112:1098–1104. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kolk A, Jubitz N, Mengele K, Mantwill K,
Bissinger O, Schmitt M, Kremer M and Holm PS: Expression of
Y-box-binding protein YB-1 allows stratification into long- and
short-term survivorsof head and neck cancer patients. Br J Cancer.
105:1864–1873. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fedchenko N and Reifenrath J: Different
approaches for interpretation and reporting of immunohistochemistry
analysis results in the bone tissue - a review. Diagn Pathol.
9:2212014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bondarenko A, Angrisani N,
Meyer-Lindenberg A, Seitz JM, Waizy H and Reifenrath J:
Magnesium-based bone implants: Immunohistochemical analysis of
peri-implant osteogenesis by evaluation of osteopontin and
osteocalcin expression. J Biomed Mater Res A. 102:1449–1457. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Su CM, Chang TY, Hsu HP, Lai HH, Li JN,
Lyu YJ, Kuo KT, Huang MT, Su JL and Chen PS: A novel application of
E1A in combination therapy with EGFR-TKI treatment in breast
cancer. Oncotarget. 7:63924–63936. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xue ZX, Wen WX, Zhuang Y, Hua ZJ and Xia
YN: Comparison of the efficacy of icotinib in patients with
non-small-cell lung cancer according to the type of epidermal
growth factor receptor mutation. Mol Clin Oncol. 5:265–268. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Grandis JR, Zeng Q, Drenning SD and
Tweardy DJ: Normalization of EGFR mRNA levels following restoration
of wild-type p53 in a head and neck squamous cell carcinoma cell
line. Int J Oncol. 13:375–378. 1998.PubMed/NCBI
|
|
26
|
Baschnagel AM, Tonlaar N, Eskandari M,
Kumar T, Williams L, Hanna A, Pruetz BL and Wilson GD: Combined
CD44, c-MET, and EGFR expression in p16-positive and p16-negative
head and neck squamous cell carcinomas. J Oral Pathol Med.
46:208–213. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hrustanovic G, Lee BJ and Bivona TG:
Mechanisms of resistance to EGFR targeted therapies. Cancer Biol
Ther. 14:304–314. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Scott AM, Allison JP and Wolchok JD:
Monoclonal antibodies in cancer therapy. Cancer Immun.
12:142012.PubMed/NCBI
|
|
29
|
Andreadis C, Vahtsevanos K, Sidiras T,
Thomaidis I, Antoniadis K and Mouratidou D: 5-Fluorouracil and
cisplatin in the treatment of advanced oral cancer. Oral Oncol.
39:380–385. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pasini F, Fraccon AP, Modena Y, Bencivenga
M, Giacopuzzi S, La Russa F, Gusella M and de Manzoni G: Targeted
therapies for advanced and metastatic adenocarcinoma of the
gastroesophageal junction: Is there something new? Gastric Cancer.
20:31–42. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Neumair P, Joos L, Warschkow R, Dutly A,
Ess S, Hitz F, Früh M, Brutsche M, Baty F, Krähenbühl S, et al:
Erlotinib has comparable clinical efficacy to chemotherapy in
pretreated patients with advanced non-small cell lung cancer
(NSCLC): A propensity-adjusted, outcomes research-based study. Lung
Cancer. 100:38–44. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Oliveira-Silva RJ, de Carvalho Carolina A,
de Souza Viana L, Carvalho AL and Reis RM: Anti-EGFR therapy:
Strategies in head and neck squamous cell carcinoma. Recent Pat
Anticancer Drug Discov. 11:170–183. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ma L, Zou B and Yan H: Identifying EGFR
mutation-induced drug resistance based on alpha shape model
analysis of the dynamics. Proteome Sci. 14:122016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jin Y, Zhang W, Wang H, Zhang Z, Chu C,
Liu X and Zou Q: EGFR/HER2 inhibitors effectively reduce the
malignant potential of MDR breast cancer evoked by P-gp substrates
in vitro and in vivo. Oncol Rep. 35:771–778. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Steinway SN, Dang H, You H, Rountree CB
and Ding W: The EGFR/ErbB3 pathway acts as a compensatory survival
mechanism upon c-met inhibition in human c-Met+ hepatocellular
carcinoma. PLoS One. 10:e01281592015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sato H, Hatanaka KC, Hatanaka Y,
Hatakeyama H, Hashimoto A, Matsuno Y, Fukuda S and Sabe H: High
level expression of AMAP1 protein correlates with poor prognosis
and survival after surgery of head and neck squamous cell carcinoma
patients. Cell Commun Signal. 12:172014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Aboshanif M, Kawasaki Y, Omori Y, Suzuki
S, Honda K, Motoyama S and Ishikawa K: Prognostic role of
regenerating gene-I in patients with stage-IV head and neck
squamous cell carcinoma. Diagn Pathol. 11:792016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gupta R, Chetty C, Bhoopathi P, Lakka S,
Mohanam S, Rao JS and Dinh DE: Downregulation of uPA/uPAR inhibits
intermittent hypoxia-induced epithelial-mesenchymal transition
(EMT) in DAOY and D283 medulloblastoma cells. Int J Oncol.
38:733–744. 2011.PubMed/NCBI
|
|
39
|
Yamada S, Yanamoto S, Kawasaki G, Mizuno A
and Nemoto TK: Overexpression of cortactin increases invasion
potential in oral squamous cell carcinoma. Pathol Oncol Res.
16:523–531. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu HS, Lu HH, Lui MT, Yu EH, Shen W, Chen
YP, Chang KW and Tu HF: Detection of copy number amplification of
cyclin D1 (CCND1) and cortactin (CTTN) in oral carcinoma and oral
brushed samples from areca chewers. Oral Oncol. 45:1032–1065. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Pu YS, Huang CY, Kuo YZ, Kang WY, Liu GY,
Huang AM, Yu HJ, Lai MK, Huang SP, Wu WJ, et al: Characterization
of membranous and cytoplasmic EGFR expression in human normal renal
cortex and renal cell carcinoma. J Biomed Sci. 16:822009.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Michikawa C, Uzawa N, Sato H, Ohyama Y,
Okada N and Amagasa T: Epidermal growth factor receptor gene copy
number aberration at the primary tumour is significantly associated
with extracapsular spread in oral cancer. Br J Cancer. 104:850–855.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gonzales CB, De La Chapa JJ, Saikumar P,
Singha PK, Dybdal-Hargreaves NF, Chavez J, Horning AM, Parra J and
Kirma NB: Co-targeting ALK and EGFR parallel signaling in oral
squamous cell carcinoma. Oral Oncol. 59:12–19. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Stewart EL, Tan SZ, Liu G and Tsao MS:
Known and putative mechanisms of resistance to EGFR targeted
therapies in NSCLC patients with EGFR mutations-a review. Transl
Lung Cancer Res. 4:67–81. 2015.PubMed/NCBI
|
|
45
|
Martini V, Gattazzo C, Frezzato F,
Trimarco V, Pizzi M, Chiodin G, Severin F, Scomazzon E, Guzzardo V,
Saraggi D, et al: Cortactin, a Lyn substrate, is a checkpoint
molecule at the intersection of BCR and CXCR4 signalling pathway in
chronic lymphocytic leukaemia cells. Br J Haematol. 178:81–93.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang ZN, Liu D, Yin B, Ju WY, Qiu HZ, Xiao
Y, Chen YJ, Peng XZ and Lu CM: High expression of PTBP1 promote
invasion of colorectal cancer by alternative splicing of cortactin.
Oncotarget. 8:36185–36202. 2017.PubMed/NCBI
|