Introduction
Articular cartilage injury is a common clinical
orthopedic disease. Self-repair of articular cartilage is difficult
at pathogenic sites, and there are no specific methods to repair
articular cartilage injury in clinical practice (1). In recent years, with rapid developments
in modern biology and advanced materials, the application of
cartilage tissue engineering has promoted the emergence of new
methods to repair articular cartilage injury (2). Articular cartilage is rich in type-II
collagen, and the synthesis and secretion of type-II collagen are
specific indicators of articular chondrocytes to maintain their
differentiated phenotype. In addition, they are essential bases for
the evaluation of cartilage tissue engineering (3). In cartilage tissue engineering,
commonly used accelerants include growth factors, such as exogenous
growth factors, which are expensive and can easily biodegrade.
Moreover, they may have immunogenicity and other problems, limiting
their clinical application (4).
Therefore, it is necessary to actively explore more effective
accelerants with improved bio-performance. Type-I collagen is
widely distributed. Studies have confirmed that (5) type-I collagen has good biocompatibility
and biodegradability, and has been used in hemostasis, and in
artificial skin and corneal repair. Previous studies showed that
(6) type-I collagen scaffolds have
good prospects in cartilage tissue engineering and cartilage
repair. However, there are few studies on the effects of type-I
collagen hydrogel of different concentrations on chondrocyte growth
and differentiation. To further optimize the concentration of
type-I collagen in collagen hydrogel, its effects on articular
chondrocytes from New Zealand white rabbits were analyzed.
Materials and methods
Experimental animals
Three newborn New Zealand white rabbits (male or
female) aged 1 week were selected. The average weight was 0.17 kg.
The rabbits were provided by the animal center of the Fifth
People's Hospital of Jinan. The study was approved by Ethics
Committee of the the Fifth People's Hospital of Jinan.
Reagents
Type-I collagen was extracted from calf skin, and
prepared by the pathology laboratory of the Fifth People's Hospital
of Jinan; α-MEM medium (Shanghai Huiying Biotechnology Co., Ltd.);
trypsin (Beijing Geyuan Tianrun Biotechnology Co., Ltd.); type-II
collagenase (Shanghai Shize Biotechnology Co., Ltd.); fluorescein
diacetate (FDA)/propidium iodide (PI) dye (Shanghai Hualan Chemical
Technology Co., Ltd.); DNA extraction kit (Shenzhen Baoankang
Biology Co., Ltd.); ReactTra Ace Qpcr RT kit [Toyobo (Shanghai)
Biotechnology Co., Ltd.]; acetic acid, NaOH, formaldehyde, and
other chemical reagents were from Sinopharm Group Chemical Reagent
Co., Ltd.
Preparation of collagen hydrogel
Appropriate amounts of type-I collagen and 0.5 mol/l
acetic acid aqueous solution were weighed and mixed. The pH of the
solution was adjusted to approximately 7.5 using 2 mol/l NaOH
solution at 4°C, and the final concentration of type-I collagen was
10 mg/ml. The solution was then diluted to 7 and 5 mg/ml,
respectively. Approximately 0.5 ml of collagen solutions of
different concentrations were placed in a water bath at constant
temperature (37°C) for 30 min to prepare the collagen hydrogel with
type-I collagen concentrations of 10, 7, and 5 mg/ml.
Chondrocyte extraction and
inoculation
Articular cartilages from three New Zealand white
rabbits were harvested, and the chondrocytes were separated by
enzyme digestion, and cultured. Chondrocytes were then collected,
and appropriate amounts of α-MEM medium were added to prepare cell
suspensions. Cell suspensions were seeded in culture dishes at the
density of 1×105 cells/ml, and then cultured in an
illumination incubator. After cells were confluent, 0.25% trypsin
was used for digestion, followed by subculture. The morphology of
second-generation chondrocytes was observed under an inverted
fluorescence phase-contrast microscope.
Second-generation chondrocytes were collected and
cultured in vitro with 10, 7, and 5 mg/ml type-I collagen
hydrogel, respectively. The above three sterile collagen solutions
were mixed and the suspensions had a concentration of
5×106 cells/ml. The cell suspensions were then cultured
in an incubator and added to the 300 µl collagen
hydrogel-chondrocyte complex after the collagen solution produced
hydrogel, denoted as groups A, B, and C with 10, 7, and 5 mg/ml
type-I collagen hydrogel, respectively. The complex continued to be
cultured in an incubator for 2 weeks, and the solution was replaced
every 2 days.
Observation of cell viability
The collagen hydrogel-chondrocyte complexes (one in
each group) were selected after in vitro culture for 1 day,
and stained with fluorescein diacetate (FDA)/propidium iodide (PI).
Cell viability was observed under a laser confocal microscope.
Histological observation
The collagen hydrogel-chondrocyte complexes were
selected after in vitro culture for 2 weeks and fixed using
10% neutral formaldehyde, followed by paraffin embedding and
sectioning at 5 µm. Sections were then stained with H&E and
toluidine blue to observe the cell distribution.
Real-time quantitative RT-PCR
The collagen hydrogel-chondrocyte complexes were
selected after in vitro culture for 2 weeks. Real-time
quantitative RT-PCR was used to measure the mRNA levels of type-II
collagen, polymerized proteoglycan, type-I collagen, type-X
collagen, Sox9, and other chondrocyte-related genes. The
housekeeping gene, GAPDH, was used to normalize the expression of
each gene. mRNA was extracted from samples using a specific
extraction kit, followed by reverse transcription into cDNA using a
ReverTra Ace Qpcr RT kit. The cDNA was then amplified using
SsoFast™ EvaGreen® Supermix reagent (Beijing Changlitong
Technology Co., Ltd.) in a CFX960 real-time fluorescence
quantitative PCR instrument. The expression of each gene was
analyzed, and each sample was measured three times. Related primers
were designed and synthesized by Shanghai Gongda Co., Ltd.
Statistical analysis
SPSS 20.0 (IBM, New York, NY, USA) statistical
software was used for data analysis. Numerical data are presented
as mean ± SD, and compared using the paired samples t-test. The
Chi-square test was used for comparisons of numerical data.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Chondrocyte inoculation and
observation of viability
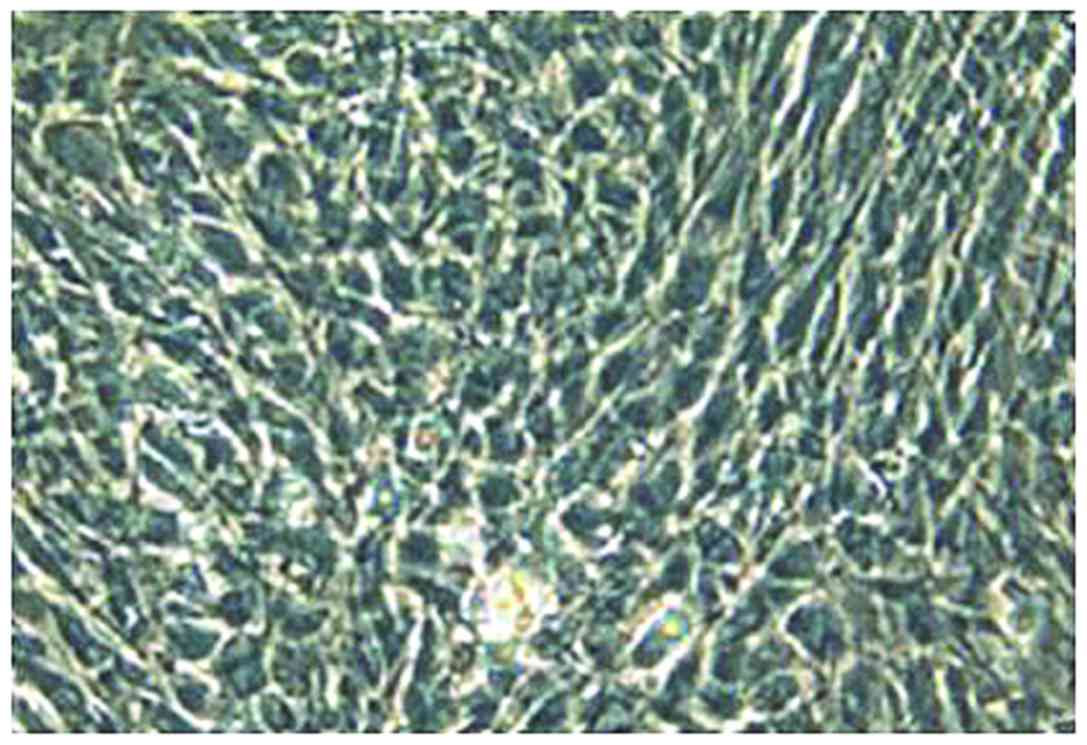
Observation by inverted fluorescence phase-contrast
microscopy showed that the second-generation chondrocytes were
polygonal with diameter of approximately 8–10 µm, and had good
growth status (Fig. 1). After
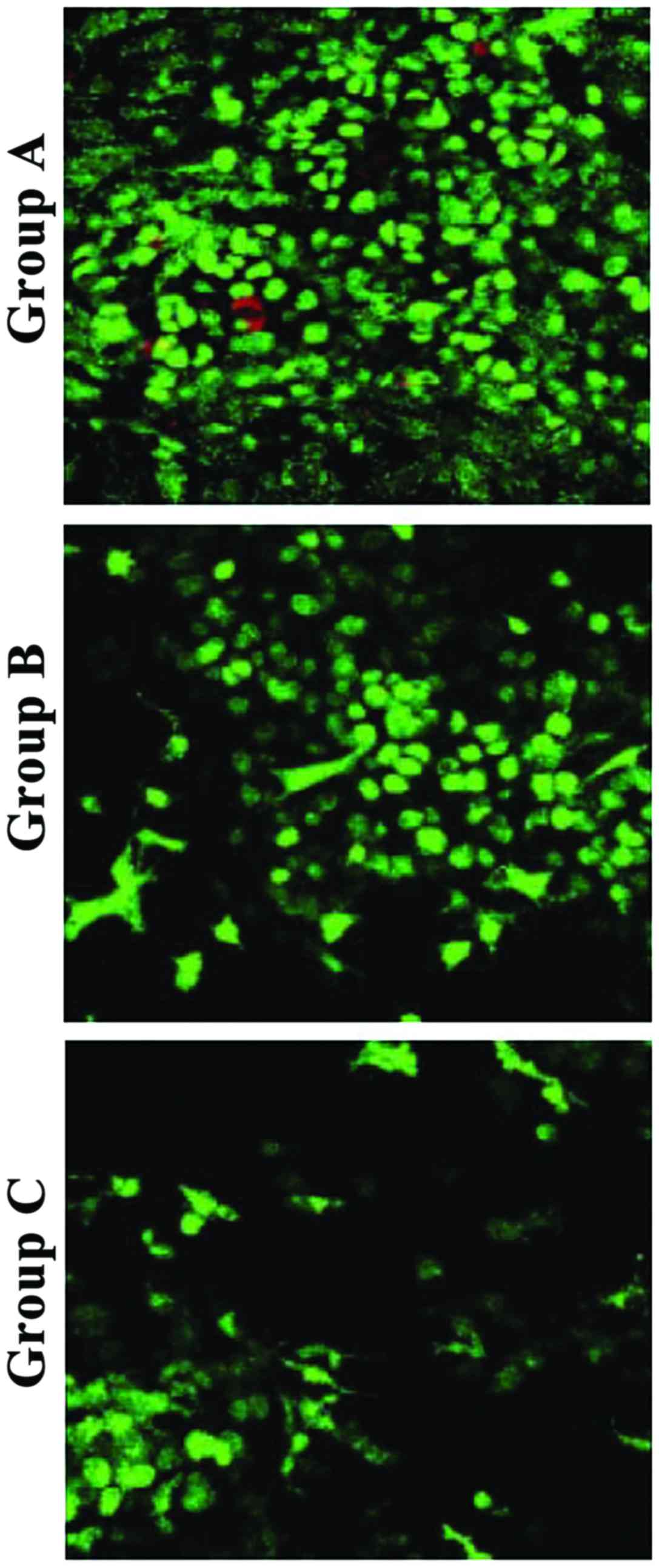
collagen hydrogel-chondrocyte complexes in groups A, B, and C were
cultured in vitro for 1 day, and stained with FDA/PI, laser
confocal microscopy showed that the distribution of chondrocytes
was uniform. Most were spherical, while some were polygonal in
shape, with a tendency to proliferate and aggregate. Most of the
chondrocytes in collagen hydrogels of groups A, B, and C were
stained green, while a few were stained red. The cell density of
group A was significantly higher than that of group B and C
(Fig. 2).
Histological observation
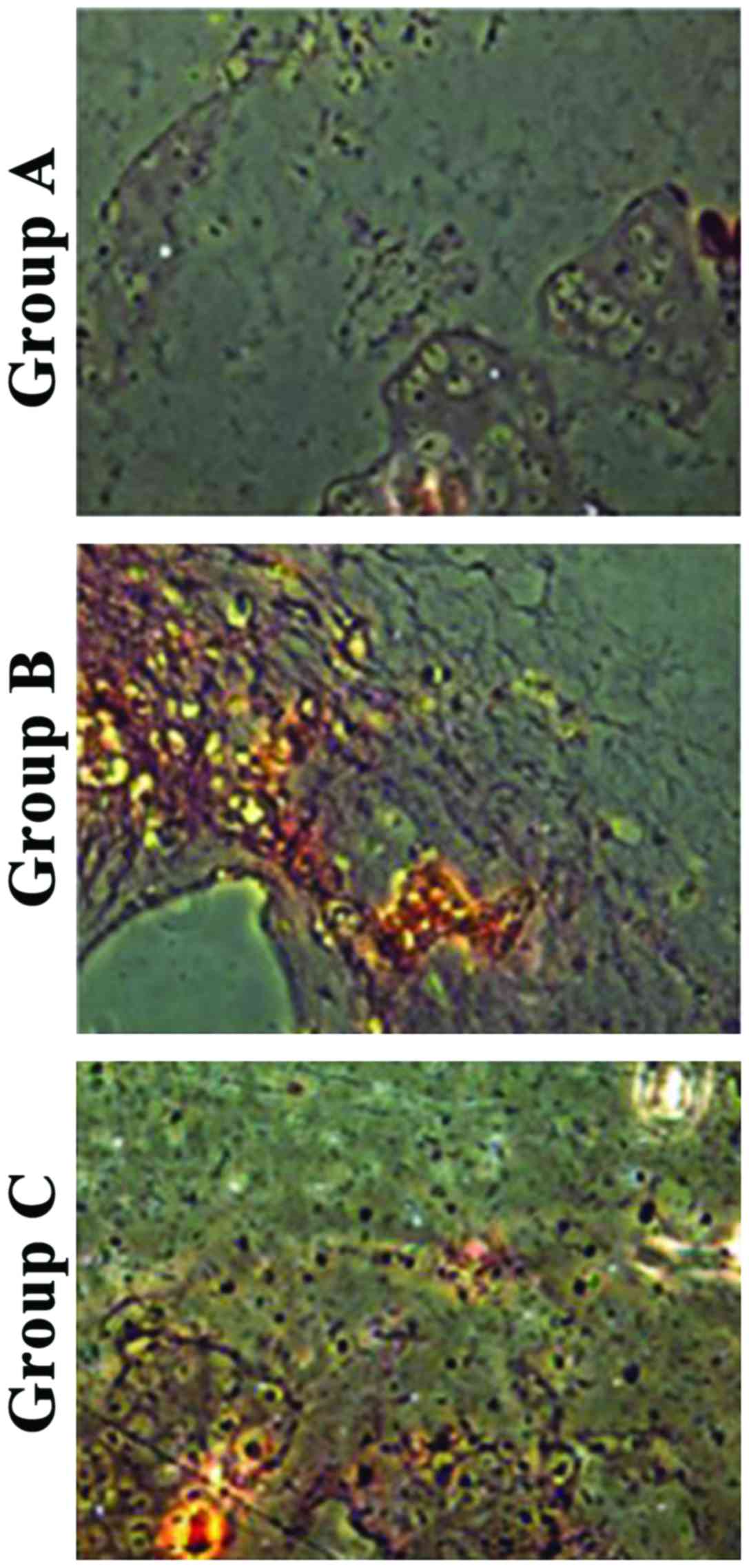
H&E staining showed that chondrocytes showed
obvious aggregation in group A, partial proliferation and
aggregation in group B, and uniform distribution in group C
(Fig. 3).
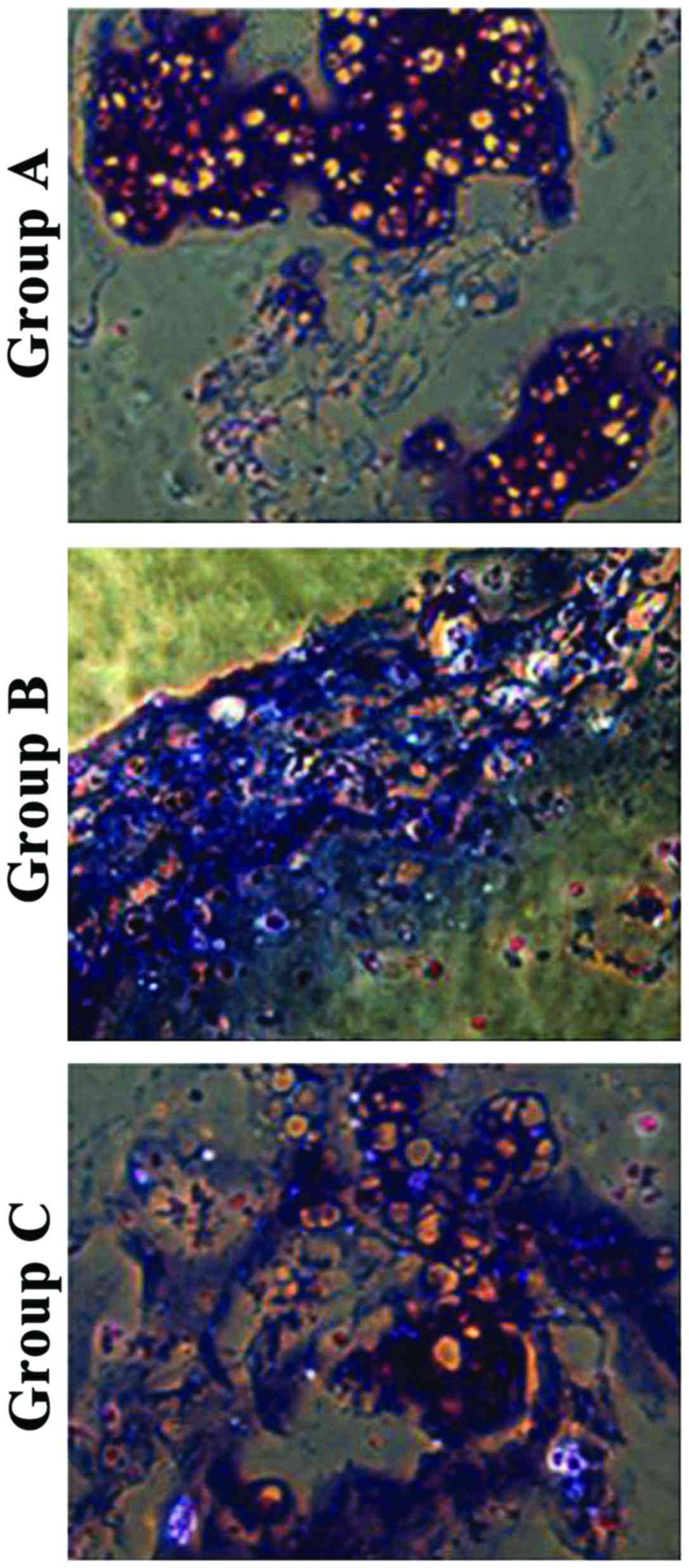
Toluidine blue staining showed that chondrocytes in
group A had aggregation areas and some were stained purple-red. In
group B, fewer chondrocytes were aggregated and had different
staining color and intensity around them, and aggregation was not
obvious. In group C, the distribution of chondrocytes was uniform
with different staining (Fig.
4).
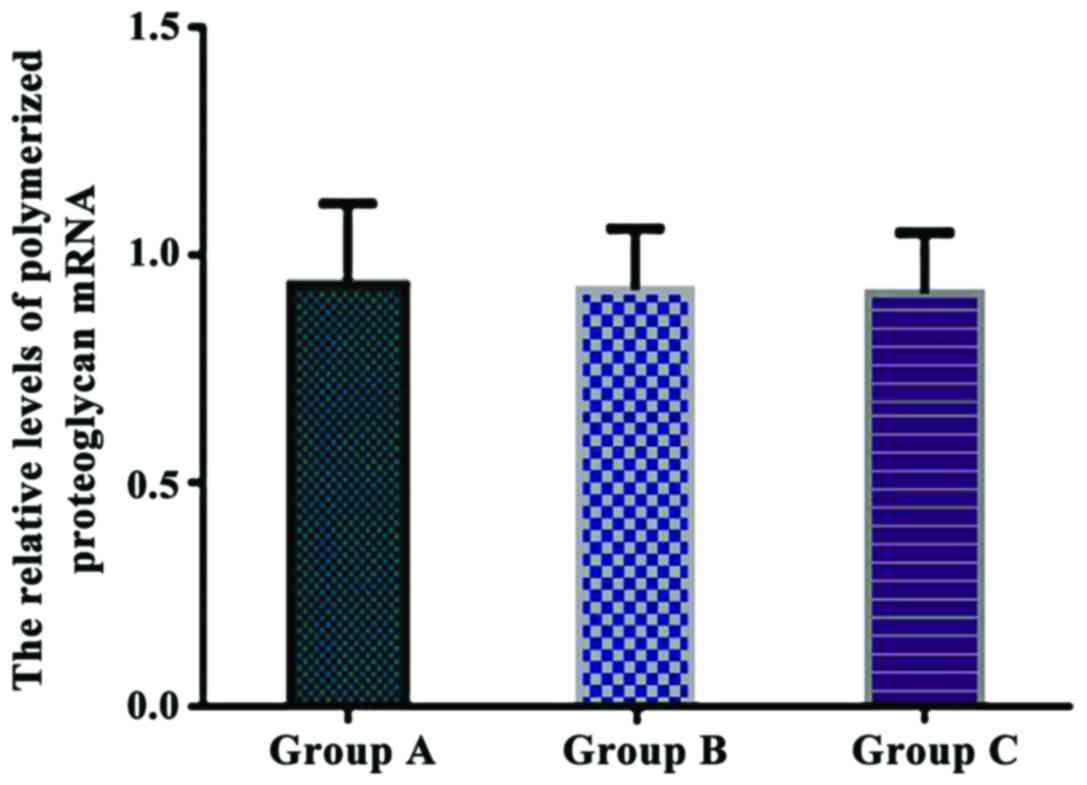
Comparison of the relative levels of
polymerized proteoglycan mRNA in each group
The relative levels of polymerized proteoglycan mRNA
were 0.936±0.178 in group A, 0.925±0.136 in group B, and
0.917±0.135 in group C. There were no significant differences in
the relative levels of polymerized proteoglycan mRNA between the
three groups (P>0.05) (Fig.
5).
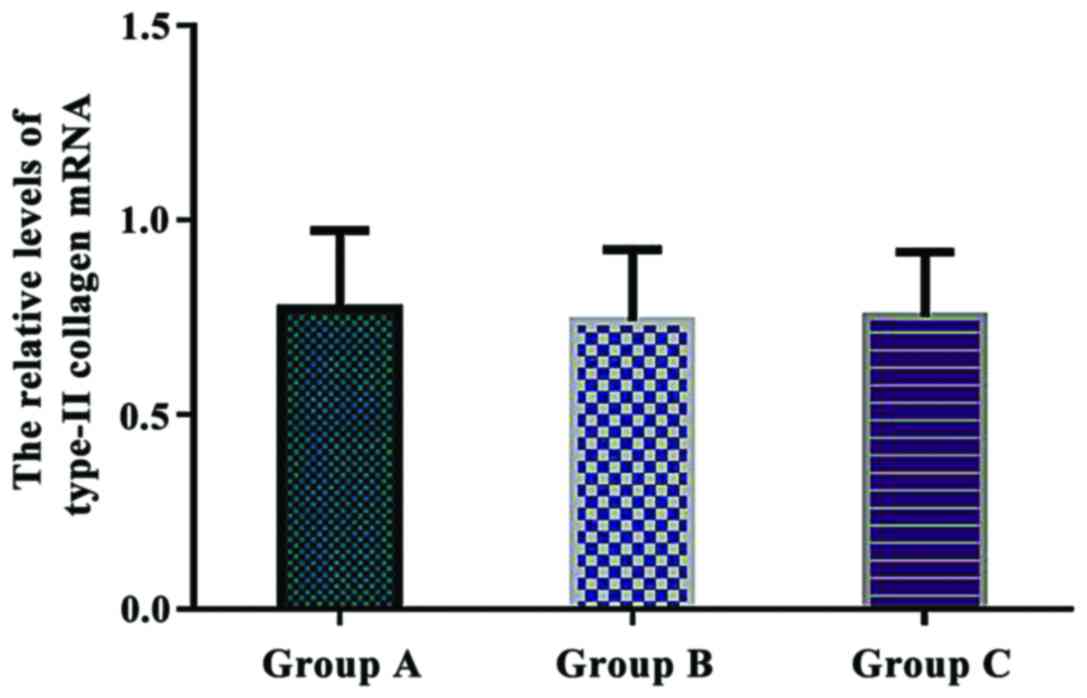
Comparison of the relative levels of
type-II collagen mRNA in each group
The relative levels of type-II collagen mRNA were
0.772±0.201 in group A, 0.741±0.183 in group B, and 0.752±0.165 in
group C. There were no significant differences in the relative
levels of type-II collagen mRNA between the three groups
(P>0.05) (Fig. 6).
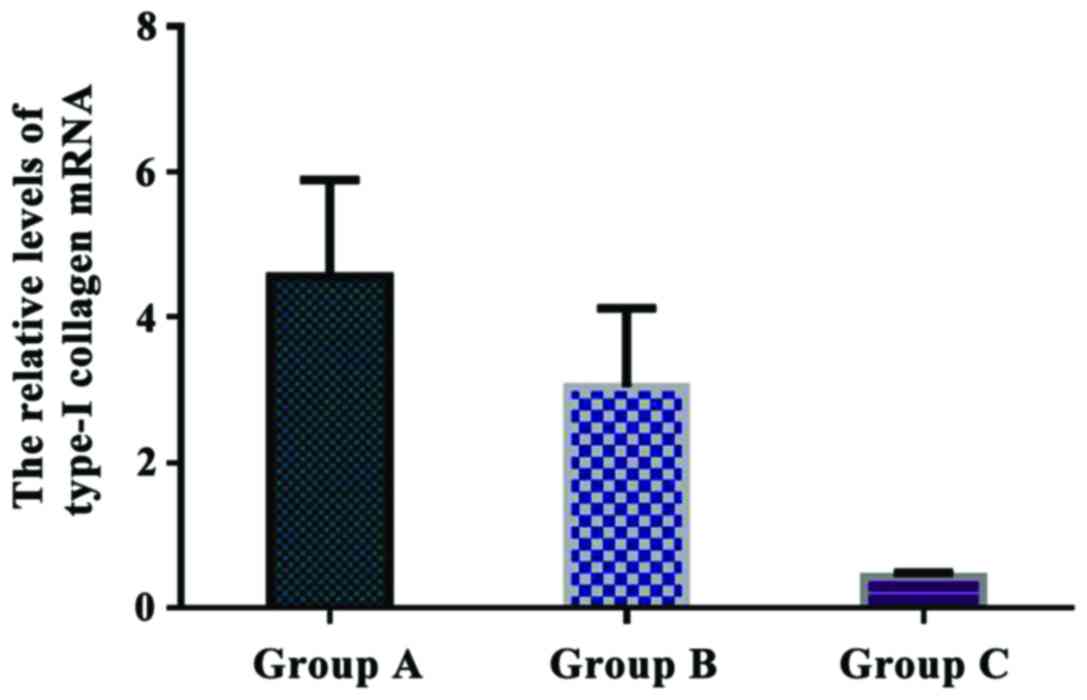
Comparison of the relative levels of
type-I collagen mRNA in each group
The relative levels of type-I collagen mRNA were
4.561±1.327 in group A, 3.046±1.075 in group B, and 0.432±0.058 in
group C. The relative expression of type-I collagen mRNA in group A
was significantly higher than that in group B and C (P<0.05).
Additionally, there was a significant difference in type-I collagen
mRNA expression between group B and C (P<0.05) (Fig. 7).
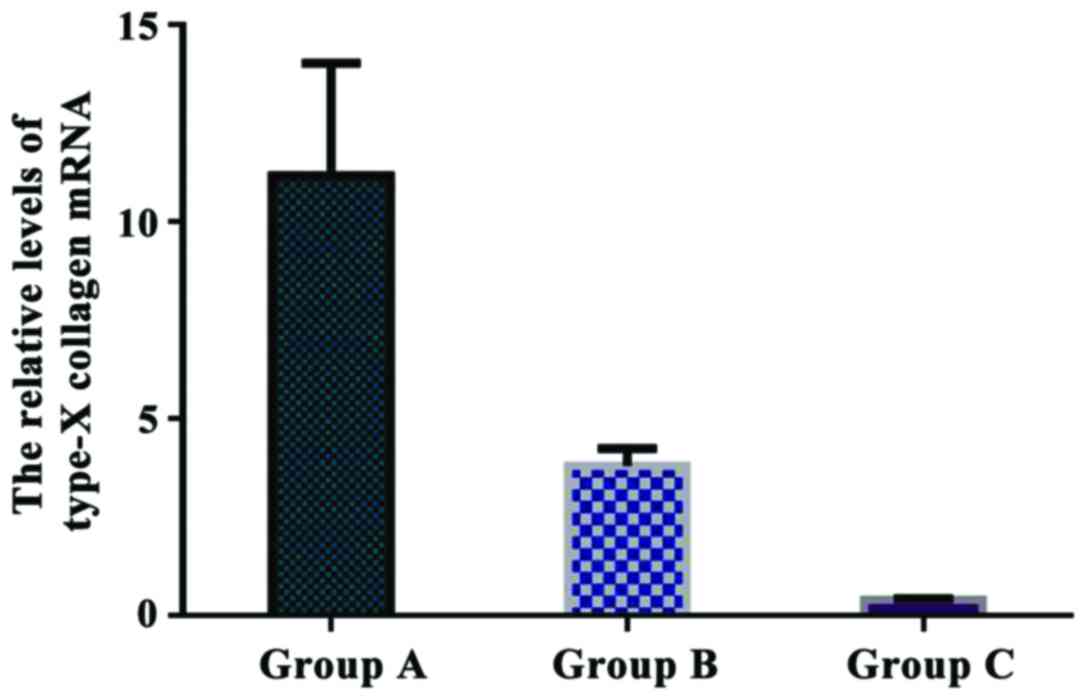
Comparison of the relative levels of
type-X collagen mRNA in each group
The relative levels of type-X collagen mRNA were
11.172±2.845 in group A, 3.801±0.446 in group B, and 0.403±0.027 in
group C. The relative expression of type-X collagen mRNA in group A
was significantly higher than that in group B and C (P<0.05).
There was a significant difference in type-X collagen expression
between group B and C (P<0.05) (Fig.
8).
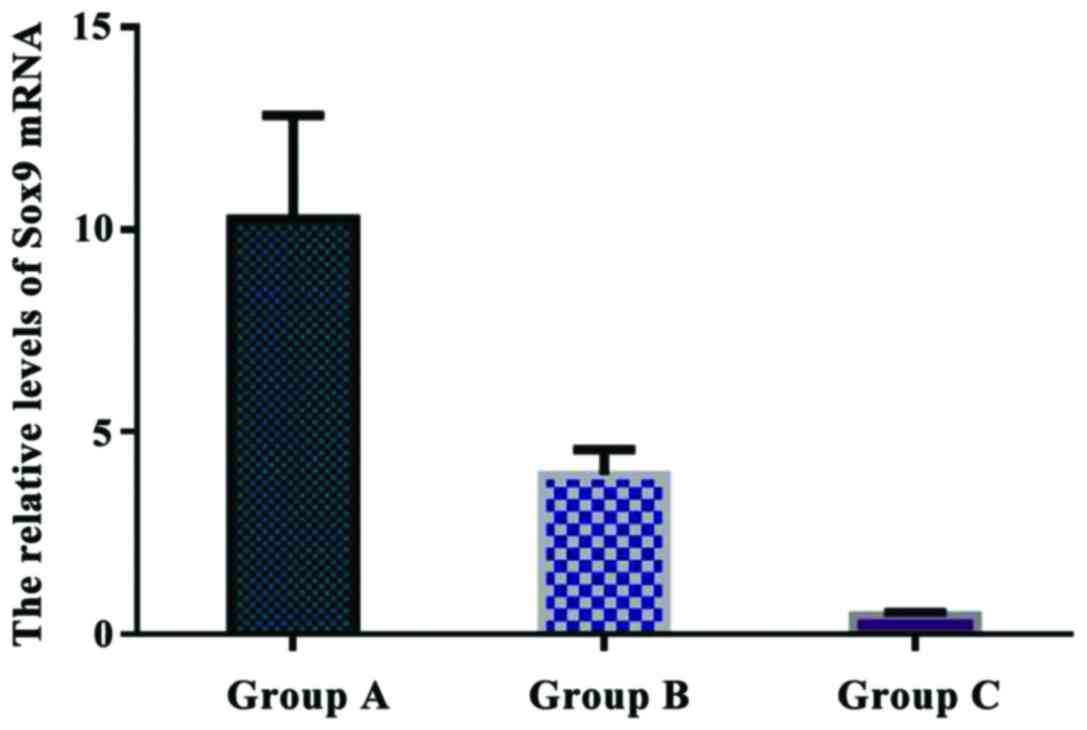
Comparison of the relative levels of
Sox9 mRNA in each group
The relative levels of Sox9 mRNA were 10.263±2.551
in group A, 3.942±0.628 in group B, and 0.477±0.085 in group C. The
relative expression of Sox9 mRNA in group A was significantly
higher than that in group B and C (P<0.05). There was a
significant difference in Sox9 mRNA expression between group B and
C (P<0.05) (Fig. 9).
Discussion
Collagen hydrogel is a form of hydrogel that is
sensitive to temperature. It is a form of collagen fiber formed by
continuous aggregation of collagen molecules of a neutral collagen
solution under heating conditions or at 37°C (7–9). Related
studies showed that (10) collagen
concentration is positively correlated with collagen fiber density,
which is an important factor for several physical and chemical
indexes, such as the mechanical properties and water absorption of
collagen gel. Related data showed that (11–13)
collagen molecules have biological activity, and can actively
participate in signal transduction and cellular behavior at the
molecular level. Collagen concentration in the collagen hydrogel
therefore plays an important role in its biological function. The
biological functions of collagen hydrogels containing different
concentrations of collagen may be different (12,14,15).
Herein, we aimed to investigate the effects of type-I collagen
hydrogel of different concentrations on the growth and
differentiation of rabbit chondrocytes.
Related data showed that (16) collagen phenotype plays a highly
important role in maintaining the normal structure of cartilage. At
different stages of chondrocyte differentiation, the expression of
collagen phenotype is different. In the process of differentiation,
articular cartilage and epiphyseal cartilage mainly synthesize
type-II, VI, IX, and XI collagen, whereas hypertrophic chondrocytes
specifically synthesize type-X and II collagen. Type-X and I
collagen are synthesized in the process from chondrocyte
hypertrophy to differentiation into osteoblast-like cells (17). Type-II collagen and Aggrecan are
markers of chondrocytes (18,19). The
type-I collagen gene is expressed by fibrous tissues, and can be
used to determine whether chondrocytes are differentiated into
fibrous tissues (20–22). Sox9 also belongs to the group of
chondrocyte fibrosis-related genes, and the type-X collagen gene is
an important marker of hypertrophic chondrocytes (23,24).
This study showed that the levels of Aggrecan and type-II collagen
mRNA in groups A, B, and C were not significantly different after
in vitro culture for 2 weeks (P>0.05). The levels of
type-I collagen, type-X collagen, and Sox9 mRNA in group A were
significantly higher than those in group B and C (P<0.05). The
levels of type-I collagen, type-X collagen, and Sox9 mRNA were
upregulated with increasing concentration of type-I collagen,
suggesting that the increase of type-I collagen concentration may
induce chondrocyte fibrosis and hypertrophy, thus affecting the
formation of normal cartilage tissue. H&E staining showed that
chondrocytes were aggregated significantly in group A, some
chondrocytes showed proliferation and aggregation in group B, and
the distribution of chondrocytes was relatively uniform in group C,
indicating that cell differentiation had occurred.
In conclusion, high concentration of type-I collagen
hydrogel can promote chondrocyte fibrosis and upregulation of the
expression of type-I collagen, type-X collagen, and Sox9 mRNA. High
concentration type-I collagen hydrogel can result in hypertrophy of
articular chondrocytes in a premature manner, which may promote the
development of articular cartilage to osteoarthritis.
References
|
1
|
Mahapatra C, Jin GZ and Kim HW:
Alginate-hyaluronic acid-collagen composite hydrogel favorable for
the culture of chondrocytes and their phenotype maintenance. Tissue
Eng Regen Med. 13:538–546. 2016. View Article : Google Scholar
|
|
2
|
Foldager CB, Pedersen M, Ringgaard S,
Bünger C and Lind M: Chondrocyte gene expression is affected by
very small iron oxide particles-labeling in long-term in vitro MRI
tracking. J Magn Reson Imaging. 33:724–730. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ge D, Zhang QS, Zabaleta J, Zhang Q, Liu
S, Reiser B, Bunnell BA, Braun SE, O'Brien MJ, Savoie FH, et al:
Doublecortin may play a role in defining chondrocyte phenotype. Int
J Mol Sci. 15:6941–6960. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsai WB, Chen CH, Chen JF and Chang KY:
The effects of types of degradable polymers on porcine chondrocyte
adhesion, proliferation and gene expression. J Mater Sci Mater Med.
17:337–343. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xin W, Heilig J, Paulsson M, Zaucke F and
Collagen II: Regulates integrin expression profile and chondrocyte
differentiation. Connect Tissue Res. 56:307–314. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gurusinghe S, Young P, Michelsen J and
Strappe P: Suppression of dedifferentiation and hypertrophy in
canine chondrocytes through lentiviral vector expression of Sox9
and induced pluripotency stem cell factors. Biotechnol Lett.
37:1495–1504. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dou Y, Li N, Zheng Y and Ge Z: Effects of
fluctuant magnesium concentration on phenotype of the primary
chondrocytes. J Biomed Mater Res A. 102:4455–4463. 2014.PubMed/NCBI
|
|
8
|
Yu L, Ferlin KM, Nguyen BN and Fisher JP:
Tubular perfusion system for chondrocyte culture and szp
expression. J Biomed Mater Res A. 103:1864–1874. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ko AR, Huh YH, Lee HC, Song WK, Lee YS and
Chun JS: Identification and characterization of arginase II as a
chondrocyte phenotype-specific gene. IUBMB Life. 58:597–605. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Duval E, Leclercq S, Elissalde JM, Demoor
M, Galéra P and Boumédiene K: Hypoxia-inducible factor 1alpha
inhibits the fibroblast-like markers type I and type III collagen
during hypoxia-induced chondrocyte redifferentiation: Hypoxia not
only induces type II collagen and aggrecan, but it also inhibits
type I and type III collagen in the hypoxia-inducible factor
1alpha-dependent redifferentiation of chondrocytes. Arthritis
Rheum. 60:3038–3048. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Johnson JS, Morscher MA, Jones KC, Moen
SM, Klonk CJ, Jacquet R and Landis WJ: Gene expression differences
between ruptured anterior cruciate ligaments in young male and
female subjects. J Bone Joint Surg Am. 97:71–79. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kontturi LS, Järvinen E, Muhonen V, Collin
EC, Pandit AS, Kiviranta I, Yliperttula M and Urtti A: An
injectable, in situ forming type II collagen/hyaluronic acid
hydrogel vehicle for chondrocyte delivery in cartilage tissue
engineering. Drug Deliv Transl Res. 4:149–158. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Heo J, Koh RH, Shim W, Kim HD, Yim HG and
Hwang NS: Riboflavin-induced photo-crosslinking of collagen
hydrogel and its application in meniscus tissue engineering. Drug
Deliv Transl Res. 6:148–158. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Park H, Guo X, Temenoff JS, Tabata Y,
Caplan AI, Kasper FK and Mikos AG: Effect of swelling ratio of
injectable hydrogel composites on chondrogenic differentiation of
encapsulated rabbit marrow mesenchymal stem cells in vitro.
Biomacromolecules. 10:541–546. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lien SM, Ko LY and Huang TJ: Effect of
pore size on ECM secretion and cell growth in gelatin scaffold for
articular cartilage tissue engineering. Acta Biomater. 5:670–679.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Galván JA, García-Martínez J,
Vázquez-Villa F, García-Ocaña M, García-Pravia C,
Menéndez-Rodríguez P, González-del Rey C, Barneo-Serra L and de los
Toyos JR: Validation of COL11A1/procollagen 11A1 expression in
TGF-β1-activated immortalised human mesenchymal cells and in
stromal cells of human colon adenocarcinoma. BMC Cancer.
14:8672014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Faikrua A, Wittaya-areekul S, Oonkhanond B
and Viyoch J: A thermosensitive chitosan/corn starch/β-glycerol
phosphate hydrogel containing TGF-β1 promotes differentiation of
MSCs into chondrocyte-like cells. Tissue Eng Regen Med. 11:355–361.
2014. View Article : Google Scholar
|
|
18
|
Jin R, Teixeira LS Moreira, Krouwels A,
Dijkstra PJ, van Blitterswijk CA, Karperien M and Feijen J:
Synthesis and characterization of hyaluronic acid-poly(ethylene
glycol) hydrogels via Michael addition: An injectable biomaterial
for cartilage repair. Acta Biomater. 6:1968–1977. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schindler OS: Current concepts of
articular cartilage repair. Acta Orthop Belg. 77:709–726.
2011.PubMed/NCBI
|
|
20
|
Li P, Wei X, Guan Y, Chen Q, Zhao T, Sun C
and Wei L: MicroRNA-1 regulates chondrocyte phenotype by repressing
histone deacetylase 4 during growth plate development. FASEB J.
28:3930–3941. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tew SR, Li Y, Pothacharoen P, Tweats LM,
Hawkins RE and Hardingham TE: Retroviral transduction with SOX9
enhances re-expression of the chondrocyte phenotype in passaged
osteoarthritic human articular chondrocytes. Osteoarthritis
Cartilage. 13:80–89. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Darling EM and Athanasiou KA: Retaining
zonal chondrocyte phenotype by means of novel growth environments.
Tissue Eng. 11:395–403. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang M and Wang J: Epigenetic regulation
of gene expression in osteoarthritis. Genes Dis. 2:69–75. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Balakrishnan B and Banerjee R:
Biopolymer-based hydrogels for cartilage tissue engineering. Chem
Rev. 111:4453–4474. 2011. View Article : Google Scholar : PubMed/NCBI
|