Introduction
Uterine fibroids are the most common benign tumor
type of the female genital tract in women of reproductive age
(1). It mostly occurs in women
between of 35 and 50 years of age and the estimated prevalence in
the United States is 20–40% (2). The
most frequent clinical symptoms are menorrhagia, lower abdominal
pain, a sensation of pelvic fullness and infertility (3,4).
Available treatments are medication, surgery (including
hysterectomy and myomectomy) and less invasive therapies. Surgeries
may cause severe trauma and undesirable co-morbidities (5). Medical therapy only an effect on the
clinical symptoms based on long-term administration, and has
adverse effects (6). A large variety
of less invasive alternatives to hysterectomy have been developed,
such as uterine artery embolization (UAE), high-intensity focused
ultrasound (HIFU) and radiofrequency thermal ablation (7–9).
Exposure to X-ray radiation is a persistent problem of UAE and may
cause numerous complications (7).
HIFU may be time-consuming and less effective in large uterine
fibroids despite its non-invasiveness (8). Microwave ablation (MWA) has a high
thermal efficiency and has gained wide use in treating solid tumors
in organs other than the uterus (10,11).
MWA has now become an important alternative for
treating uterine fibroids, and is usually performed under the
guidance of ultrasound (US) and magnetic resonance imaging (MRI).
MWA has several advantages over other available techniques; for
instance, it is non-invasive, feasible and safe, and it only
requires a short downtime with no radiation-induced injuries
(12). It is of great value in
treating patients with gynecological diseases who require
non-invasive treatments with organ function preservation (12,13).
However, optimal imaging techniques are important in evaluating the
necrosis of fibroids after MWA.
US is one of the most commonly used techniques for
detecting uterine fibroids. However, conventional US has
limitations in assessing the mid- and long-term results of
treatments (14). Contrast-enhanced
US (CEUS) has become the focus of medical research on US and has
become more reliable in evaluating the effects of MWA due to the
improvement of high-frequency probe and color Doppler flow imaging
(CDFI) techniques (14).
Furthermore, MRI is considered to be the most precise imaging
technique to reach a diagnosis of uterine fibroids as well as to
locate and evaluate the lesion; it is also able to distinguish
uterine fibroids from adenomyosis (15). Furthermore, the signal intensity on
T2-weighted imaging (T2WI) provides abundant information on uterine
fibroids.
In the present study, CEUS was performed on enrolled
patients with uterine fibroids to assess the effectiveness of MWA
in treating uterine fibroids.
Patients and methods
Patients
Between October 2013 and September 2015, a total of
60 consecutive patients with uterine fibroids who received MWA
treatment at Ningbo No. 2 Hospital (Ningbo, China), who were in
their two-year follow-up period, were enrolled in the experimental
group. The inclusion criteria were as follows: i) Female patients
of reproductive age, diagnosed with uterine fibroids by US and MRI,
who had completed childbearing, declined hysterectomy and
experienced symptoms such as menorrhagia, abdominal pain, pelvic
pain and anemia; ii) pre-menopausal women with no desire for
fertility; iii) intramural or subserous myoma of >5 cm in
diameter or submucous myoma with a diameter of >3 cm, or an
uterine junctional zone of >5 mm in width and a lesion of >3
cm in thickness; iv) patients with complete data from conventional
and enhanced MRI examinations pre- and post-MWA therapy; and v) no
history of treatments including UEA and HIFU.
As the control group, 60 consecutive patients were
recruited on their follow-up visit at least two years after MWA
treatment for uterine fibroids. The common clinical symptoms were
progressive dysmenorrhea, prolonged menstruation, menorrhagia and
pelvic bulge. Of the 78 fibroids included in the control group, 57
were single and 21 were multiple (Table
I). The diameter of the fibroid ranged from 1.5 to 4.0 cm with
a mean of 3.2 cm.
 | Table I.Demographic and clinical data of
patients with uterine fibroids prior to treatment. |
Table I.
Demographic and clinical data of
patients with uterine fibroids prior to treatment.
| Parameter | Experimental group
(n=60) | Control group
(n=60) |
|---|
| Age, years
(range) | 45.8±6.7 | 40.3±15.7 |
|
| (38–55) | (30–60) |
| Clinical symptoms
(%) |
|
|
|
Progressive dysmenorrhea | 60 (100) | 59 (98.3) |
|
Menorrhagia | 56
(93.3) | 52 (56.6) |
| Pelvic
bulge | 49
(81.6) | 41 (68.3) |
| Prolonged
menstruation | 42
(70.0) | 37 (61.6) |
| Shortened
menstruation | 34
(56.6) | 28 (46.6) |
| Fibroid location
(%) |
|
|
| Anterior
wall | 53
(88.3) | 50 (83.3) |
|
Cervix | 10
(16.6) | 12 (20.0) |
| Posterior
wall | 15
(25.0) | 16 (26.6) |
The Institutional Review Boards of Ningbo No. 2
Hospital approved the present study and all participants provided
written informed consent prior to enrollment.
CEUS examination
Ultrasonography was performed using an Acuson
Sequoia 512 US system (Siemens Medical Solutions, Mountain View,
CA, USA) with the mechanical index varying from 0.15 to 0.20. The
frequency of the transducers ranged from 2.0 to 4.0 MHz. The
contrast agent used for CEUS was SonoVue (Bracco Imaging Co.,
Gorizia, Italy) dissolved in 5 ml 0.9% sodium chloride solution.
Gray-scale US was first performed to identify the morphology,
location, size, echo and resistance index of uterine fibroids.
Subsequently, CEUS was performed for a continuous observation >3
min after a rapid intravenous bolus administration of contrast
medium through the antecubital vein. The fibroid volume was
calculated using the following formula: V=1/6πxD1xD2xD3 (16), where D1, D2 and D3 are the
longitudinal, transverse and anteroposterior diameters of the
fibroid, respectively, on CEUS. Two experienced sonographers
reviewed the images.
Sonographic evaluation was repeated at one week
prior to MWA therapy and at one and six months as well as one and
two years following the procedure.
MRI examination
Images were acquired on a 3.0T system (GE Signa
Profile; GE Healthcare, Little Chalfont, UK). The imaging protocols
included a T1W spin echo (SE) sequence with a repetition time (TR)
of 360 msec and an echo time (TE) of 16 msec, and a T2W SE sequence
with TR of 3,000 msec and a TE of 90 msec in transverse and
sagittal planes (slice thickness, 1 mm; field of view, 34 mm;
number of excitations, 4; matrix, 256×192).
MRI was performed in all patients at one week prior
to the procedure and at one and six months as well as one and two
years following the procedure.
MWA procedure
MWA therapy was performed using a microwave device
(KV2000; Kangyou Microwave Energy Sources Institute, Nanjing,
China) with a frequency of 2,450 MHz. The patient adopted a
lithotomy position and underwent the procedure after local
anesthesia. A single percutaneous microwave electrode was rapidly
placed into the fibroids under UA guidance. The output energy of
the microwave was set between 50 and 60 W, while the treatment time
was set at 480–1,440 sec, according to the volume and sonographic
changes of the fibroid. In a three-dimensional manner, the
electrode was placed from shallow to deep and from back to front.
The ablation region involved the fibroids and the normal tissue
within 1 cm around the lesion to prevent recurrence (17).
Perfusion parameters on CEUS and
MRI
The perfusion parameters derived from the
time-intensity curve (TIC) included mean transit time, start time
(ST), rise time (RT), peak enhancement (PE), wash-in rate (WiR) and
total area under the curve (AUC) during the enhancement period of
fibroid tissue and vessel, the ST, RT and AUC differences between
fibroid vessel and tissue, and the RT, PE, WiR and AUC ratios of
fibroid vessel to tissue. CEUS was repeated at different
postoperative times to assess the enhancement inside or around the
lesion at different phases. Pre-operative enhanced MRI was applied
to determine the enhancement inside the targeted area. After the
operation, MRI was repeated to detect necrosis and residues of
fibroids.
Adverse events
Adverse events during the MWA therapy were recorded,
and they included pain in the sacrococcygeal, hip and targeted
regions as well as skin burns. Immediately after the therapy, vital
signs, skin conditions, the amount and color of vaginal discharge
and the activity of extremities were examined. Patients were
followed up during the first postoperative month to record any
adverse events.
Statistical analysis
Data were analyzed using SPSS 17.0 statistical
software (SPSS, Inc., Chicago, IL, USA). Values are expressed as
the mean ± standard deviation. Student's t-test was performed to
compare between groups and the chi-square test was used to analyze
enumeration data. P<0.05 was considered to indicate a
statistically significant difference.
Results
Characteristics and parameters of
uterine fibroids on CEUS
Prior to MWA therapy, CEUS was performed to
investigate the variations of parameters during different phases.
As presented in Table II,
significant differences were observed in the WiR of the fibroid
tissue, STD, RTR and WiR ratio between the experimental and control
groups. However, the WiR of fibroid vessels, AUC of fibroid vessels
and tissues and RTD did not differ significantly between the two
groups. Parameters on pre-operative CEUS may provide reliable
information on the perfusion of uterine fibroids to help evaluate
the regression area and remnants of fibroid objectively.
 | Table II.Quantitative perfusion parameters
derived from the time-intensity curve. |
Table II.
Quantitative perfusion parameters
derived from the time-intensity curve.
| Parameter | Experimental
group | Control group | T-value | P-value |
|---|
| Fibroid tissue |
|
|
|
|
| MTT
(sec) |
9.32±2.62 |
13.21±4.35 | −3.562 | 0.036a |
| AUC |
64.32±25.36 |
70.32±36.23 | −0.465 | 0.596 |
| WiR |
1.43±0.36 |
0.92±0.46 | 2.732 | 0.027a |
| Fibroid vessel |
|
|
|
|
| AUC |
136.56±48.32 |
149.36±69.62 | 1.363 | 0.136 |
| WiR |
3.21±1.22 |
3.37±1.36 | −0.163 | 0.865 |
| RTR |
1.35±0.19 |
1.96±0.24 | 1.362 | 0.032a |
| WiRR |
0.56±0.23 |
0.29±0.13 | 2.763 | 0.018a |
| RTD
(sec) |
1.32±0.92 |
3.56±1.12 | −1.362 | 0.635 |
| STD
(sec) |
1.32±0.32 |
2.32±0.92 | −1.369 | 0.041a |
Volume and signal intensity changes of
fibroids on MRI after MWA therapy
All clinical symptoms were alleviated or removed,
and fibroids were either reduced in volume or cured by MWA in
patients with uterine fibroids. As presented in Table III, the reductions in volume of
hypointense, isointense and hyperintense fibroids were 62.42±18.13,
53.27±10.05 and 47.43±9.56%, respectively, on T1WI. On T2WI, the
reductions were 67.32±32.63, 59.36±19.36 and 42.63±10.37% in
hypointense, isointense and hyperintense fibroids, respectively.
The higher the signal intensity on T1WI and T2WI, the lower the
reduction in volume. It is indicative that different blood supply
for fibroids results in different ablation. The reductions were
significantly higher in the experimental group than that in the
control group.
 | Table III.Reductions in volume of hypointense,
isointense and hyperintense uterine fibroids on T1WI and T2WI. |
Table III.
Reductions in volume of hypointense,
isointense and hyperintense uterine fibroids on T1WI and T2WI.
| Signal type | Experimental group
(%) | Control group
(%) | P-value |
|---|
| T1WI |
|
|
|
| Hypointense |
62.42±18.13 |
53.12±21.27 | <0.01a |
| Isointense |
53.27±10.05 |
47.13±9.12 | 0.07 |
| Hyperintense |
47.43±9.56 |
40.23±10.35 | 0.09 |
| T2WI |
|
|
|
| Hypointense |
67.32±32.63 |
55.08±13.37 | 0.02a |
| Isointense |
59.36±19.36 |
54.66±17.24 | 0.13 |
| Hyperintense |
42.63±10.37 |
34.84±13.19 | 0.06 |
Adverse events
Among 60 patients in the experimental group, 32
(53.3%) and 26 (43.3%) patients complained about pain at the
treated site and in the sacrococcygeal region, respectively, while
7 patients experienced radiating pain during the MWA therapy. Skin
burns were reported in 38 cases (63.4%) and improved after focus
position adjustment, treatment rhythm control and water cooling.
After the therapy, lower abdominal and sacrococcygeal pain were
reported in 9 (15.0%) and 4 (6.6%) cases, and disappeared one week
later. There were no major complications, indicating that MWA is
reliable for treating uterine fibroids due to its
noninvasiveness.
Comparison of CEUS and MRI in pre- and
post-MWA assessment
CEUS clearly revealed the non-perfused area of the
fibroids prior to and after MWA (Fig.
1). In a representative subject from the experimental group,
CEUS performed one week prior to MWA displayed complete contrast
perfusion inside the fibroids with distinct boundaries, regular
shape and homogeneous echo (Fig.
1A). On CEUS performed one month after MWA, there was no echo
inside the fibroids and an inhomogeneous echo was present around
the lesion, which was caused by contrast perfusion. This indicated
the presence of a residue inside the fibroids, which required
re-ablation (Fig. 1B). Uterine
fibroids displayed a rapid wash-in and slow wash-out enhancement,
as seen from the TIC of CEUS (Fig.
1C). The reason for this was that the RT of uterine fibroids
was shorter than that of normal uterine muscle.
In a representative subject of the control group,
conventional US prior to MWA revealed a circular lump with clear
margins and homogeneous echo in the uterus. CDFI displayed striped
or ring-shaped blood signals inside the fibroids (Fig. 1D). On conventional US performed half
a year after MWA, fibroids appeared with an inhomogeneous echo
inside and no blood signal, indicating that the lesion had been
completely ablated (Fig. 1E).
From these results, it may be deduced that CEUS
provides information on the blood supply and perfusion of fibroids
prior to MWA. This may be used to evaluate the effectiveness of MWA
immediately after the therapy. Re-ablation is required if remnants
of fibroids are verified.
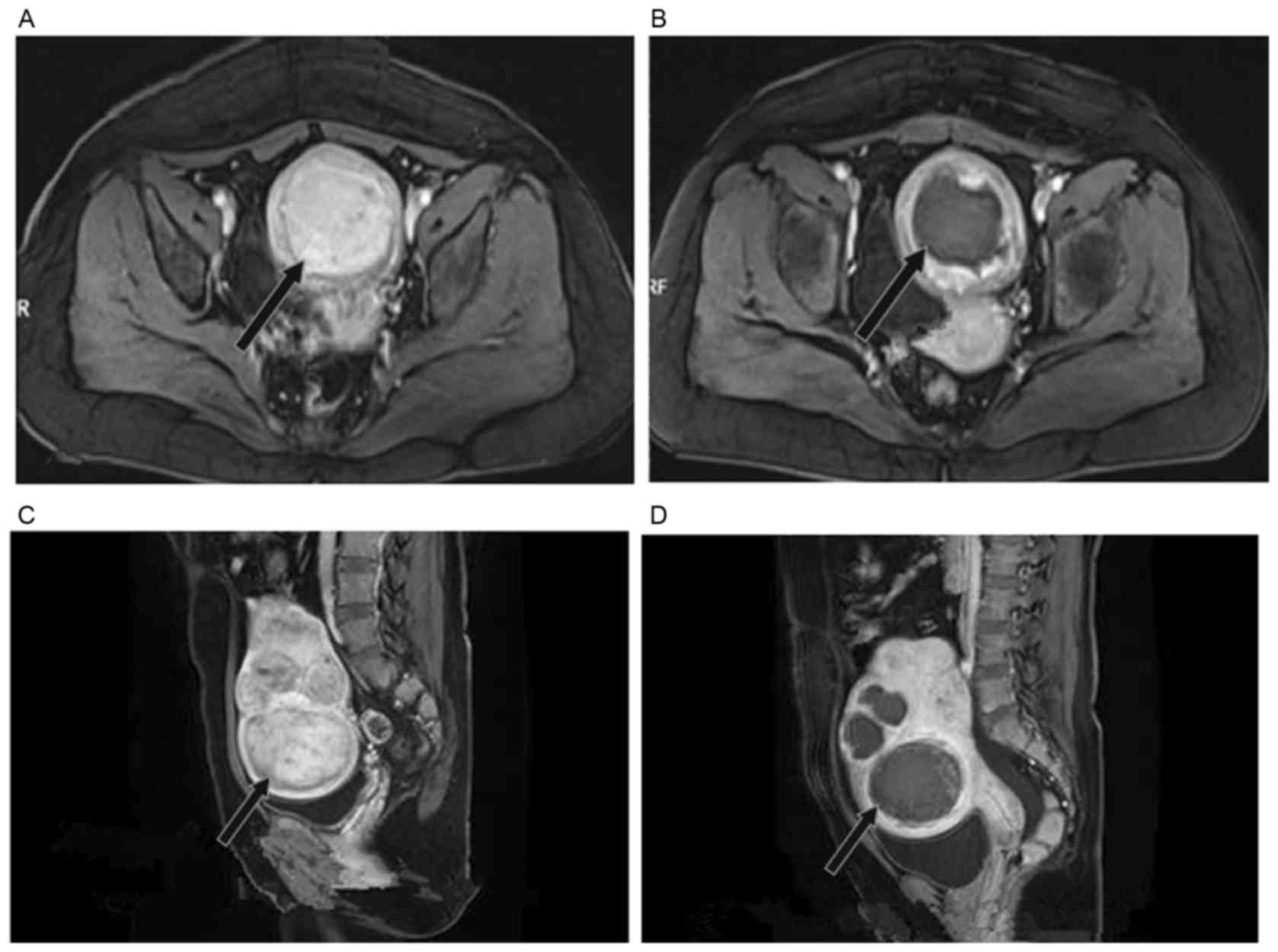
On MRI, the non-perfused area of the fibroids prior
to and after MWA was distinctly displayed (Fig. 2). In a representative subject from
the experimental group, uterine fibroids demonstrated high signal
intensity with distinct boundaries and regular shapes on
contrast-enhanced T1WI one week prior to MWA (Fig. 2A). One month after the MWA therapy,
contrast-enhanced T1WI displayed fibroids with hypointensity inside
and periphery enhancement (Fig.
2B).
In a representative subject from the control group,
multiple uterine fibroids measuring 45×40×43 mm with mixed high
signal intensity were detected on sagittal T2WI one week prior to
MWA (Fig. 2C). Fibroids appeared as
hypointense with clear boundaries and no remnant was seen on T2WI
half a year after MWA. The reduction of fibroids in volume was
estimated to be 80% (Fig. 2D).
The signal intensity on MRI was closely associated
with the proton component of the lesion. Therefore, MRI cannot
present real-time blood supply of the lesion and it is not optimal
for immediate evaluation after MWA.
Effectiveness of CEUS in the follow-up
of patients after MWA
A total of 78 single or multiple fibroids were
confirmed by pre-operative CEUS. Three months after MWA, CEUS
revealed no perfusion inside or around the lesion in 71 fibroids.
Contrast-enhanced MRI displayed no enhancement in the targeted area
in 67 fibroids, indicating a complete inactivation. However,
abnormal contrast perfusion was found in 7 fibroids, while mild
enhancement was verified in 11 fibroids, indicating incomplete
ablation and partial remission.
At the end of the follow-up period, significant
differences were observed between residual ratios on MRI and CEUS
in each group and complete fibroid regression was observed
(Table IV). However, no significant
difference was noted between the experimental and control groups in
the regression and residual ratios, suggesting that MVA is
effective in treating uterine fibroids. It was noted that CEUS is
better than MRI in evaluating regression and residual ratios.
 | Table IV.Evaluation of the effect of microwave
ablation on uterine fibroids by CEUS and MRI. |
Table IV.
Evaluation of the effect of microwave
ablation on uterine fibroids by CEUS and MRI.
|
| Experimental group
(n) | Control group
(n) |
|---|
|
|
|
|
|---|
| Imaging method | Total fibroids | Complete fibroid
regression | Residualfibroid | Total fibroids | Complete fibroid
regression | Residualfibroid |
|---|
| CEUS | 78 | 71 | 7 | 83 | 71 | 12 |
| MRI | 78 | 67 | 11 | 83 | 64 | 19 |
| P-value | – | 0.01 | <0.01 | – | 0.03 | 0.01 |
| χ2 |
| 8.14 |
|
| 3.27 |
|
Discussion
Conventional two-dimensional US and CDFI are
commonly used to assess the efficacy of MWA treatment of uterine
fibroids through displaying the size, internal echo changes and
blood supply of fibroids (18).
However, the limitations lie in determining the area of fibroid
necrosis as well as fibroid regression and residue. CEUS and
contrast-enhanced MRI reveal the perfusion changes inside the
treated area and blood supply of the fibroids, evaluate the fibroid
regression and effectiveness objectively and guide targeted
touch-up procedures in a timely manner to obtain better results
(19).
In the present study, CEUS and MRI were performed
for a comparative investigation on the perfusion in the targeted
region and the ablation area after MWA (20). According to the results obtained by
examining parameters during different phases on CEUS, a significant
difference was observed in the STD between vessel and tissue in
fibroids, WiR of fibroid tissue and RT and WiR ratios of vessel to
tissue in fibroids. The intensity of fibroid tissue had a steeply
increasing curve, arrived at the PE rapidly and then slowly
decreased on TIC. It displayed a fast wash-in and slow wash-out
enhancement on CEUS due to the anatomical link with the surrounding
normal muscle fibers and perfusion of fibroids. The enhancement
inside the fibroids was greatly reduced after MWA compared with
that prior to MWA. The higher the signal intensity on T1WI and
T2WI, the lower the reduction in volume. A significant difference
was noted in the volume reduction of fibroids with different blood
supply and signal intensity.
Based on the results of the present study, signal
intensity changes in the targeted region one month after MWA
demonstrated coagulation necrosis in fibroids. The CEUS findings,
including the maximum diameter and volume of fibroids and
non-perfused region, were consistent with the MRI findings
regarding the necrotic area. However, CEUS is advantageous over
MRI, as it effectively displays the maximum diameter and volume of
fibroids following MWA. Therefore, CEUS is an effective and
reliable imaging technique in evaluating short-term results of
MWA.
The incidence of pain in the sacrococcygeal and
treated regions, and skin burns encountered in the present study
conformed with those reported by previous studies (21). These adverse events are common after
MWA, and should be paid attention to and avoided. No complications
were observed in the present study, indicating that CEUS it is safe
for real-time evaluation of the treatment of uterine fibroids by
MWA.
The present study revealed a significant difference
in fibroid regression and residual ratios between CEUS and MRI.
Treatment results were associated with the fibroid size, ablation
time and the surgeon. Large fibroids usually present with abundant
internal blood supply and fast blood flow. Heat loss during MWA
leads to fibroid residue. Real-time evaluation during MWA therapy
is required to re-ablate fibroid residue in order to obtain better
results.
In conclusion, CEUS is advantageous over MRI, as it
safely provides real-time information on the perfusion. It reliably
provides information on the ablation area and fibroid residue after
MWA. Therefore, it is of great value in clinical practice and may
be utilized for assessing the efficacy of MWA treatment of uterine
fibroids.
Acknowledgements
This study was supported by The Regional Special
Disease Center Construction Project of Zhejiang Province (no.
2014-98).
References
|
1
|
Talaulikar VS and Manyonda I: Progesterone
and progesterone receptor modulators in the management of
symptomatic uterine fibroids. Eur J Obstet Gynecol Reprod Biol.
165:135–140. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Olejek A, Olszak-Wąsik K and
Czerwinska-Bednarska A: Long-term intermittent pharmacological
therapy of uterine fibroids-a possibility to avoid hysterectomy and
its negative consequences. Prz Menopauzalny. 15:48–51.
2016.PubMed/NCBI
|
|
3
|
Carrafiello G, Recaldini C, Fontana F,
Ghezzi F, Cuffari S, Laganà D and Fugazzola C: Ultrasound guided
radiofrequency thermal ablation of uterine fibroids: Medium-term
follow-up. Cardiovasc Intervent Radiol. 33:113–119. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ankem K: Information-seeking behaviour of
women in their path to an innovative alternate treatment for
symptomatic uterine fibroids. J Med Libr Assoc. 95:164–172. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Estrade-Huchon S, Bouhanna P, Limot O,
Fauconnier A and Bader G: Severe life-threatening hemoperitoneum
from posttraumatic avulsion of a pedunculated uterine leiomyoma. J
Minim Invasive Gynecol. 17:651–652. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ellens N and Hynynen K: Simulation study
of the effects of near- and far-field heating during focused
ultrasound uterine fibroid ablation using an electronically focused
phased array: A theoretical analysis of patient safety. Med Phys.
41:0729022014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen XM and Luo PF: Current status,
questions and challenges of transcatheter uterine artery
embolization for the treatment of uterine fibroids. J Int Radio.
15:449–450. 2006.
|
|
8
|
Ren XL, Zhou XD, Zhang J, He GB, Han ZH,
Zheng MJ, Li L, Yu M and Wang L: Extracorporeal ablation of uterine
fibroids with high-intensity focused ultrasound: Imaging and
histopathologic evaluation. J Ultrasound Med. 26:201–212. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ghezzi F, Cromi A, Bergamini V, Scarperi
S, Bolis P and Franchi M: Midterm outcome of radiofrequency thermal
ablation for symptomatic uterine myomas. Surg Endosc. 21:2081–2085.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dong BW, Liang P, Yu XL, Yu DJ, Zhang J,
Feng L, Cheng ZG, Wang Y and Wang ZL: Long-term results of
percutaneous sonographically-guided microwave ablation therapy of
early-stage hepatocellular carcinoma. Zhonghua Yi Xue Za Zhi.
86:797–800. 2006.(In Chinese). PubMed/NCBI
|
|
11
|
Liang P, Wang Y, Zhang D, Yu X, Gao Y and
Ni X: Ultrasound guided percutaneous microwave ablation for small
renal cancer: Initial experience. J Urol. 180:844–848. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang J, Feng L, Zhang B, Ren J, Li Z, Hu
D and Jiang X: Ultrasound-guided percutaneous microwave ablation
for symptomatic uterine fibroid treatment-a clinical study. Int J
Hyperthermia. 27:510–516. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang J, Feng L, Zhang BS, Ren JT, Li ZC,
Wen B, Hu DM, Jiang X and Du LD: The study of percutaneous
microwave coagulation for uterine myomas. Chin J Med Ultrasound
(Electronic Edition). 8:40–43. 2011.
|
|
14
|
Wang F, Zhang J, Han ZY, Cheng ZG, Zhou
HY, Feng L and Hu DM: Imaging manifestation of conventional and
contrast-enhanced ultrasonography in percutaneous microwave
ablation for the treatment of uterine fibroids. Eur J Radiol.
81:2947–2952. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang JL, Xiong WL, Deng LW and Cheng GX:
The diagnostic value of MRI in adenomyosis. Chin J CT & MRI.
23:1417–1419. 2015.
|
|
16
|
Wei CF, Hu B and Jiang LX: Evaluation of
uterine adenomyoma treatment with high intensity focused ultrasound
by contrast-enhanced ultrasound. Chin J Med Ultrasound (Electronic
Edition). 7:54–59. 2010.
|
|
17
|
Zhu Li, Chen WZ, Chen JY, Wen HY, Deng YB,
Zhang R and Wang ZB: Study on the relationship between ultrasound
ablation for uterine fibroids and signal of MRI. Acta Acad Med
Militaris Tertiae. 14:1370–1373. 2009.
|
|
18
|
Hindley J, Gedroyc WM, Regan L, Stewart E,
Tempany C, Hynyen K, Mcdannold N, Inbar Y, Itzchak Y, Rabinovici J,
et al: MRI guidance of focused ultrasound therapy of uterine
fibroids: Early results. AJR Am J Roentgenol. 183:1713–1719. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou XD, Ren XL, Zhang J, He GB, Zheng MJ,
Tian X, Li L, Zhu T, Zhang M, Wang L and Luo W: Therapeutic
response assessment of high intensity focused ultrasound therapy
for uterine fibroid: Utility of contrast-enhanced ultrasonography.
Eur J Radiol. 62:289–294. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang X, Peng S, Chen JY and Zhang RT:
Investigation on influence factors of ultrasound ablation in
treating multiple uterine fibroids. J Minimally Invasive Med.
03:2014.
|
|
21
|
Yan LM, He J, Huang GH, He M, Li KQ and
Zhang L: High intensity focused ultrasound ablation for uterine
fibroids in patients with retroposition of uterus. Zhongguo Chao
Sheng Yi Xue Zazhi. 28:72–74. 2012.
|