Introduction
As an inflammatory spine and joint disease,
pathogenesis of ankylosing spondylitis (AS) is mainly correlated
with human leukocyte antigen B27 (HLA-B27). Inflammatory factors
can invade synovial joints, articular cartilage, tendons, ligaments
and attachment points of ligament to cause fibrous and bony
rigidity (1,2). With the high morbidity, AS seriously
affects human health. However, etiology and pathogenesis of AS are
very complex, and studies have shown that the occurrence of AS is
closely correlated with genetic factors, inflammatory factors,
autoimmune disorders and other factors (3).
MicroRNAs (miRNAs) are endogenous non-coding
single-stranded small molecule RNAs with a length of ~18–25
nucleotides. miRNAs plays pivotal roles in cell development,
proliferation, differentiation and apoptosis, and carcinogenic
process through the regulation of gene expression at
post-transcriptional level, and expression of miRNAs is closely
correlated with the development of various human diseases (4). Studies have shown that human genome
encodes >1,000 miRNAs, and these miRNAs can regulate the
expression of 60% of all protein-encoding genes (5). miRNAs can act on 3′-untranslated region
of target mRNA, which in turn leads to the silencing of the target
gene (6).
Studies have shown that miRNAs play important roles
in the development and progression of rheumatic diseases, and the
most studied miRNA is miR-146a (7).
It has been shown that Toll-like receptor ligands such as
lipopolysaccharide, lipoprotein and inflammatory factors such as
tumor necrosis factor-α (TNF-α) and interleukin-1β (IL-1β) can
increase the expression of miR-146a by activating nuclear factor-κB
(NF-κB) activity, while overexpression of miR-146a can inhibit the
activity of NF-κB pathway by inhibiting the expression of TNF
receptor associated factor 6 (TRAF-6) and interleukin 1
receptor-associated kinase-1 (IRAK-1) (8,9). Studies
have shown that NF-κB can affect the production of TNF-α, IL-1β and
IL-6 (10), so we speculate that
miR-146a can affect the levels of inflammatory factors in patients
with AS through NF-κB pathway. In this study, expression level of
miR-146a in peripheral blood mononuclear cell (PBMC) and levels of
TNF-α, IL-1β and IL-6 in serum of AS patients were detected. In
addition, the correlation between expression of miR-146a and levels
of TNF-α, IL-1β and IL-6, and clinical indicators including bath
ankylosing spondylitis disease activity index (BASDAI), C-reactive
protein (CRP), erythrocyte sedimentation rate (ESR) and duration
morning stiffness were analyzed.
Materials and methods
Materials
Forty-five patients with AS who were admitted by the
Department of Rheumatology of Weifang Hospital were selected from
June, 2014 to January, 2016. Among the patients, there were 33
males and 12 females with an average age of 27.42±6.85 years. All
patients with AS were diagnosed according to the New York standard
established by American College of Rheumatology in 1984 (11), all patients were HLA-B27 positive.
Exclusion criteria: Patients received intra-articular and
glucocorticoid treatment before admission, patients suffering from
severe heart, brain, kidney and other vital organs dysfunction and
systemic disease, pregnant and lactating women. A total of 30
normal healthy people who received physical examination in Weifang
Hospital were selected as normal control group. Control group
included 21 males and 9 females with an average age of 26.73±5.36
years. There was no significant difference in gender and age
between AS group and normal control group. Clinical parameters of
AS patients including BASDAI, ESR, CRP and duration of morning
stiffness were collected. Sample collections were approved by the
Ethics Committee of Weifang People's Hospital. All participants
signed informed consent.
RPMI-1640 medium and fetal bovine serum (FBS)
(HyClone Laboratories, Logan, UT, USA); Ficoll paque plus
lymphocyte isolation solution (GE Healthcare, Bethesda, MD, USA);
RNA extraction kit (Invitrogen, Carlsbad, CA, USA); primer
synthesis, reverse transcription kit, and quantitative real-time
PCR (qRT-PCR) kit (Takara, Dalian, China); IL-1β, IL-6 and TNF-α
enzyme-linked immunosorbent assay (ELISA) kits (Beyotime Institute,
Nantong, China) were used.
Isolation of PBMC from patients and
cell culture
Fasting venous blood (5 ml) was extracted from AS
patients and healthy control in the morning and
ethylenediaminetetraacetic acid (EDTA) was added for
anticoagulation. PBMCs were isolated using Ficoll-Paque Plus
lymphocyte isolation solution. PBMCs were cultured with RPMI-1640
medium containing 10% autologous serum in an incubator (37°C, 5%
CO2) for 2 h. After that, cells were washed and
suspended cells were removed to obtain adherent cells (PBMCs).
Culture medium was replaced every 24 h, and subculture was
performed when cell fusion was reached.
qRT-PCR to detect the expression of
miR-146a in PBMC
PBMCs were collected and total RNA was extracted
using RNA extraction kit according to the instruction.
Concentration and purity of total RNA were measured using UV-Vis
spectrophotometer (Hitachi, Tokyo, Japan) and only samples with a
ratio of A260/A280 between 1.8 and 2.0 were used. Reverse
transcription was then performed according to the instructions of
reverse transcription kit to synthesize cDNA. qRT-PCR was performed
according to the instruction of qRT-PCR kit using cDNA as template
and U6 RNA as endogenous control. The primers for miR-146a and U6
are listed in Table I. Reaction
conditions were 95°C for 10 min, followed by 40 cycles of 95°C for
15 sec and 60°C for 1 min. Ct values were processed using
2−ΔCt method according to the following formula: ΔCt
(target gene) = Ct (target gene)-Ct (control gene).
 | Table I.Primers used for qRT-PCR. |
Table I.
Primers used for qRT-PCR.
| Genes | Primer sequences |
|---|
| miR-146a | F:
5′-TGAGAACTGAATTCCATGGGTT-3′ |
|
| R:
5′-GCTGTCAACGATACGCTACGTAACG-3′ |
| U6 | F:
5′-GCTTCGGCAGCACATATACTAAAAT-3′ |
|
| R:
5′-CGCTTCACGAATTTGCGTGTCAT-3′ |
ELISA method to detect the levels of
IL-1β, IL-6 and TNF-α in supernatant of PBMC medium and serum
PBMCs were collected at logarithmic growth phase,
and 2 ml (2×105 cells/ml) PBMC cell suspension was
transferred to a 6-well plate, followed by incubation for 24 h.
Supernatant (50 µl) of culture medium was collected to measure the
levels of IL-1β, IL-6 and TNF-α using ELISA according to the
instructions. Fasting venous blood (5 ml) was extracted from AS
patients and healthy controls in the morning. After water bath at
37°C for 10 min, blood samples were centrifuged to collect serum.
Serum sample were stored at −20°C before use. Serum contents of
IL-1β, IL-6 and TNF-α were measured using ELISA according to the
instructions.
Statistical analysis
Data were analyzed using SPSS 17.0 software (IBM
Corp., Armonk, NY, USA). Measurement data were expressed as mean ±
standard deviation, and comparisons between two groups were
performed using t-test. Comparisons of count data between two
groups were performed using χ2. Pearson's correlation
analysis was used to analyze the correlation between variables.
P<0.05 was considered to be statistically significant.
Results
Comparison of general information
between AS disease group and control group
In this study, 45 patients with AS were collected,
including 33 males and 12 females with an average age of 27.42±6.85
years. There were 30 healthy people in healthy control group,
including 21 males and 9 females with an average age of 26.73±5.36
years. There was no significant difference in gender and age
between the two groups (P>0.05). General information, BASDAI
score, ESR, CRP and duration of morning stiffness of AS patients
and normal controls are listed in Table
II.
 | Table II.General information of subjects. |
Table II.
General information of subjects.
| Characteristics | AS group (n=45) | Normal control
group | P-value |
|---|
| Sex
(male/female) | 33/12 | 21/9 | P>0.05 |
| Age (years) | 27.42±6.85 | 26.73±5.36 | P>0.05 |
| BASDAI score | 4.97±1.39 | NA | NA |
| ESR (mm/h) | 21.08±19.33 | NA | NA |
| CRP (mg/l) | 17.35±15.76 | NA | NA |
| Duration of morning
stiffness (min) | 30.58±27.57 | NA | NA |
Expression level of miR-146a in PBMC
detected by qRT-PCR
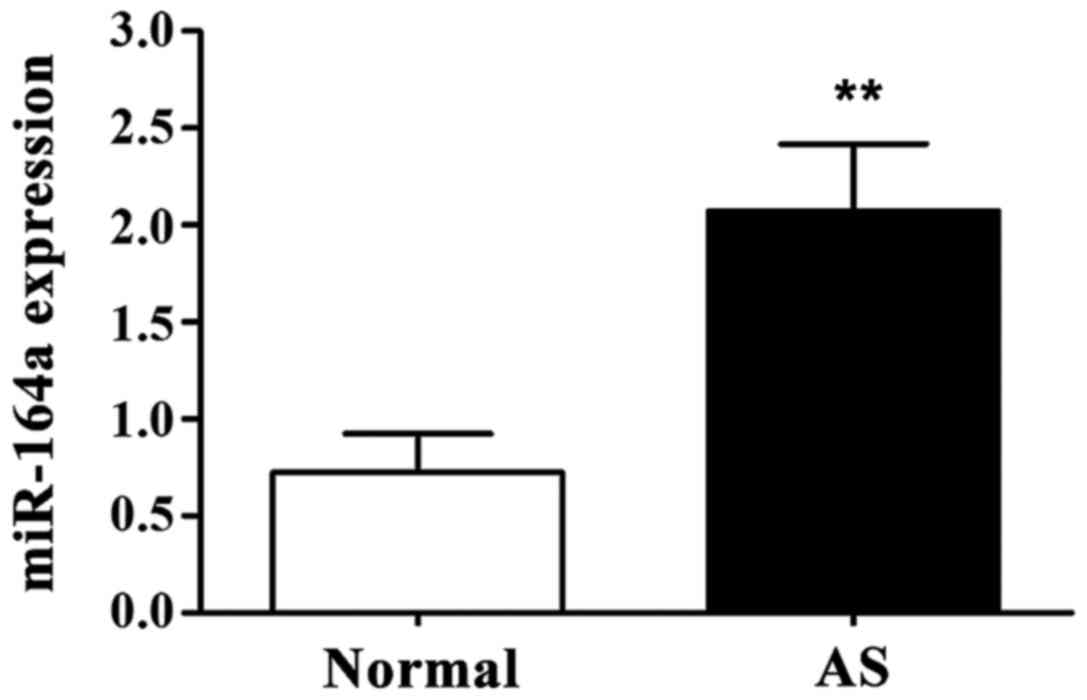
As shown in Fig. 1,
expression level of miR-146a in PBMC of AS group was significantly
higher than that of normal control group (P<0.01).
Levels of TNF-α, IL-1β and IL-6 in
supernatant of PBMC culture medium
Levels of TNF-α, IL-1β and IL-6 in supernatant of
PBMCs derived from AS patients were significantly higher than those
in normal control group (P<0.01) (Fig. 2).
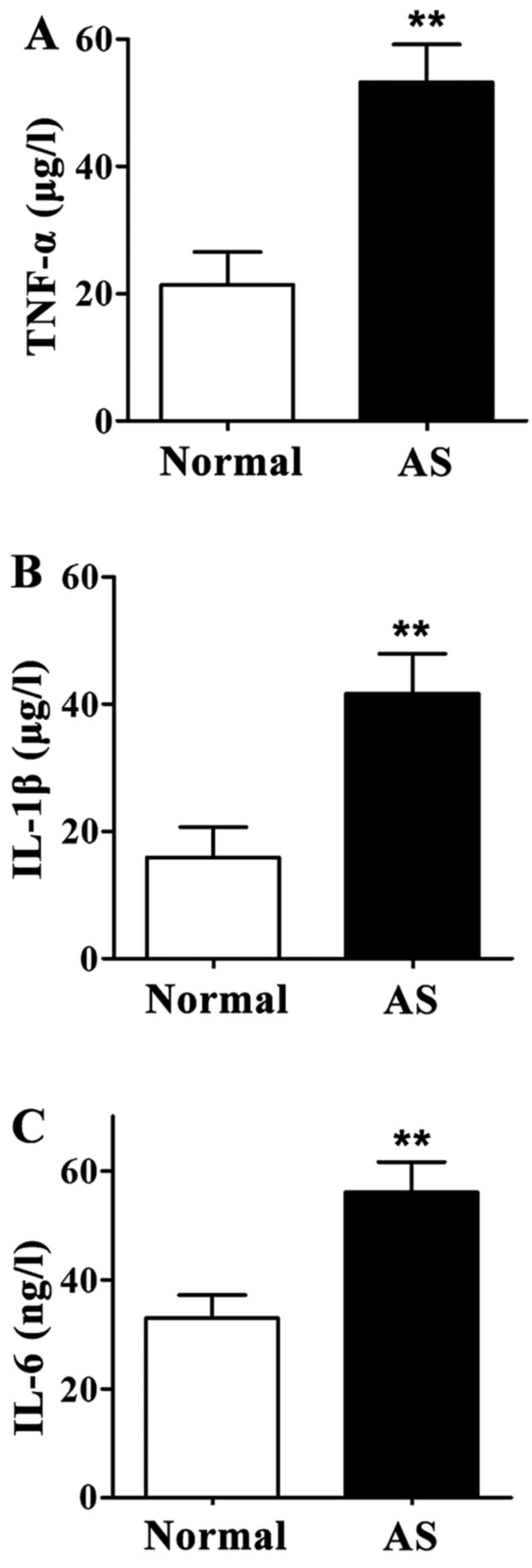
Levels of TNF-α, IL-1β and IL-6 in
serum
Levels of TNF-α, IL-1β and IL-6 in serum of AS
patients were significantly higher than those of healthy controls
(P<0.01) (Fig. 3).
Correlation between miR-146a
expression and serum inflammatory factors in patients with AS
Correlation between miR-146a expression and levels
of TNF-α, IL-1β and IL-6 in serum were analyzed by Pearson's
correlation analysis. As shown in Table III, relative expression levels of
miR-164a in PBMCs of AS patients were positively correlated with
the levels of TNF-α, IL-1β and IL-6 in serum (P<0.01).
 | Table III.Correlation between miR-146a
expression and serum inflammatory factors in patients with AS. |
Table III.
Correlation between miR-146a
expression and serum inflammatory factors in patients with AS.
| Serum inflammatory
factors | TNF-α | IL-1β | IL-6 |
|---|
| miR-146a | r=0.632 | r=0.574 | r=0.483 |
| P-value | P<0.01 | P<0.01 | P<0.01 |
Correlation between miR-146a
expression and clinical indicators of AS patients
Pearson's correlation analysis (Table IV) showed that relative expression
level of miR-164a in PBMC of AS patients was positively correlated
with BASDAI, ESR, CRP and duration of morning stiffness
(P<0.01).
 | Table IV.Correlation between miR-146a
expression and clinical indicators of AS patients. |
Table IV.
Correlation between miR-146a
expression and clinical indicators of AS patients.
| Clinical
indicators | BASDAI | ESR | CRP | Duration of morning
stiffness |
|---|
| miR-146a | r=0.551 | r=0.738 | r=0.685 | r=0.497 |
| P-value | P<0.01 | P<0.01 | P<0.01 | P<0.01 |
Discussion
AS is a kind of chronic progressive inflammatory
disease with the main clinical manifestations of waist, back, neck,
buttocks and hip pain, joint swelling and pain, difficulties in
movement and eye, lung, kidney and other organ damage, seriously
affecting the life quality of patients (12–14).
miRNAs are noncoding small RNAs that can regulate
gene expression. Mature miRNA binds to proteins to form RNA-induced
silencing complexes, which can inhibit mRNA translation to reduce
translation under imperfect base pairing. Under perfect base
pairing, RNA-induced silencing complexes can cause mRNA
degradation. Therefore, miRNA can regulate a series of
physiological processes in cells by inhibiting mRNA degradation or
cutting mRNA (15). miR-146 family
has two members: miR-146a and miR-146b. miR-146a can specifically
inhibit the expression of bridging molecules (such as TRAF-6 and
IRAK-1) between Toll-like receptor and NF-κB, so as to indirectly
inhibit the activity of NF-κB and reduce the production of IL-1β,
IL-6 and other inflammatory factors. miR-146 plays a key role in
the pathogenesis of systemic rheumatic diseases such as systemic
lupus erythematosus and systemic scleroderma (16,17).
Studies have shown that the activation of NF-κB pathway can lead to
the production of a huge amount of inflammatory factors such as
TNF-α, IL-1β and IL-6, which may be associated with the high
expression level of miR-146a (18).
As common inflammatory factors, TNF-α, IL-1β and
IL-6 have anti-infection, anti-tumor, immune regulation and other
biological effects. Under disease conditions, increased levels of
inflammatory factors can further cause tissue damage and
exacerbations (19,20). As an autoimmune inflammatory disease,
the development of AS is closely related with Inflammatory factors.
It has been reported that TNF-α, IL-1β and IL-6 are involved in the
pathogenesis of AS (21,22).
Results of this study showed that expression level
of miR-146a in PBMCs of AS patients was significantly higher than
that of healthy adults. In addition, levels of TNF-α, IL-1β and
IL-6 in serum and supernatant of PBMC culture medium were
significantly higher in healthy AS patients than in healthy adults,
and the expression level of miR-146a was positively correlated with
levels of TNF-α, IL-1β and IL-6. In addition, BASDAI, ESR, CRP and
duration of morning stiffness were the most common indicators of
AS. This study showed that expression level of miR-146a was
positively correlated with BASDAI, ESR, CRP and duration of morning
stiffness in AS patients. These results suggest that miR-146a may
play an important role in the development and progression of AS
disease.
In conclusion, expression level of miR-146a in PBMC
of AS patients was positively correlated with the levels of TNF-α,
IL-1β and IL-6, and clinical indicators of patients, suggesting
that miR-146a may be involved in AS by influencing the expression
of inflammatory factors. Therefore, miR-146a may provide a new
direction for AS diagnosis and treatment.
References
|
1
|
Braun J and Sieper J: The sacroiliac joint
in the spondyloarthropathies. Curr Opin Rheumatol. 8:275–287. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Braun J, Bollow M, Remlinger G, Eggens U,
Rudwaleit M, Distler A and Sieper J: Prevalence of
spondylarthropathies in HLA-B27 positive and negative blood donors.
Arthritis Rheum. 41:58–67. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xiang YJ and Dai SM: Prevalence of
rheumatic diseases and disability in China. Rheumatol Int.
29:481–490. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bartel DP: MicroRNAs: Target recognition
and regulatory functions. Cell. 136:215–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Siomi H and Siomi MC: On the road to
reading the RNA-interference code. Nature. 457:396–404. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Panera N, Gnani D, Crudele A, Ceccarelli
S, Nobili V and Alisi A: MicroRNAs as controlled systems and
controllers in non-alcoholic fatty liver disease. World J
Gastroenterol. 20:15079–15086. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Taganov KD, Boldin MP, Chang KJ and
Baltimore D: NF-kappaB-dependent induction of microRNA miR-146, an
inhibitor targeted to signaling proteins of innate immune
responses. Proc Natl Acad Sci USA. 103:pp. 12481–12486. 2006;
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bhaumik D, Scott GK, Schokrpur S, Patil
CK, Campisi J and Benz CC: Expression of microRNA-146 suppresses
NF-kappaB activity with reduction of metastatic potential in breast
cancer cells. Oncogene. 27:5643–5647. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pedersen I and David M: MicroRNAs in the
immune response. Cytokine. 43:391–394. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu Z, Xiao B, Tang B, Li B, Li N, Zhu E,
Guo G, Gu J, Zhuang Y, Liu X, et al: Up-regulated microRNA-146a
negatively modulate Helicobacter pylori-induced inflammatory
response in human gastric epithelial cells. Microbes Infect.
12:854–863. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Van der Linden S, Valkenburg HA and Cats
A: Evaluation of diagnostic criteria for ankylosing spondylitis. A
proposal for modification of the New York criteria. Arthritis
Rheum. 27:361–368. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Han GW, Zeng LW, Liang CX, Cheng BL, Yu
BS, Li HM, Zeng FF and Liu SY: Serum levels of IL-33 is increased
in patients with ankylosing spondylitis. Clin Rheumatol.
30:1583–1588. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fan D, Ding N, Yang T, Wu S, Liu S, Liu L,
Hu Y, Duan Z, Xia G, Xu S, et al: Single nucleotide polymorphisms
of the interleukin-33 (IL-33) gene are associated with ankylosing
spondylitis in Chinese individuals: A case-control pilot study.
Scand J Rheumatol. 43:374–379. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ciccia F, Alessandro R, Rizzo A,
Accardo-Palumbo A, Raimondo S, Raiata F, Guggino G, Giardina A, De
Leo G, Sireci G, et al: Macrophage phenotype in the subclinical gut
inflammation of patients with ankylosing spondylitis. Rheumatology
(Oxford). 53:104–113. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Denli AM, Tops BB, Plasterk RH, Ketting RF
and Hannon GJ: Processing of primary microRNAs by the
microprocessor complex. Nature. 432:231–235. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zilahi E, Tarr T, Papp G, Griger Z, Sipka
S and Zeher M: Increased microRNA-146a/b, TRAF6 gene and decreased
IRAK1 gene expressions in the peripheral mononuclear cells of
patients with Sjögrens syndrome. Immunol Lett. 141:165–168. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rostom S, Dougados M and Gossec L: New
tools for diagnosing spondyloarthropathy. Joint Bone Spine.
77:108–114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gregory RI, Yan KP, Amuthan G, Chendrimada
T, Doratotaj B, Cooch N and Shiekhattar R: The Microprocessor
complex mediates the genesis of microRNAs. Nature. 432:235–240.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Feldmann M, Brennan FM and Maini RN: Role
of cytokines in rheumatoid arthritis. Annu Rev Immunol. 14:397–440.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Butler DM, Maini RN, Feldmann M and
Brennan FM: Modulation of proinflammatory cytokine release in
rheumatoid synovial membrane cell cultures. Comparison of
monoclonal anti TNF-alpha antibody with the interleukin-1 receptor
antagonist. Eur Cytokine Netw. 6:225–230. 1995.PubMed/NCBI
|
|
21
|
Park MC, Chung SJ, Park YB and Lee SK:
Pro-inflammatory effect of leptin on peripheral blood mononuclear
cells of patients with ankylosing spondylitis. Joint Bone Spine.
76:170–175. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Claudepierre P, Rymer JC, Authier FJ,
Allanore Y, Larget-Piet B, Gherardi R and Chevalier X: A
relationship between TGF-beta 1 or IL-6 plasm Levels and clinical
features of ankylosing spondylitis. Br J Rheumatol. 36:400–401.
1997. View Article : Google Scholar : PubMed/NCBI
|