Introduction
With the development of computed tomography (CT) and
positron emission tomography (PET), the number of patients who are
characterized by ground-glass nodule (GGN) of CT has increased
(1). GGN is a pulmonary nodule
characterized by ground-glass opacity, which refers to high-density
shadows on CT images and visible pulmonary bronchial or pulmonary
vessels of the lung (2). Numerous
pulmonary diseases are characterized by GGN on CT, such as early
lung cancer, pneumonia, alveolar hemorrhage (3). However, the differential diagnosis of
benign and malignant lesions is currently a focus of research due
to the complexity of the imaging of pulmonary GGN (4). GGN occurs most frequently in
bronchioloalveolar carcinoma, adenocarcinoma and atypical
adenomatous hyperplasia. However, it also occurs in inflammatory
and focal lesions, focal hemorrhage or fibrosis (4). Therefore, pulmonary GGN are still
difficult to identify.
PET-CT is able to observe the metabolic changes of
diseases from the molecular level, and has broad application
prospects in cancer (5). PET-CT
could analyze the distribution range, appearance, shape and
companion of GGN. It could also identify accompanying signs and
dynamic characteristics (6).
Therefore, it will help to narrow the scope of diagnosis and
provide clues for the diagnosis. At present, it is widely used in
the diagnosis, staging, therapeutic evaluation and recurrence
monitoring of cancer (7).
In the present study, the PET-CT examination results
of 54 patients with pulmonary GGN were studied and compared with
the pathological observations with the purpose of evaluating the
application value of PET-CT in the diagnosis of pulmonary GGN.
Materials and methods
General information
In total, 54 patients with pulmonary GGN that were
identified by a PET-CT examination were selected and confirmed by
pathological examination and clinical diagnosis in the First
Affiliated Hospital of Zhengzhou University (Zhengzhou, China)
between April 2014 and April 2015. The patients included 32 males
and 22 females, aged 32–68 years. Inclusion criteria for patients
were as follows: i) Pulmonary nodules identified by CT; ii) no
previous history of cancer; and iii) blood sugar levels were
maintained at normal levels. Exclusion criteria were as follows: i)
Diabetic patients whose glucose could not be controlled in normal
range; and ii) pregnant or lactating females.
All diagnoses were confirmed by the examination of
pathological slices, which were acquired through CT-guided needle
biopsy or thoracoscopic local excision of the lesion. All patients
were required to fast for 4–6 h prior to the examination. Blood
sugar, height and body mass were detected before examination. The
patients were requested to be in repose for 40–60 min after
intravenous injection. Then, the clavicle, axillary and double
pulmonary fields were scanned by spiral CT. If the image of the
internal and edge of GGN was poor, target scan or high resolution
CT scan was performed. The present study was ethically approved by
the Ethics Committee of The First Affiliated Hospital of Zhengzhou,
and written informed consent was obtained from the patients.
Examination methods
A Siemens Biograph Truepoint 64 (52 rings) PET-CT
instrument (Siemens AG, Munich, Germany) with a 52-ring lutetium
oxyorthosilicate crystal detector and with 32,448 detection units
was used. The axial field-of-view was 216 mm and the axial
resolution was 2 mm. The integrated CT system had a 64-layer data
acquisition system and an instantaneous time resolution of 83 msec.
The imaging agent used was 18F-fluorodeoxyglucose
(18F-FDG), which was produced by Beijing Science and
Technology Co., Ltd. (Beijing, China) using a Sumitomo HM-20
medical cyclotron with PET-FDG IT-1 automatic synthesis modules
(Sumitomo Heavy Industries. Ltd., Tokyo, Japan). The radioactive
chemical purity was >95%. All patients were required to fast for
4–6 h prior to the examination and the fasting blood glucose was
required to be kept within the normal range (blood sugar
concentration was controlled at 7.0 mmol/l). Following the
intravenous administration of the 18F-FDG imaging agent
at a dose of 0.12 mCi/kg (8), the
patient rested in a quiet and dark environment for 60 min, then
emptied his/her bladder and drank 800–1,000 ml warm water prior to
the scan.
Following confirmation that the PET and CT axial
images matched each other through a positioning scan, the patients
underwent a whole-body CT scan with the following conditions: 120
kV voltage, 50–80 mA current and 3 mm layer thickness. The patients
then underwent a PET scan. Finally, a processing workstation with
TrueD software (Xeleris Workstation v3.0; GE Healthcare, Chicago,
IL, USA) was used to conduct image fusion and obtain the axial,
coronal and sagittal views for PET, CT and image fusion.
Image evaluation
A total of three radiology doctors who had >6
years of experience in the imaging diagnosis of chest diseases
judged the position, size, shape, edge, interface and standardized
uptake value of pulmonary lesions and divided these into pure and
mixed type GGN according to whether the nodule excluded or
contained solid components, respectively. The uptake of
18F-FDG in the nodules was observed and compared with
the uptake of the pulmonary background. An uptake that was higher
than that of the pulmonary background was evaluated as a
malignancy, while an uptake lower than or equal to the pulmonary
background was evaluated as benign. This result, combined with the
imaging findings of GGN lesions, was used to make a diagnosis.
Data analysis
Data were analyzed using t-test and χ2
tests on SPSS statistical analysis software, version 17.0 (SPSS,
Inc., Chicago, IL, USA). Data were given as mean ± standard
deviation. All the results were repeated three times. P<0.05 was
considered to indicate a statistically significant result.
Results
General information
The 54 patients included 32 male and 22 female cases
and the age range of the patients was 32–68 years. All patients had
solitary pulmonary GGN, which were distributed in the upper lobe of
the left lung in 12 cases, in the lower lobe of the left lung in 8
cases, in the upper lobe of the right lung in 24 cases, in the
middle lobe of the right lung in 3 cases and in the lower lobe of
the right lung in 7 cases. The nodule size range was 0.6–2.0
cm.
The 54 cases of GGN included 44 cases where the GGN
had a diameter >1 cm. Among these, 2 cases were pure GGN without
elevated radioactivity uptake and the remaining 42 cases were mixed
type GGN with an elevated metabolism compared with the pulmonary
background. There were 10 cases of GGN with a diameter of <1 cm,
including 5 cases of mixed nodules and 5 cases of pure GGN, all of
which did not have elevated radioactive uptake (Table I).
 | Table I.Association of the size and type of
GGN with benign and malignant lesions according to their metabolism
determined using positron emission tomography-computed
tomography. |
Table I.
Association of the size and type of
GGN with benign and malignant lesions according to their metabolism
determined using positron emission tomography-computed
tomography.
|
| Elevated metabolism
(n) | Normal metabolism
(n) |
|---|
|
|
|
|
|---|
| GGN properties | Benign | Malignant | Benign | Malignant |
|---|
| Size (cm) |
|
|
|
|
| ≥1 | 8 | 34 | 0 | 2 |
|
<1 | 0 | 0 | 5 | 5 |
| Type |
|
|
|
|
| Pure
ground-glass type | 0 | 0 | 2 | 4 |
| Mixed
type | 8 | 34 | 3 | 3 |
Association between pathological type
and metabolic observations
The association between pathological type and nodule
metabolism is demonstrated in Table
II. Positive rate refers to the PET-CT diagnosis of malignant
lesions as a proportion of malignant cases; detection rate refers
to the PET-CT diagnosis of benign lesions as a proportion of cases;
false positive rate refers to the PET-CT diagnosis of benign cases
as a proportion of malignant cases. By analysis of the data in this
table, the detection rate of PET-CT for malignant lesions in the
study population can thus be calculated to be 62.9%, with a
positive rate of 82.9% and a false positive rate of 58.3%.
 | Table II.Association between the pathological
type of ground-glass nodule and PET-CT results. |
Table II.
Association between the pathological
type of ground-glass nodule and PET-CT results.
|
| PET-CT (n) |
|---|
|
|
|
|---|
| Pathological
type | Elevated
metabolism | Normal
metabolism |
|---|
| Adenocarcinoma | 34 | 7 |
| Atypical adenomatous
hyperplasia | 2 | 0 |
| Inflammation | 5 | 2 |
| Fungal infection | 0 | 4 |
Associations of the size and type of
GGN with metabolic observations and pathology
The associations of the size of the GGN with the
metabolic observations provided by PET-CT and the pathology results
were analyzed and are presented in Table
I. Amongst the lesions with a diameter >1 cm, the PET-CT of
42 cases indicated a high metabolism, of which 81% (34/42) were
malignant and 19% (8/42) were benign. Additionally, the PET-CT
scans of 2 malignant cases did not reveal elevated metabolism.
There were 10 lesions with a diameter <1 cm, of which 50% (5/10)
were malignant and 50% (5/10) were benign. None of these 10 lesions
exhibited elevated metabolism.
Results analyzed according to the type of GGN are
also presented in Table I. There
were 7 cases of pure GGN without elevated metabolism, among which 2
cases were benign and 5 cases were malignant. The remaining 47
cases were mixed nodules, and 42 of these cases had a high PET-CT
metabolism, 8 of which were benign and 34 of which were malignant.
Among the 5 cases of mixed GGN without elevated metabolism, 3 cases
were benign and 2 cases were malignant.
Association of imaging observations
with nodule malignancy
The associations between imaging observations and
the benign and malignant features of local nodules are demonstrated
in Table III. From these results,
it may be observed that the mean size of the malignant GGN was
greater than that of those that were benign. Furthermore, nodules
with a regular shape were more likely to be malignant while those
with a smooth edge were more likely to be benign. By further
analyzing the imaging features of the GGN, the presence of a
vacuole sign, pleural indentation signs, lobulation and burr-like
appearance indicated a higher possibility of the lesion being
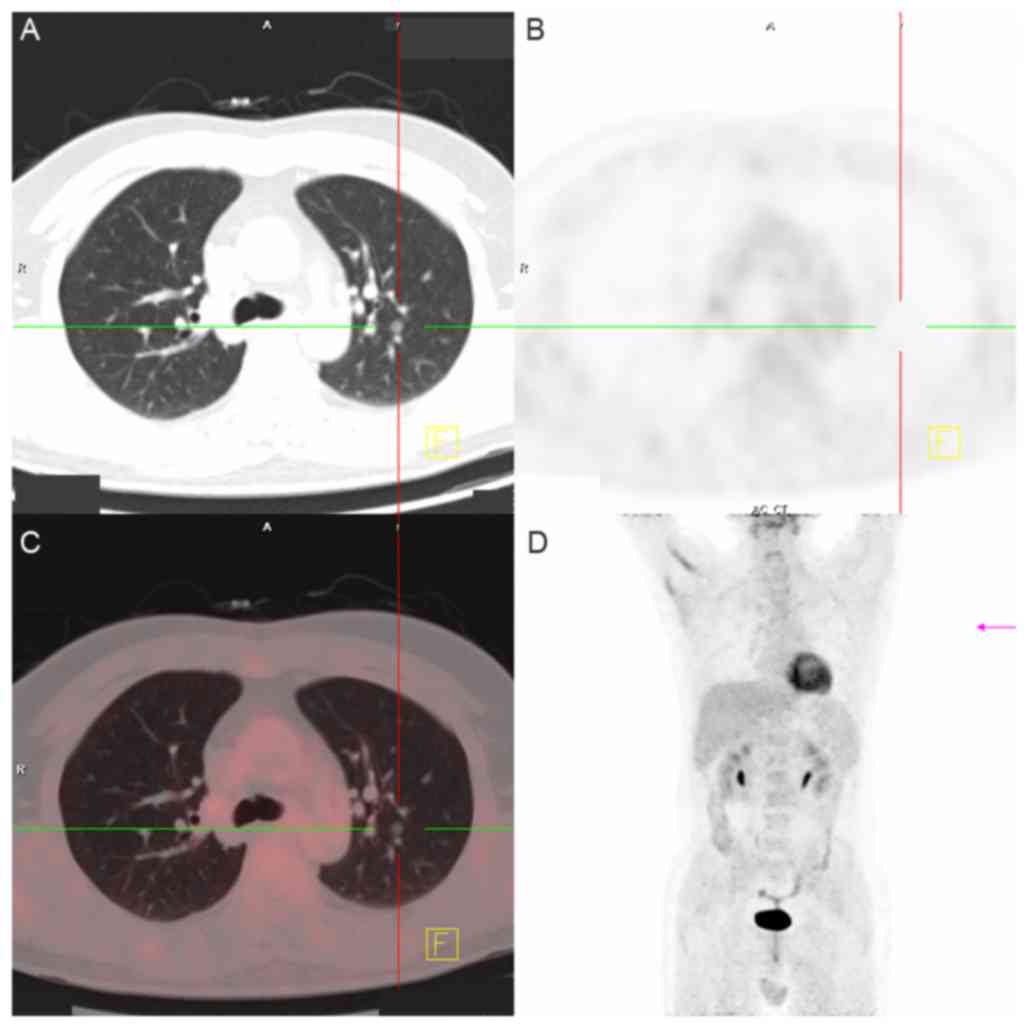
malignant rather than benign. Examples of imaging scans for various
patients are shown in Figs.
1–4.
 | Table III.Association between imaging
observations and the benign and malignant nature of the
nodules. |
Table III.
Association between imaging
observations and the benign and malignant nature of the
nodules.
| Observations | Malignant | Benign |
χ2-test | P-value |
|---|
| Size (cm) | 1.2±0.8 | 1.0±0.4 |
|
|
| Regular shape | 27 | 16 | 5.993 | 0.014 |
| Burr-like | 32 | 1 | 20.559 | <0.01 |
| Lobulated | 24 | 3 | 4.964 | 0.026 |
| Vascular bundle | 34 | 8 | 2.612 | 0.106 |
| Pleural
indentation | 26 | 2 | 9.120 | 0.003 |
| Smooth edges | 7 | 23 | 4.567 | 0.033 |
| Vacuole sign | 22 | 2 | 5.856 | 0.016 |
Discussion
With the popularization and application of high-end
CT scanning and the enhancement of people's health consciousness,
the detection rate of pulmonary nodules is increasing (9). A multinomial study reported that the
sensitivity and specificity of the diagnosis of primary lung cancer
by PET-CT were 84–97 and 76–95%, respectively (10). However, the diagnosis of GGN is
challenging due to the poor specificity of their imaging
performance. In 1997, the Nomenclature Committee of the
International Union of Biochemistry defined GGN as a fuzzy density
where the bronchi or lung blood vessels remain visible with
high-resolution CT (11).
Additionally, it refers to a class of non-specific CT signs that
have a slightly increased density in high-resolution CT images
(12). With regard to pathology, GGN
has been identified for a variety of inflammatory conditions,
edema, bleeding, fibrosis and tumor lesions (13–17).
For smaller pulmonary GGN lesions, the National
Comprehensive Cancer Network (18)
suggest that lesions that are >0.8 cm and persistently exist
should be dealt with as soon as possible, while the lesions that
are <0.8 cm should be dynamically observed and surgically
biopsied. Therefore, the judgment of whether the imaging shows
benign or malignant GGN lesions directly affects the patient's
treatment options. As PET-CT is becoming more widely used, the
research on its use in the diagnosis of pulmonary GGN is increasing
(1). In vitro and in
vivo experiments have demonstrated that lung cancer cells have
higher levels of glucose metabolism as compared with normal tissue
cells (19). Furthermore, as an
effective means of identification and diagnosis, 18F-FDG
PET-CT is important in the diagnosis of primary lung cancer
(20). Previous studies revealed the
sensitivity and specificity for 18F-FDG PET (PET-CT) in
the diagnosis of lung cancer primary lesions to be 84–97 and
76–95%, respectively (21–23). Additionally, the diagnostic value of
PET-CT has been affirmed by numerous researchers, particularly for
nodular lesions that were otherwise difficult to characterize
(7,24).
At present, 18F-FDG is the most widely
used imaging agent in PET-CT imaging in the clinic. Tumors use
glucose mainly in glycolysis to obtain energy, even when adequate
oxygen is available. Furthermore, the use of glycolysis as the main
method of obtaining energy by tumors is associated with the
existence of intermittent hypoxia in tumor tissues (25). Hypoxia induces an increase in the
expression of the glucose transporter-1, thereby increasing glucose
uptake (26). Tumor hypoxia may be
associated with numerous other factors, including abnormal
morphology and function of the microvasculature that feeds the
tumor, an increased distance between nutrient vessels and tumor
cells, reduction of the oxygen transfer capacity of red blood cells
induced by the disease itself or therapy, immature tumor cell
differentiation and a low capacity of oxygen utilization (27). Tumor cells have high metabolic
activity, which causes them to consume a large amount of FDG
(28). The ability of cancer cells
to undergo glycolysis is associated with the degree of malignancy
and the proliferation rate of the tumor; specifically, tumor cells
with a high degree of malignancy, strong invasiveness and high
proliferation rate have greater glycolytic activity (29).
In the present study, there were 54 GGN lesions,
ranging in diameter from 0.6 to 2.0 cm. Among these cases, all 10
lesions with a diameter of <1 cm exhibited no evident increase
in metabolic uptake, despite them including 5 malignant cases
exhibiting an adherent growth type of adenocarcinoma (primary
bronchial alveolar carcinoma). Furthermore, they were all
considered to be pure GGN, indicating that the lower degree of
malignancy may be associated with a lower glucose uptake. In the
present study, the accuracy of PET-CT was analyzed according to the
nodule diameter and the type of nodule. It was concluded that the
detection rate of PET-CT was low for smaller GGN, and there was no
clear advantage for pure GGN, while the detection rate of PET-CT
was high for larger GGN. However, due to the small number of cases
with a diameter <1 cm, expansion of the sample size is necessary
for this conclusion to be verified.
In conclusion, PET-CT is an examination method that
combines pathological metabolic patterns with imaging
manifestations and has been widely used in the examination of
malignant diseases. When PET-CT is used to judge the benign and
malignant nature of pulmonary GGN, a comprehensive diagnosis should
be performed by taking into account the nodule size, imaging
performance, glucose metabolism and clinical data. The sample size
used in the current study is small, and for smaller diameter, pure
GGN, the number of cases examined requires expansion in order to
clarify the findings.
Acknowledgements
The authors would like to express their gratitude to
all those who helped during the writing up of the present
study.
References
|
1
|
Gao YJ and Zsolt Szabo: Metabolic and
anatomic characteristics of bronchioloalveolar carcinoma on 18F-FDG
PET/CT. Chin J Nucl Med. 29:238–241. 2009.
|
|
2
|
Naidich DP, Bankier AA, MacMahon H,
Schaefer-Prokop CM, Pistolesi M, Goo JM, Macchiarini P, Crapo JD,
Herold CJ, Austin JH and Travis WD: Recommendations for the
management of subsolid pulmonary nodules detected at CT: A
statement from the fleishchner society. Radiology. 266:304–317.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim H, Park CM, Koh JM, Lee SM and Goo JM:
Pulmonary subsolid nodules: What radiologists need to know about
the imaging features and management strategy. Diagn Interv Radiol.
20:47–57. 2014.PubMed/NCBI
|
|
4
|
Park CM, Goo JM, Lee HJ, Lee CH, Chun EJ
and Im JG: Nodular Ground-glass opacity at thin-section CT:
Histologic correlation and evaluation of change at follow up.
Radiographics. 27:391–408. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Eglinton T, Luck A, Bartholomeusz D,
Varghese R and Lawrence M: Positron-emission tomography/computed
tomography (PET/CT) in the initial staging of primary rectal
cancer. Colorectal Dis. 12:667–673. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jung W, Jang JY, Kang MJ, Chang YR, Shin
YC, Chang J and Kim SW: The clinical usefulness of
18F-fluorodeoxyglucose positron emission tomography-computed
tomography (PET-CT) in follow-up of curatively resected pancreatic
cancer patients. HPB (Oxford). 18:57–64. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mac Manus MP and Hicks RJ: PET scanning in
lung cancer: Current status and future directions. Semin Surg
Oneol. 21:149–155. 2003. View Article : Google Scholar
|
|
8
|
Liu W, Li X, Quan J, Ouyang X and Zheng H:
Diagnostic value of 18F-FDG PET/CT for solitary nodular-type
bronchoalveolar carcinoma. Nan Fang Yi Ke Da Xue Xue Bao.
33:114–116. 2013.(In Chinese). PubMed/NCBI
|
|
9
|
Dalli A, Sen H Selimoglu, Coskunsel M,
Komek H, Abakay O, Sergi C and Tanrikulu A Cetin: Diagnostic value
of PET/CT in differentiating benign from malignant solitary
pulmonary nodules. J BUON. 18:935–941. 2013.PubMed/NCBI
|
|
10
|
Christensen JA, Nathan MA, Mullan BP,
Hartman TE, Swensen SJ and Lowe VJ: Characterization of the
solitary pulmonary nodule: 18F FDG PET versus nodule-enhancement
CT. AJR Am J Roentgenol. 187:1361–1367. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Collins J and Stern EJ: Ground-glass
opacity at CT: The ABCs. AJR Am J Roentgenol. 169:355–367. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kandemir Z, Sentürk A, Ozdemir E, Yildirim
N, Hasanoğlu HC, Keskin M and Türkölmez S: The evaluation of
hypermetabolic mediastinal-hilar lymph nodes determined by PET/CT
in pulmonary and extrapulmonary malignancies: Correlation with
EBUS-TBNA. Turk J Med Sci. 45:1234–1242. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Park CM, Goo JM, Lee HJ, Lee CH, Chun EJ
and Im JG: Nodular ground-glass opacity at thin-section CT:
Histologic correlation and evaluation of change at follow-up.
Radiographics. 27:391–408. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lei Z, Xu N, Zhang J, et al: MSCT
diagnosis of unusual interstitial pneumonia. Chin J Mod Med.
22:76–79. 2012.
|
|
15
|
Qi YG, Huang Y, Zheng JS, et al: The
Features and diagnostic value of CT and PET in pneumonia type of
bronchioloalveolar carcinoma. J Clin Radic. 29:187–190. 2010.
|
|
16
|
Guo G, Chen N, Li GF, et al: Complete
video-assisted thoracoscopic surgery for the diagnosis and
treatment of focal ground-glass opacity. Chin J Min Inv Surg.
12:641–643. 2012.
|
|
17
|
Jiang BG and Li L: Characteristics of thin
slice spiral CT imaging in 45 cases of solitary pulmonary nodule.
Chin J Geront. 31:1541–1543. 2011.
|
|
18
|
Porcu P: A look at the national
comprehensive cancer network guidelines for cutaneous lymphomas.
Clin Lymphoma Myeloma Leuk. 10 Suppl 2:S109–S111. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lin CC, Chen LC, Tseng VS, Yan JJ, Lai WW,
Su WP, Lin CH, Huang CY and Su WC: Malignant pleural effusion cells
show aberrant glucose metabolism gene expression. Eur Respir J.
37:1453–1465. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Erasmus JJ, McAdams HP, Rossi SE, Goodman
PC, Coleman RE and Patz EF: FDG PET of pleural effusions in
patients with non-small cell lung cancer. AJR Am J Roentgenol.
175:245–249. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Endo K, Oriuchi N, Higuchi T, Iida Y,
Hanaoka H, Miyakubo M, Ishikita T and Koyama K: PET and PET/CT
using 18 F-FDG in the diagnosis and management of cancer patients.
Int J Clin Oncol. 11:286–296. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Glaudemans AW, de Vries EF, Galli F,
Dierckx RA, Slart RH and Signore A: The use of (18)F-FDG-PET/CT for
diagnosis and treatment monitoring of inflammatory and infectious
diseases. Clin Dev Immunol. 2013:6230362013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mi B, Wan W, Yu C, You X, Jiang F and You
Q: The value of extra-lung lesions on ¹8F-FDG PET/CT in improving
diagnosis of lung cancer. Zhongguo Fei Ai Za Zhi. 15:78–83.
2012.(In Chinese). PubMed/NCBI
|
|
24
|
Birim O, Kappetein AP, Stijnen T and
Bogers AJ: Meta-analysis of positron emission tomographic and
computed tomographic imaging in detecting mediastinal lymph node
metastases in nonsmall cell lung cancer. Ann Thorac Surg.
79:375–382. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Waki A, Fujibayashi Y, Yonekura Y, Sadato
N, Ishii Y and Yokoyama A: Reassessment of FDG uptake in tumor
cells: High FDG uptake as a reflection of oxygen-independent
glycolysis dominant energy production. Nucl Med Biol. 24:665–670.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Macheda ML, Rogers S and Best JD:
Molecular and cellular regulation of glucose transporter (GLUT)
proteins in cancer. J Cell Physiol. 202:654–662. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Arabi M and Piert M: Hypoxia PET/CT
imaging: Implications for radiation oncology. Q J Nucl Mol Imaging.
54:500–509. 2010.
|
|
28
|
Chan WK, Au WY, Wong CY, Liang R, Leung
AY, Kwong YL and Khong PL: Metabolic activity measured by F-18 FDG
PET in natural killer-cell lymphoma compared to aggressive B- and
T-cell lymphomas. Clin Nucl Med. 35:571–575. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Koiso K, Kakizoe T, Murahashi I, Umeda T
and Ueno A: Studies on the metabolic activities of the bladder
tumors. 2. Studies on the energy metabolism of the bladder tumors
(author's transl). Nihon Hinyokika Gakkai Zasshi. 68:22–32.
1977.(In Japanese).
|