Introduction
Acute bowel injury (ABI) is a condition caused by
bowel ischemia, edema and increased permeability resulting from
sepsis, trauma or major surgery, which is also referred to as acute
intestinal distress syndrome (1).
Increased intestinal permeability in ABI results in the
translocation of large amounts of intestinal bacteria, thus
initiating or aggravating systematic inflammatory response
syndrome. ABI may lead to the development of multiple organ
dysfunction syndrome (MODS) (2).
Current treatment for sepsis to prevent MODS requires rapid
diagnosis to allow timely treatment with antibiotics and the
required interventions, such as resuscitation (3). Therefore, early and effective
inhibition of the production of pro-inflammatory factors may
protect against the intestinal damage caused by excessive
inflammation, in addition to preventing and treating sepsis and
MODS.
A number of studies undertaken in China have
demonstrated that electroacupuncture on Zusanli acupoints
significantly decreases levels of tumor necrosis factor-α (TNF-α)
in the liver, kidney and jejunum tissues in rat models of ABI
(4,5). Electroacupuncture may improve blood
flow and perfusion of the tissues, thus reducing organ edema and
dysfunction (6). In a previous
clinical study, it was demonstrated that electroacupuncture on
Zusanli acupoints significantly reduces the urinary
lactulose/mannitol (L/M) ratio and levels of plasma D-lactic acid.
This suggests it may improve the function of the intestinal mucosal
barrier, thus decreasing intestinal permeability. It was
demonstrated that electroacupuncture accelerated intestinal
function recovery and achieved early target feeding in sepsis
patients. In addition, serum levels of inflammatory factors,
including C-reactive protein, interleukin (IL)-6 and TNF-α, were
significantly reduced, as was the APACHE II score, while immune
functions significantly improved (7).
Previous studies have indicated that ghrelin may
improve the intestinal mucosal barrier in patients with sepsis
(8,9). Ghrelin is an endogenous brain-gut
peptide primarily secreted by endocrine cells in gastric mucosa
(primarily gastric X/A-like cells) and serves as an endogenous
ligand for the growth hormone secretagogue receptor (GHS-R).
Ghrelin has multiple physiological functions. It increases
appetite, stimulates the secretion of growth hormone (GH), promotes
fat deposition, increases gastrointestinal motility and increases
gastric acid secretion. Ghrelin therefore promotes growth,
development and weight gain (10).
Furthermore, ghrelin inhibits the production of pro-inflammatory
factors including TNF-α, IL-6 and high mobility group box 1 (HMGB1)
and protects organ function in patients with sepsis (9,11,12).
The present study hypothesized that the mechanisms
involved in the protective effects of electroacupuncture on ABI may
be associated with the increased secretion of ghrelin and
upregulated expression of GSH-R. Therefore, in the present study,
cecal ligation and puncture (CLP) was performed to produce septic
ABI rat models, electroacupuncture on Zusanli acupoints was
conducted and TNF-α and HMGB1 levels in the serum were measured.
Additionally, myeloperoxidase (MPO) and diamine oxidase (DAO)
activities, water content and pathological changes in the bowel
tissues were measured to evaluate the protective effects of
electroacupuncture on Zusanli acupoints on ABI. In addition,
changes in the ghrelin and GHS-R levels in serum and bowel tissues
were also evaluated to investigate the mechanisms involved in the
protective effects of electroacupuncture on ABI, and thus provide
theoretical basis for the clinical treatment of ABI and further
experimental studies. The results suggest that electroacupuncture
decreases the production of TNF-α and HMGB1, which are increased in
ABI. This protective process involves increased ghrelin expression
and is inhibited by the blocking of GHS-R.
Materials and methods
Animals
Male specific pathogen free (SPF) level
Sprague-Dawley rats (n=48) between 10 and 12-weeks old, with a body
weight of 295±22 g, were obtained from Shanghai Sippr BK laboratory
animals, Ltd. (Shanghai China; license number, SCXK[Hu]2008-0016).
All rats were acclimatized in housing conditions at 25±2°C, 55±2%
humidity and a 12 h light/dark cycle. Rats were fed with
conventional laboratory feed and provided with water ad
libitum. Following acclimatization, rats were fasted for 12 h
and water fasted for 4 h prior to surgery. All procedures and
animal experiments were approved by the Animal Care and Use
Committee of Zhejiang Chinese Medical University (No.
2014-K-183-01).
A random number table method was used to assign the
rats into 4 groups (n=12 each group): Sham group, ABI group,
ABI+electroacupuncture (EA) group, and ABI+GHRA+EA group.
Acute bowel injury rat models induced
by cecal ligation and puncture
ABI rat models induced by severe abdominal infection
were induced according to standard cecal ligation and puncture
(CLP) methods (13,14). The rats were anesthetized by
intraperitoneal injection of 3% sodium pentobarbital solution (45
mg/kg, Chinese Pharmaceutical Group Shanghai Chemical Reagent
Company, Shanghai, China), aseptic operation was performed to make
an incision along the midline of the abdomen. When the cecum was
exposed and the root was ligated (to avoid the ligation of the
blood vessels at the root of ileum and cecum), a size 16 needle was
used to puncture the cecum 4 times, then the cecum was put back
into the abdominal cavity and the abdominal incision was closed
layer by layer. For the rats in the Sham group, the incision along
the midline of the abdomen was made and closed immediately.
Electroacupuncture intervention
For the rats in the ABI+EA group, electroacupuncture
was performed 20 min following CLP. In brief, electroacupuncture
was performed by 7 mm perpendicular insertion of a needle on the
bilateral Zusanli acupoints (5 mm below the capitula fibula,
posterior-lateral side of the knee joint), then an
electroacupuncture apparatus (G6805-I; Qingdao Xinsheng Industry
Co., Ltd., Qingdao, China) was connected to the needle and rats
stimulated for 30 min with a current of 3 mA and frequency of 2–100
Hz.
For the rats in the ABI+GHRA+EA group, a
micro-injection pump (Shanghai Alcott biotech Co., Ltd., Shanghai,
China) was used to inject GSH-R blocker
[D-Arg1,D-Phe5,D-Trp7.9,
Leu11] (Shanghai Absin Bioscience, Inc., Shanghai,
China) substance P (700 ng/kg, diluted with 0.9% NaCl to obtain a
solution of 1 ml) for 1 h at the tail vein. Electroacupuncture on
Zusanli acupoints was performed 20 min following the injection
following the same procedure as used for the ABI+EA group.
Specimen collection
For rats in each group, blood was collected 12 h
following CLP. The blood was centrifuged at 4°C at 380 × g for 20
min and the serum was collected and stored at −80°C until
examination of serum TNF-α, HMGB1 and ghrelin levels. Prior to
sacrifice, the 48 rats were anesthetized by intraperitoneal
injection of 3% sodium pentobarbital solution (45 mg/kg). Rats were
sacrificed by cervical dislocation immediately following blood
collection. The abdomen was incised and bowel tissue ~3 cm in
length was quickly collected 2, 5, 8, and 11 cm from the ileocecal
junction. The mesentery was separated, intestinal fecal material
was removed and then the tissue was rinsed with PBS three times.
The samples 2 cm from the ileocecal junction were fixed with 10%
formaldehyde at room temperature for 48 h, stained with hematoxylin
(at room temperature for 5 min) and eosin (at room temperature for
1 min) (H&E) and subsequently prepared for immunohistochemical
staining. The tissues 5 cm from the ileocecal junction were
prepared for western blot analysis. The tissues obtained 8 cm from
the ileocecal junction, were stored at −80°C until measurement of
MPO and DAO activities. Tissues obtained 11 cm from the ileocecal
junction were analyzed for water content.
Measuring the level of serum TNF-α,
HMGB1 and ghrelin
TNF-α (cat. no. CSB-E11987r), HMGB1 (cat. no.
CSB-E08224r) and ghrelin ELISA kits (cat. no. CSB-E09816r) were
used for the measurements of serum TNF-α, HMGB1 and ghrelin
(Shanghai Westang Bio-tech Co., Ltd., Shanghai, China). Optical
density (OD) was measured using a microplate reader (Multiskan EX,
V. 2.3; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at 450 nm
within 30 min. Serum TNF-α, HMGB1 and ghrelin levels were then
calculated according to the OD value and standard curves.
Measuring MPO and DAO activity in the
bowel tissues
MPO and DAO activities were measured using detection
MPO biochemical kits and DAO biochemical kits (Beijing ComWin
Biotech Co., Ltd., Beijing, China). After 100 mg of bowel tissue
was cut into small sections, 1 ml 0.5% hexadecyltrimethylammonium
bromide (Beijing Solarbio Science & Technology Co., Ltd.,
Beijing, China) was added, and the tissue was homogenized. The
homogenate was then centrifuged at 1,520 × g at 4°C for 10 min, and
the supernatant was collected for the measurement of MPO
activity.
A further 200 mg bowel tissue was cut into small
sections, a 7-fold volume of phosphate-buffered saline (PBS; 0.1
mol, pH=7.2) was added, the tissue was homogenized, then
centrifuged at 9,500 × g at 4°C for 10 min and the supernatant was
collected to measure DAO activity. OD was measured with a
microplate reader at 450 nm within 30 min of each assay and the
activities of MPO and DAO in the bowel tissues were estimated using
standard curves.
Measuring water content in bowel
tissues
Water content in bowel tissues was measured using
the wet-dry weighting method. In brief, fresh bowel tissue was
obtained and blotting paper was used to remove surface water. The
specimen was weighed on an electronic analytical balance (1/100,000
g) and this was recorded as the wet weight. The specimen was then
placed in a draught drying cabinet and dried at 90°C for 72 h and
weighed again; recorded as the dry weight. Water content (%) was
measured as (wet weight - dry weight) / wet weight × 100%.
Pathological changes of the bowel
tissues
Intestinal mucosa injury was scored using the Chiu
method as reported by Bao et al (15) to grade the severity of the intestinal
mucosa injury. Once the bowel tissue was obtained, the ileal
tissues were fixed with 10% formaldehyde at 25°C for 48 h, paraffin
embedded, sliced and stained with H&E. Subsequently,
pathological changes in the intestinal tissues were evaluated and
graded using the Chiu's score (16,17).
Chiu's score grading was as follows: Grade 0, normal mucosal villi;
Grade 1, development of subepithelial Gruenhagen's space, usually
observed at the apex of the villus, often with capillary
congestion; Grade 2, extension of the subepithelial Gruenhagen's
space with moderate lifting of epithelial layer from the lamina
propria; Grade 3, massive epithelial lifting down the sides of
villi, with a few tips being denuded; Grade 4; denuded villi with
lamina propria and dilated capillaries exposed, increased
cellularity of lamina propria; and Grade 5, digestion and
disintegration of lamina propria, hemorrhage, and ulceration.
Measuring ghrelin and GSH-R protein
expression in bowel tissues
Ghrelin, GSH-R and HMGB1 protein expression in bowel
tissues were measured by western blot analysis. In brief, 20 mg of
the bowel tissues were cut into small sections, 200 µl
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Haimen, China; 50 mM TrisHCl pH 7.4, 1% NP-40, 0.5%
Na-deoxycholate, 0.1% SDS, 150 mM NaCl, 2 mM EDTA, 50 mM NaF) was
added to homogenize the tissues, and then they were centrifuged at
13,680 × g at 4°C for 5 min. The supernatant was collected and
protein concentration was measured using the bicinchoninic acid
(BCA) method (Beijing ComWin Biotech Co., Ltd.). Then, 30 µg of the
total protein underwent 10% SDS-PAGE, separated proteins were
transferred to a nitrocellulose membrane (Beyotime Institute of
Biotechnology) and blocked with 5% skimmed milk powder for 2 h at
25°C. The membrane was then rinsed with Tris buffered saline, 0.05%
Tween-20 (TBST) buffer 3 times. Ghrelin (cat. no. LS-C149239;
1:500, LifeSpan BioSciences, Inc., Seattle, WA, USA), GSH-R (cat.
no. LS-C136920; 1:500, LifeSpan BioSciences, Inc.) or HMGB1 (cat.
no. ab79823; 1:500; Abcam, Cambridge, UK) primary antibodies
(1:500; Abcam, Cambridge, UK) were added and incubated at 4°C
overnight. Then, horseradish peroxidase (HRP) labeled goat
anti-rabbit secondary antibody (cat. no. CW0103s; 1:2,000; Beijing
ComWin Biotech Co., Ltd.) was added and incubated at 25°C for 2 h.
ECL reagent (Beyotime Institute of Biotechnology) was then added
according to the manufacturer's protocol. Antibodies against
β-actin (cat. no. CW0096M; 1:2,000; Beyotime Institute of
Biotechnology) were used as the internal reference. The Gel-Doc gel
imaging system (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was
used to capture images and the VSD Gel Image Analysis System
(Bio-Rad Laboratories, Inc.) was used for the semi-quantitative
analysis of the bands. Relative protein expression was normalized
to β-actin.
Immunohistochemistry
Ghrelin and GSH-R positive cells in the bowel
tissues were measured by immunohistochemical examination. The
tissues were fixed with 4% paraformaldehyde for 48 h at room
temperature and then embedded in paraffin, sliced to a thickness of
4 µm and placed on slides covered with polylysine. They were
deparaffinized with dimethyl benzene, followed by gradient alcohol
dehydration. Then, tissues were rinsed with 1× PBS (5 min ×3),
underwent 10 min incubation at 25°C with 3%
H2O2 and a further 3 rinses with 1×PBS (5
min) were performed. Samples were blocked with 10% fetal bovine
serum (FBS; Beijing ComWin Biotech Co., Ltd.) for 30 min at 25°C
and then ghrelin (cat. no. LS-C149239) or GSH-R antibodies (cat.
no. LS-C136920; both 1:100; both LifeSpan BioSciences, Inc.) were
added and incubated overnight at 4°C. The slides were then washed
with 1xPBS (5 min ×3). HRP labeled secondary antibody (cat. no.
CW0103s; 1:200; Beijing ComWin Biotech Co., Ltd.) was added
followed by incubation for 1 h at 25°C and another 3 washes with
PBS (5 min) were performed. Staining with 3,3′-Diaminobenzidine and
hematoxylin was performed. The section was then cleared with
dimethyl benzene (30 min ×2) and mounted with neutral resins, prior
to observation and imaging with a CP62-JSM-6390LV microscope
(Midwest Technology Co., Ltd., Beijing, China). Images were
analyzed with Image-Pro Plus image analysis software (Media
Cybernetics, Version 4.1, USA). For each slide, 10 non-overlapping
vision fields were selected under magnification: ×400, then the
cumulative integrated optical density and the area of detection in
each visual field was recorded under the same light intensity.
Statistical analysis
SPSS 17.0 software (SPSS Inc., Chicago, IL, USA) was
used for the statistical analysis. Quantitative data are presented
as the mean ± standard deviation. Statistical significance was
evaluated by independent sample t-test or one-way analysis of
variance with least significant difference (LSD) test for post hoc
analysis. Chiu's scoring (16,17) was
presented as median ± interquartile Range (IQR), and analyzed by
Kruskal-Wallis H test. P<0.05 was considered to indicate a
statistically significant difference.
Results
Electroacupuncture promotes ghrelin
and GSH-R expression in rats with ABI
Compared with rats in the Sham group, ABI rats had
85% of the level of serum ghrelin, (86.7±6.4 ng/ml vs. 102.4±4.9
ng/ml), 53% of the bowel ghrelin level (0.08±0.01 vs. 0.15±0.01)
and 30% of the GSH-R level (0.03±0.01 vs. 0.10±0.06; all P<0.05;
Fig. 1A and B). In ABI+EA rats,
serum ghrelin, bowel ghrelin and GSH-R were all significantly
higher than in the ABI group (P<0.05). Compared with rats in the
Sham group, ABI+GHRA+EA rats had 89% of the serum ghrelin levels
(91.3±6.0 ng/ml), 53% of the bowel ghrelin (0.08±0.01) and 20% of
the GSH-R levels (0.02±0.00; all P<0.05; Fig. 1A and B).
 | Figure 1.Effects of EA on the level of serum
and protein GSH, GSH-R in the bowel tissues of ABI rat models. ABI
rat models were induced by CLP. For rats in the ABI+EA group, EA
was performed for 30 min, 20 min after CLP. For rats in the
ABI+GHRA+EA group, a microinjection pump was used to inject GHRA
for 1 h via the tail vein following CLP but prior to EA. (A)
Protein expression of ghrelin and its receptor were determined by
western blot analysis. β-actin was used as an internal control. (B)
The level of serum ghrelin was determined by ELISA. *P<0.05 vs.
ABI group; ∆P<0.05 vs. Sham group;
#P<0.05 vs. ABI+EA group. Data are presented as mean
± standard deviation (n=12 each group). EA, electroacupuncture;
ABI, acute bowel injury; CLP, cecal ligation and puncture; GHRA,
GSH-R Blocker; GSH, ghrelin; GSH-R, ghrelin receptor. |
The level of serum ghrelin in bowel tissue,
determined by western blot analysis, was supported by the
immunohistochemistry results for ghrelin and GSH-R (Fig. 2).
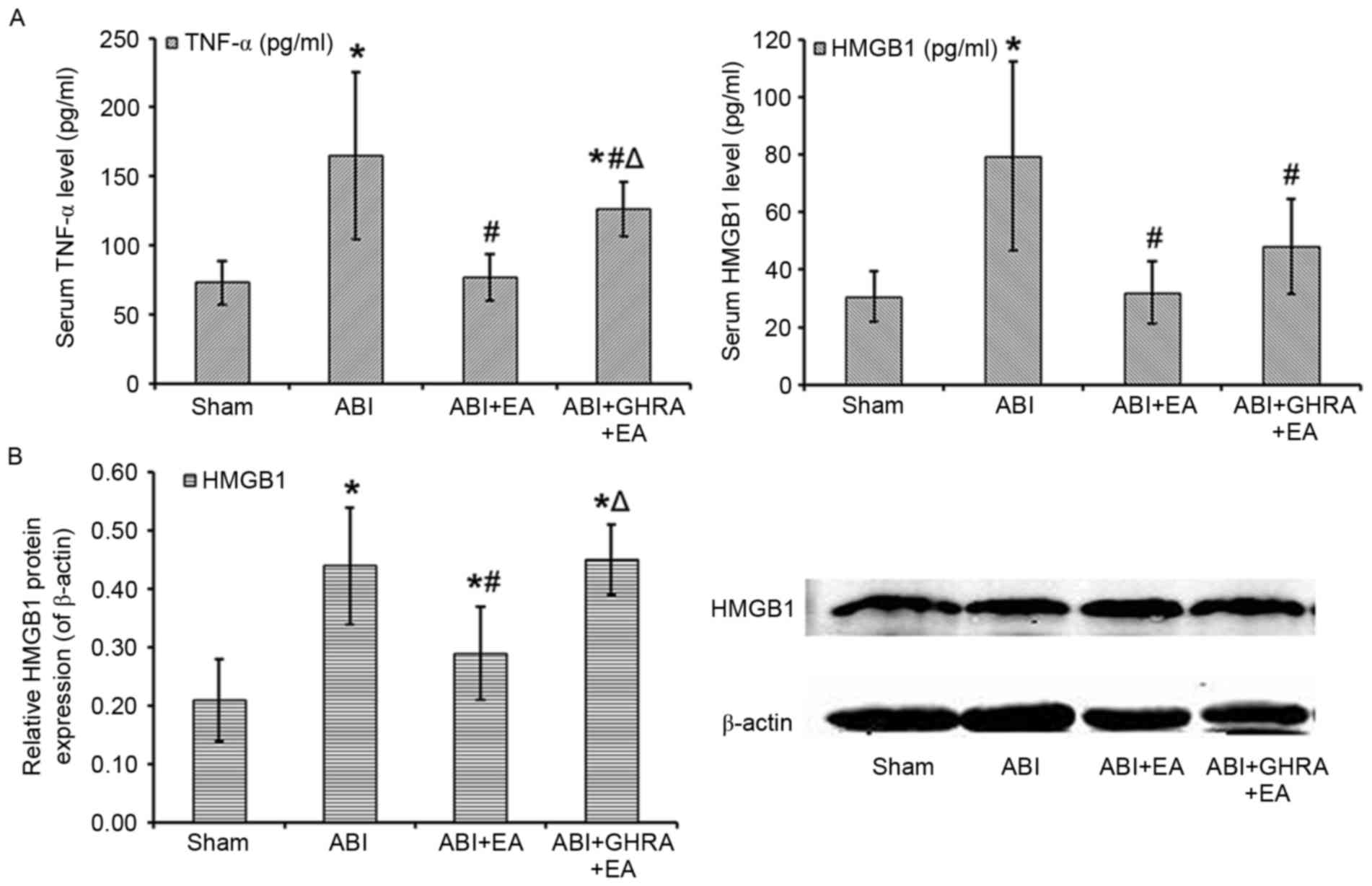
Electroacupuncture reduces serum TNF-α
and HMGB1 levels by promoting ghrelin secretion in rats with
ABI
Serum TNF-α and HMGB1 levels in each group are
presented in Fig. 3. Compared with
the Sham group, rats in the ABI group exhibited a significant 226%
increase in the level of serum TNF-α (164.9±60.3 pg/ml vs.
72.9±15.8 pg/ml; P<0.05; Fig.
3A), a significant 258% increase in the level of serum HMGB1
(79.5±32.8 pg/ml vs. 30.8±8.7 pg/ml; P<0.05; Fig. 3A) and a significant 210% increase in
the level of HMGB1 in bowel tissue (0.44±0.10 vs. 0.21±0.07;
P<0.05; Fig. 3B). Compared with
ABI rats, the ABI+EA rats had significantly lower TNF-α levels
(105% of Sham, 76.8±16.6 pg/ml; Fig.
3A), serum HMGB1 (104% of Sham, 32.1±10.8 pg/ml; Fig. 3A) and bowel tissue HMGB1 (138% of
Sham, 0.29±0.08; Fig. 3B; all
P<0.05). Compared with the ABI+EA group, the ABI+GHRA+EA group
had significantly higher TNF-α levels (173% of Sham, 126.1±19.6
pg/ml; P<0.05; Fig. 3A) and bowel
tissue HMGB1 (214% of Sham, 0.45±0.06; P<0.05; Fig. 3B). There were also higher levels of
serum HMGB1 in this group (156% of Sham, 48.1±16.5 pg/ml; Fig. 3A), though this difference was not
significant compared with the ABI+EA group.
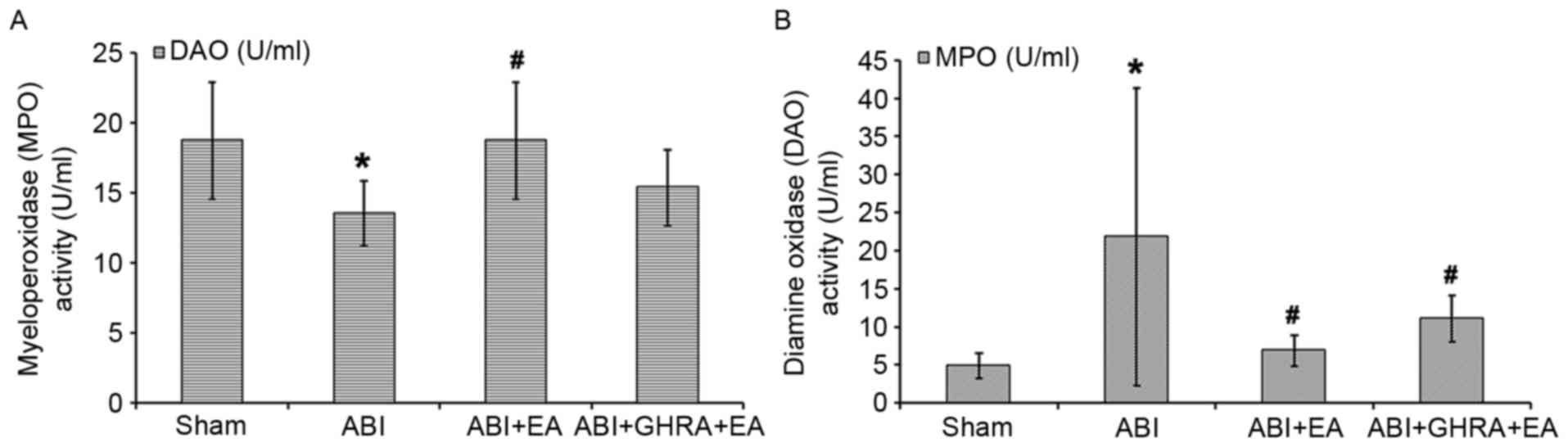
Electroacupuncture decreased MPO
activity but increased DAO activity in bowel tissues by promoting
ghrelin secretion
The results of the MPO and DAO activity
investigations are presented in Fig.
4. The ABI group exhibited a decrease in DAO activity compared
with the Sham group (13.52±2.33 U/ml vs. 18.74±4.18 U/ml; Fig. 4A) and a 446% increase in MPO activity
(21.83±19.55 U/ml vs. 4.89±1.68 U/ml; Fig. 4B; both P<0.05). Compared with the
ABI group, DAO activity in the ABI+EA group increased back to
levels similar to the Sham group (18.74±4.18 U/ml; P<0.05;
Fig. 4A), whereas MPO activity
significantly decreased, to 140% of the Sham group (6.89±2.05 U/ml)
compared with the ABI group (P<0.05; Fig. 4B). In the ABI+GHRA+EA group, DAO
activity was 82% of the Sham group (15.38±2.69 U/ml) and MPO
activity was 227% of the Sham group value (11.09±3 U/ml).
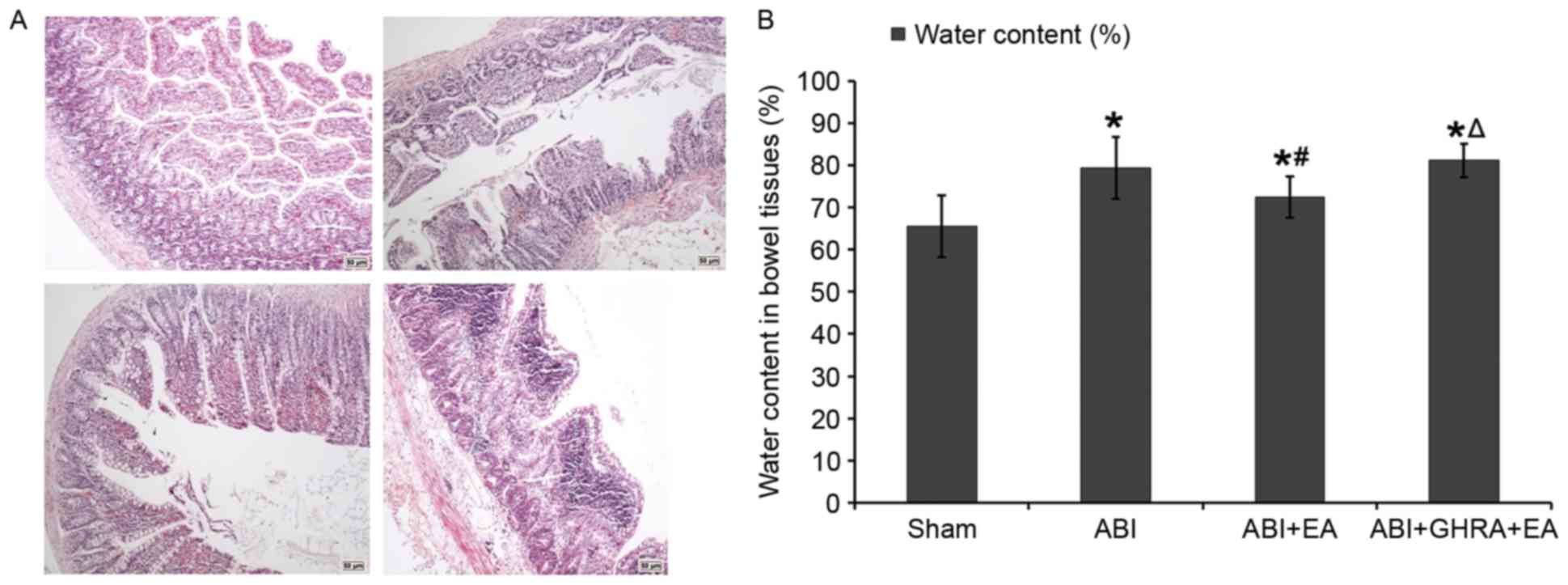
Electroacupuncture improved intestinal
mucosal barrier and reduced water content in bowel tissues by
promoting ghrelin secretion
The pathological changes in the bowel were analyzed
histologically and representative samples are presented in Fig. 5A. The following characteristics were
observed: In the Sham group, the villus was neat, perivascular
structurally normal, there was no evidence of significant bleeding,
the muscle fibers were arranged in neat rows and the serosa was
normal. In the ABI group, there was intestinal villi damage,
significant swelling, ulcers, clear perivascular bleeding,
neutrophil infiltration and basal fractures. In the ABI+EA group, a
small amount of intestinal villi defects, a small amount of
necrosis, a small amount of bleeding and a small amount of
neutrophil infiltration was detected. In the ABI+GHRA+EA group,
there were clearly damaged intestinal villi and swelling,
accompanied by bleeding and ulceration.
Pathological changes in the intestinal tissues were
evaluated according to the method reported by Chiu et al
(16) (Table I). Chui's score (median ±
interquartile range) in the Sham, ABI, ABI+EA and ABI+GHRA+EA
groups were 0, 4±0, 2±1 and 4±0, respectively. Chiu's score in the
ABI+EA group was significantly lower than that of the ABI group
(P<0.05), however Chiu's score in the ABI+GHRA+EA group was
significantly higher than that of the ABI+EA group (P<0.05).
 | Table I.EA improved intestinal mucosal barrier
in bowel tissues by promoting ghrelin secretion in ABI rat
models. |
Table I.
EA improved intestinal mucosal barrier
in bowel tissues by promoting ghrelin secretion in ABI rat
models.
| Groups | Chiu's score
(16) |
|---|
| Sham | 0 |
| ABI | 4±0a |
| ABI+EA | 2±1a,b |
| ABI+GHRA+EA | 4±0a,c |
Bowel water content was also measured. Water content
in the ABI group was significantly increased by 121% compared with
the Sham group (79.42±7.36% vs. 65.55±7.37%; P<0.05). The water
content in the ABI+EA group was significantly reduced compared with
the ABI group (72.41±4.89%; P<0.05) and the water content in the
ABI+GHRA+EA group was significantly greater than that of the ABI+EA
group (81.13±3.94%; P<0.05; Fig.
5B).
Discussion
The aim of the present study was to investigate the
role of electroacupuncture in assisting the recovery of rats from
ABI and determine whether ghrelin was involved in the recovery
process. The results of the current study suggest that
electroacupuncture promoted ghrelin and GSH-R expression and
decreased the inflammatory response, indicated by lower levels of
the pro-inflammatory factors TNF-α and HMGB1 following
electroacupuncture. The function of the gastrointestinal barrier
was also improved following electroacupuncture, suggested by the
restoration of DAO activity levels and the decrease in MPO activity
towards levels observed in the Sham group. The recovery of rats
following ABI by electroacupuncture was also inhibited by
GSH-R.
The inflammatory response that culminates in MODS is
mediated by a complex interaction of pro-inflammatory factors
called cytokines, two of which are TNF-α and HMGB1 (18). It has been suggested that HMGB1 may
be a marker of subclinical intestinal inflammation (19). A potential marker for intestinal
damage is DAO (20), which is
primarily expressed in the small intestine and its expression and
activity decrease following intestinal injury. The opposite is
observed with MPO, a peroxidase enzyme identified primarily in
neutrophils, as its activity increases as a result of neutrophil
infiltration of the intestine (21).
The present study revealed that electroacupuncture reversed the
increase in TNF-α and HMGB1 levels in rats that had undergone ABI
and improved the function of the intestinal mucosal barrier,
indicated by the restoration of DAO activity levels and the
decrease in MPO towards levels observed in the Sham group. These
results for TNF-α and improved function of the intestinal mucosal
barrier have been observed in a previous clinical study (7), and similar results have also been
obtained in previous studies investigating the liver, kidney and
jejunum tissues in CLP rats (6,22).
Electroacupuncture significantly inhibits TNF-α levels in the
liver, kidney and jejunum tissues in ABI rat models.
Electroacupuncture improves blood flow and perfusion of the
tissues, thus reducing organ edema and dysfunction (6). Electroacupuncture applied to the
bilateral ST-25 and ST-36 in an acute pancreatitis rat model also
demonstrated an improved intestinal propulsion rate, reduced
pro-inflammatory markers and improved acute lung injury (22). Acupuncture may not only inhibit the
release of pro-inflammatory factors but also increase the secretion
of gastrointestinal hormones. Wu et al (23), assessed the effects of
electroacupuncture on mast cells, substance P (SP) and vasoactive
intestinal peptide (VIP) in rat models of irritable bowel syndrome,
and revealed that the sensitivity threshold in the rat models was
significantly lower than the rats in the control group, whereas the
mast cell count and secretion of SP and VIP were significantly
higher. Lin et al (24)
indicated that acupuncture on Zusanli acupoints may improve gastric
motility, blood flow of gastric mucosa and the secretion of
gastrointestinal hormones, including motilin.
The present study demonstrated that ghrelin and
GSH-R levels decreased in the rats with ABI, in accordance with
previous findings (25,26). Das et al (8) revealed that the level of ghrelin
decreased significantly with the progression of sepsis, aggravation
of gastrointestinal dysfunction and occurrence of gastric secretion
disorders. Another study demonstrated that ghrelin was associated
with the prognosis of patients with sepsis and that ghrelin levels
were significantly higher in patients that survived compared with
those that succumbed (12). In
addition, levels of ghrelin and GSH-R were closely associated with
impaired gastrointestinal mucosal function and the severity of this
impairment (27). In animal models
of acute renal injury, induced by intraperitoneal injection of
lipopolysaccharide, an injection of ghrelin either prior to or
following injury reduced serum levels of TNF-a, interleukin
(IL)-1β, and IL-6, thus partially restoring the impaired glomerular
filtration rate (28).
Administration of exogenous ghrelin also significantly increased
the degree of vascular relaxation and inhibited the nuclear
factor-κB (NF-κB) pathway. This improved tissue perfusion and the
permeability of the intestinal mucosa, inhibited the production of
inflammatory mediators, reduced intestinal wall edema, decreased
bacterial translocation, delayed sepsis progress into shock and
finally served important roles in improving septic intestinal
dysfunction in rat models of sepsis (29). In rat models of acute pulmonary
injuries induced by CLP, ghrelin increased the blood flow in the
lungs, inhibited the activation of NF-κB, down-regulated
pro-inflammatory factors, decreased the damage to the pulmonary
tissues, and thus greatly reduced the mortality of the rats
(30).
In addition, the present study demonstrated that
electroacupuncture promoted the expression of ghrelin and GSH-R in
rats with ABI. To the best of our knowledge, this is the first
study to identify the effects of electroacupuncture on the
increased secretion of ghrelin and GSH-R. GSH-R blocker was
administered to the rats following CLP but prior to the stimulation
of electroacupuncture. The protective effects of electroacupuncture
on the intestinal mucosal barrier were reversed and there was an
increase in serum TNF-α and HMGB1 levels, suggesting that the
effects of Zusanli acupoints electroacupuncture on ABI are
associated with ghrelin and GSH-R levels. Therefore,
electroacupuncture on Zusanli acupoints may increase ghrelin levels
in the body by increasing the expression of ghrelin and GSH-R in
bowel tissues. Ghrelin may, in turn, inhibit the release of
pro-inflammatory factors, improve the perfusion of bowel tissues
and protect the bowel mucosal barrier. Future studies are required
to investigate the detailed mechanisms involved in increasing
ghrelin and GSH-R expression by Zusanli acupoint electroacupuncture
in rats with ABI. Electroacupuncture ameliorated ABI in rats by
promoting the secretion of ghrelin and upregulating the expression
of GSH-R. This information may assist with the use of
electroacupunture as a clinical therapy for ABI in the early
treatment of sepsis.
Acknowledgements
The present study was supported by Zhejiang
provincal key program for traditional Chinese medicine in
prevention and treatment of major diseases (grant no.
2012ZGG001).
References
|
1
|
Malbrain ML and De Laet I: AIDS is coming
to your ICU: Be prepared for acute bowel injury and acute
intestinal distress syndrome. Intensive Care Med. 34:1565–1569.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
De-Souza DA and Greene LJ: Intestinal
permeability and systemic infections in critically ill patients:
Effect of glutamine. Crit Care Med. 33:1125–1135. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Seoane L, Winterbottom F, Nash T,
Behrhorst J, Chacko E, Shum L, Pavlov A, Briski D, Thibeau S,
Bergeron D, Rafael T and Sundell E: Using quality improvement
principles to improve the care of patients with severe sepsis and
septic shock. Ochsner J. 13:359–366. 2013.PubMed/NCBI
|
|
4
|
Hu S, Song Q, Wang HB, Lü Y, Yu Y, Wang L,
Zhou GY, Shi X and Sheng ZY: Study on the protective effect and
mechanism of electroacupuncturing at Zusanli point (ST 36) on
endotoxin induced hepatic injury in rats. Zhong Guo Zhong Xi Yi Jie
He Ji Jiu Za Zhi Bian Ji Bu. 14:296–298. 2007.(In Chinese).
|
|
5
|
Song Q, Hu S, Wang H, Lv Y, Shi X, Sheng Z
and Sheng W: Electroacupuncturing at Zusanli point (ST36)
attenuates pro-inflammatory cytokine release and organ dysfunction
by activating cholinergic anti-inflammatory pathway in rat with
endotoxin challenge. Afr J Tradit Complement Altern Med.
11:469–474. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Song XM, Li JG, Wang YL, Hu ZF, Zhou Q, Du
ZH and Jia BH: The protective effect of the cholinergic
anti-inflammatory pathway against septic shock in rats. Shock.
30:468–472. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang LC and Wu JN: The clinical efficacy
of sepsis with early intervention of electroacupuncture. Life Sci
J. 10:1900–1903. 2013.
|
|
8
|
Das UN: Relationship between gut and
sepsis: Role of ghrelin. World J Diabetes. 2:1–7. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu R, Dong W, Qiang X, Wang H, Blau SA,
Ravikumar TS and Wang P: Orexigenic hormone ghrelin ameliorates gut
barrier dysfunction in sepsis in rats. Crit Care Med. 37:2421–2426.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kojima M and Kangawa K: Ghrelin: Structure
and function. Physiol Rev. 85:495–522. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li WG, Gavrila D, Liu X, Wang L,
Gunnlaugsson S, Stoll LL, McCormick ML, Sigmund CD, Tang C and
Weintraub NL: Ghrelin inhibits proinflammatory responses and
nuclear factor-kappaB activation in human endothelial cells.
Circulation. 109:2221–2226. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Koch A, Sanson E, Helm A, Voigt S,
Trautwein C and Tacke F: Regulation and prognostic relevance of
serum ghrelin concentrations in critical illness and sepsis. Crit
Care. 14:R942010. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chaudry IH, Tabata Y, Schleck S and Baue
AE: Effect of splenectomy on reticuloendothelial function and
survival following sepsis. J Trauma. 20:649–656. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rittirsch D, Huber-Lang MS, Flierl MA and
Ward PA: Immunodesign of experimental sepsis by cecal ligation and
puncture. Nat Protoc. 4:31–36. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bao J, Tan S, Yu W, Lin Z, Dong Y, Chen Q,
Shi J, Duan K, Bai X, Xu L, et al: The effect of peritoneal air
exposure on intestinal mucosal barrier. Gastroenterol Res Pract.
2014:6748752014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chiu CJ, Mcardle AH, Brown R, Scott HJ and
Gurd FN: Intestinal mucosal lesions in low flow states. I. A
morphological, hemodynamic and metabolic reappraisal. Arch Surg.
101:478–483. 1970. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Surbatovic M, Veljovic M, Jevdjic J,
Popovic N, Djordjevic D and Radakovic S: Immunoinflammatory
response in critically ill patients: Severe sepsis and/or trauma.
Mediators Inflamm. 2013:3627932013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Palone F, Vitali R, Cucchiara S,
Pierdomenico M, Negroni A, Aloi M, Nuti F, Felice C, Armuzzi A and
Stronati L: Role of HMGB1 as a suitable biomarker of subclinical
intestinal inflammation and mucosal healing in patients with
inflammatory bowel disease. Inflamm Bowel Dis. 20:1448–1457. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhao L, Luo L, Jia W, Xiao J, Huang G,
Tian G, Li J and Xiao Y: Serum diamine oxidase as a hemorrhagic
shock biomarker in a rabbit model. PLoS One. 9:e1022852014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mondello S, Galuppo M, Mazzon E, Domenico
I, Mondello P, Carmela A and Cuzzocrea S: Glutamine treatment
attenuates the development of ischaemia/reperfusion injury of the
gut. Eur J Pharmacol. 643:304–315. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Guo H, Zhu SF, Zhang RR, Zhao XL, Wan MH
and Tang WF: Electroacupuncture ameliorates acute lung injury
through promoting gastrointestinal motility in rats with acute
pancreatitis. Evid Based Complement Alternat Med. 2014:9435962014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu HG, Jiang B, Zhou EH, Shi Z, Shi DR,
Cui YH, Kou ST and Liu HR: Regulatory mechanism of
electroacupuncture in irritable bowel syndrome: Preventing MC
activation and decreasing SP VIP secretion. Dig Dis Sci.
53:1644–1651. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lin YP, Yi SX, Yan J and Chang XR: Effect
of acupuncture at Foot-Yangming Meridian on gastric mucosal blood
flow, gastric motility and brain-gut peptide. World J
Gastroenterol. 13:2229–2233. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cheyuo C, Jacob A and Wang P:
Ghrelin-mediated sympathoinhibition and suppression of inflammation
in sepsis. Am J Physiol Endocrinol Metab. 302:E265–E272. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jacob A, Wu R, Zhou M, Coppa GF and Wang
P: Mechanism of the inhibitory effect of ghrelin in sepsis. Hepat
Med. 2:33–38. 2010.PubMed/NCBI
|
|
27
|
Neto A Serpa, Veelo DP, Peireira VG, de
Assunção MS, Manetta JA, Espósito DC and Schultz MJ: Fluid
resuscitation with hydroxyethyl starches in patients with sepsis is
associated with an increased incidence of acute kidney injury and
use of renal replacement therapy: A systematic review and
meta-analysis of the literature. J Crit Care.
185.e1-72014.PubMed/NCBI
|
|
28
|
Arnes L, Hill JT, Gross S, Magnuson MA and
Sussel L: Ghrelin expression in the mouse pancreas defines a unique
multipotent progenitor population. PLoS One. 7:e520262012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu R, Dong W, Zhou M, Cui X, Simms H Hank
and Wang P: Ghrelin improves tissue perfusion in severe sepsis via
downregulation of endothelin-1. Cardiovasc Res. 68:318–326. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wu R, Dong W, Zhou M, Zhang F, Marini CP,
Ravikumar TS and Wang P: Ghrelin attenuates sepsis-induced acute
lung injury and mortality in rats. Am J Respir Crit Care Med.
176:805–813. 2007. View Article : Google Scholar : PubMed/NCBI
|