Introduction
With the globalization and younger-age trend of
obesity, non-alcoholic fatty liver disease (NAFLD) has become one
of the most prevalent chronic liver diseases among children in
developed countries and wealthy Chinese families, particularly in
coastal regions (1–5). Dipeptidyl peptidase IV (DPPIV) is a
type of transmembrane serine protease, T cell activation protein
and adenosine deaminase binding protein, which is also known as
cluster of differentiation 26. Besides the hydrolytic activity of
peptidase, it also has regulating effects on fat metabolism,
immunity and inflammation (6). The
results of previous studies have revealed that the activity of
DPPIV is associated with chronic liver diseases, including NAFLD
(7–9). Previously, Machado et al
(10) and Qu et al (11) effectively combined the powerful
antioxidant N-acetylcysteine (NAC) and activated carbon for
medicine with good adsorption properties and biocompatibility, and
prepared an NAC-activated carbon sustained-release microcapsule
(ACNAC). ACNAC reduced various side effects of NAC and enhanced the
half-life of drugs and bioavailability. Based on previous studies
(12–15), the present study aimed to further
explore the effects of ACNAC on young rats with NAFLD by
establishing a model of young rats with NAFLD in order to detect
the serum DPPIV activity level and the expression of DPPIV protein
in the liver. The present study discusses whether ACNAC is a DPPIV
inhibitor, and whether it is able to protect young rats with
NAFLD.
Materials and methods
Preparation of non-alcoholic fatty
liver model and sample collection
A total of 64 healthy, clean and weaned
Sprague-Dawley male rats (weight, 51.93±4.28 g; 21 days old) were
purchased from the Animal Center of Zhejiang Academy of Medical
Sciences [Hangzhou, China; animal license number: SCXK (Zhe)
2014-0001]. The rats had ad libitum access to a standard
commercial diet and water, with the exception of preoperative
fasting and were kept in rooms maintained at 22±1°C, 40–60%
relative humidity with a 12 h light/dark cycle throughout the
experiments. All experiments were performed in accordance with the
National Institutes of Health Guide for the Care and Use of
Laboratory Animals, and ethical approval was granted by the Animal
Care Committee of Xixi Hospital Affiliated to Zhejiang University
of Traditional Chinese Medicine (Hangzhou, China). Following 1 week
of acclimatization to these conditions, rats were randomly divided
into the following groups (n=8 per group): Normal group, fed
standard diets; model group; fed high-fat diets (69% basic feed,
10% lard oil, 2% cholesterol, 5% sugar, 0.5% cholate, 10% yolk
powder, 3% yeast powder and 0.5% decavitamin); polyene phosphatidyl
choline group; administered 60 mg/kg PPC (Sanofi-aventis; Beijing,
China) by gastric perfusion daily and fed high-fat diets; NAC
group, administered 60 mg/kg NAC (Whu hoyo Co., Ltd, Wuhan, Hubei)
by gastric perfusion daily and fed high-fat diets; activated carbon
release microcapsule group, administered 60 mg/kg activated carbon
release microcapsule (Zhejiang Hangzhou Wood industry Co., Ltd.,
Huzhou, Zhejiang) and fed high-fat diets; and ACNAC low-, medium-
and high-dose groups, fed high-fat diets and administered 15, 30
and 60 mg/kg of ACNAC [made in-house as described previously
(12–15)], respectively by gastric perfusion
daily. Following 7 weeks of these treatments, the young rats were
sacrificed, and their blood and livers were collected and stored at
−80°C for future use.
Calculation of liver index
The liver-wet weight and the body weight of the
young rats were calculated. The liver index was calculated
according to the following formula: Liver index (%) = liver-wet
weight / body weight × 100.
Observation of the degree of fatty
degeneration in the liver tissue of young rats by hematoxylin and
eosin (HE) staining
Fresh liver tissues from rats of each group were
treated in 4% paraformaldehyde for 24 h at room temperature,
dehydrated with alcohol prepared according to a set gradient (75,
85, 90, 95 and 100%) and then embedded into paraffin blocks. A
total of 5 blocks were selected at random and each one was cut into
three sections on a paraffin microtome with a thickness of 4-µm.
Sections were deparaffinaged in dimethylbenzene, embedded in xylene
I for 20 min, xylene II for 20 min, absolute ethyl alcohol I for 10
min, absolute ethyl alcohol II for 10 min, 95% alcohol for 5 min,
90% alcohol for 5 min, 80% alcohol for 5 min and 70% alcohol for 5
min successively prior to washing with water. Nuclei staining with
hematoxylin: Sections were stained with hematoxylin for 3–8 min and
eosin 1–3 min. The liver tissue structures were observed with a
light microscope (magnification, ×400; Nikou Corporation; Tokyo,
Japan).
Blood from celiac vein was used to
determine the biochemical indicators
A Hitachi 7060 automatic biochemical analyzer
(Hitachi, Ltd., Tokyo, Japan) was used to detect serum alanine
transaminase (ALT; Wako Pure Chemical Industries, Ltd., Osaka,
Japan), aspartate transaminase (AST; Wako Pure Chemical Industries,
Ltd., Osaka, Japan), total cholesterol (TC; Beijing Homa Biological
Engineering Co., Ltd., Beijing, China), total triglycerides (TG;
Beijing Homa Biological Engineering Co., Ltd.), high-density
lipoprotein cholesterol (HDL-C; Medicalsystem Biotechnology Co.,
Ltd., Ningbo, Zhejiang), low-density lipoprotein cholesterol
(LDL-C; Medicalsystem Biotechnology Co., Ltd.), fasting blood
glucose (FBG; Autec Diagnostic; Baden-Württemberg, Germany). An
abbott i2000 (Abbott Molecular Inc., Chicago, IL, USA) was used to
detect serum fasting insulin (FINS; Abbott Molecular Inc.).
Determination of the activity of DPPIV
by a Gly-Pro-7-amino-4-methylcoumarin (AMC) fluorescence
method
The operations were conducted according to the
manufacturer's instructions of a DPPIV kit (AAT Bioquest Inc.,
California, USA). A total of 5 mg of Gly-Pro-AMC (a sensitive
fluorogenic substrate; molecular weight, 443.37) was introduced
into 563.5 µl of dimethyl sulfoxide (DMSO) to prepare 20 mM
substrate DMSO stock solution. The substrate DMSO stock solution
were diluted into 100 µM substrate DMSO stock solution with 50 mM
Tris-HCl, and mixed with rat serum (150 µl:150 µl) for 1 h at room
temperature. The fluorescence intensity was measured with an
enzyme-labeled instrument (Biotek Corporation; Broadview, IL, USA)
at Ex/Em=380/500 nm. The activity inhibition rates of DPPIV were
calculated with the following equation (16): Activity inhibition rate of DPPIV =
(fluorescence intensity of negative control group - fluorescence
intensity of each administration group) / fluorescence intensity of
negative control group × 100%.
Detection of DPPIV protein expression
by an immunohistochemical staining method
Liver tissue were fixed with formaldehyde and
hydrated with gradient ethanol as described in HE staining.
Following citrate buffer antigen retrieval, slices were incubated
with 3% deionized water for 20 min and then washed with PBS for 5
min, 3 times. Following blocking with 5–10% normal goat serum
(Boster Biological Technology Co., Ltd.) for 25 min at room
temperature, 4 µm thick slices were incubated with DPPIV primary
antibody (cat. no. 10940_1_ap; 1:100; Proteintech group, Inc.,
Wuhan, Hubei) at 4°C overnight. Subsequently, samples were
incubated with goat anti-rabbit secondary antibody (cat. no.
GB23303; 1:200; Wuhan Goodbio Technology Co., Ltd., Wuhan, Hubei)
for 50 min at room temperature. Following color development with
3,3′-diaminobenzidine reagent for 5 min at room temperature, the
sections were mounted and observed under a light microscope
(magnification, ×400; Nikou Corporation), and the appearance of a
brown-yellow liver cell membrane indicated positive expression. A
total of five non-overlapping fields of view were selected and the
immunohistochemical scores (IHS) method (17) was used to score samples according to
the percentage of positive cells and their staining intensity, and
the results were analyzed with Image Pro-Plus version 6.0 (Media
Cybernetics, Rockville, Maryland, USA).
Detection of the expression of DPPIV
protein in liver tissues by western blot analysis
Total protein was extracted from the liver tissue.
The total sample protein was extracted using the holoprotein
extraction kit (Nanjing KeyGEN Biotech Co., Ltd., Nanjing, China).
Liver tissue (100 mg) was placed in a petri dish and cut into
blocks (~3×3 mm), 0.5–1 ml of 0–4°C Lysis Buffer were added, which
contained 5 µl of phosphatase inhibitor, 1 µl of protease inhibitor
and 5 µl 100 mM phenylmethylsulfonyl fluoride. The mixture was
homogenized 15 times under 4°C prior to centrifugation at 10,625 ×
g for 15 min at 4°C. The supernatants were collected and protein
quantification were performed using the BCA method: and the
absorbance was read at 562 nm with a ultraviolet spectrophotometer.
In total, 20 µg protein was loaded into each well and subjected to
5% SDS-PAGE at 60 mA. The proteins were then transferred onto a
polyvinylidene difluoride membrane at 100 V constant voltage for 65
min, and incubated using 5% skimmed milk powder overnight at 4°C.
The PVDF membrane was incubated with DPPIV and β-actin primary
antibody (1:500, Abcam; Cambridge, UK) for 2 h at room temperature,
and then washed three times for 10 min with phosphate buffered
saline with Tween 20 (PBST). The PVDF membrane were incubated with
horseradish peroxidase labeled goat anti-rabbit secondary antibody
(cat. no. ab97200; 1:1,000; Abcam, Cambridge, England) for 1 h at
room temperature and washed three times for 10 min with PBST. The
diaminobenzidine developer were prepared before use. The washed
PVDF membrane was put into the developer for 3 min, and then the
diaminobenzidine reaction was terminated with water. The membrane
was imaged with a full-automatic digital gel image analysis system
(Shanghai Tanon Technology Co., Ltd., Shanghai, China) and the
results were analyzed with Image J version 1.48 (National
Institutes of Health, Bethesda, USA).
Statistical methods
Data are presented as the mean ± standard deviation.
Comparison among groups was performed using one-way analysis of
variance and the least significant difference method was used for
pairwise comparison among groups when the variances were equal. In
addition, the Games-Howell method was used for pairwise comparison
among groups when the variances were not equal. SPSS version 18.0
software (SPSS, Inc., Chicago, IL, USA) was used for the
statistical analysis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Liver index results of young rats
Compared with the normal group, the liver index in
the model group increased significantly (P<0.05). Compared with
the model group, the liver index in the ACNAC groups were
significantly decreased (P<0.05), and the most marked decrease
was observed in the high-dose group. No significant difference was
observed in liver index between the ACNAC high-dose and PPC groups
(Fig. 1).
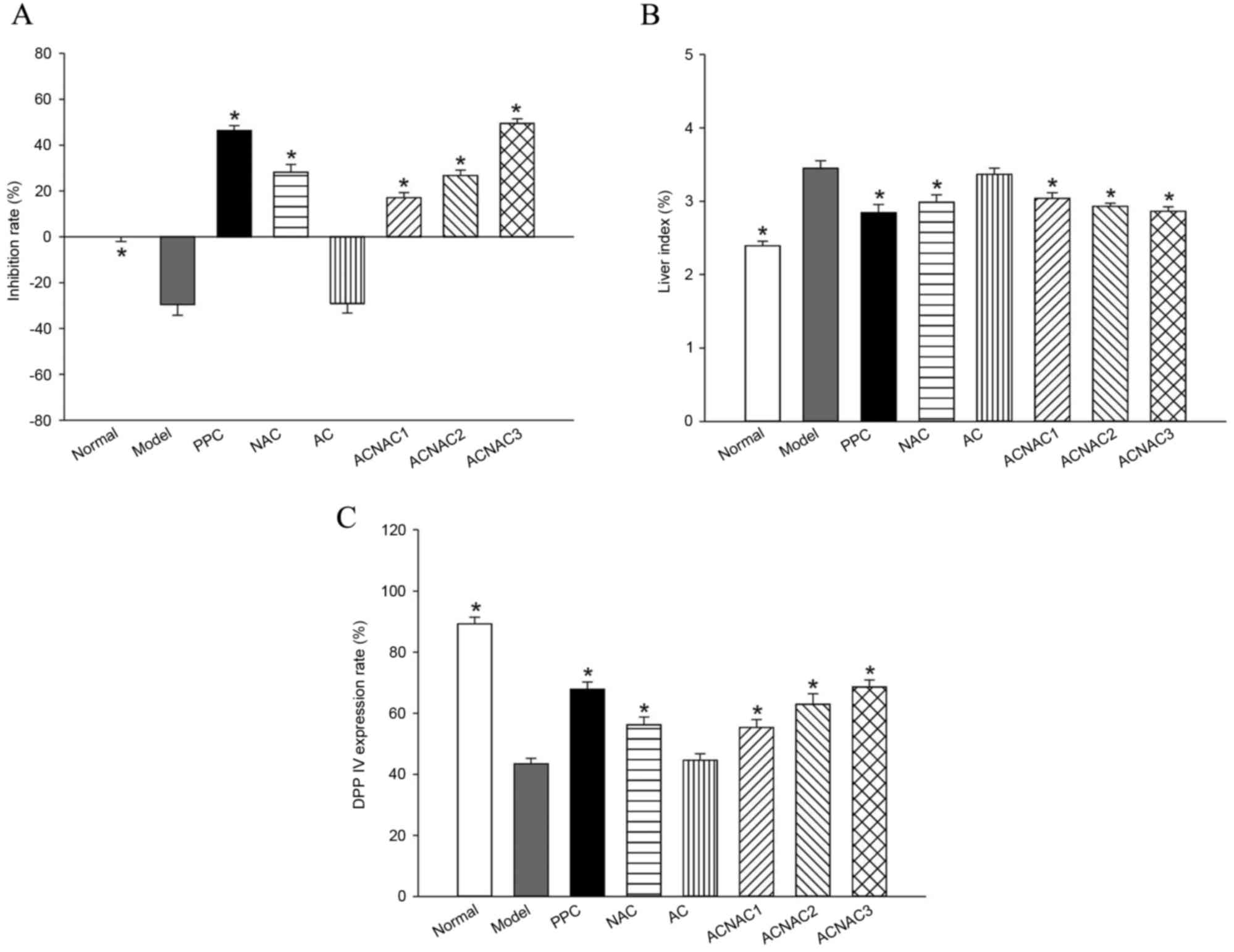 | Figure 1.Comparative results of liver index,
serum DPPIV activity inhibition ratio and DPPIV expression rate in
the young rats of each group. (A) Liver index, (B) DPPIV inhibition
and (C) DPPIV expression rates. Bars represent the mean ± standard
deviation of results obtained per experimental group. *P<0.05
vs. the model group determined by one-way analysis of variance and
the least significant difference method. Normal, normal group;
model, model group; PPC, polyene phosphatidyl choline group; NAC,
N-acetylcysteine control group; AC, activated carbon release
microcapsule control group; ACNAC1, N-acetylcysteine
activated carbon release microcapsule low-dose group; ACNAC2,
N-acetylcysteine activated carbon release microcapsule
middle-dose group; ACNAC3, N-acetylcysteine activated carbon
release microcapsule high-dose group; DPPIV, dipeptidyl peptidase
IV. |
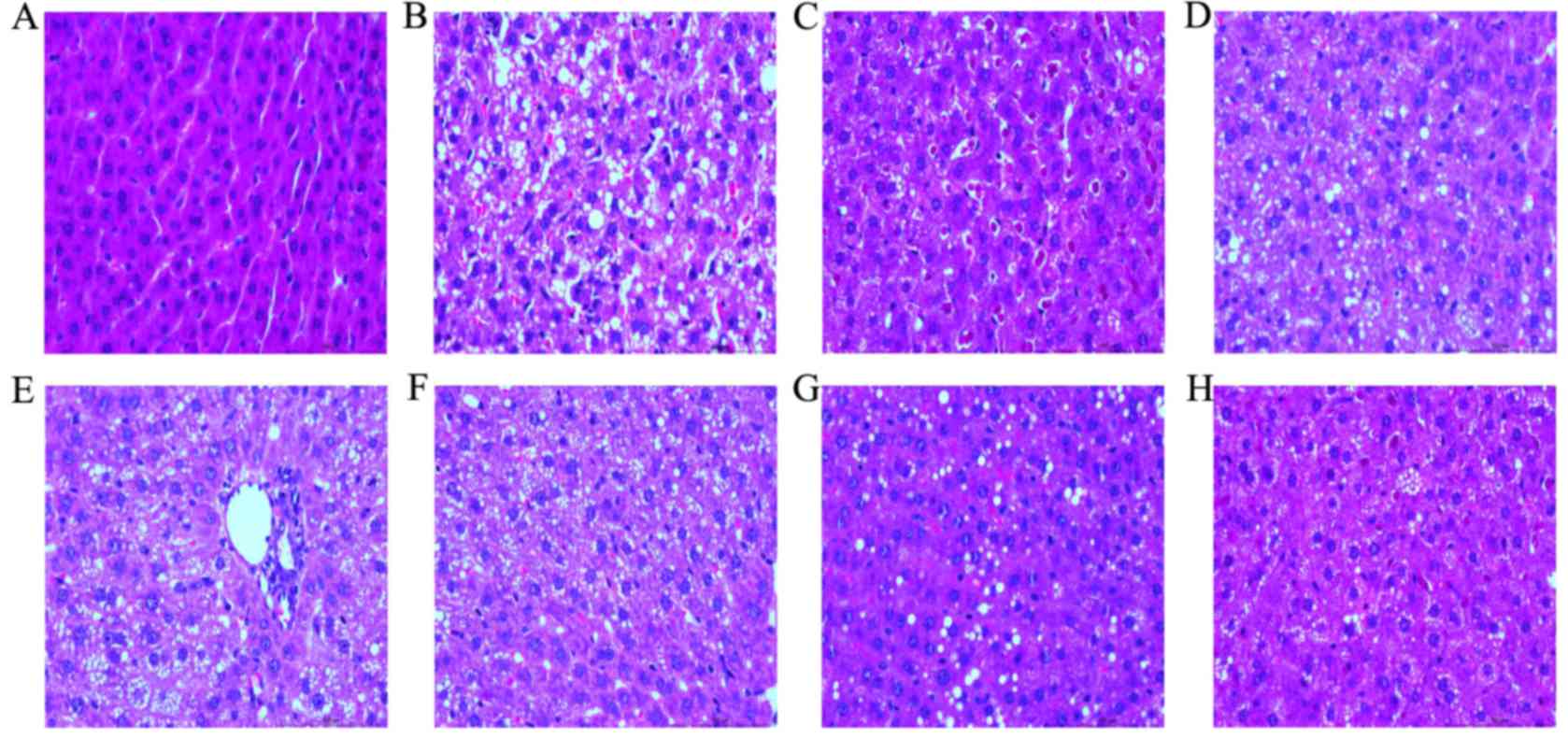
HE staining of liver tissue of young
rats
In the normal group and under a light microscope the
structure of liver tissues of young rats was clear and complete.
Additionally, the structure of the liver lobule was normal and the
liver cells were distributed around the central vein in a
radioactive arrangement. In the model group, fatty degeneration in
liver cells of young rats was markedly increased. Furthermore,
diffuse hepatocellular fatty degeneration was accompanied with
ballooning degeneration, and there were areas of spotty necrosis of
liver cells accompanied with hepatic fibrosis around the central
veins. Compared with the model group, in the ACNAC groups,
particularly the high-dose group, fatty degeneration and the degree
of ballooning degeneration of liver tissue of young rats was
markedly alleviated, with no evident inflammatory cell infiltration
or focal necrosis occurring in the central veins. Additionally,
compared with the model group, the PPC group has no evident fatty
degeneration and occasional microvesicular fat droplet vacuoles.
Finally, there were no marked differences observed between the PPC
and ACNAC high-dose groups (Fig.
2).
Serum biochemical indices in young
rats
Compared with the model group, the high-dose ACNAC
group had significantly reduced ALT, AST, TC, TG, LDL-C, FBG and
FINS and increased HDL-C levels (P<0.05). Furthermore, no
significant differences were observed in the TC, TG, LDL-C and
HDL-C levels between the ACNAC high-dose and PPC groups (P>0.05;
Figs. 3 and 4).
 | Figure 3.Comparative results of ALT, AST, FBG
and FINS serums in young rats of each group. (A) ALT, (B) AST, (C)
FBG and (D) FINS. Bars represent the mean ± standard deviation of
the results obtained per experimental group. *P<0.05 vs. the
model group, as determined by one-way analysis of variance and the
least significant difference method. Normal, normal group; model,
model group; PPC, polyene phosphatidyl choline group; NAC,
N-acetylcysteine control group; AC, activated carbon release
microcapsule control group; ACNAC1, N-acetylcysteine
activated carbon release microcapsule low-dose group; ACNAC2,
N-acetylcysteine activated carbon release microcapsule
middle-dose group; ACNAC3, N-acetylcysteine activated carbon
release microcapsule high-dose group; ALT, alanine transaminase;
AST, aspartate transaminase; FBG, fasting blood glucose; FINS,
fasting insulin. |
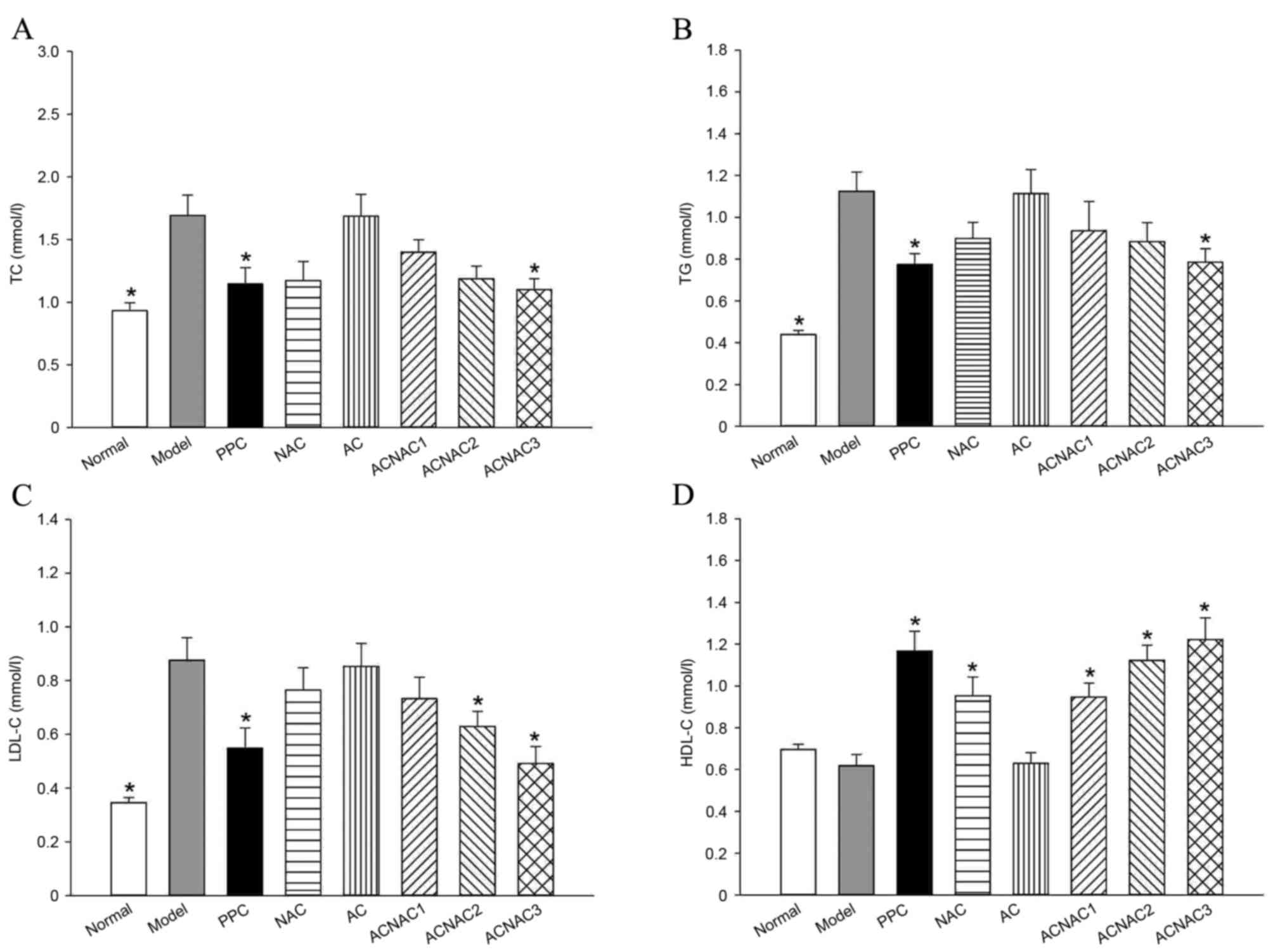 | Figure 4.Comparative results of TC, TG, HDL-C
and LDL-C serums in the young rats of each group. (A) TC, (B) TG,
(C) LDL-C and (D) HDL-C. Bars represent the mean ± standard
deviation of the results obtained per experimental group.
*P<0.05 vs. the model group, as determined by one-way analysis
of variance and least significant difference method. Normal, normal
group; Model, model group; PPC, polyene phosphatidyl choline group;
NAC, N-acetylcysteine control group; AC, activated carbon
release microcapsule control group; ACNAC1, N-acetylcysteine
activated carbon release microcapsule low-dose group; ACNAC2,
N-acetylcysteine activated carbon release microcapsule
middle-dose group; ACNAC3, N-acetylcysteine activated carbon
release microcapsule high-dose group; TC, total cholesterol; TG,
total triglycerides; LDL-C, low density lipoproteins cholesterol;
HDL-C, high density lipoproteins cholesterol. |
Determination of inhibition rate of
DPPIV activity via the Gly-Pro-AMC fluorescence method
Serum was added to a fully functional enzyme
standard instrument to detect the inhibitory effect of ACNAC on the
activity of DPPIV. Compared with the normal group, the model group
exhibited a significantly decreased inhibition rate of DPPIV
activity of −29.54±13.32% in the serum (P<0.05). Compared with
the model group, the high-dose ACNAC group exhibited a
significantly increased inhibition rate of DPPIV activity of
49.50±5.56% in the serum (P<0.05). In comparison with the PPC
group, which had an inhibition rate of 46.42±5.88%, the ACNAC
high-dose group had an increased inhibition rate of DPPIV activity
in the serum and the intergroup differences; however, this was not
statistically significant (P>0.05; Fig. 1).
Detection of DPPIV protein expression
by immunohistochemical staining
Positive expression of DPPIV is indicated by a
brown-yellow liver cell membrane. Compared with the percentage of
DPPIV-positive cells of the liver cell membrane in the normal group
(89.19±6.23%) the expression of positive cells in the model group
(43.48±4.99%) was significantly decreased (P<0.05). Compared
with the model group, a significantly higher percentage of
DPPIV-positive cells were detected in the liver cell membranes of
the ACNAC groups (P<0.05), and the positive expression of DPPIV
increased with the decrease of fatty degeneration of liver tissue,
particularly in the high-dose ACNAC group in which the percentage
(68.57±6.48%) of positive cells was most markedly increased. In
comparison with the PPC group (67.90±6.45%), the percentage of
positive cells of DPPIV-positive cells in the ACNAC high-dose group
was slightly increased; however there was no statistical
significant (P>0.05; Figs. 1 and
5).
Results of DPPIV protein expression
detection by western blot analysis
In comparison with the normal group, the DPPIV
protein expression in the model group was significantly decreased
(P<0.05). Compared with the model group, DPPIV protein
expression in the low-, medium- and high-dose ACNAC groups
significantly increased in a dose-dependent manner (P<0.05;
Fig. 6).
Discussion
NAFLD has become the most common liver disease
influencing the health of children and adolescents (18–20).
According to a previous study, NAFLD patients most likely account
for 30% among 70 million individuals in the USA and up to 8% among
children and adolescents between 2 and 19 years old (20). The pathogenesis in children may be
different from adults; however, the overall incidence trend is
simple fatty liver, fatty liver hepatitis, liver fibrosis and even
liver cirrhosis (21). Consequently,
it is necessary to screen the children with such a disease risk as
soon as possible and to study the pathogenesis (22,23). For
obese children with NAFLD, any unhealthy dietary and lifestyle
habits should be corrected (24–26),
aerobic exercise encouraged, and their weight should be controlled
to reduce insulin resistance so that liver damage may be reversed
at an early stage (27–29). If necessary, drug intervention with
low levels of toxicity and side effects, and evident curative
effects may be used to effectively reduce the occurrence of NAFLD
and other chronic metabolic diseases in children (30,31).
At present, the main issues facing medication for
children are imperfect drug safety information and the lack of
medicinal varieties (32),
specification and special formulations, which results in
adultification of medicine in children and various disadvantages
(33). In order to solve these
problems, China has increased the input of oral sustained-release
therapies, controlled release preparations, orally disintegrating
tablets and transdermal absorption preparations, with the aim of
developing preparations that conform to the characteristics of
medication in infants and children and that have a low toxicity,
few side effects and a high bioavailability (34). For the present study, the advantage
of adsorptive behavior and in vitro release properties of
activated carbon for medicine were considered, and activated carbon
NAC sustained-release microcapsules were studied in order to
improve in oxidizability and bioavailability of NAC, reduce the
dose in clinical use, increase drug stability and elucidate the
ideal pharmaceutical preparation for infants and children.
DPPIV is widely found in organisms and its
expression is predominantly in epithelial cells, lymphocytes,
endothelial cells and fibroblasts. Previous studies have
demonstrated that DPPIV has a multiple-effect on biological
activity (35,36), particularly the protease in known
DPPIV affects fat metabolism, predominantly lipid metabolism of an
organism through neuropeptide Y and inactivation of other
polypeptides.
The present study used a high-fat diet to induce a
model of young rats with NAFLD and combines children's dietary
structure and habits in order to establish a young animal model
with abdominal obesity, lipid metabolism disorder and elevated
liver enzyme levels that are similar to children with NAFLD. The
results demonstrated that compared with the normal group, the model
group exhibited weight gain, liver index increase, visceral fat
deposition, evident liver volume increase, and the livers were
grayish-yellow color and had a greasy surface area in young rats.
Additionally, different degrees of fatty changes and ballooning
degeneration were observed via HE pathological staining. In
addition, vacuoles of different sizes were filled in the cells, and
various areas were accompanied by inflammatory cell infiltration
and occasional focal necrosis. Furthermore, the activity of ALT,
AST, TC, TG, LDL-C, FBG, FINS and DPPIV in the serum was evidently
increased and the expression of HDL-C and DPPIV protein in the
liver cell membrane were markedly decreased.
A previous study by Balaban et al (37) demonstrated that the activity of DPPIV
in the serum in patients with non-alcoholic steatohepatitis was
markedly higher than those in the normal group. Furthermore, the
degree of pathological change of liver tissue and degree of fat
change of liver cells of NAFLD were associated with the serum DPPIV
activity and the intensity of protein expression. It is thought
that DPPIV may be closely associated with the pathogenesis of NAFLD
(38). It has previously been
revealed that the DPPIV inhibitor may improve fatty degeneration of
the liver in rats (39).
Furthermore, a previous clinical experiment demonstrated that the
DPPIV inhibitor may also improve transaminase levels and ballooning
degeneration of liver cells in patients with NAFLD (40). The present experimental results
demonstrated that, compared with the model group, the ACNAC groups
(particularly the high-dose group) exhibited different degrees of
improvement in the indexes detailed above, which reduced the
activity of ALT, AST and DPPIV and the content of TC, TG, LDL-C,
FBG and FINS, increased the content of HDL-C and strengthened DPPIV
protein expression in the liver cell membrane. This is consistent
with the results of the study performed by Balaban et al
(37). These findings suggest that
ACNAC may have the effect of a DPPIV inhibitor in the treatment of
NAFLD in young rats, which provides novel rationale for the use of
NAC in studies of NAFLD/non-alcoholic steatohepatitis in young
rats, and provides theoretical knowledge for ideal pharmaceutical
preparations for infants and children. However, the underlying
mechanism still remains to be investigated in future
experiments.
Acknowledgements
The present study was supported by Hangzhou Science
and Technology Development projects (grant nos. 20130633B09,
20140633B29 and 20142013A60).
References
|
1
|
Roth CL, Elfers CT, Figlewicz DP, Melhorn
SJ, Morton GJ, Hoofnagle A, Yeh MM, Nelson JE and Kowdley KV:
Vitamin D deficiency in obese rats exacerbates nonalcoholic fatty
liver disease and increases hepatic resistin and Toll-like receptor
activation. Hepatology. 55:1103–1111. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fan JG: Epidemiology of alcoholic and
nonalcoholic fatty liver disease in China. J Gastroenterol Hepatol.
28 Suppl 1:S11–S17. 2013. View Article : Google Scholar
|
|
3
|
Fan JG and Zeng MD: Fatty liver disease.
Version 2. Beijing: People's Medical Publishing House 20; 2013
|
|
4
|
Farrell GC, McCullough AJ and Day CP:
Non-Alcoholic Fatty Liver Disease: A Practical Guide.
Wiley-Blackwell; Oxford: pp. 2162013
|
|
5
|
Loomba R, Sirlin CB, Schwimmer JB and
Lavine JE: Advances in pediatric nonalcoholic fatty liver disease.
Hepatology. 50:1282–1293. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gorrell MD: Dipeptidyl peptidase IV and
related enzymes in cell biology and liver disorders. Clin Sci
(Lond). 108:277–292. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fimeisz G, Varga T, Lengyel G, Fehér J,
Ghyczy D, Wichmann B, Selmeci L, Tulassay Z, Rácz K and Somogyi A:
Seram dipeptidyl peptidase-4 activity in insulin resistant patients
with nonalcoholic fatty liver disease: A novel liver disease
biomarker. PLoS One. 5:e122262010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ragab D, Laird M, Duffy D, Casrouge A,
Mamdouh R, Abass A, Shenawy DE, Shebl AM, Elkashef WF, Zalata KR,
et al: CXCL10 antagonism and plasma sDPPIV correlate with
increasing liver disease in chronin HCV genotype 4 infected
patients. Cytokine. 63:105–112. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chielle Ottobelli E, de Souza WM, da Silva
TP, Moresco RN and Moretto MB: Adipocytokines, inflammatory and
oxidative stress markers of clinical relevance altered in young
overweight/obese subjects. Clin Biochem. 49:548–553. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Machado MV, Kruger L, Jewell ML,
Michelotti GA, Pereira Tde A, Xie G, Moylan CA and Diehl AM:
Vitamin B5 and N-acetylcysteine in nonalcoholic steatohepatitis: A
preclinical study in a dietary mouse model. Dig Dis Sci.
61:137–148. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qu QL, Zhang YG, Yang LZ and Sun L:
Intraperitoneal chemotherapy with mitomycin C bound to activated
carbon nanoparticles for nude mice bearing human gastric carcinoma.
Zhonghua Zhong Liu Za Zhi. 28:257–260. 2006.(In Chinese).
PubMed/NCBI
|
|
12
|
Wang FG, Zhuang RX, Xi JJ, Fang HY and Gao
JQ: Preparation and distribution in mice of acetylcysteine
nanoparticles. Zhongguo Yiyao Gongye Zazhi. 43:572–576. 2012.(In
Chinese).
|
|
13
|
Wang FG, Shen YQ, Zhuang RX, Xi JJ and
Fang HY: Study on preparation of acetylcysteine nanoparticles.
Strait Pharmaceut J. 25:15–17. 2013.
|
|
14
|
Jia L, Wang J, Shi JP, Wang F, Gang H, Zhu
M and Lou G: The effect of N-acetylcysteine nano-carbon on
anti-oxidative capacity in non-alcoholic steatohepatitis rats.
Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi. 28:4–6. 2014.(In
Chinese).
|
|
15
|
Fang HY, Zhang RX, Xi JJ, Sun JJ, Shao YD,
Si ZZ, Pan XW and Wang FG: Role of treatment on liver fibrosis of
activated carbon N-acetylcysteine microcapsule. Zhong Guo Lin
Chuang Yao Li Xue Yu Zhi Liao Xue Bian Ji Bu. 20:976–980. 2015.(In
Chinese).
|
|
16
|
Pei Z, Li X, Longenecker K, von Geldern
TW, Wiedeman PE, Lubben TH, Zinker BA, Stewart K, Ballaron SJ,
Stashko MA, et al: Discovery, structure-activity relationship, and
pharmacological evaluation of
(5-substituted-pyrrolidinyl-2-carbonyl)-2-cyanopyrrolidines as
potent dipeptidyl peptidase IV Inhibitors. J Med Chem.
49:3520–3535. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Soslow RA, Dannenberg AJ, Rush D, Woerner
BM, Khan KN, Masferrer J and Koki AT: COX-2 is expressed in human
pulmonary, colonic, and mammary tumors. Cancer. 89:2637–2645. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lavine JE and Schwimmer JB: Nonalcoholic
fatty liver disease in the pediatric population. Clin Liver Dis.
8(549–558): viii–ix. 2004.
|
|
19
|
Fraser A, Longnecker MP and Lawlor DA:
Prevalence of elevated alanine aminotrasferase among US adolescents
and associated factors: NHANES 1999–2004. Gastroenterology.
133:1814–1820. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schwimmer JB, Deutsch R, Kahen T, Lavine
JE, Stanley C and Behling C: Prevalence of fatty liver in children
and adolescents. Pediatric. 118:1388–1393. 2006. View Article : Google Scholar
|
|
21
|
Kohli R, Boyd T, Lake K, Dietrich K,
Nicholas L, Balistreri WF, Ebach D, Shashidhar H and Xanthakos SA:
Rapid progression of NASH in childhood. J Pediatr Gastroenterol
Nutr. 50:453–456. 2010.PubMed/NCBI
|
|
22
|
Holterman A, Gurria J, Tanpure S and
DiSomma N: Nonalcoholic fatty liver disease and bariatric surgery
in adolescents. Semin Pediatr Surg. 23:49–57. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alterio A, Alisi A, Liccardo D and Nobili
V: Non-alcoholic fatty liver and metabolic syndrome in children: A
vicious circle. Horm Res Paediatr. 82:283–289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ozhan B, Ersoy B, Kiremitci S, Ozkol M and
Taneli F: Insulin sensitivity indices: Fasting versus
glucose-stimulated indices in pediatric non-alcoholic fatty liver
disease. Eur Rev Med Pharmacol Sci. 19:3450–3458. 2015.PubMed/NCBI
|
|
25
|
Anderson EL, Howe LD, Jones HE, Higgins
JP, Lawlor DA and Fraser A: The prevalence of non-alcoholic fatty
liver disease in children and adolescents: A systematic review and
meta-analysis. PLoS One. 10:e01409082015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jogo R, Ness AR, Emmett P, Mattocks C,
Jones L and Riddoch CJ: Obesogenic diet and physical activity:
Independent or associated behaviours in adolescents? Public Health
Nutr. 13:673–681. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Monteiro PA, Antunes Bde M, Silveira LS,
Christofaro DG, Fernandes RA and Junior Freitas IF: Body
composition variables as predictors of NAFLD by ultrasound in obese
children and adolescents. BMC Pediatr. 14:252014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Manco M, Bedogni G, Marcellini M, Devito
R, Ciampalini P, Sartorelli MR, Comparcola D, Piemonte F and Nobili
V: Waist circumference correlates with liver fibrosis in children
with non-alcoholic steatohepatitis. Gut. 57:1283–1287. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schwimmer JB, Celedon MA, Lavine JE, Salem
R, Campbell N, Schork NJ, Shiehmorteza M, Yokoo T, Chavez A,
Middleton MS and Sirlin CB: Heritability of nonalcoholic fatty
liver disease. Gastroenterology. 136:1585–1592. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Alisi A and Nobili V: Non-alcoholic fatty
liver disease in children now: Lifestyle changes and pharmacologic
treatments. Nutrition. 28:722–726. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Black LJ, Jacoby P, She Ping-Delfos WC,
Mori TA, Beilin LJ, Olynyk JK, Ayonrinde OT, Huang RC, Holt PG,
Hart PH, et al: Low serum 25-hydroxyvitamin D concentrations
associate with non-alcoholic fatty liver disease in adolescents
independent of adiposity. J Gastroenterol Hepatol. 29:1215–1222.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Abdel-Rahman SM, Amidon GL, Kaul A,
Lukacova V, Vinks AA and Knipp GT: Summary of the national
institute of child health and human development-best
pharmaceuticals for children act pediatric formulation initiatives
workshop-pediatric biopharmaceutics classification system working
group. Clin Ther. 11:S11–S24. 2012. View Article : Google Scholar
|
|
33
|
Ma LX, Liu X, Yan GQ, et al: Problems and
thoughts on children drug use. Chin Hospitals. 16:45–46. 2012.
|
|
34
|
Hu N: Present situation and development
strategy of paediatric formulations. Med Sci. 1:2392015.
|
|
35
|
Drucker DJ and Nauck MA: The incretin
system: Glucagons-like peptide-l receptor agonists and dipeptidyl
peptidase-4 inhibitors in type 2 diahetes. Lancet. 368:1696–1705.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Holst JJ: Glucagon-like peptide-1: From
extract to agent. The claude bernard lecture, 2005. Diabetologia.
49:253–260. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Balaban YH, Korkusuz P, Simsek H, Gokcan
H, Gedikoglu G, Pinar A, Hascelik G, Asan E, Hamaloglu E and Tatar
G: Dipeptidyl peptidaseIV (DPPIV) in NASH patients. Ann Hepatol.
6:242–250. 2007.PubMed/NCBI
|
|
38
|
Han Q: The study of the relationship
between DPPIV and fatty liver disease inobese children and young
rats. Tianjin Med Uni. 5:2011.
|
|
39
|
Shirakawa J, Fujii H, Ohnuma K, Sato K,
Ito Y, Kaji M, Sakamoto E, Koganei M, Sasaki H, Nagashima Y, et al:
Diet-induced adipose tissue inflammation and liver steatosis are
prevented by DPP-4 inhibition in diabetic mice. Diabetes.
60:1246–1257. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yilmaz Y, Yonal O, Deyneli O, Celikel CA,
Kalayci C and Duman DG: Effects of sitagliptin in diabetic patients
with nonalcoholic steatohepatitis. Acta Gastroenterol Belg.
75:240–244. 2012.PubMed/NCBI
|