Introduction
Peyronie's disease (PD) is a penile fibrotic disease
characterized by the presence of tunical plaques which can
clinically lead to penile pain, penile deformity including
curvature, narrowing and hourglass, and potential erectile
dysfunction (1). In almost all
fibrotic diseases, one of most important mechanisms is that
fibroblasts transdifferentiate intoα-smooth muscle actin
(αSMA)-positive myofibroblasts (2).
In Peyronie's disease, tunica albuginea myofibroblasts (MFs) were
mainly derived from tunica albuginea fibroblasts (TAFs) induced by
TGF-β (3). Previously, Vernet D
found thatαSMA positive myofibroblasts content was considerably
increased in the human Peyronie's disease and TGFβ1-induced rat
plaques as compared to control tunica albuginea by
Immunohistochemistry (4). Furtherly
when compared the gene expression profiling between the PD plaques
and the nomal penile tunica abuginea, Magee TR that the mainly
up-regulated genes were involved in the function of myofibroblasts,
such as myofibroblast differentiation and collagen synthesis, while
the down-regulated genes were that inhibit these processes,
collagenase and matrix metalloproteinases (MMPs) (5,6). On the
one hand myofibroblasts could secrete extracellular matrix (ECM)
components (particularly collagen), and in the other hand, MFs had
the function of automatic contraction (7,8). These
processes could resulted in the PD plaques and the penile
deformity.
MSCs were a kind of stem cells with the function of
self-renewal and multi-directional differentiation. In mounting
experimental or pre-clinical studies, researchers found that
mesenchymal stem cells (MSCs) could prevent the development of
tissue fibrosis (9,10). Previously studies proved that MSCs
could slow the progression of fibrosis, and reverse functional
remodeling in heart, liver, kidney, and lung tissues (11–15).
Adipose tissue-derived stem cells (ADSCs) belonged to the family of
MSCs. Because of abundant adipose tissues and simplely obtained
method, ADSCs were the mainly source of adult stem cells.
Furthermore, compared with other stem cells, ADSCs had fewer
ethical problems and lower immunogenicity (16). Therefore, there were a lot of studies
involved in the roles of ADSCs in attenuating fibrotic disease,
such as liver cirrhosis, idiopathic pulmonary fibrosis and kidney
fibrosis (17–19). It will be highlighted that ADSCs were
progressively used to recover the animal model of Peyronie's
disease. The mechanisms of anti-fibrosis by ADSCs had not been
completely elucidated (20,21), while the paracrine signaling is
considered as one of the main underlying mechanisms of the
therapeutic effects of MSCs (12).
Otherwise, the balance of activation vs. inactivation and
proliferation vs. apoptosis of TAMFs played the important role in
Peyronie's disease. Furthermore, MMPs participated in the
regulation and clearance of ECM secreted by MFs, and automatic
contraction of MFs was mainly involved by the RhoA/ROCK signaling
pathway (22,23).
Therefore, we performed the following experiments by
using the transwell coculture of ADSCs and tunica albuginea MFs.
Firstly, we assess whether ADSCs regulated the secretion of
collagen by MFs and the contraction of MFs. Additionally, we
explored the proliferation vs. apoptosis of MFs when cocultured
with ADSCs. Finally, we investigated MMPs and RhoA/ROCK signaling
pathway of TAMFs when cocultured with ADSCs.
Materials and methods
Cell culture
We used 3 male Sprague-Dawley rats (from the Animal
Feeding Center of Nanjing Medical University, Jiangsu, China) to
isolate the ADSCs and tunica albuginea fibroblasts (TAFs) In
independent experiments. Under 4% chloral hydrate anesthesia,
penile tissue paratesticular fat and penile tunica albuginea were
harvested to isolate the ADSCs and TAFs, then all rats were
euthanized. All procedures were approved by the Institutional
Animal Care and Use Committee of Nanjing University.
Monoculture. ADSCs
ADSCs were isolated from paratesticular fat of SD
rats and cultured as described previously (24). Rat paratesticular adipose tissues
were minced and incubated with 0.1% collagenase I (Catalog No.
17100-017, Life Technologies, GIBCO) for 1 h at 37°C. The digested
tissues were filtered through a 75-µm mesh, then centrifuged at 200
× g for 5 min, and the cell precipitates were re-suspended in
Dulbecco's modified Eagle's medium (DMEM) containing 10% fetal
bovine serum (FBS; both from Gibco, Carlsbad, CA, USA) and 1%
antibiotics (100 mg/l streptomycin and 100 U/ml penicillin) at 37°C
in a humidified 95% air/5% CO2 environment. Surface
markers (such as CD90, CD34, CD45, CD44) were identified by flow
cytometric analysis of passage 3 ADSCs as peformed in our previous
study (25). The passage 3–8 ADSCs
were starved with serum-free medium with 5 ng/ml TGF-β1 for 24 h
and used to the following experiments.
Preparate and monoculture MFs
Primary TAFs were isolated, cultured and identified
as previously described (26).
Briefly, TAFs from Sprague-Dawley rats were cultured in DMEM,
supplemented with 10% FBS (both from Gibco, Carlsbad, CA, USA) and
1% antibiotics (100 mg/l streptomycin and 100 U/ml penicillin) at
37°C in a humidified 95% air/5% CO2 environment. The
TAFs were then treated with TGF-β1 (10 ng/ml; Sigma) for 24 h. As
described in our previous study, TAFs were transformated into
myofibroblasts which had high expression of αSMA (3). Then myofibroblasts were maintained with
5 ng/ml TGF-β1 for following experiments.
Transwell cocluture of MFs and
ADSCs
The transwell coculture delivered a great
environment that both types of cells shared culture medium but did
not directly contact (27). Cells
were digested with trypsin, re-suspended in a serum-free DMEM
medium with 5 ng/ml TGF-β1. ADSCs were plated into the matrigel
coated Transwell chambers (Corning, NY, USA) and MFs were planted
in 12-well plates. Then Chambers were inserted into 12-well plates
filled with serum-free DMEM medium with 5 ng/ml TGF-β1. The system
was incubated at the temperature of 37°C for 24, 48 or 72 h of
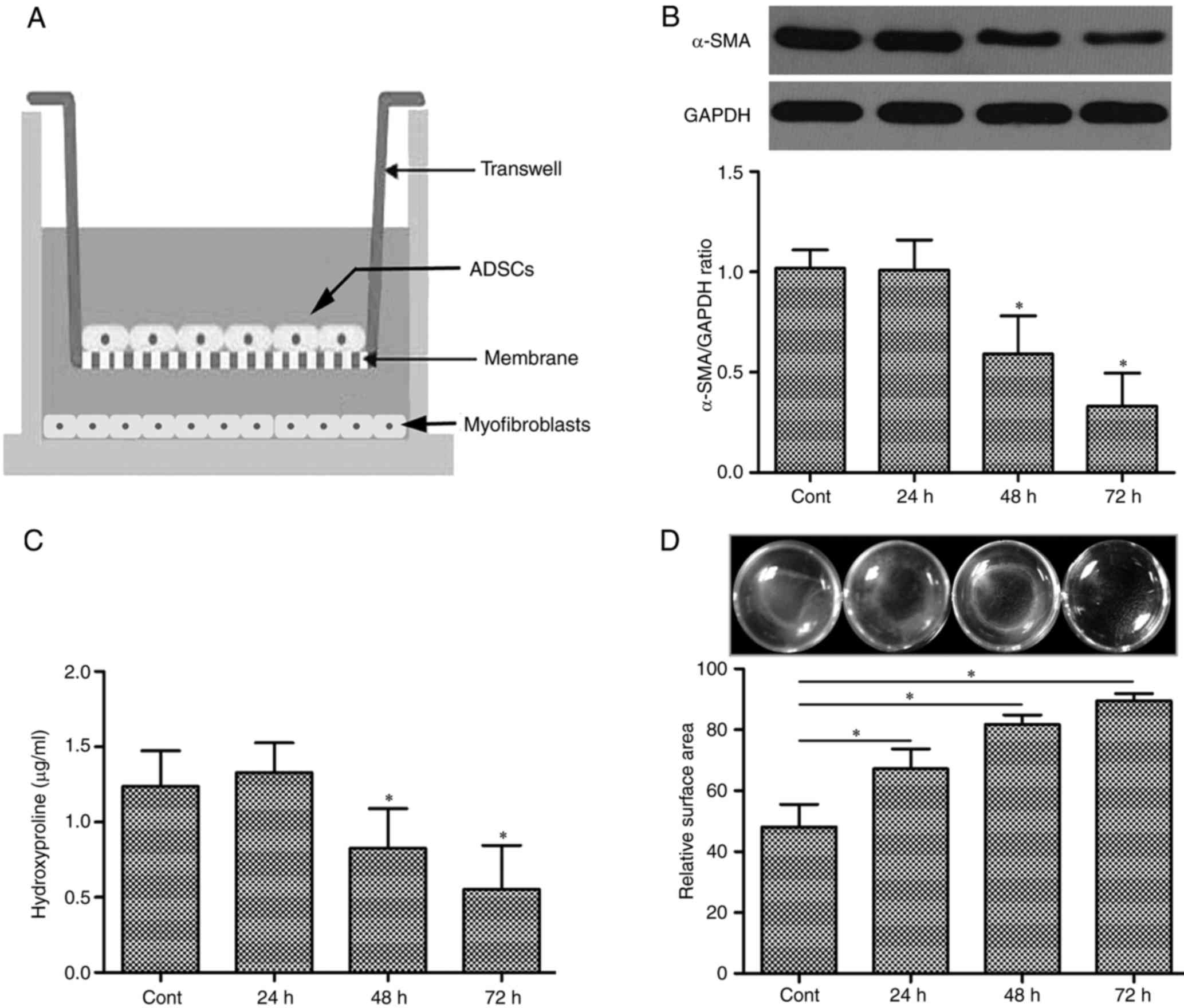
incubation (Fig. 1A).
Measurement of hydroxyproline
concentration
Hydroxyproline was used to estimate the secretion of
total collagen in the medium according to the method described by
Woessner (28) and the protocol
included in the hydroxyproline kit (A030-1; Nanjing Jiancheng
Bioengineering Institute, Jiangsu, China). Duplicate 300-µl
aliquots of medium were taken from each sample and transferred to
microtiter plates, and the absorbance of each was determined at 550
nm by spectrophotometry. The hydroxyproline concentrations of the
sample pending to be tested were calculated using a linear standard
curve and are presented as µg/ml medium.
Collagen gel contraction assay
All experimental operation according to the protocol
(3), Collagen gels were prepared
using 2 mg/ml of rat tail collagen I (Wobio, Nanjing, China) that
was neutralized with 1 M NaOH and supplemented with DMEM. After
cocultured with ADSCs for 24, 48 or 72 h, MFs were digested with
trypsin, re-suspended and seeded at a density of 3×105 cells/ml in
microtiter plates that were lubricated with FBS. Following
lubrication, 0.5 ml of the final collagen gel was incubated at 37°C
in a humidified 95% air/5% CO2 environment for 24 h.
Images were acquired using an Odyssey Scanning System (LI-COR
Biosciences, Lincoln, NE, USA), and the surface areas were
quantified using ImageJ software (NIH, Bethesda, MD, USA).
Western blot analysis
Cells were harvested at scheduled time and washed in
phosphate-buffered saline (PBS) and then lysed in RIPA buffer
(Sigma). Total protein concentrations were measured using
Bicinchoninic acid (BCA) reagent (Beyotime Biotech, Jiangsu, China)
were used to determine the protein concentration. Western blot was
performed as previously described (29). Briefly, Proteins were separated in
10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis
(SDS-PAGE) and were then electrophoretically transferred onto
polyvinylidene fluoride (PVDF) membranes at 200 mA for 60–120 min.
The membranes were blocked 5% non-fat milk in TBST at 37°C for 1 h.
Then membranes were incubated at 4°C overnight with the primary
antibodies, [anti-αSMA (Rabbit, 1:1,000, Sigma), anti-Smad2
(Rabbit, 1:1,000, Sigma), anti-phosphorylated (p-)Smad2 (Rabbit,
1:1,000, Sigma), anti-RhoA (Mouset, 1:1,000, Sigma), anti-ROCK1
(Rabbit, 1:1,000, Sigma), anti-ROCK2 (Rabbit, 1:1,000, Sigma),
anti-Col1 (Rabbit, 1:1,000; Abcam), MMP-2 (Rabbit, 1:1,000; CST),
MMP-3 (Rabbit, 1:1,000; CST), MMP-9 (Rabbit, 1:1,000; CST), MMP-13
(Rabbit, 1:1,000; Abcam), anti-GAPDH (Mouse, 1:1,000, Sigma)].
After washing the membranes with TBST, the membranes were then
incubated with the corresponding secondary antibody (horseradish
peroxidase conjugated goat anti-rabbit/mouse IgG; 1:10,000, Wuhan
Boster Biological Technology Ltd., Wuhan, China) at room
temperature for 2 h. The immunoreactive traces of membranes were
detected by chemiluminescence (ECL) Kit (Beyotime Biotech) and an
Odyssey Scanning System (LI-COR Biosciences). Finally, ImageJ
software was used to quantify the expression levels of the target
proteins by calculating the ratio of the mean intensity of each
target protein to GAPDH.
Statistical analysis
All data were obtained from at least 3 individual
experiments and expressed as the mean values ± standard deviation
(SD). Statistical analysis was performed by Student's t-tests using
SPSS 16.0 software. A P-value <0.05 was considered to a
statistically significant.
Results
ADSCs attenuated the expression of
α-SMA in tunica albuginea MFs
Since the hallmarker of activated myofibroblasts is
αSMA, western blots were performed to analyse the effects of ADSCs
on the αSMA protein levels of tunical abluginea MFs. Compared to
monocultured MFs in the presence of TGF-β1 (5 ng/ml), the αSMA
protein level of MFs was reduced when transwell coculture with
ADSCs in a time-dependent manner in the presence of TGF-β1 (5
ng/ml; Fig. 1B).
ADSCs could reduces the levels of
hydroxyproline in the culture mediam of MFs
Hydroxyproline was an general amino acid which was
specificly expressed in collagen proteins. Therefore hydroxyproline
was often used to measure secretion and deposition of collagen
protein (28). As shown in Fig. 1C, cocultured with ADSCs, the levels
of hydroxyproline was suppressed in the culture medium of MFs
(CM-MFs) when compared with monocultured MFs, although TGF-β1 (5
ng/ml) was existed in MFs with or without ADSCs (P<0.01).
Otherwise, the attenuate effect of hydroxyproline in CM-MFs was
also in a time-dependent manner (Fig.
1C).
The collagen gel contraction inducing
by MFs is suppressed when cocultured with ADSCs
Collagen gel contraction assay was carried out to
analyze the inhibitory effects of ADSCs on the contractile process
of MFs. The MFs mixed with 5 ng/ml TGF-β1 were applied to collagen
gels and incubated with or without culture mediam of ADSCs
(CM-ADSCs) for 24, 48 and 72 h. MFs could induce contraction of
collagen gel as shown in control wells. Compared to control wells,
CM-ADSCs significantly suppressed gel contraction in a
time-dependent manner (P<0.01). Futhermore, CM-ADSCs could
almost completely reversed this contraction at 72 h (Fig. 1D).
ADSCs attenuated the activation of
Smad signaling pathway in MFs
The Smad signaling pathway is a classical TGF-β1
signaling pathway, which promotes transcription, translation, and
synthesis of collagen. Smad2 is the key factor in the TGF-β1/Smad
signaling pathway (30). In this
study, collagen I expression was inhibited in MFs co-cultured with
ADSCs compared with in monocultured MFs at 48 h and 72 h (Fig. 2). Furthermore, compared with
monocultured MFs, p-Smad2, the activated state of Smad 2, were down
regulated in MFs co-cultured with ADSCs, although TGF-β1 (5 ng/ml)
was supplemented. Additionally, no significant differences in total
Smad2 protein levels were observed between MFs co-cultured with
ADSCs and monocultured MFs (Fig.
3).
ADSCs could promote the expression of
matrix metalloproteinases (MMPs) in MFs
One of the functions of MMPs was to degradate
collagen fibers. Therefore, MMP-2, −3, −9, and −13 were determined
by western blot from monocultured MFs, monocultured ADSCs or
co-cultures of MFs with ADSCs. Compared with monocultured MFs or
ADSCs, the expressions of MMP-2, −3, −9, and −13 were markedly
increased in the presence of MFs coclutured with ADSCs (Fig. 4).
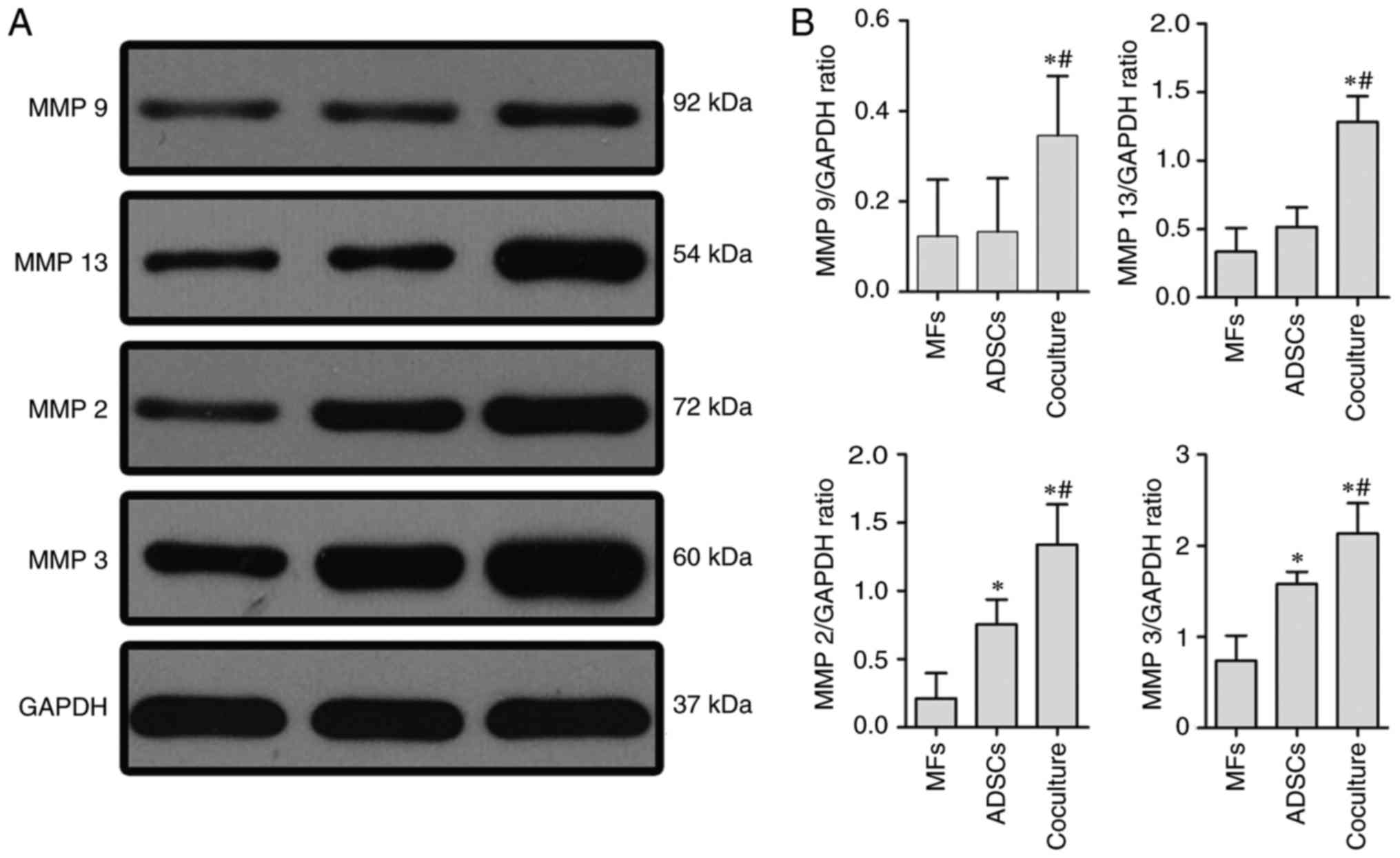 | Figure 4.Expression of MMP-2, −3, −9, and −13,
following co-culture of myofibroblasts with adipose tissue-derived
stem cells (ADSCs) in 5 ng/ml TGF-β1 CM for 72 h. (A)
Representative western blots showing the protein levels of MMP-2,
−3, −9 and −13 in MFs by monoculture or co-culture with ADSCs, and
monocultured ADSCs, respectively. (B) The relative levels of MMP-2,
−3, −9 and −13 to GAPDH are indicated by the corresponding bar
chart. Comparisons are made with monocultures of MFs and ADSCs in 5
ng/ml TGF-β1 for 72 h. Data are presented as means ± SD. Three
independent experiments were performed. *P<0.01, vs.
monocultured MFs; #P<0.01, vs. monocultured ADSCs.
MMP, matrox mettaloproteinase; CM, culture medium. |
Activation of the Rho/ROCK pathway by
MFs was inhibited via cocultured with ADSCs
The Rho/ROCK signaling pathway also play a major
role in fibrotic disease by promoting cell contraction and
migration (24–26). To elucidate the molecular mechanisms
of the MFs contraction, we thus performed an in vitro
experiment in which the cells were pre-treated with TGF-β1 (5
ng/ml) for 24 h and then with or without ADSCs cocultured. Compared
to the monocultured MFs, the expression of RhoA was significantly
lower after 72 h in MFs cocultured with ADSCs, and the expression
of ROCK1 or ROCK2, which were the receptor of RhoA, were slightly
decreased when cocultured with ADSCs (Fig. 5).
ADSCs could promote apoptosis of MFs
by increasing expression or activation of caspase3 and
caspase9
Most members of caspase family play a important role
in apoptosis and were often considered as the protein hallmarks of
cell apoptosis. Caspases associated apoptosis are subcategorised as
initiator caspases (eg. caspase9) and executioner caspases (eg.
caspase 3). In the present study, we investigated whether ADSCs
were able to regulate the expression of caspase3 and caspase9 in
MFs. Compared to monocultured MFs, the expression of caspase3 and
caspase9 of MFs were significantly elevated when cocultured with
ADSCs. A similar differential effect of cleaved-caspase3 and
cleaved-caspase9 were also seen in co-cultures of MFs with ADSCs or
monocultured MFs (Fig. 6).
Discussion
As a kind of fibrotic dieases, the pathophysiologic
process of Peyronie's disease (PD) was that the normal architecture
of penile TA was damaged by repeated micro-truama and replaced by a
large number of redundant ECM. ADSCs derived from rat
paratesticular fat exhibited fibroblastic and spindle-shaped
morphology, and expressed typical mesenchymal markers such as CD29,
CD44, CD73, and CD90, and negtively expression of hematopoietic
hullmarks CD45 and CD34 (25,31).
As we all known, collagen was the main component of
ECM. In this study, we identified that ADSCs could exerted an
antifibrotic function via reducing collagen secretion and promoting
collagen degradation. Consistent with our results, Harn H et
al found that ADSCs could reduce the expresion of α-smooth
muscle actin (αSMA), which was the marker of hepatic stellate
cells, a precursor of MFs. Further, ADSCs could inhibit the
production of collagen fiber. Otherwise, ADSCs could degrade
collagen fiber by increasing the expression of matrix
metalloproteinase-9. Therefore, ADSCs could abrogate
chemical-induced liver fibrosis (32). Moreover, after ADSC or CM-ADSCs
injected to scars, Zhang Q confirmed that the regular collagen
architecture was recovered and that the expression of αSMA and
collagen type I were also decreased by histomorphometric and
real-time quantitative polymerase chain reaction analysis (33).
Furthermore, Our results indicated that ADSCs had
the ability of synthesis and secretion of MMPs, including MMP2, 3,
9, 13, whose major function was the degradation of collagen. In
normal physiological process, the component of ECM was regulated by
MMPs and its inhibitor, TIMP. In addition, we also found that ADSCs
could elevate the expression of MMPs in myofibroblasts. Previously,
Hattori et al presented that MMP-2, 3, 9, 13 were detected
at higher levels in media from co-cultures with ADSCs and
inflammatory cells when compared with monocultured inflammatory
cells (34). Moreover, Yu et
al found that ADSCs could increase the expression of MMP2 and
MMP9, while down-regulated the expression of TIMP-1, meanwhile
decrease the αSMA expression was in pancreatic stellate cells
(27). A particularly important
factor in the mechanism of matrix accumulation is the transforming
growth factor-b (TGF-b), whose production is highly induced in many
fibrotic diseases, including atherosclerosis and fibrosis of the
kidney, liver and lung (35–37). Overexpressed TGF-b is proposed to
stimulate fibroblast proliferation, matrix production and
granulation tissue formation by TGF-b/Smad signaling pathway
(38). In our study, ADSCs could
reduced transnuclear expression of phospho-Smad2, which was the
crucial step for initiation of TGF-b signal transduction. These
results indicated that ADSCs could decrease synthetise of collagen
via TGF-b/Smad signaling pathway.
RhoA is a small guanosine triphosphate (GTP) ase,
belong to a member of the Ras homolog gene family. The mainly
function of Rho GTPase was to regulate the actin cytoskeleton.
Previous studies have suggested that RhoA and its downstream
receptors, ROCK (including ROCK1 and ROCK2), were one of key
signaling plathway in numerous fibrotic diseases (39). Ca2+ sensitization and myosin light
chain (MLC) phosphorylation were induced by the progress of Rho
GTPase transform to Rho GDPase, which initiated the contraction of
various cells (40). In our previous
study, we found that the contractility of MFs was involved in the
activation of RhoA/ROCK pathway. By contrast, in current study,
ADSCs could clearly attenuate the expression of RhoA, and its ROCK1
and ROCK2 in MFs. This discovery indicates that ADSCs had the
ability to inhibit the contraction of MFs by modulating the
RhoA/ROCK signaling pathway. Moreover, as reported in the
literature, the relaxation of smooth muscle in the penis results
from the inbibition of RhoA/ROCK pathway (41).
Myofibroblasts was the most important cell in PD
plaque and was derived from transformation of penile TAFs, which
was the largest class of cells that make up the normal TA (42). The persistence activation of
myofibroblasts facilitated the progress of fibrosis, which led the
penile structural remodeling. The expression of αSMA was the mark
of activated myofibroblasts. Therefore, preventing myofibroblast
activation is a potential therapeutic strategy to PD (43). In this study, we employed western
blot analysis and found that ADSCs inhibited tunica albuginea MFs
activation, which was indicated by a time-dependent decrease in
αSMA expression. Apoptosis induced by loss of adhesion or
adhesion-mediated signalling (termed ‘anoikis’) is a main pathway
of tunica albuginea MFs clearance (44). Although the precise mechanism of
myofibroblast apoptosis in the resolution of wound healing and
tissue repair are not well defined, anoikis is likely a relevant
model to study apoptotic mechanisms since cell adhesion and
biomechanical tension unloading appear to play important roles in
this physiological (45). In this
study, ADSCs could inhibit the expression of caspase3 (cleaved
caspase3) and caspase9 (cleaved caspase9) which were the markers of
cell apotosis. Previous study was undertaken to determine the
regulatory mechanisms of TGF-β1 to induce anoikis-resistance in any
cell type by PI3K-AKT pathway (46).
Otherwise, the apoptosis of myofibroblasts was induced by growing
factor, such as HGF, whereas molecular mechanisms had yet to be
fully understood (47).
In summary, we had shown that ADSCs could inhibit
the activation of tunical albuginea MFs, reduce the expression of
collagen in MFs through TGF-β1-Smad signaling pathway, suppress the
contraction of myofibroblasts by RhoA-ROCK signaling pathway. In
the other hand, ADSCs could degrade collagen by paracrine or
promoting MFs autocrine MMPs. Furthermore, ADSCs could induce
apoptosis of MFs, though the detail molecular mechanism was
confusing. These findings suggested that ADSCs attenuated the
development of PD (Fig. 7). The
anti-fibrotic ability of ADSCs will be considered in future
treatment approach of PD.
Acknowledgements
The present study was supported by grants from the
National Natural Science Foundation of China (no. 81671452).
References
|
1
|
Sherer BA and Levine LA: Contemporary
review of treatment options for Peyronie's disease. Urology.
95:16–24. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wynn TA and Ramalingam TR: Mechanisms of
fibrosis: Therapeutic translation for fibrotic disease. Nat Med.
18:1028–1040. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jiang HS, Zhu LL, Zhang Z, Chen H, Chen Y
and Dai YT: Estradiol attenuates the TGF-β1-induced conversion of
primary TAFs into myofibroblasts and inhibits collagen production
and myofibroblast contraction by modulating the Smad and Rho/ROCK
signaling pathways. Int J Mol Med. 36:801–807. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vernet D, Ferrini MG, Valente EG, Magee
TR, Bou-Gharios G, Rajfer J and Gonzalez-Cadavid NF: Effect of
nitric oxide on the differentiation of fibroblasts into
myofibroblasts in the Peyronie's fibrotic plaque and in its rat
model. Nitric Oxide. 7:262–276. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Magee TR, Qian A, Rajfer J, Sander FC,
Levine LA and Gonzalez-Cadavid NF: Gene expression profiles in the
Peyronie's disease plaque. Urology. 59:451–457. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zorba OU, Sirma S, Ozgon G, Salabas E,
Ozbek U and Kadioglu A: Comparison of apoptotic gene expression
profiles between Peyronie's disease plaque and tunica albuginea.
Adv Clin Exp Med. 21:607–614. 2012.PubMed/NCBI
|
|
7
|
Powell DW, Mifflin RC, Valentich JD, Crowe
SE, Saada JI and West AB: Myofibroblasts. I. Paracrine cells
important in health and disease. Am J Physiol. 277:C1–C9. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gelbard M: Myofibroblasts and
mechanotransduction: Do forces in the tunica albuginea contribute
to Peyronie's disease? J Sex Med. 5:2974–2976. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Arno AI, Amini-Nik S, Blit PH, Al-Shehab
M, Belo C, Herer E and Jeschke MG: Effect of Human Wharton's Jelly
Mesenchymal Stem Cell Paracrine Signaling on Keloid Fibroblasts.
Stem Cells Transl Med. 3:299–307. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kocher AA, Schlechta B, Gasparovicova A,
Wolner E, Bonaros N and Laufer G: Stem cells and cardiac
regeneration. Transpl Int. 20:731–746. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Salibian AA, Widgerow AD, Abrouk M and
Evans GR: Stem cells in plastic surgery: A review of current
clinical and translational applications. Arch Plast Surg.
40:666–675. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li L, Zhang S, Zhang Y, Yu B, Xu Y and
Guan Z: Paracrine action mediate the antifibrotic effect of
transplanted mesenchymal stem cells in a rat model of global heart
failure. Mol Biol Rep. 36:725–731. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Iwamoto T, Terai S, Hisanaga T, Takami T,
Yamamoto N, Watanabe S and Sakaida I: Bone-marrow-derived cells
cultured in serum-free medium reduce liver fibrosis and improve
liver function in carbon-tetrachloride-treated cirrhotic mice. Cell
Tissue Res. 351:487–495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Gatti S, Bruno S, Deregibus MC, Sordi A,
Cantaluppi V, Tetta C and Camussi G: Microvesicles derived from
human adult mesenchymal stem cells protect against
ischaemia-reperfusion-induced acute and chronic kidney injury.
Nephrol Dial Transplant. 26:1474–1483. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ortiz LA, Gambelli F, McBride C, Gaupp D,
Baddoo M, Kaminski N and Phinney DG: Mesenchymal stem cell
engraftment in lung is enhanced in response to bleomycin exposure
and ameliorates its fibrotic effects. Proc Natl Acad Sci USA.
100:pp. 8407–8411. 2003; View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou Y, Yuan J, Zhou B, Lee AJ, Lee AJ,
Ghawji M Jr and Yoo TJ: The therapeutic efficacy of human adipose
tissue-derived mesenchymal stem cells on experimental autoimmune
hearing loss in mice. Immunology. 133:133–140. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tang WP, Akahoshi T, Piao JS, Narahara S,
Murata M, Kawano T, Hamano N, Ikeda T and Hashizume M: Splenectomy
enhances the therapeutic effect of adipose tissue-derived
mesenchymal stem cell infusion on cirrhosis rats. Liver Int.
36:1151–1159. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tzouvelekis A, Paspaliaris V, Koliakos G,
Ntolios P, Bouros E, Oikonomou A, Zissimopoulos A, Boussios N,
Dardzinski B, Gritzalis D, et al: A prospective, non-randomized, no
placebo-controlled, phase Ib clinical trial to study the safety of
the adipose derived stromal cells-stromal vascular fraction in
idiopathic pulmonary fibrosis. J Transl Med. 11:1712013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Burgos-Silva M, Semedo-Kuriki P,
Donizetti-Oliveira C, Costa PB, Cenedeze MA, Hiyane MI,
Pacheco-Silva A and Câmara NO: Adipose Tissue-Derived Stem Cells
Reduce Acute and Chronic Kidney Damage in Mice. PLoS One.
10:e01421832015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yun IS, Jeon YR, Lee WJ, Lee JW, Rah DK,
Tark KC and Lew DH: Effect of human adipose derived stem cells on
scar formation and remodeling in a pig model: A Pilot Study.
Dermatol Surg. 38:1678–1688. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lam MT, Nauta A, Meyer NP, Wu JC and
Longaker MT: Effective delivery of stem cells using an
extracellular matrix patch results in increased cell survival and
proliferation and reduced scarring in skin wound healing. Tissue
Eng Part A. 19:738–747. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lauer-Fields JL, Juska D and Fields GB:
Matrix metalloproteinases and collagen catabolism. Biopolymers.
66:19–32. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Schofield AV and Bernard O: Rho-associated
coiled-coil kinase (ROCK) signaling and disease. Crit Rev Biochem
Mol Biol. 48:301–316. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Qiu X, Villalta J, Ferretti L, Fandel TM,
Albersen M, Lin G, Dai Y, Lue TF and Lin CS: Effects of intravenous
injection of adipose-derived stem cells in a rat model of radiation
therapy-induced erectile dysfunction. J Sex Med. 9:1834–1841. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu LL, Zhang Z, Jiang HS, Chen H, Chen Y
and Dai YT: Superparamagnetic iron oxide nanoparticle targeting of
adipose tissue-derived stem cells in diabetes-associated erectile
dysfunction. Asian J Androl. 19:425–432. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ahuja SK, Sikka SC and Hellstrom WJ:
Stimulation of collagen production in an in vitro model for
Peyronie's disease. Int J Impot Res. 11:207–212. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu FX, Su LF, Dai CL, Wang Y, Teng YY, Fu
JH, Zhang QY and Tang YH: Inhibition of pancreatic stellate cell
activity by adipose-derived stem cells. Hepatobiliary Pancreat Dis
Int. 14:215–221. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Woessner JF Jr: The determination of
hydroxyproline in tissue and protein samples containing small
proportions of this imino acid. Arch Biochem Biophys. 93:440–447.
1961. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sun Z, Wang C, Shi C, Sun F, Xu X, Qian W,
Nie S and Han X: Activated Wnt signaling induces myofibroblast
differentiation of mesenchymal stem cells, contributing to
pulmonary fibrosis. Int J Mol Med. 33:1097–1109. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xu F, Liu C, Zhou D and Zhang L:
TGF-β/SMAD Pathway and Its Regulation in Hepatic Fibrosis. J
Histochem Cytochem. 64:157–167. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li Y, Zhang W, Gao J, Liu J, Wang H, Li J,
Yang X, He T, Guan H, Zheng Z, et al: Adipose tissue-derived stem
cells suppress hypertrophic scar fibrosis via the p38/MAPK
signaling pathway. Stem Cell Res Ther. 7:1022016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Harn HJ, Lin SZ, Hung SH, Subeq YM, Li YS,
Syu WS, Ding DC, Lee RP, Hsieh DK, Lin PC and Chiou TW:
Adipose-derived stem cells can abrogate chemical-induced liver
fibrosis and facilitate recovery of liver function. Cell
Transplant. 21:2753–2764. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang Q, Liu LN, Yong Q, Deng JC and Cao
WG: Intralesional injection of adipose-derived stem cells reduces
hypertrophic scarring in a rabbit ear model. Stem Cell Res Ther.
6:1452015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hattori H and Ishihara M: Altered protein
secretions during interactions between adipose tissue- or bone
marrow-derived stromal cells and inflammatory cells. Stem Cell Res
Ther. 6:702015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gagliano N, Arosio B, Grizzi F, Masson S,
Tagliabue J, Dioguardi N, Vergani C and Annoni G: Reduced
collagenolytic activity of matrix metalloproteinases and
development of liver fibrosis in the aging rat. Mech Ageing Dev.
123:413–425. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Huang YR, Wei QX, Wan YG, Sun W, Mao ZM,
Chen HL, Meng XJ, Shi XM, Tu Y and Zhu Q: Ureic clearance granule,
alleviates renal dysfunction and tubulointerstitial fibrosis by
promoting extracellular matrix degradation in renal failure rats,
compared with enalapril. J Ethnopharmacol. 155:1541–1552. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
García-Alvarez J, Ramirez R, Checa M,
Nuttall RK, Sampieri CL, Edwards DR, Selman M and Pardo A: Tissue
inhibitor of metalloproteinase-3 is up-regulated by transforming
growth factor-beta 1 in vitro and expressed in fibroblastic foci in
vivo in idiopathic pulmonary fibrosis. Exp Lung Res. 32:201–214.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fang S, Xu C, Zhang Y, Xue C, Yang C, Bi
H, Qian X, Wu M, Ji K, Zhao Y, et al: Umbilical cord-derived
mesenchymal stem cell-derived exosomal MicroRNAs suppress
myofibroblast differentiation by inhibiting the transforming growth
factor-beta/SMAD2 pathway during wound healing. Stem Cells Transl
Med. 5:1425–1439. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Thumkeo D, Watanabe S and Narumiya S:
Physiological roles of Rho and Rho effectors in mammals. Eur J Cell
Biol. 92:303–315. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fukata Y, Kimura K, Oshiro N, Saya H,
Matsuura Y and Kaibuchi K: Association of the myosin-binding
subunit of myosin phosphatase and moesin: Dual regulation of moesin
phosphorylation by Rho-associated kinase and myosin phosphatase. J
Cell Biol. 141:409–418. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sopko NA, Hannan JL and Bivalacqua TJ:
Understanding and targeting the Rho kinase pathway in erectile
dysfunction. Nat Rev Urol. 11:622–628. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kwon KD, Choi MJ, Park JM, Song KM, Kwon
MH, Batbold D, Yin GN, Kim WJ, Ryu JK and Suh JK: Silencing histone
deacetylase 2 using small hairpin RNA induces regression of
fibrotic plaque in a rat model of Peyronie's disease. BJU Int.
114:926–936. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gonzalez-Cadavid NF and Rajfer J:
Mechanisms of disease: New insights into the cellular and molecular
pathology of Peyronie's disease. Nat Clin Pract Urol. 2:291–297.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Golan-Gerstl R, Wallach-Dayan SB, Zisman
P, Cardoso WV, Goldstein RH and Breuer R: Cellular FLICE-like
inhibitory protein deviates myofibroblast fas-induced apoptosis
toward proliferation during lung fibrosis. Am J Respir Cell Mol
Biol. 47:271–279. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Rodgers KD, Rao V, Meehan DT, Fager N,
Gotwals P, Ryan ST, Koteliansky V, Nemori R and Cosgrove D:
Monocytes may promote myofibroblast accumulation and apoptosis in
Alport renal fibrosis. Kidney Int. 63:1338–1355. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Horowitz JC, Rogers DS, Sharma V, Vittal
R, White ES, Cui Z and Thannickal VJ: Combinatorial activation of
FAK and AKT by transfonning growth factor-beta1 confers an
anoikis-resistant phenotype to myofibroblasts. Cell Signal.
19:761–771. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Iekushi K, Taniyama Y, Azuma J, Sanada F,
Kusunoki H, Yokoi T, Koibuchi N, Okayama K, Rakugi H and Morishita
R: Hepatocyte growth factor attenuates renal fibrosis through
TGF-β1 suppression by apoptosis of myofibroblasts. J Hypertens.
28:2454–2461. 2010.PubMed/NCBI
|