Introduction
Chiari malformation (CM) is a hindbrain disorder
that is associated with deformity and elongation of the cerebellar
tonsils. It is specifically characterized by the descent of the
cerebellar tonsils >5 mm below the foramen magnum into the
spinal canal (1,2). CM can be divided into four types, among
which CM type I (CM-I) is the most common. Although CM-I occurs in
pediatric and adult patients, the prevalence of CM-I is not well
defined (2). CM-I often becomes
clinically apparent when the patient is aged 20–39. Thus, in the
past, CM-I had been diagnosed primarily during adolescence or
adulthood (2,3). With the advent of magnetic resonance
imaging (MRI), however, the number of pediatric patients diagnosed
with CM-I is increasing (4).
Previous studies have described the clinical features of CM-I in
pediatric populations (2,5,6).
CM-I is usually associated with ventricular
dilation, syringomyelia and scoliosis, but the relevant
pathogenesis is not clearly understood. Moreover, the incidence of
these conditions co-presenting with CM-I has varied considerably in
previous studies (7–10).
Management of CM-I and concomitant syringomyelia and
scoliosis in children remains controversial. Some authors have
recommended syringoperitoneal shunting for treating syringomyelia
(11,12). The treatment of scoliosis consists of
serial observation, bracing and/or corrective spinal surgery. It is
yet to be established whether aggressive surgical treatment is
necessary, and the ideal therapeutic option is undetermined
(13). It is widely accepted,
however, that posterior fossa decompression should be included in
the treatment protocol for CM-I as it can lead to both clinical and
radiological improvement (8,14,15). The
use of specific surgical procedures and related issues, however,
remain controversial; these include the use of craniectomy, the
size of the bone window, whether the arachnoid should be opened and
whether a cerebellar tonsil should be manipulated (14,15).
The current study evaluated a consecutive case
series of 92 children who were diagnosed with CM-I. Their clinical
manifestations, concomitant ventricular dilation, syringomyelia,
basilar invagination, platybasia and scoliosis were analyzed. The
study also evaluated the surgical outcomes of small-bone-window
posterior fossa decompression (SPFD) with autologous-fascia
duraplasty (AFD) in the treatment of pediatric CM-I.
Patients and methods
Patient recruitment
A search of medical records was conducted to
identify pediatric patients (aged 0–18 years at their initial
presentation to the neurosurgery clinic) with CM-I who had been
operated on in the Department of Neurosurgery at Beijing Tiantan
Hospital, Capital Medical University (Beijing, China). The cohort
included 92 children (female:male ratio, 1.00:1.42; mean age ±
standard deviation, 10.0±4.5) with congenital CM-I who were treated
with SPFD + AFD between January 2001 and January 2015. CM-I was
diagnosed according to the following criteria (1): i) Herniation of tonsils ≥5 mm was below
the plane of the foramen magnum; or ii) tonsillar herniation of 3–5
mm accompanied by other CM-I features, such as syringomyelia.
Exclusion criteria were as follows: i) Occipitocervical
instability; ii) congenital spinal bifida; iii) diastematomyelia;
iv) concurrent intraspinal tumors or myelitis; v) acquired CM-I as
a complication of cerebrospinal fluid (CSF) diversion procedures or
chronic CSF leakage; and vi) incomplete data. The study protocol
was approved by the Institutional Review Board and Ethics Committee
of Beijing Tiantan Hospital, Capital Medical University.
Clinical presentation
Subjective clinical symptoms and physical
examinations were documented. Symptoms recorded included headache,
foramen magnum nerve compression symptoms (occipitocervical
headaches, neck or shoulder pain), sensory disturbance, motor
dysfunction (weakness or muscle atrophy), lower cranial nerve
dysfunction (dysphagia, hoarseness or coughing), cerebellar
syndrome (truncal and appendicular ataxia) and scoliosis. Some
patients also had bradycardia. Following the exclusion of endocrine
dysfunction and organic heart disease by comprehensive evaluations,
including laboratory, electrophysiological and radiological
examinations, these symptoms were suspected to be associated with
CM-I or with concomitant syringomyelia.
Imaging
Preoperative plain radiographs and perioperative MRI
scans of the cervical spine were available for all patients. The
descending distance between the inferior pole of the cerebellar
tonsil and the level of the foramen magnum was measured on sagittal
T1-weighted images in a picture archiving and communication system
workstation (OsiriX software; version 6.0; Pixmeo SARL, Bernex,
Switzerland). The degree of cerebellar descent was classified into
three groups according to the cerebellar tonsillar descent (CTD)
grading system (16): Grade I, the
tonsil descends over the foramen magnum but does not reach the C1
arch; Grade II, the tonsil descends to the C1 arch level; and Grade
III, the tonsil descends below the C1 arch. The location of the
syringomyelia was determined on sagittal MRI images, and its size
was determined by measuring the longitudinal and transverse
diameters.
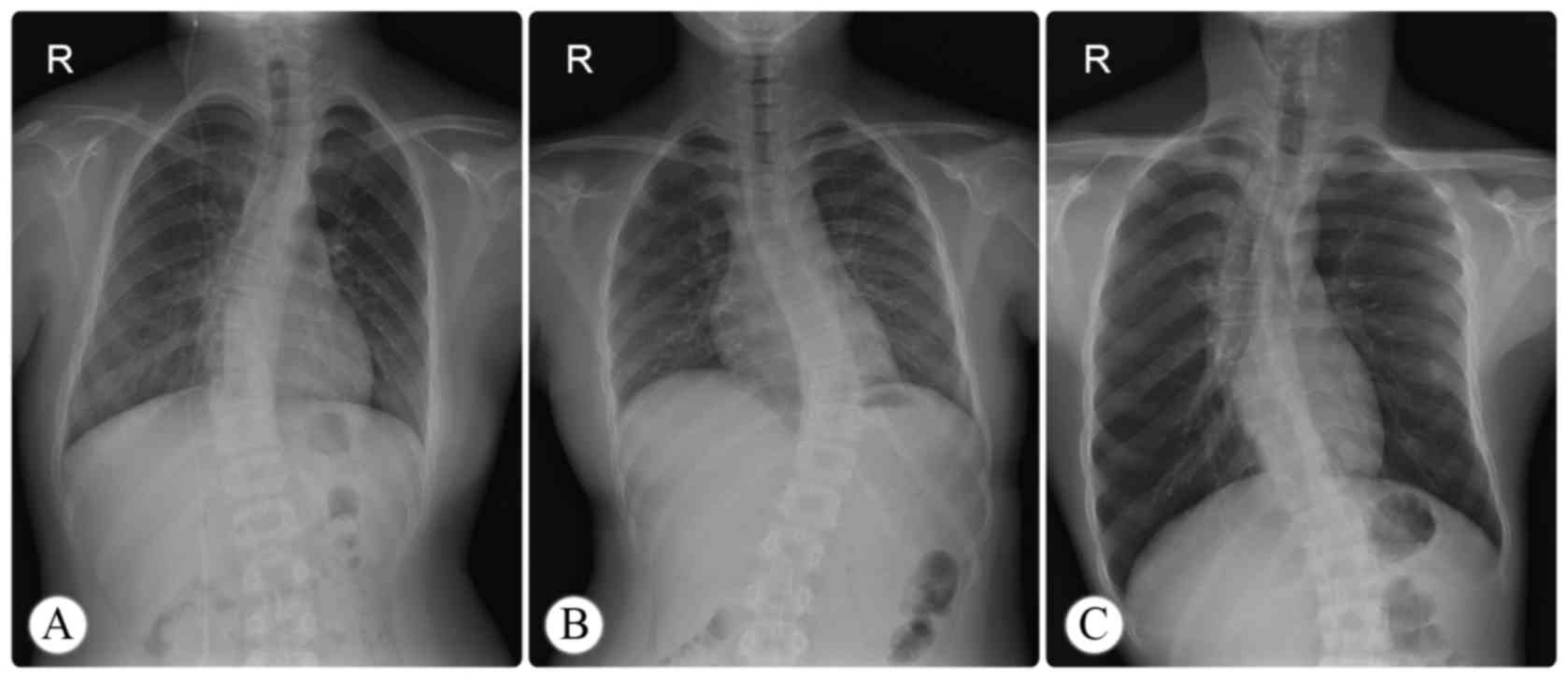
The data for concomitant scoliosis were collected
from standing posteroanterior radiographs (Fig. 1), and coronal curvature measurements
were performed according to the Cobb method (17). Scoliosis was defined as a Cobb's
angle of ≥10°. Curve severity was defined as follows: mild, 10–25°;
moderate, 26–40°; severe, ≥41°. In cases of S-shaped scoliosis, the
major curve was selected for analysis and the minor curves were
considered compensatory.
Other associated abnormalities were also recorded,
such as ventricular dilation, platybasia and basilar invagination.
Ventricular dilation was defined by an Evans' index of >0.30
(18).
Surgical approach
Surgery was performed on all children with a
combined strategy that included SPFD and AFD. After patients were
positioned left-laterally, a posterior midline skin incision was
made from 1 cm below the inion to the spinous process of C4. An
autologous graft (2×2 cm) was resected from the fascia and
reserved. The SPFD procedure, which included a small-bone-window
suboccipital craniectomy (diameter, 2.0–2.5 cm) and C-1 laminectomy
(1.5 cm), was performed. The thick constraining dural band found at
the occipitocervical junction was resected. When the dura mater was
opened, the surgeon aimed to keep the arachnoid intact. The dura
mater was subsequently grafted with the autologous graft to enlarge
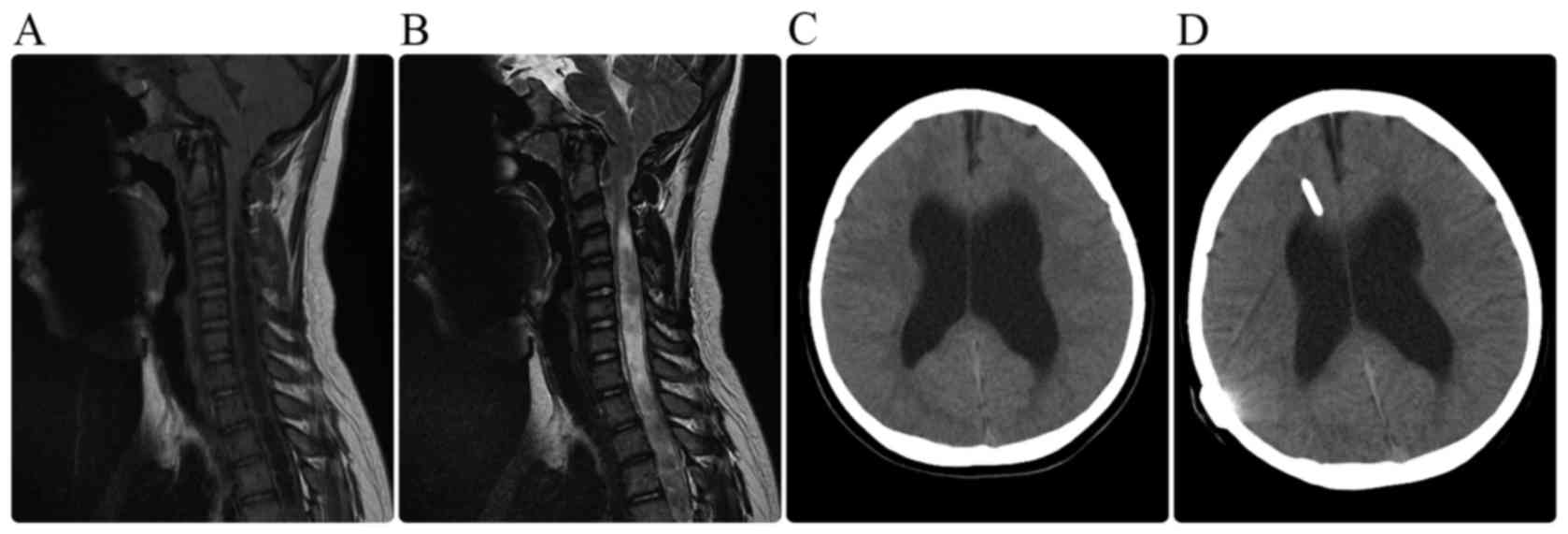
the cisterna magna. Six children presented with progressive
hydrocephalus and severe intracranial hypertension, and an
emergency ventriculoperitoneal shunt was implanted in these cases
(Fig. 2).
Follow-up
Follow-up data for all children were available in
their records. The data had been obtained during individual office
visits or telephone interviews, with a mean follow-up time of 88.6
months (standard deviation, 46.2; range, 10–166 months). Clinical
outcomes were qualitatively categorized as follows: i) Improved,
the patients experienced partial or complete relief of their chief
complaints; or ii) not improved, no notable change in their chief
complaints, or deterioration of their clinical status. Furthermore,
the clinical outcomes were quantitatively evaluated according to
the Chicago Chiari Outcome Scale (CCOS; Table I) (19).
 | Table I.Chicago Chiari outcome scale. |
Table I.
Chicago Chiari outcome scale.
|
| Score |
|---|
|
|
|
|---|
| Characteristic | 1 | 2 | 3 | 4 |
|---|
| Pain | Worse | Unchanged and
refractory to medication | Improved or
controlled with medication | Resolved |
| Non-pain | Worse | Unchanged or
improved but impaired | Improved and
unimpaired | Resolved |
| Functionality | Unable to
attend | Moderate impairment
(<50% attendance) | Mild impairment
(>50% attendance) | Fully
functional |
| Complication | Persistent
complication, poorly controlled | Persistent
complication, well-controlled | Transient
complication | Uncomplicated
course |
Statistical analysis
SPSS 22.0 software (IBM SPSS, Armonk, NY, USA) was
used for statistical analyses. Chi-squared test, continuity
correction test or Fisher's exact probability test were used to
screen the potential risk factors, including age at onset, age at
diagnosis, sex, duration of symptoms, CTD grades, concomitant
syringomyelia, hydrocephalus, basilar invagination, platybasia and
scoliosis. Logistic regression analysis was performed to identify
the risk factors for clinical outcomes (binary-classification
qualitative data). Furthermore, to verify the association between
potential risk factors and clinical outcomes, CCOS scores
(continuous variables) were compared using one-way analysis of
variance (ANOVA) with a Bonferroni post hoc test test for
multi-group comparisons and Mann-Whitney U test for two-group
comparison. P≤0.05 was considered to indicate a statistically
significant significance.
Results
Patient population and clinical
characteristics
Over the 14-year span of this study, 98 pediatric
patients were diagnosed with CM-I and underwent SPFD + AFD. Six
patients were lost to follow-up and excluded. This case series
consisted of 38 females and 54 males, with a female:male ratio of
1.00:1.42. The average age at the time of surgery was 10.0±4.5
years (range, 1–18 years). In total, 11 (12.0%) children were
asymptomatic. In the symptomatic group (n=81), the duration of
symptoms preceding the initial diagnosis ranged from 2 weeks to 4
years (mean, 16.8 months). A total of 59 children presented with
headache (72.8% of all symptomatic children), 17 with foramen
magnum nerve compression symptoms (21.0%), 29 with sensory
disturbance (35.8%), 36 with motor dysfunction (44.4%), 7 with
lower cranial nerve dysfunction (8.6%) and 16 with cerebellar
syndrome (19.8%). In addition, the results of electrocardiography,
which is performed routinely upon admission, indicated that 4
patients had atrioventricular block (3 patients with second-degree
and 1 patient with third-degree block), with no relevant underlying
disease detected. None of the children complained of bowel or
bladder dysfunction.
Preoperative MRI
The distance of tonsillar herniation ranged from 6.0
to 18.4 mm (mean, 11.2±3.2 mm). On the preoperative MRI, platybasia
was observed in 5 children (5.4%) and basilar invagination in 12
children (13.0%). Associated ventricular dilation was observed in
24 children (26.1%). Concomitant syringomyelia was observed in 72
children (78.3%): Cervical syringomyelia (n=4; 5.6% of concomitant
syringomyelia cases), cervicothoracic syringomyelia (n=67; 93.0%)
and holocord syringomyelia (n=1; 1.4%).
Scoliosis evaluation
A total of 44 children (47.8%; 19 females and 25
males) showed scoliosis on plain films: 35 children had C-shaped
thoracic scoliosis in a single curve; 8 children had S-shaped
thoracic scoliosis with two adjacent curves; and 1 child had
S-shaped thoracolumbar scoliosis. According to the severity
classification, 26 children were diagnosed with mild scoliosis, 12
with moderate scoliosis and 6 with severe scoliosis. Further
analysis indicated that all children with scoliosis had concomitant
syringomyelia. In total, 36 (81.8%) of the children with scoliosis
also had a unilateral sensory disturbance and/or motor disturbance
or unilateral muscle atrophy. These data are summarized in Table II.
 | Table II.Characteristics of coexisting
scoliosis. |
Table II.
Characteristics of coexisting
scoliosis.
| Characteristic | Number | Percentage (%) |
|---|
| Sex |
|
|
|
Male | 25 | 56.8 |
|
Female | 19 | 43.2 |
| Curve type |
|
|
|
C-shaped thoracic
scoliosis | 35 | 79.5 |
|
S-shaped thoracic
scoliosis | 8 | 18.2 |
|
S-shaped thoracolumbar
scoliosis | 1 | 2.3 |
| Curve
orientation |
|
|
| Convex
to the left | 20 | 45.5 |
| Convex
to the right | 24 | 54.5 |
| Severity |
|
|
| Mild
(10–25°) | 26 | 59.1 |
|
Moderate (26–40°) | 12 | 27.3 |
| Severe
(>41°) | 6 | 13.6 |
| Total | 44 | 100 |
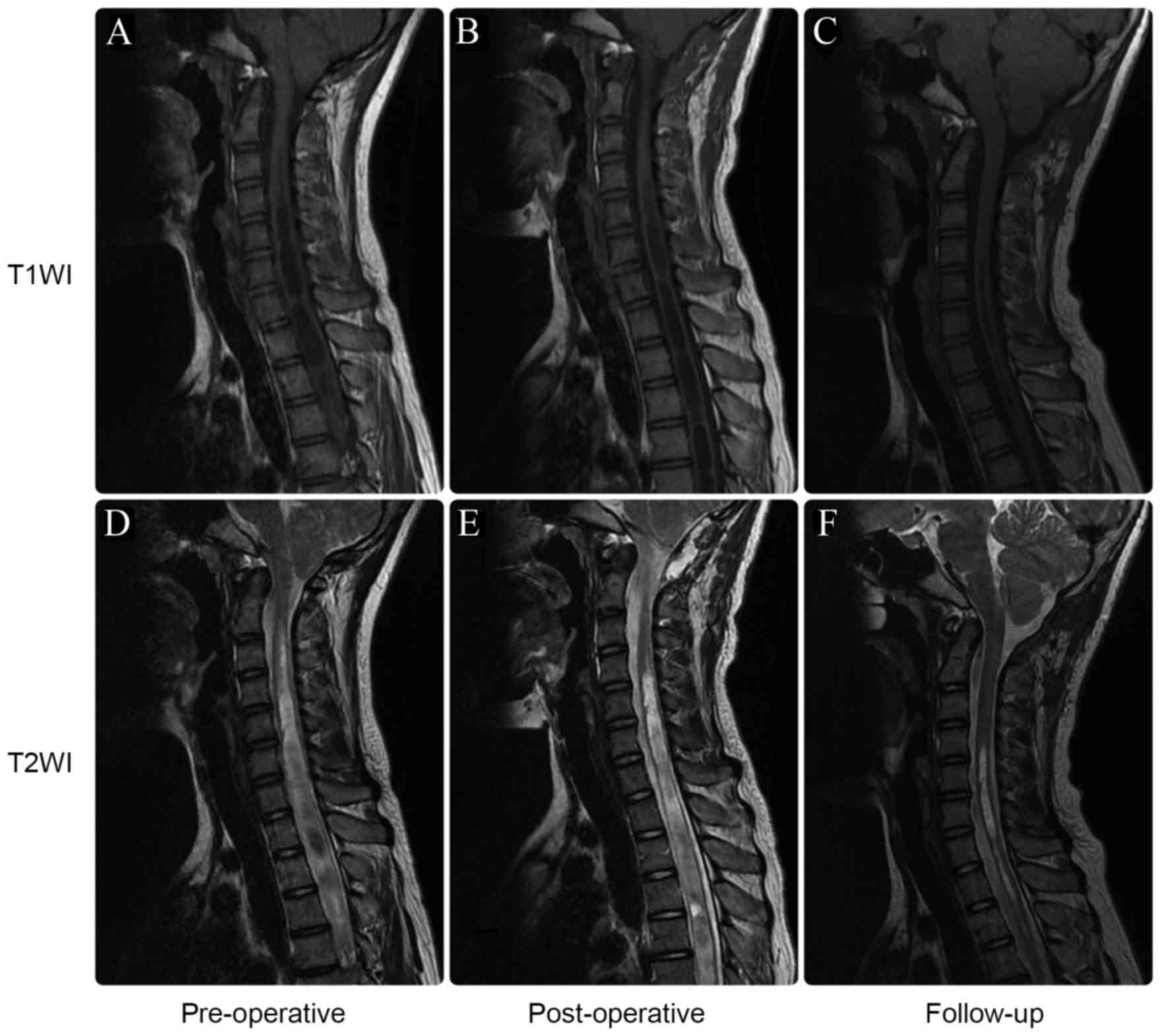
Complications and follow-up
There were no deaths in this series. Central nervous
system infection occurred postoperatively in four children.
Antibiotics were prescribed and the infections were effectively
treated. Suboccipital hydrops was noted in two children (Fig. 3). During the follow-up period,
symptoms were alleviated in 66 patients (81.5% of all the
symptomatic children), remained unchanged in 12 patients (14.8%)
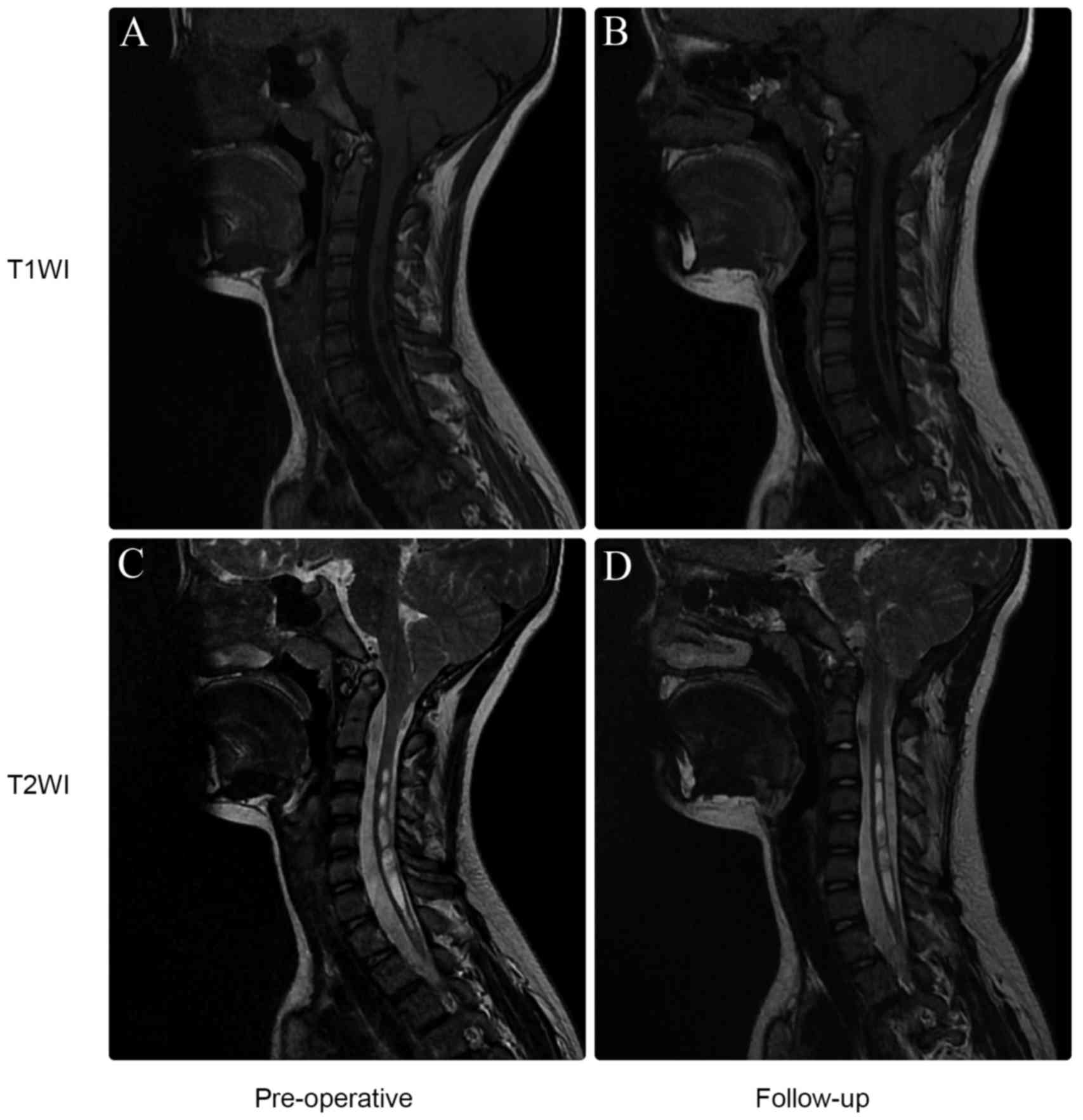
and progressed in 3 patients (3.7%). Preoperatively, 72 patients
had concomitant syringomyelia. According to follow-up MRI scans,
syringomyelia was absent or markedly reduced in 56 of these
patients (77.8%; Fig. 4). Analysis
of these results using Chi-squared tests indicated that CTD grades
and the incidence of basilar invagination (Fig. 5) and platybasia were associated with
clinical outcomes (P<0.05); the statistical data are summarized
in Table III. Logistic regression
analysis indicated that a high CTD grade [P=0.016; odds ratio
(OR)=3.675; 95% confidence interval (CI), 1.138–8.527], basilar
invagination (P=0.008; OR=3.489; 95% CI, 1.048–10.754) or
platybasia (P=0.002; OR=3.981; 95% CI, 1.654–9.386) significantly
increased the likelihood of poor clinical outcomes. A comparison of
CCOS scores by ANOVA also indicated that CTD grade, and basilar
invagination and platybasia by Mann-Whitney U tests are predictors
of poor clinical prognosis (P<0.05; Table IV).
 | Table III.Results of Chi-squared tests. |
Table III.
Results of Chi-squared tests.
| Characteristic | Chi-square
value | P-value |
|---|
| Age at onset
(<10 vs. >10 years) | 0.305 | 0.581 |
| Age at diagnosis
(<10 vs. >10 years) | 0.007 | 0.932 |
| Sex (male vs.
female) | 0.268 | 0.605 |
| Duration (<16.8
vs. >16.8 months) | 1.522 | 0.217 |
| CTD (Grade
I–III) | 14.224 | 0.001 |
| Syringomyelia
(present vs. absent) | 0.683a | 0.408a |
| Hydrocephalus
(present vs. absent) | 1.658a | 0.198a |
| Basilar
invagination (present vs. absent) | 11.864a | 0.001a |
| Platybasia (present
vs. absent) | – | 0.004b |
| Scoliosis (present
vs. absent) | 1.897 | 0.168 |
 | Table IV.Results of statistical analyses. |
Table IV.
Results of statistical analyses.
|
| CTD (Grade
I–III) | Basilar
invagination (present vs. absent) | Platybasia (present
vs. absent) |
|---|
|
|
|
|
|
|---|
| Clinical CCOS
assessment | F value | P-value | Z value | P-value | Z value | P-value |
|---|
| Composite CCOS
score | 3.580 | 0.032 | −4.927 | 0.000 | −3.601 | 0.000 |
| Pain subscore | 0.139 | 0.871 | −6.215 | 0.000 | −3.996 | 0.000 |
| Non-pain
subscore | 3.459 | 0.036 | −3.629 | 0.000 | −3.451 | 0.001 |
| Functionality
subscore | 3.942 | 0.023 | −3.578 | 0.000 | −4.125 | 0.000 |
| Complications
subscore | 0.984 | 0.378 | −1.177 | 0.239 | −1.573 | 0.116 |
Discussion
CM-I is a craniocervical junction disorder
characterized by a cerebellar tonsil descending below the foramen
magnum into the spinal canal. This was first described by Hans
Chiari in 1891 (1). The incidence of
CM-I has been reported to be 0.5–3.5% in the general population
(20). A previous study reported a
slight female predominance (female/male ratio 1.3:1.0) (20), although this is inconsistent with the
findings in the current study (female/male ratio 1.00:1.52). CM-I
is generally considered a congenital neurological condition,
although in recent years its acquired form has been identified as a
complication of CSF diversion procedures or chronic CSF leakage
(21,22).
CM-I is thought to be a multifactorial condition,
although the pathogenesis is still undetermined. A well-accepted
hypothesis is that the hindbrain tissues are dislocated into the
spinal canal because of an overcrowded posterior cranial fossa, due
to underdevelopment of the mesodermal occipital somite (23). Previous morphometric results have
supported this postulation. The posterior fossa volume in CM-I
patients was reported to be smaller compared with the control
group, although the difference was not statistically significant
(23,24). In addition, some familial cases
suggest a genetic component (25).
The incidence of syringomyelia in CM-I patients has
ranged widely in previous reports, from 30 to 70% (20). The exact prevalence of syringomyelia
in pediatric patients remains unclear. In the current study, a high
syringomyelia occurrence rate (78.3%) was observed in CM-I
patients. There are several hypotheses concerning the development
of syringomyelia in the CM-I context, including the ‘water-hammer’
mechanism proposed by Gardner (26)
and the ‘pressure dissociation’ mechanism proposed by Williams
(27). However, most authors agree
that partial obstruction in the foramen magnum area, which blocks
the normal circulation of CSF, is the pacing factor in the
development of syringomyelia (28).
Diagnosis of CM-I depends on MRI (29). For a long time, the diagnostic
criteria have been under debate. Barkovich et al (30) proposed that 3 mm below the foramen
magnum was the lowest extent of tonsillar descent in normal
patients, and defined CM-I as >3 mm of tonsillar descent. Others
have proposed that 5 mm of tonsillar ectopia should be adopted as
the cutoff for diagnosing CM-I (2).
In addition, Mikulis et al (31) reported that age affects the normal
position of cerebellar tonsils and proposed age-based diagnostic
criteria. These were as follows: In patients aged 0–9, the cutoff
distance for tonsillar descent should be 6 mm; in patients aged
10–29, it should be 5 mm; in patients aged 30–79 years it should be
4 mm; and at >79 years of age, it should be 3 mm (31). Considering the fact that patients
with lesser degrees of tonsillar ectopia (3–5 mm) may also develop
classic neurological symptoms and syringomyelia that are amenable
to neurosurgical intervention, the current study adopted the more
flexible comprehensive diagnostic criteria proposed by Tubbs et
al (1).
Patients with CM-I can be asymptomatic or can
present with a variety of signs and symptoms ranging from headache
to severe myelopathy and brain stem compression (29). With the increasing availability of
diagnostic MRI, more asymptomatic patients are being identified
(4). In the adult population, 15–30%
of CM-I patients are asymptomatic (20). In the current study, 11 (12.0%)
asymptomatic children were identified. Innate bias, however, is
inevitable in an in-hospital cohort. Sleep apnea and feeding
problems are reported to be more common in pediatric patients,
although other clinical manifestations appear to be similar in
different age groups (6). As
previously reported, the most common presenting symptom of CM-I is
headache (4), which is consistent
with the current findings. In infants and children who are unable
to communicate verbally, headaches may manifest as crying and
irritability. Other common symptoms include non-radicular pain in
the shoulder, back and extremities, motor and sensory disturbances,
clumsiness, ataxia and lower cranial nerve dysfunction (29). In the current cohort, two patients
with atrioventricular block were also identified.
There is no effective non-surgical strategy for
treating patients with CM-I (12).
Posterior fossa decompression, with or without dural opening, and
with or without arachnoid opening or dissection, is most commonly
used for the surgical treatment of CM-I and can change its clinical
course (13,32). However, the size of the bone window
required for posterior fossa decompression is still under debate
(33,34). Furthermore, it has not yet been
established whether a difference exists in the operative techniques
used for pediatric and adult patients. Large-bone-window posterior
fossa decompression effectively enlarges the posterior cranial
fossa and therefore relieves symptoms (35). However, there may be a higher
incidence of complications, such as CSF leak, pseudocysts,
meningitis and hydrocephalus (35).
Furthermore, excessive removal of the squamous part of the
occipital bone could lead to downward and backward displacement of
the cerebellum and brain stem, leading to long-term complications.
According to a previous study, small-bone-window craniectomy is
enough to achieve favorable clinical outcomes, and the incidence of
complications is reduced (35). The
procedure described in the aforementioned study relieves the
compression of the occipitocervical bones and preserves enough bone
to support the cerebellum and brain stem. In the current study, all
patients underwent a small-bone-window approach, with which high
rates of clinical (81.5%) and radiological (77.8%) improvement were
achieved.
Whether it is necessary to open the arachnoid space
is another focus of controversy. Some authors assert that
CSF-related complications, such as CSF leak or meningitis, are
directly associated with opening the arachnoid (36). A previous study has indicated that
the incidence of CSF leaks is not higher in the arachnoid-opening
group, provided that watertight duraplasty is performed (37). Furthermore, reconstruction of the
cisterna magna and tonsillar manipulation could contribute to a
better prognosis (37). It is the
current authors’ opinion that arachnoid opening is unnecessary in
most patients; SPFD and duraplasty without arachnoid incision is
sufficient to relieve compression and alleviate clinical symptoms.
It should be noted that the incidence of CSF-related complications
was low in the pediatric cohort of the current study.
The prevalence of scoliosis in the general pediatric
population is 2–4%, while in children with CM-I the prevalence of
scoliosis is >4-fold increase (9,38).
Progressive scoliosis is a relatively common manifestation of CM-I
when there is coexistent syringomyelia (38). In the current study, the incidence of
scoliosis was markedly higher (47.8%) in CM-I patients compared
with the reported prevalence in the general pediatric population.
Nokes et al (39) identified
3 patients with CM-I and scoliosis, but without syringomyelia. In
the current cohort, all scoliotic children had coexistent
syringomyelia. Considering the relatively mild severity of
pediatric scoliosis, spinal orthopedic surgery was not recommended
for the majority of these patients. It was considered that brace
treatment could effectively reverse scoliotic progression. Eule
et al (40) conducted a
20-year review of surgical and nonsurgical treatment in a pediatric
cohort with CM-I associated with syringomyelia and scoliosis. It
was found that early decompression of CM-I helped stabilize or
alleviate the associated scoliosis, avoiding the requirement for
orthopedic spinal surgery.
The current study had several limitations.
Preoperatively, the scoliosis evaluations were based only on
standing posteroanterior radiographs. The absence of side-bending
radiographs limited a three-dimensional assessment. During the
follow-up period, only MRI scans were performed. Plain radiography
was not performed in all of the patients. Therefore, the present
study did not track the progression of scoliosis. Furthermore, for
ethical reasons, there were no control groups undergoing
large-bone-window posterior fossa decompression or arachnoid
opening in the current study.
In conclusion, early recognition and surgical
treatment of pediatric CM-I leads to good outcomes in the majority
of patients. SPFD with duraplasty was demonstrated to be an
effective, safe treatment option with a low complication rate. High
CTD grade, basilar invagination and platybasia were indicated to be
predictors of poor clinical prognosis.
References
|
1
|
Tubbs RS, Lyerly MJ, Loukas M, Shoja MM
and Oakes WJ: The pediatric Chiari I malformation: A review. Childs
Nerv Syst. 23:1239–1250. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Aitken LA, Lindan CE, Sidney S, Gupta N,
Barkovich AJ, Sorel M and Wu YW: Chiari type I malformation in a
pediatric population. Pediatr Neurol. 40:449–454. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dones J, De Jesús O, Colen CB, Toledo MM
and Delgado M: Clinical outcomes in patients with Chiari I
malformation: A review of 27 cases. Surg Neurol. 60:142–148. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Poretti A, Ashmawy R, Garzon-Muvdi T,
Jallo GI, Huisman TA and Raybaud C: Chiari type 1 deformity in
children: Pathogenetic, clinical, neuroimaging, and management
aspects. Neuropediatrics. 47:293–307. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Albert GW, Menezes AH, Hansen DR, Greenlee
JD and Weinstein SL: Chiari malformation Type I in children younger
than age 6 years: Presentation and surgical outcome. J Neurosurg
Pediatr. 5:554–561. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Amin R, Sayal P, Sayal A, Massicote C,
Pham R, Al-Saleh S, Drake J and Narang I: The association between
sleep-disordered breathing and magnetic resonance imaging findings
in a pediatric cohort with Chiari 1 malformation. Can Respir J.
22:31–36. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tubbs RS, Doyle S, Conklin M and Oakes WJ:
Scoliosis in a child with Chiari I malformation and the absence of
syringomyelia: Case report and a review of the literature. Childs
Nerv Syst. 22:1351–1354. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ono A, Suetsuna F, Ueyama K, Yokoyama T,
Aburakawa S, Numasawa T, Wada K and Toh S: Surgical outcomes in
adult patients with syringomyelia associated with Chiari
malformation type I: The relationship between scoliosis and
neurological findings. J Neurosurg Spine. 6:216–221. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Krieger MD, Falkinstein Y, Bowen IE, Tolo
VT and McComb JG: Scoliosis and Chiari malformation type I in
children. J Neurosurg Pediatr. 7:25–29. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Strahle J, Smith BW, Martinez M, Bapuraj
JR, Muraszko KM, Garton HJ and Maher CO: The association between
Chiari malformation type I, spinal syrinx, and scoliosis. J
Neurosurg Pediatr. 15:607–611. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Isik N, Elmaci I, Isik N, Cerci SA,
Basaran R, Gura M and Kalelioglu M: Long-term results and
complications of the syringopleural shunting for treatment of
syringomyelia: A clinical study. Br J Neurosurg. 27:91–99. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Morina D, Petridis AK, Fritzsche FS,
Ntoulias G and Scholz M: Syringomyelia regression after shunting of
a trapped fourth ventricle. Clin Pract. 3:e12013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Whitson WJ, Lane JR, Bauer DF and Durham
SR: A prospective natural history study of nonoperatively managed
Chiari I malformation: Does follow-up MRI surveillance alter
surgical decision making. J Neurosurg Pediatr. 16:159–166. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Guyotat J, Bret P, Jouanneau E, Ricci AC
and Lapras C: Syringomyelia associated with type I Chiari
malformation. A 21-year retrospective study on 75 cases treated by
foramen magnum decompression with a special emphasis on the value
of tonsils resection. Acta Neurochir (Wien). 140:745–754. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Beecher JS, Liu Y, Qi X and Bolognese PA:
Minimally invasive subpial tonsillectomy for Chiari I
decompression. Acta Neurochir (Wien). 158:1807–1811. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yilmaz A, Kanat A, Musluman AM, Colak I,
Terzi Y, Kayacı S and Aydin Y: When is duraplasty required in the
surgical treatment of Chiari malformation type I based on tonsillar
descending grading scale? World Neurosurg. 75:307–313. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morrissy RT, Goldsmith GS, Hall EC, Kehl D
and Cowie GH: Measurement of the Cobb angle on radiographs of
patients who have scoliosis. Evaluation of intrinsic error. J Bone
Joint Surg Am. 72:320–327. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ambarki K, Israelsson H, Wåhlin A,
Birgander R, Eklund A and Malm J: Brain ventricular size in healthy
elderly: Comparison between Evans index and volume measurement.
Neurosurgery. 67:94–99. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yarbrough CK, Greenberg JK, Smyth MD,
Leonard JR, Park TS and Limbrick DD Jr: External validation of the
Chicago Chiari outcome scale. J Neurosurg Pediatr. 13:679–684.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arnautovic A, Splavski B, Boop FA and
Arnautovic KI: Pediatric and adult Chiari malformation Type I
surgical series 1965–2013: A review of demographics, operative
treatment, and outcomes. J Neurosurg Pediatr. 15:161–177. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Atkinson JL, Weinshenker BG, Miller GM,
Piepgras DG and Mokri B: Acquired Chiari I malformation secondary
to spontaneous spinal cerebrospinal fluid leakage and chronic
intracranial hypotension syndrome in seven cases. J Neurosurg.
88:237–242. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Riffaud L, Moughty C, Henaux PL, Haegelen
C and Morandi X: Acquired Chiari I malformation and syringomyelia
after valveless lumboperitoneal shunt in infancy. Pediatr
Neurosurg. 44:229–233. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Urbizu A, Poca MA, Vidal X, Rovira A,
Sahuquillo J and Macaya A: MRI-based morphometric analysis of
posterior cranial fossa in the diagnosis of chiari malformation
type I. J Neuroimaging. 24:250–256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Furtado SV, Reddy K and Hegde AS:
Posterior fossa morphometry in symptomatic pediatric and adult
Chiari I malformation. J Clin Neurosci. 16:1449–1454. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shimojima K, Okamoto N, Tamasaki A, Sangu
N, Shimada S and Yamamoto T: An association of 19p13.2
microdeletions with Malan syndrome and Chiari malformation. Am J
Med Genet A. 167A:724–730. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gardner WJ: Hydrodynamic mechanism of
syringomyelia: Its relationship to myelocele. J Neurol Neurosurg
Psychiatry. 28:247–259. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Williams B: On the pathogenesis of
syringomyelia: A review. J R Soc Med. 73:798–806. 1980.PubMed/NCBI
|
|
28
|
Koyanagi I and Houkin K: Pathogenesis of
syringomyelia associated with Chiari type 1 malformation: Review of
evidences and proposal of a new hypothesis. Neurosurg Rev.
33:271–285. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
McVige JW and Leonardo J: Neuroimaging and
the clinical manifestations of Chiari malformation type I (CMI).
Curr Pain Headache Rep. 19:182015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Barkovich AJ, Wippold FJ, Sherman JL and
Citrin CM: Significance of cerebellar tonsillar position on MR.
AJNR Am J Neuroradiol. 7:795–799. 1986.PubMed/NCBI
|
|
31
|
Mikulis DJ, Diaz O, Egglin TK and Sanchez
R: Variance of the position of the cerebellar tonsils with age:
Preliminary report. Radiology. 183:725–728. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chotai S and Medhkour A: Surgical outcomes
after posterior fossa decompression with and without duraplasty in
Chiari malformation-I. Clin Neurol Neurosurg. 125:182–188. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Menezes AH: Current opinions for treatment
of symptomatic hindbrain herniation or Chiari type I malformation.
World Neurosurg. 75:226–228. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Abd-El-Barr M and Groff MW: Less is more:
Limiting the size of posterior fossa decompressions in Chiari I
malformations. World Neurosurg. 81:706–707. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bao C, Yang F, Liu L, Wang B, Li D, Gu Y,
Zhang S and Chen L: Surgical treatment of Chiari I malformation
complicated with syringomyelia. Exp Ther Med. 5:333–337. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Navarro R, Olavarria G, Seshadri R,
Gonzales-Portillo G, McLone DG and Tomita T: Surgical results of
posterior fossa decompression for patients with Chiari I
malformation. Childs Nerv Syst. 20:349–356. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Alfieri A and Pinna G: Long-term results
after posterior fossa decompression in syringomyelia with adult
Chiari Type I malformation. J Neurosurg Spine. 17:381–387. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Godzik J, Dardas A, Kelly MP, Holekamp TF,
Lenke LG, Smyth MD, Park TS, Leonard JR and Limbrick DD: Comparison
of spinal deformity in children with Chiari I malformation with and
without syringomyelia: Matched cohort study. Eur Spine J.
25:619–626. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nokes SR, Murtagh FR, Jones JD III,
Downing M, Arrington JA, Turetsky D and Silbiger ML: Childhood
scoliosis: MR imaging. Radiology. 164:791–797. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Eule JM, Erickson MA, O'Brien MF and
Handler M: Chiari I malformation associated with syringomyelia and
scoliosis: A twenty-year review of surgical and nonsurgical
treatment in a pediatric population. Spine (Phila Pa 1976).
27:1451–1455. 2002. View Article : Google Scholar : PubMed/NCBI
|