Introduction
N,N-dimethylformamide (DMF) is a major solvent
predominantly used in the chemical industry. Wenzhou is the largest
synthetic leather-producing district in the world, producing ~70%
of synthetic leather goods in the domestic market and 50% worldwide
(1,2). The primary toxic effects following DMF
exposure are gastric irritation, skin eruption and hepatotoxicity.
DMF may be metabolized by mixed-function oxidase in the liver to
toxic metabolites, including N-acetyl-S-(N-methylcarbamoyl)
cysteine, which is one of the principal substances associated with
liver injury (3,4).
Previous studies have reported that the severity of
liver injury induced by DMF may be directly related to exposure
dosage, exposure time and the patient's liver function prior to
exposure (5,6). There have been previous case reports of
hepatic dysfunction occurring in workers as a result of DMF alone
or in combination with other organic solvents (3). DMF-induced liver toxicities include
hepatitis, fibrosis, cirrhosis and cancer (3). Treatment strategies for DMF-induced
hepatic dysfunction include hepatoprotection, symptomatic treatment
and life support (7). However, acute
hepatic failure induced by DMF is rare. The present case report
describes a patient with acute hepatic failure following exposure
to high DMF levels via respiratory tract inhalation and skin
absorption over a short period of time. The patient recovered
satisfactorily following artificial liver support therapy and other
treatments. The clinical characteristics, polymorphisms and
therapeutic strategy of DMF poisoning are discussed herein.
Case report
Patient information
The patient was a fit and healthy 38-year-old female
who did not consume alcohol. The patient started a job at a
synthetic leather factory that manufactured belts using DMF as a
solvent in September, 2014. One week later, the patient had a
routine physical check-up, including laboratory tests and an
abdominal ultrasound, the results of which were all within normal
levels.
After 2.5 months, the patient was referred to
Pingyang County Hospital (Wenzhou, China) due to symptoms of
fatigue, poor appetite, abdominal distention, nausea and jaundice.
No specific treatment was administered at this time. The patient's
condition worsened 3 days after this hospital visit. The patient
subsequently visited The First Affiliated Hospital of Wenzhou
Medical University (Wenzhou, China), at which point a physical
examination indicated stable vital signs, consciousness, jaundiced
skin and sclera, abdominal shifting dullness and edema of both
lower limbs; however, there was no splenomegaly. Laboratory data
are presented in Table I. The
clinical data indicated a serum bile acid level of 435 µmol/l
(normal range, 0–12 µmol/l) and hyaluronidase >2,000 ng/ml
(normal range, 0–100 ng/ml). The patient tested negative for viral
infections with hepatitis A-E. The patient exhibited polymorphisms
for the glutathione S-transferase mu-1 (GSTM1)-null genotype and
the glutathione S-transferase theta-1 (GSTT1)-positive genotype.
The GSTM1 and GSTT1 genotypes were determined by co-amplification
of both genes using polymerase chain reaction (PCR). DNA was
extracted from whole peripheral blood using a whole blood genomic
DNA extraction kit (Tiangen Biotech Co., Ltd., Beijing, China)
according to the manufacturer's protocol. PCR was performed in a 25
µl mixture containing 100 ng genomic DNA and 1 µl each of forward
and reverse primers as previously described (8). Primers were as follows: GSTM1 forward,
5′-GAACTCCCTGAAAAGCTAAAGC-3′ and reverse,
5′-GTTGGGCTCAAATATACGGTGG-3′; GSTT1 forward,
5′-TTCCTTACTGGTCCTCACATCTC-3′ and reverse,
5′-TCACCGGATCATGGCCAGCA-3′. Thermocycling conditions were as
follows: 94°C for 4 min followed by 35 cycles of 94°C for 40 sec,
62°C for 30 sec and 72°C for 40 sec, with a final extension at 72°C
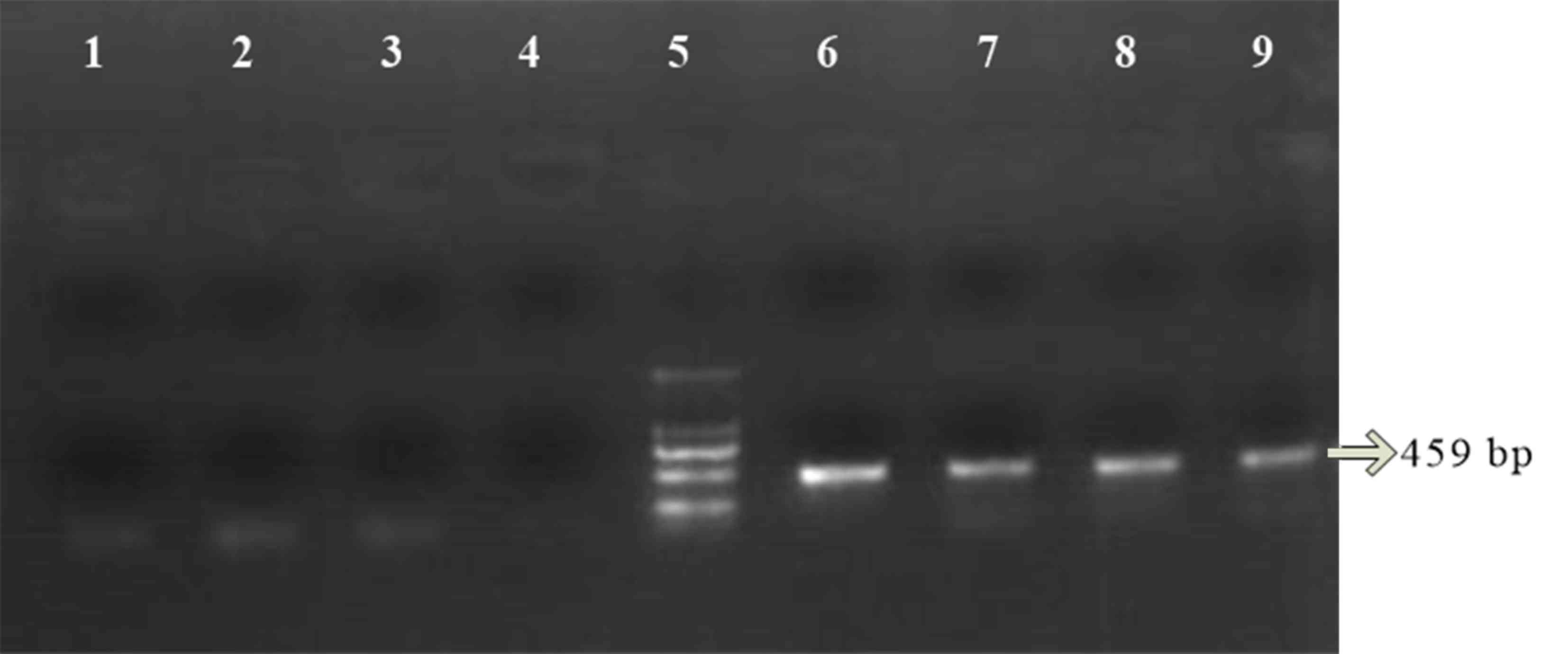
for 10 min. The PCR products were determined by electrophoresis
using 2% agarose gels. The amplified fragment length of GSTM1 was
219 bp and the amplified fragment length of GSTT1 was 459 bp
(Fig. 1). The Child-Pugh score was
13, which was categorized as class C (9). An abdominal computed tomography (CT)
scan was performed on admission, and the results are shown in
Fig. 2A. On day 7 after admission,
abdominal ultrasound indicated shrinkage of the liver,
echogenicity, ascites and splenomegaly.
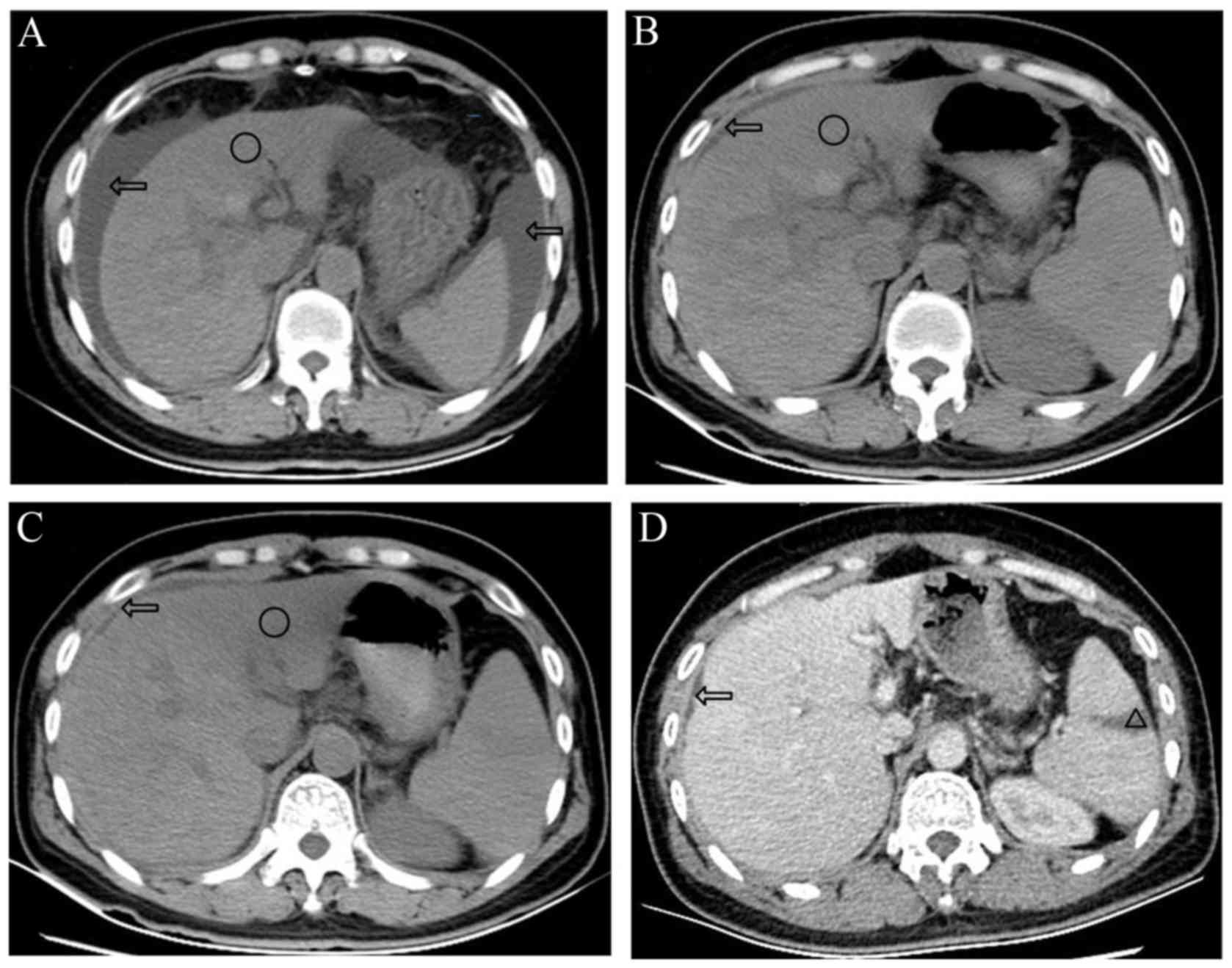 | Figure 2.Abdominal CT scans of the patient. (A)
Abdominal CT scan on day 5 after admission indicated liver
deformation, shrinkage of the liver, border irregularity, a
low-density shadow with border blurring (circle), ascites (arrows)
and splenomegaly. (B) Abdominal CT scan on day 21 indicated that
the size of the liver had recovered, ascites (arrows) were
decreased and the low-density shadow (circle) was similar to the
previous CT. (C) Abdominal CT on day 52, indicated that the
low-density shadow (circle) had decreased and its border was clear,
and ascites (arrows) were not present. (D) Contrast-enhanced CT
scan on day 54 indicated hepatic cirrhosis, liver deformation, an
infarction of the spleen (triangle) and an absence of ascites
(arrows). Compared with previous CT scans, the low-density shadow
was not evident. CT, computed tomography. |
 | Table I.Sequential laboratory data for the
patient. |
Table I.
Sequential laboratory data for the
patient.
|
|
|
Weeks after
the patient started her job |
|---|
|
|
|
|
|---|
| Item | Normal range | 10.7 | 10.9 | 11.3 | 11.4 | 12.1 | 13 | 14.6 | 16 | 18.1 | 19.3 |
|---|
| TBil (µmol/l) | 0–20 | Admitted to
Pingyang | 289 | Admitted to EICU
of | 350 | 344 | 206 | Transferred to | 135 | 63 | Discharged from |
| ALT (U/l) | 7–40 | County Hospital | 621 | The First
Affiliated | 231 | 58 | 21 | general ward | 17 | 26 | hospital |
| AST (U/l) | 13–35 |
| 572 | Hospital of
Wenzhou | 150 | 53 | 34 |
| 32 | 42 |
|
| Alb (g/l) | 40–55 |
| 29.4 | Medical
University | 25.5 | 30.8 | 40.8 |
| 33.3 | 29.6 |
|
| PT (sec) | 11.7–14.8 |
| 28.4 |
| 28.8 | 24.4 | 21.9 |
| 19.9 | 17.3 |
|
Following admission, the treatment for this patient
included daily intravenous infusions of liver protection agents
[magnesium isoglycyrrhizinate (200 mg; Jiangsu Chia Tai Tianqing
Pharmaceutical Co., Ltd., Jiangsu, China), polyene phosphatidyl
choline injection (10 mg; Chengdu Tiantaishan Pharmaceutical Co.,
Ltd., Chengdu, China) and ademetionine injection (1,000 mg; Pfizer,
Inc., New York, NY, USA)] to alleviate hepatic dysfunction in
addition to plasma, blood platelet and albumin transfusions for
support and symptomatic treatment. Vital signs and liver function
were monitored. The patient's liver function deteriorated despite
supportive treatment. On days 8, 12 and 16, an artificial liver
support system (ALSS) was implemented. On day 17, the Child-Pugh
score was 9, which was categorized as class B (9). From day 9, the patient exhibited a
fever, and blood culture and empiric antimicrobial therapy with
piperacillin-tazobactam were initiated. The patient reported
feeling better on day 22. Her temperature was reduced and liver
function had markedly improved. CT re-examination showed an
improvement compared with the initial scan (Fig. 2A and B). During hospitalization, the
presence of viral infections, including hepatitis A-C was excluded,
as were other medication-related causes of hepatitis. At 1 month
following the last CT, CT re-examination revealed the low-density
shadow had decreased and its border was clear (Fig. 2C). The low-density shadow did not
appear on a contrast-enhanced CT scan (Fig. 2D). The patient was discharged 2
months after admission.
Workplace evaluation
A visit to the patient's workplace was conducted in
January, 2015. It was observed that the factory comprised a
four-floor building that primarily produced raw belts. There were
four major areas in the work field: A wet process line on the first
floor, a dry process line on the second floor, a storage room on
the third floor and a multifunctional room on the fourth floor. The
patient had been employed for ~2.5 months on the wet process floor.
The wet process involved using large quantities of DMF. The work
areas emitted a strong odor and the room was poorly ventilated.
Workers were not well equipped with personal protective devices.
The patient worked ~10 h per day. There were 10 workers
simultaneously working at the same place. Several co-workers had
similar symptoms, such as dizziness, fatigue, abdominal distention
and nausea. These workers were also sent to Pingyang County
Hospital, where supportive care was administered. After resting for
1 to 2 days, the workers returned to work. Policemen and officials
from the Chinese Center for Disease Control and Prevention
investigated the factory while the workers were hospitalized. The
factory was shut down after it was found that the air DMF
concentration in the workplace was 2- to 3-fold above the national
standard.
Discussion
There have been several previous reports of hepatic
injury from occupational exposure to DMF; however, few reports have
described acute hepatic failure induced by DMF. Only 5 cases have
been reported in previous articles (Table II) (10–14). In
the present case, the diagnosis of DMF-induced acute hepatic
failure was based on clinical history (no history of medication use
or alcohol intake), symptoms and signs, abnormal liver function and
negative hepatitis viral series, as well as high DMF concentrations
in the work environment that exceeded legal limits. Several
co-workers of the patient also suffered variable degrees of liver
dysfunction. Therefore, based on the national standards for
occupational disease (7,15), although the body concentration of DMF
and pathology were not measured, a relationship between the acute
hepatic failure and DMF intoxication was strongly suspected.
 | Table II.Cases of DMF-induced acute hepatic
failure. |
Table II.
Cases of DMF-induced acute hepatic
failure.
| Author and year | Sex (M/F) | Age (years) | Exposure time
(months) | Exposure field
(process line) | Air concentration of
DMF (mg/m3) | Clinical
characteristics | LF recovery time
(months) | ALSS | Outcome | (Refs.) |
|---|
| Shi et al,
2004 | M | 39 | 3.0 | Wet | 23.7 | Liver dysfunction,
poor appetite, jaundice, coma, abdominal distention, nausea,
vomiting, and CT indicated low-density shadow in the liver and
ascites | 1.5 | No | Survived | (10) |
| Liu et al,
2009 | F | 40 | 2.3 | – | 41.4–131 | Liver dysfunction,
fatigue, poor appetite, jaundice, coma, and CT indicated uneven
liver density, splenomegaly and ascites | N/A | No | Succumbed | (11) |
| Ding et al,
2011 | F | 38 | 0.6 | Dry | 42 | Liver dysfunction,
fatigue, poor appetite, jaundice, abdominal pain | N/A | No | Succumbed | (12) |
| Tong et al,
2014 | M | 23 | 2.5 | – | 157.1 | Liver dysfunction,
fatigue, poor appetite, jaundice, disseminated intravascular
coagulation, and CT indicated splenomegaly and ascites | N/A | Yes | Succumbed | (13) |
| Zhang et al,
2015 | F | 40 | 2.0 | – | 131 | Liver dysfunction,
poor appetite, nausea, fatigue, vomiting, jaundice, and CT
indicated uneven liver density, splenomegaly and ascites | N/A | No | Succumbed | (14) |
| Present case | F | 38 | 2.5 | Wet | 40–60 | Liver dysfunction,
fatigue, poor appetite, nausea, jaundice, abdominal distention, and
CT indicated low-density shadow in the liver, spleen infarction and
ascites | 2 | Yes | Survived | N/A |
It has previously been reported that the severity of
liver damage induced by DMF is directly associated with the
exposure dosage and time (5,6). According to the current study (Table II), the exposure dosage of this
patient was 2- to 3-fold the standard dose; however, the exposure
time was relatively short. Moreover, other co-workers who worked in
the same work field did not have comparable hepatic dysfunction.
The contradiction between DMF-induced severe hepatic damage and low
concentrations of environmental DMF and short-term exposure may be
explained by individual susceptibility to DMF (6,16). It
has previously been reported that DMF susceptibility may be related
to polymorphisms of GSTM1 and GSTT1 (5,8). In the
present case, the polymorphism of GSTM1 indicated a null genotype.
This was not consistent with the study by Xu et al (8), in which induced liver function injury
in patients with the GSTM1-positive genotype was 2.3-fold as high
as that of individuals with the GSTM1-null genotype in workers
exposed to DMF. In the present case, the polymorphism of GSTM1
indicated a positive genotype. This was inconsistent with a
previous study that demonstrated that the occurrence of induced
liver function injury in patients with the GSTT1-null genotype was
4.4-fold higher compared with patients with the GSTT1-positive
genotype (5). Thus, severe hepatic
injury may be not associated with polymorphisms of GSTM1 and GSTT1.
Whether there were other susceptible factors in the current patient
requires further investigation.
The clinical presentation of occupational liver
disease may be acute/subacute or chronic; however, it is often
insidious (17). The common clinical
symptoms of DMF poisoning in patients include liver injury,
fatigue, nausea, poor appetite, jaundice and ascites (10–14).
However, acute hepatic failure may result in high mortality
(Table II). Serum liver enzymes,
including alanine transaminase, aspartate transaminase and
bilirubin levels, are routinely used as indicators of
hepatotoxicity (14,17). All of these parameters exhibited high
serum concentrations in the current case. However, the most notable
indicators in the current patient were serum bile acid and
hyaluronidase levels. It has previously been reported that both of
these factors are potential indicators of early hepatic fibrosis
activities in occupational workers (17,18). The
abdominal CT scan of the current patient indicated large,
low-density shadow at admission, which markedly improved as the
patient received supportive therapy (Fig. 2A-C). A contrast-enhanced CT scan
indicated that the low-density shadow had been fully reduced by day
54 (Fig. 2D). It was postulated that
the low-density shadow may have been due to fatty degeneration and
fibrosis. Different degrees of fatty degeneration and fibrosis are
characteristics of toxic hepatitis, which leads to cirrhosis
(14,17).
The treatments recommended for acute hepatic failure
induced by DMF are non-specific. ALSS has previously been reported
to be a safe, effective and important modality for acute and
acute-on-chronic liver failure via plasma exchange or absorption
mechanisms (19,20). Chen et al (21) reported that the initiation of ALSS in
patients with liver failure following acute poisoning for an
average of 2.7 times every 4 to 5 days improved the survival rate
(76.9 vs. 38.9%). A novel method (preconcentration method) has been
proposed to increase the adsorption of protein-bound toxins onto
adsorbents in ALSS, which may simultaneously reduce cost and
shorten the treatment time by two-thirds (22). The combination of active supportive
treatment and ALSS provided a good prognosis for the present
patient.
In conclusion, DMF is a major solvent predominately
used in the chemical industry. The mortality of DMF-induced acute
severe hepatitis is high. There is no specific treatment for
DMF-induced acute hepatic failure; however, ALSS may be an
effective clinical treatment method for these patients.
References
|
1
|
Zhang Q, Huang C, Wei Y, Zhu Q, Tian W and
Wang C: Risk assessment of N,N-dimethylformamide on residents
living near synthetic leather factories. Environ Sci Pollut Res
Int. 21:3534–3539. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wei YM, Tian WL, Zhang QY, Zheng YY, Yang
YK, Wu ZC, Zhu Q, Zhou L and Fang SM: Evaluating population
exposure to N,N-dimethylformamide in a small industrial area
accounting for population movement. J Zhejiang Univ-Sci A.
12:794–806. 2011. View Article : Google Scholar
|
|
3
|
Kim TH and Kim SG: Clinical outcomes of
occupational exposure to n,n-dimethylformamide: Perspectives from
experimental toxicology. Saf Health Work. 2:97–104. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rui D, Daojun C and Yongjian Y: Liver and
heart toxicity due to 90-day oral exposure of ICR mice to
N,N-dimethylformamide. Environ Toxicol Pharmacol. 31:357–363. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Luo JC, Cheng TJ, Kuo HW and Chang MJW:
Abnormal liver function associated with occupational exposure to
dimethylformamide and glutathione S-transferase polymorphisms.
Biomarkers. 10:464–474. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nomiyama T, Uehara M, Miyauchi H, Imamiya
S, Tanaka S and Seki Y: Causal relationship between a case of
severe hepatic dysfunction and low exposure concentrations of
N,N-dimethylformamide in the synthetics industry. Ind Health.
39:33–36. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
GBZ 85–2002Diagnostic Criteria of
Occupational Acute Dimethylformamide Poisoning. http://www.nhfpc.gov.cn/cmsresources/zwgkzt/wsbz/zybzdbz/zyb/zyb/085.pdf(published
on 8, April, 2002).
|
|
8
|
Xu CM, Qian YL, Zhu LJ, Xian JX, Chai JR,
Ruan Z and Zhang X: Abnormal liver function associated with
polymorphism of GSTT1, GSTMI and CYP2El in workers exposed to
N,N-dimethylformamide. Zhonghua Lao Dong Wei Sheng Zhi Ye Bing Za
Zhi. 27:333–337. 2009.(In Chinese). PubMed/NCBI
|
|
9
|
Kumbasar A, Navdar M, Ataoglu E, Uzunhasan
I, Ergen K, Poturoglu S, Basinoglu F, Yilmaz F, Yenigun M, Sar F
and Tanriverdi O: N-Terminal pro-B-Type natriuretic peptide levels
are linked with modified child-pugh classification in patients with
nonalcoholic cirrhosis [NT-ProBNP and Liver Cirrhosis]. Cell
Biochem Biophys. 75:111–117. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shi MG, Li L and Sui YQ: Acute DMF
poisoning causes subacute hepatic necrosis: A case report. Chin J
Ind Hyg Occup Dis. 22:234. 2004.
|
|
11
|
Liu Q, Du F, Tan L, Wang BW and Zhou YW:
Occupational DMF poisoning causes death: A case report. Chin J
Forensic Med. 24:60–61. 2009.
|
|
12
|
Ding YL, Ying GX and Jin LP: Occupational
DMF poisoning causes death: A case report. Occup Health Emerg
Rescue. 29:107–108. 2011.
|
|
13
|
Tong Z, Shi J, Zhu X and Zhu B: Death
after exposure to dimethylformamide: A case report. Chin J Ind Hyg
Occup Dis. 32:285–286. 2014.
|
|
14
|
Zhang H, Liu Q, Duan Y, Dong H and Zhou Y:
Chronic occupational N,N-dimethylformamide poisoning induced death:
A case report. Forensic Sci Med Pathol. 11:584–588. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
GBZ 2.1-2007, . 2007, Occupational
Exposure Limits for Hazardous Agents in the Workplace. Part1:
Chemical hazardous agents. http://www.nhfpc.gov.cn/zhuz/pyl/200704/38838/files/5eb946b479124c32a6ebb8c49f483c24.pdf(published
on 12, April, 2007).
|
|
16
|
Wang C, Huang C, Wei Y, Zhu Q, Tian W and
Zhang Q: Short-term exposure to dimethylformamide and the impact on
digestive system disease: An outdoor study for volatile organic
compound. Environ Pollut. 190:133–138. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Malaguarnera G, Cataudella E, Giordano M,
Nunnari G, Chisari G and Malaguarnera M: Toxic hepatitis in
occupational exposure to solvents. World J Gastroenterol.
18:2756–2766. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
He J, Wang P, Zhu JQ, Wu G, Ji JM and Xue
Y: Role of urinary biomarkers of N,N-dimethylformamide in the early
detection of hepatic injury among occupational exposed workers. Int
Arch Occup Environ Health. 83:399–406. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Z, Zhao YC, Cheng Y, Jian GD, Pan MX
and Gao Y: Hybrid bioartificial liver support in cynomolgus monkeys
with D-galactosamine-induced acute liver failure. World J
Gastroenterol. 20:17399–17406. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou PQ, Zheng SP, Yu M, He SS and Weng
ZH: Prognosis of acute-on-chronic liver failure patients treated
with artificial liver support system. World J Gastroenterol.
21:9614–9622. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen LG, Guleng B, Ren JL, Chen JM and
Wang L: Artificial liver support system in treatment of liver
failure after acute poisoning. World J Emerg Med. 2:283–286. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ding W, Zou L, Sun S, Li W and Gao D: A
new method to increase the adsorption of protein-bound toxins in
artificial liver support systems. Artif Organs. 38:954–962. 2014.
View Article : Google Scholar : PubMed/NCBI
|