Introduction
Myocardial injury (MI) following non-cardiac surgery
is a significant international public health concern, occurring in
~8% of such surgeries and causing mortality in 10% of affected
patients within 30 days of surgery (1). The pathophysiology of perioperative MI
remains poorly understood, and reliable methods do not yet exist to
predict the risk of such injury in individual patients (2,3).
MI subsequent to non-cardiac surgery may be caused
by preoperative endothelial dysfunction, with ~58% of patients with
this dysfunction developing postoperative MI (4). Endothelial dysfunction compromises the
proper regulation of vascular tone, thrombosis and inflammation
(5), and increases the risk of
atherosclerosis and thromboembolic events (6). Coronary artery endothelial dysfunction
increases the risk of adverse cardiovascular events (2,7,8), and preoperative brachial arterial
endothelial dysfunction is independently predictive of short- and
long-term postoperative cardiovascular events in patients
undergoing vascular surgery (9,10).
Perioperative endothelial dysfunction may be a risk factor for
postoperative cardiovascular events, and assessment of endothelial
function using non-invasive measures prior to and following
non-cardiac surgery may assist clinicians to prevent and treat
postoperative MI (4).
Postoperative pain may impair the endothelial
function and increase the risk of endothelial dysfunction,
consequently leading to acute coronary syndrome (11). In addition, pain is negatively
correlated with perioperative endothelial function (11). This raises the question of whether
appropriate pain management following non-cardiac surgery is able
to reduce the risk of endothelial dysfunction. In support of this
hypothesis, postoperative pain management, such as the epidural
blockade of thoracic sympathetic segments using local anesthetics,
has been demonstrated to reduce risk of MI (12–15).
To test the hypothesis that postoperative pain
control may help reduce endothelial dysfunction, a randomized
controlled investigation was performed in the present study,
comparing the analgesic effects of tramadol against a placebo. The
results may help guide clinical practices for reducing the risk of
MI subsequent to non-cardiac surgery and may help clarify the
poorly understood mechanisms underlying this injury.
Materials and methods
Subjects
This randomized, double-blind, placebo-controlled
study was performed in Chongqing Medical University (Chongqing,
China). Patients aged 20–60 years who were undergoing laparoscopic
cholecystectomy involving standardized total intravenous anesthesia
(TIVA) at Chengdu Women and Children's Central Hospital (Chengdu,
China) between January 2014 and November 2015 were eligible to
participate in the present study (16). The exclusion criteria were as
follows: Patients undergoing emergency surgery; patients classified
as grade IV or V on the American Society of Anesthesiologists scale
(11); patients declining to be
analyzed postoperatively; or patient with unstable postoperative
vital signs.
In order to estimate an adequate sample size, it was
hypothesized that the placebo group would experience 70% reduction
in the endothelial function, while the tramadol group would
experience a 30% reduction, based on the findings of previous
studies on endothelial dysfunction (17,18).
Assuming a baseline flow-mediated dilation (FMD) of 7.0±1.5%, it
was calculated that 80 patients in each group should provide 80%
power to detect a change in the FMD at α=0.05.
Group allocation and
interventions
Patients were assigned to the tramadol or saline
(placebo) groups in a 1:1 ratio using software-driven
randomization. All subjects completed a questionnaire in order to
provide the baseline clinicodemographic data, including the present
complaints, as well as personal and family medical history. Pain
severity was assessed using a modified visual analogue scale (VAS)
(11) with scores between 0 (no
pain) and 10 (extremely painful). This scale has demonstrated a
test-retest reliability intraclass correlation of 0.87 for repeated
scoring (11). Brachial artery FMD
and endothelium-independent dilation, which is referred to as the
nitroglycerin (NTG)-induced dilation, were measured on preoperative
day 1 (baseline).
All participants received intravenous glycopyrrolate
(0.2 mg; Carbosynth Ltd., Compton, UK) as premedication, followed
by TIVA that was performed as described previously (16). Briefly, TIVA was induced using
propofol (2 mg/kg; Yichang Humanwell Pharmaceutical Co., Ltd.,
Yichang, China) and fentanyl (0.3 µg/kg; Yichang Humanwell
Pharmaceutical Co., Ltd.) target-controlled infusions (TCIs),
administered using a two-channel infusion pump. Intravenous
rocuronium (0.6 mg/kg; Yichang Humanwell Pharmaceutical Co., Ltd.)
was also administered for neuromuscular blockade. Following
tracheal intubation, propofol and fentanil TCIs were titrated to
maintain the bispectral index between 40–60, and the systolic blood
pressure and heart rate within ± 20% of their baseline values.
Participants received a single intravenous dose of ondansetron (4
mg, Yichang Humanwell Pharmaceutical Co., Ltd.) to prevent
postoperative nausea and vomiting. Routine monitoring consisted of
electrocardiography, pulse oximetry, non-invasive arterial
pressure, end-tidal carbon dioxide partial pressure and bispectral
index measurement.
For postoperative pain management, patients in the
tramadol group were administered intravenous, patient-controlled
tramadol prior to transporting to the recovery room. The delivery
settings were a bolus dose of 10 mg (Yichang Humanwell
Pharmaceutical Co., Ltd.), followed by a lock-out time of 10 min
and a basal infusion rate of 10 mg/h. Patients in the placebo group
received 0.9% saline. If VAS scores were >4, patients were given
intravenous fentanyl (20 µg) repeatedly until the score fell <4
(19). The number of patients
requiring rescue analgesia was recorded. The VAS scores, FMD and
NTG-induced dilation were again examined at 2 h, 1 day and 5 days
postoperatively.
Measurement of FMD and NTG-induced
dilation
The flow-mediated and NTG-induced dilations were
both examined in the present study, since these function through
different mechanisms. In FMD, increased blood flow stimulates the
release of vasodilators, such as nitric oxide, from the endothelium
and causes arterial dilation. By contrast, NTG functions directly
on the arterial smooth muscle and induces endothelium-independent
dilation (11).
The FMD and NTG-induced dilation were measured using
ultrasound as previously described (20,21).
Briefly, the brachial artery diameter was measured using
high-resolution B-mode ultrasound under three conditions: At rest,
in response to reactive hyperemia, and following sublingual
exposure to nitroglycerin (400 µg). An L10-5 linear array
transducer with a median frequency of 7.5 MHz and a standard ATL
HDI 5,000 ultrasound system (Philips Healthcare, Amsterdam, The
Netherlands) were used. Reactive hyperemia was induced by inflating
a pneumatic tourniquet around the forearm, which had been
positioned distal to the segment of the artery being scanned, to a
pressure of 220–240 mmHg for 4.5 min, followed by release.
Doppler-derived arterial flow was measured at rest and during
hyperemia.
These procedures were conducted in a quiet
environment, and no significant alterations in the heart rate or
blood pressure were observed. These procedures have been
demonstrated to be accurate and reproducible, with a low
interobserver error (11). In our
previous studies, excellent reliability was routinely achieved,
with the FMD presenting a mean relative difference of only 3%
within individual patients (22,23).
All measurement sessions were recorded on Super-VHS
videotape for subsequent off-line analysis by an investigator
blinded to the subject identity, group allocation and time point
(pre- or postoperative). The same investigator analyzed videos of
all participants.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Collected data were evaluated using an analysis of covariance
model. Differences in the clinical and vascular parameters were
compared with baseline among the three postoperative periods (2 h,
1 day, 5 days) and significant differences were determined by
repeated-measures analysis of variance. Multiple comparisons
between groups were performed using Student-Newman-Keuls post-hoc
test. Backward stepwise multivariate analysis of variance with
Tukey's post-hoc test was performed to estimate the major
determinants of the FMD data, including age, VAS, systolic and
diastolic blood pressures, heart rate and reactive hyperemia.
Statistical analysis was performed using SPSS software 18.0 (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Baseline characteristics
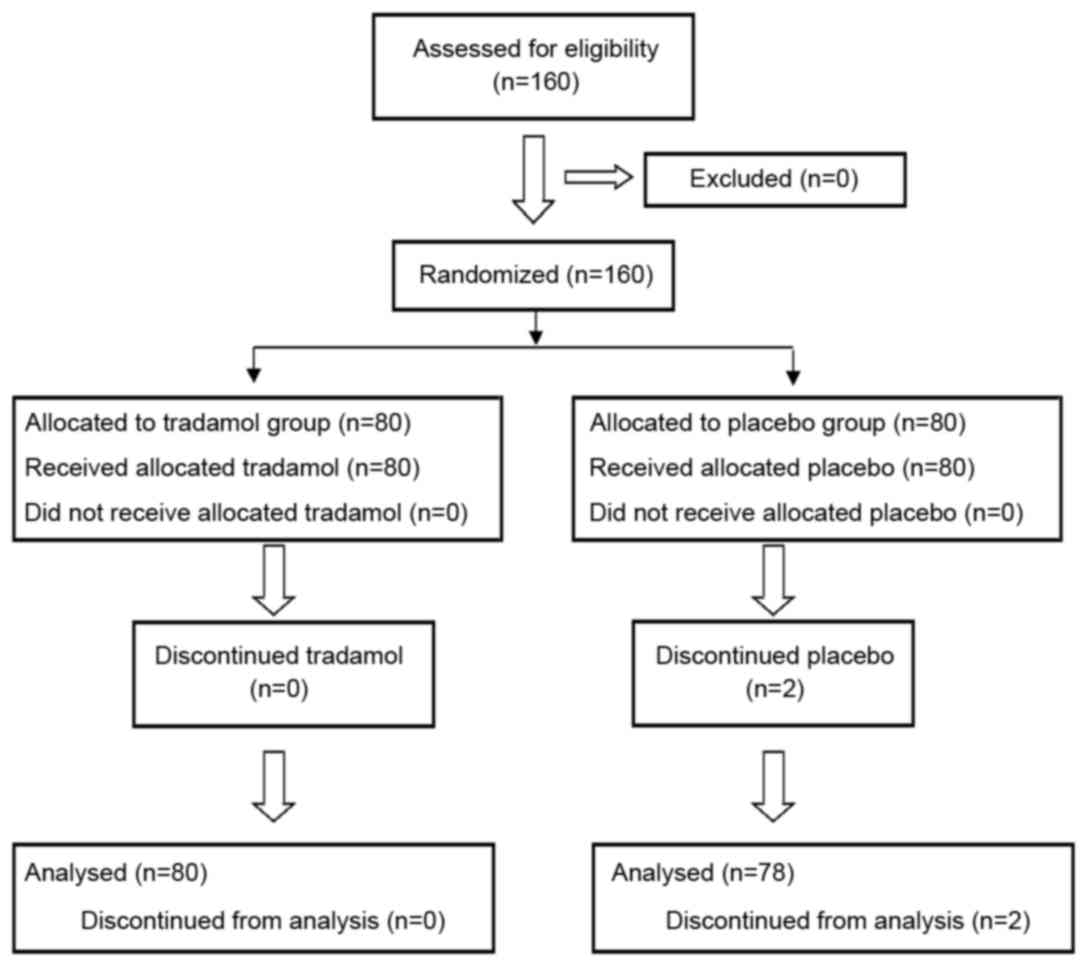
A total of 160 patients were enrolled into the
current study and randomized into the tramadol and placebo groups
(Fig. 1). However, 2 participants in
the placebo group were excluded since they had to undergo open
cholecystectomy due to dense inflammatory adhesions. Thus, 158
patients completed the study, with a mean age of 43.8±14.0 years
(range, 20–60 years). The tramadol and placebo groups were similar
with regard to their major clinicodemographic characteristics and
vascular function indicators (Table
I), including the age, body mass index (BMI), pain history,
dosage and type of anesthetic and vasoactive drugs, volume of fluid
supplementation, surgical time and volume of intraoperative
hemorrhage. No patient developed significant perioperative
cardiovascular complications.
 | Table I.Clinicodemographic characteristics and
indicators of vascular reactivity in patients receiving analgesic
or placebo treatment subsequent to laparoscopic
cholecystectomy. |
Table I.
Clinicodemographic characteristics and
indicators of vascular reactivity in patients receiving analgesic
or placebo treatment subsequent to laparoscopic
cholecystectomy.
| Characteristic | Tramadol (n=80) | Saline (n=80) |
|---|
| Age, years | 56.8±10.1 | 57.0±11.2 |
| Male: female, n | 28:32 | 26:34 |
| Risk classification,
n |
|
|
| ASA grade
I | 32 | 34 |
| ASA grade
II | 28 | 26 |
| Surgical time,
min | 65.3±10.2 | 64.2±9.6 |
| Laboratory
results |
|
|
| TC
(mmol/l) | 5.23±0.82 | 5.31±1.02 |
| TG
(mmol/l) | 1.72±1.21 | 1.68±0.65 |
| Urea
nitrogen (mmol/l) | 4.71±0.73 | 4.66±1.15 |
|
Creatinine (mmol/l) | 74.9±13.1 | 75.2±11.8 |
| Glucose
(mmol/l) | 5.28±1.10 | 4.36±3.01 |
| Vascular
reactivity |
|
|
| Brachial
FMD (%) | 7.0±1.5 | 6.9±1.4 |
| Brachial
NTG-induced dilation (%) | 16.8±2.3 | 16.3±2.7 |
| Blood flow,
ml/min | 23.6±12.8 | 20.4±9.1 |
| Shear rate,
×103/sec | 81.2±20.8 | 86.0±21.9 |
| Reactive hyperemia
(%) | 425±102 | 417.5±191.3 |
| VAS score | 0.8±1.3 | 0.6±1.1 |
| Blood pressure
(mmHg) |
|
|
|
Systolic | 113.1±15.2 | 115.4±17.6 |
|
Diastolic | 71.9±9.3 | 72.3±8.51 |
| Heart rate
(bpm) | 75.3±6.2 | 72.1±9.6 |
General clinical and vascular
parameters
The tramadol and placebo groups demonstrated similar
blood flow and reactive hyperemia at all four time points. At 2 h
postoperatively, the blood pressure and heart rate were
significantly higher in the two groups compared with these values
at the other three time points (P<0.05). In addition, at 2 h
postoperatively, the blood flow was significantly greater in
comparison with that at baseline in the two groups (P<0.05),
whereas reactive hyperemia was significantly lower (P<0.01),
although shear rate remained unchanged. The tramadol and placebo
groups demonstrated stable responses to NTG throughout surgery
(Table II).
 | Table II.Vascular parameters at different time
points in patients receiving analgesic or placebo treatment
following laparoscopic cholecystectomy. |
Table II.
Vascular parameters at different time
points in patients receiving analgesic or placebo treatment
following laparoscopic cholecystectomy.
|
| Tramadol group | Placebo (saline)
group |
|---|
|
|
|
|
|---|
| Parameter | Baseline | 2 h | 1 day | 5 days | Baseline | 2 h | 1 day | 5 days |
|---|
| Systolic blood
pressure, mmHg |
113.1±15.2 |
124.0±24.2 |
110.0±14.5 |
113.1±18.0 |
115.4±17.6 |
124.7±15.9 |
113.3±14.8 |
117.1±21.1 |
| Diastolic blood
pressure, mmHg |
71.9±9.3 |
75.8±15.2 |
72.5±11.2 |
71.8±9.5 |
72.3±8.51 |
76.2±10.4 |
73.2±7.8 |
69.8±9.3 |
| Heart rate,
bpm |
75.3±6.2 |
94.7±12.2 |
81.7±13.9 |
74.9±12.3 |
72.1±9.6 |
95.7±12.7 |
77.4±11.6 |
76.2±9.8 |
| Blood flow,
ml/min |
23.6±12.8 |
29.7±9.6 |
24.1±10.0 |
24.4±6.7 |
20.4±9.1 |
29.8±7.6 |
22.4±16.4 |
26.9±14.8 |
| Shear rate,
×103/sec |
81.2±20.8 |
84.7±16.4 |
80.2±25.5 |
82.3±21.6 |
86.0±21.9 |
83.2±22.8 |
81.2±19.8 |
83.4±23.8 |
| Hyperemia, % |
425±102 |
389±105 |
461±157 |
425±102 |
417±191 |
396±110 |
441±90 |
393±123 |
| NTG-induced
dilation, % |
16.8±2.3 |
17.2±3.1 |
17.3±3.9 |
18.6±2.6 |
16.3±2.7 |
16.4±3.2 |
16.9±4.7 |
17.1±3.7 |
FMD, NTG-induced dilation and VAS
score
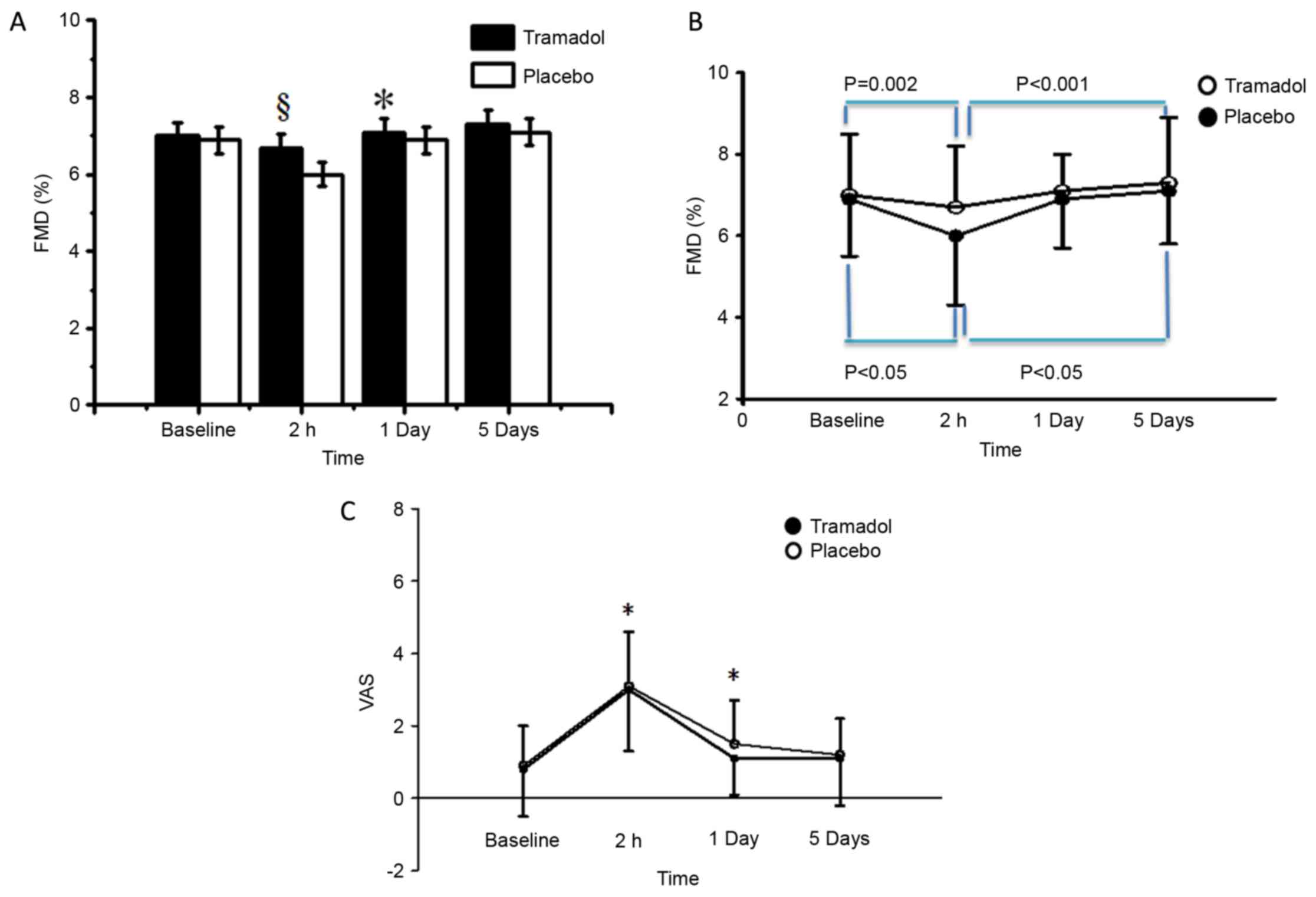
FMD was significantly higher in the tramadol group
as compared with that in the placebo group at 2 h postoperatively
(6.7±1.5 vs. 6.0±1.7%; P=0.001; Table
III) and at 1 day postoperatively (7.1±1.3 vs. 6.9±1.4%;
P=0.03; Fig. 2A). FMD decreased
significantly between the baseline and 2 h postoperatively in the
tramadol (P=0.002) and placebo groups (P<0.05; Fig. 2B). Conversely, the VAS score
increased significantly over the same period in the two groups
(P<0.05). Since the two groups had similar FMD at baseline, this
indicates that tramadol was associated with a smaller decrease in
the FMD following surgery, which later returned to similar levels
for the two groups at 5 days postoperatively. Furthermore, the
NTG-induced dilation were stable throughout the various time points
in the two groups, ranging between 16.8±2.3 and 18.6±2.6% in the
tramadol group (P>0.20; Table
II).
 | Table III.Comparison of brachial FMD and VAS
scores at different times in patients receiving analgesic or
placebo treatment following laparoscopic cholecystectomy. |
Table III.
Comparison of brachial FMD and VAS
scores at different times in patients receiving analgesic or
placebo treatment following laparoscopic cholecystectomy.
|
| Tramadol group | Placebo (saline)
group |
|---|
|
|
|
|
|---|
| Time point | VAS | FMD (%) | VAS | FMD (%) |
|---|
| Preoperative day
1 |
0.8±1.3 |
7.0±1.5 |
0.6±1.1 |
6.9±1.4 |
| Postoperative |
|
|
|
|
| 2 h |
3.0±1.7 |
6.7±1.5 |
3.1±1.5 |
6.0±1.7a |
| Day
1 |
1.1±1.0 |
7.1±0.9 |
1.1±1.2 |
6.9±1.2b |
| Day
5 |
1.1±1.3 |
7.3±1.6 |
0.9±1.0 |
7.1±1.3 |
The VAS score was significantly lower in the
tramadol group when compared with that in the placebo group at 2 h
(2.8±1.9 vs. 3.2±2.3; P=0.013) and 1 day postoperatively (1.4±1.0
vs. 1.7±1.8; P=0.031; Fig. 2C).
Across the two patient groups and all four time points, a VAS score
of ≥5 was independently associated with postoperative FMD of <7%
[odds ratio (OR), 2.7; 95% confidence interval (95% CI), 0.9–5.0;
P=0.041]. Conversely, a VAS score of <5 at 2 h postoperatively
in the tramadol group was independently associated with an FMD
value of ≥7% (OR, 2.5; 95% CI, 1.0–6.0; P=0.047). The total
fentanyl consumption was also significantly lower in the tramadol
group in comparison with that in the placebo group (37.4±36.8 vs.
53.7±37.1; P<0.05).
Backward stepwise multiple linear regression
identified several independent correlations between the
clinicodemographic variables and FMD value of patients at 2 h
postoperatively (Table IV). The FMD
value was found to be negatively correlated with the age (B=−1.403;
P=0.011), VAS score (B=−0.579; P=0.003) and BMI of patients
(B=−0.551; P=0.010). In addition, the FMD at 2 h postoperatively
was positively correlated with the FMD at the baseline (B=1.186;
P<0.001).
 | Table IV.Correlation analysis to identify the
predictors of FMD (%) at 2 h postoperatively in all patients. |
Table IV.
Correlation analysis to identify the
predictors of FMD (%) at 2 h postoperatively in all patients.
| Factor | B-value | β | P-value |
|---|
| Age | −1.403 | −0.913 | 0.011 |
| VAS score | −0.579 | −0.996 | 0.003 |
| Baseline FMD
(%) | 1.186 | 0.998 | <0.001 |
| BMI | −0.551 | −0.992 | <0.001 |
| Hyperemia (%) | 0.005 | 0.17 | 0.066 |
Discussion
The results of the present relatively small
randomized, double-blind trial suggested that analgesic treatment
following laparoscopic cholecystectomy may lead to improved
arterial endothelial function postoperatively. In addition, this
improved endothelial function appears to be correlated with a lower
incidence of MI. In particular, a VAS score of <5 appeared to be
associated with a lower risk of MI.
In the present study on laparoscopic
cholecystectomy, the FMD in the two groups significantly decreased
below the baseline value at 2 h postoperatively and remained at low
levels for at least 1 extra day, showing recovery at 5 days
postoperatively. This timescale of postoperative FMD alterations is
consistent with studies examining other types of non-cardiac
surgery (11,18,24,25). For
instance, FMD decreased significantly compared with the baseline
value during the first 24 h after knee replacement surgery,
recovering to the baseline levels by 7 days postoperatively
(18). In addition, endothelial
function significantly improved in patients within 2 weeks
postoperatively following renal transplant surgery (24), and 28 days after femoropopliteal
bypass surgery (25). These past and
present findings are consistent with the hypothesis that
alterations in the arterial endothelial function during the
perioperative period contribute to perioperative myocardial
infarction and other cardiovascular events, since these events
usually occur within 3 days after surgery (26). The current study observed that the
FMD level was significantly improved at 2 h postoperatively in the
tramadol group as compared with that in the placebo group,
suggesting that analgesic treatment may improve the arterial
endothelial function and thereby reduce the risk of MI.
Tramadol may exert these effects by reducing the
stress response to surgery and pain (14,15).
Perioperative trauma and pain cause stress reactions, as well as
the release of proinflammatory cytokines, including C-reactive
protein, tumor necrosis factor α, interleukin (IL)-1, IL-6 and
IL-8. Stress reactions and proinflammatory cytokines may then
inhibit the FMD by impairing nitric oxide production (11,27–29).
Indeed, IL-6 is a marker of endothelial dysfunction, and elevated
levels of this cytokine are associated with a low FMD in patients
suffering from myocardial infarction (29). Thoracic epidural analgesia can reduce
the stress responses to surgery and pain by blocking the
neuroendocrine pathway (14).
Micromolar concentrations of morphine inhibit the
lipopolysaccharide-induced synthesis of IL-6 (30). However, even in patients receiving
pain relief by tramadol through a patient-driven system, the FMD at
2 h postoperatively was significantly lower compared with the
baseline level, supporting the hypothesis that arterial endothelial
dysfunction in the early postoperative period may help drive MI. It
is possible that the MI observed in the tramadol group in the
current study was due to ‘non-obstructive’ vulnerable plaques
(2). These plaques cannot be
detected by myocardial studies to investigate whether postoperative
VAS scores of ≥5 may be a predictor of MI following non-cardiac
surgery.
The present study has several limitations. Larger
sample sizes and higher-risk cohorts should be examined in the
longer term in order to fully explore the correlation of analgesic
treatment and endothelial function with postoperative
cardiovascular complications. In addition, whether the included
patients took off-study drugs, antibiotics, vitamins, potassium
chloride or other antihemorrhagic treatment cannot be excluded,
which may have influenced the endothelial function and therefore
interfered with our results. However, it was confirmed that the
tramadol and placebo groups were similar in terms of the dose and
type of anesthetic and vasoactive drugs, surgery duration and
clinicodemographic characteristics.
In conclusion, the present study suggested that
analgesic treatment was able to improve arterial endothelial
function following non-cardiac surgery. This strengthens the
previously described link between postoperative pain and vascular
endothelial function; postoperative pain could impair endothelial
function, leading to postoperative MI. This could inform a new
treatment approach to decrease the incidence of postoperative acute
coronary syndrome. More prospective work on large cohorts is needed
to confirm this.
References
|
1
|
Khan J, Alonso-Coello P and Devereaux PJ:
Myocardial injury after noncardiac surgery. Curr Opin Cardiol.
29:307–311. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McIlroy DR, Chan MT, Wallace SK, Symons
JA, Koo EG, Chu LC and Myles PS: Automated preoperative assessment
of endothelial dysfunction and risk stratification for
perioperative myocardial injury in patients undergoing non-cardiac
surgery. Br J Anaesth. 112:47–56. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Deveraux PJ, Goldman L, Cook DJ, Gilbert
K, Leslie K and Guyatt GH: Perioperative cardiac events in patients
undergoing non-cardiac surgery: A review of the magnitude of the
problem, the pathophysiology of the events and methods to estimate
and communicate risk. CMAJ. 173:627–634. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Søndergaard ES, Fonnes S and Gögenur I:
Endothelial dysfunction after non-cardiac surgery: A systematic
review. Acta Anaesthesiol Scand. 59:140–146. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kwok T, Chook P, Tam L, Qiao M, Woo JLF,
Celermajer DS and Woo KS: Vascular dysfunction in Chinese
vegetarians: an apparent paradox. J Am Coll Cardiol. 46:1957–1958.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fuster V, Badimon L, Badimon JJ and
Chesebro JH: The pathogenesis of coronary artery disease and the
acute coronary syndromes (2). N Engl J Med. 326:310–318. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Suwaidi JA, Hamasaki S, Higano ST,
Nishimura RA, Holmes DR Jr and Lerman A: Long-term follow-up of
patients with mild coronary artery disease and endothelial
dysfunction. Circulation. 101:948–954. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schachinger V, Britten MB and Zeiher AM:
Prognostic impact of coronary vasodilator dysfunction on adverse
long-term outcome of coronary heart disease. Circulation.
101:1899–1906. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gokce N, Keaney JF Jr, Hunter LM, Watkins
MT, Menzoian JO and Vita JA: Risk stratification for postoperative
cardiovascular events via noninvasive assessment of endothelial
function: A prospective study. Circulation. 105:1567–1572. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gokce N, Keaney JF Jr, Hunter LM, Watkins
MT, Nedeljkovic ZS, Menzoian JO and Vita JA: Predictive value of
noninvasively determined endothelial dysfunction for long-term
cardiovascular events inpatients with peripheral vascular disease.
J Am Coll Cardiol. 41:1769–1775. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu YJ, Wei AN, Chook P, Yin Y, Cheng W, Wu
MJ, Celermajer DS and Woo KS: Impact of on-cardiovascular surgery
on reactive hyperaemia and arterial endothelial function. Clin Exp
Pharmacol Physiol. 40:466–472. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Drenger B, Fontes ML, Miao Y, Mathew JP,
Gozal Y, Aronson S, Dietzel C and Mangano DT: Investigators of the
Ischemia Research and Education Foundation; Multicenter Study of
Perioperative Ischemia Research Group: Patterns of use of
perioperative angiotensin-converting enzyme inhibitors in coronary
artery bypass graft surgery with cardiopulmonary bypass: Effects on
in-hospital morbidity and mortality. Circulation. 126:261–269.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mangano DT, Siliciano D, Hollenberg M,
Leung JM, Browner WS, Goehner P, Merrick S and Verrier E:
Postoperative myocardial ischemia. Therapeutic trials using
intensive analgesia following surgery. The Study of Perioperative
Ischemia (SPI) Research Group. Anesthesiology. 76:342–353. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wink J, Veering BT, Aarts LP and Wouters
PF: Effect of increasing age on the haemodynamic response to
thoracic epidural anaesthesia: An observational study. Eur J
Anaesthesiol. 31:597–605. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Guo W, Liu F, Fu L, Qu R, Wang G and Zhang
C: Effects of high thoracic epidural sympathetic blockade for the
treatment of severe chronic heart failure due to dilated
cardiomyopathy. Acta Cardiol. 67:533–539. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim JY, Park SY, Chang HS, Nam SK and Min
SK: The efficacy of the time-scheduled decremental continuous
infusion of fentanyl for postoperative patient-controlled analgesia
after total intravenous anesthesia. Korean J Anesthesiol.
65:544–551. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
de Boer AG, van Lanschot JJ, Stalmeier PF,
van Sandick JW, Hulscher JB, de Haes JC and Sprangers MA: Is a
single-item visual analogue scale as valid, reliable and responsive
as multi-item scales in measuring quality of life? Qual Life Res.
13:311–320. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bukal K, Ruzić A, Bazdarić K, Sokolić J,
Vukić-Dugac A, Velcić-Brumnjak S, Sestan B, Gulan JR and Gulan G:
Total knee replacement surgery is followed by transitory
endothelial dysfunction. Coll Antropol. 36:611–616. 2012.PubMed/NCBI
|
|
19
|
Park JK, Cheong SH, Lee KM, Lim SH, Lee
JH, Cho K, Kim MH and Kim HT: Does dexmedetomidine reduce
postoperative pain after laparoscopic cholecystectomy with
multimodal analgesia? Korean J Anesthesiol. 63:436–440. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jabbour-Khoury SI, Dabbous AS, Gerges FJ,
Azar MS, Ayoub CM and Khoury GS: Intraperitoneal and intravenous
routes for pain relief in laparoscopic cholecystectomy. JSLS.
9:316–321. 2005.PubMed/NCBI
|
|
21
|
Celermajer DS, Sorensen KE, Georgakopoulos
D, Bull C, Thomas O, Robinson J and Deanfield JE: Cigarette smoking
is associated with dose-related and potentially reversible
impairment of endothelium-dependent dilation in healthy young
adults. Circulation. 88:2149–2155. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Woo KS, Yip TW, Chook P, Kwong SK, Szeto
CC, Li JK, Yu AW, Cheng WK, Chan TY, Fung KP and Leung PC:
Cardiovascular Protective Effects of Adjunctive Alternative
Medicine (Salvia miltiorrhiza and Pueraria lobata) in High-Risk
Hypertension. Evid Based Complement Alternat Med. 2013:1329122013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Woo KS, Chook P, Leong HC, Huang XS and
Celermajer DS: The impact of heavy passive smoking on arterial
endothelial function in modernized Chinese. J Am Coll Cardiol.
36:1228–1232. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kocak H, Ceken K, Yavuz A, Yucel S, Gurkan
A, Erdogan O, Ersoy F, Yakupoglu G, Demirbas A and Tuncer M: Effect
of renal transplantation on endothelial function in haemodialysis
patients. Nephrol Dial Transplant. 21:203–207. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Unal O, Karatepe O, Ugurlucan M, Koc B,
Filizcan U and Aksoy M: Effects of lower extremity
revascularization on the endothelial functions measured with
noninvasive brachial artery flow-mediated dilatation. Ann Vasc
Surg. 25:969–974. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fleischmann KE, Goldman L, Young B and Lee
TH: Association between cardiac and noncardiac complication in
patients undergoing noncardiac surgery. Outcomes and effects on
length of stay. Am J Med. 115:515–520. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bhagat K and Vallance P: Inflammatory
cytokines impair endothelium-dependent dilatation in human veins in
vivo. Circulation. 96:3042–3047. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chia S, Qadan M, Newton R, Ludlam CA, Fox
KA and Newby DE: Intra-arterial tumor necrosis factor-alpha impairs
endothelium-dependent vasodilatation and stimulates local tissue
plasminogen activator release in humans. Arterioscler Thromb Vasc
Biol. 23:695–3701. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Erzen B, Sabovic M, Sebestjen M, Keber I
and Poredos P: Interleukin-6 correlates with endothelial
dysfunction in young post-myocardial infarction patients.
Cardiology. 107:111–116. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Roy S, Cain KJ, Chapin RB, Charboneau RG
and Barke RA: Morphine modulates NF kappa B activation in
macrophages. Biochem Biophys Res Commun. 245:392–396. 1998.
View Article : Google Scholar : PubMed/NCBI
|