Introduction
Type 2 diabetes mellitus (T2DM) is a common complex
disease defined by hyperglycemia (1). The prevalence of T2DM is increasing
year by year around the world and is becoming a serious global
public health problem (1,2). However, identifying the pathogenesis
and mechanism is difficult due to heterogeneous phenotypes and a
broad spectrum of pathophysiological processes (3). A previous study indicated that genetic
variation and various postnatal factors (including smoking,
physical activity and education) are associated with T2DM (4). However, A recent study reported that
low birth weight, which reflects the intrauterine nutrient
conditions, was associated with metabolic disorders after birth,
such as obesity and insulin resistance (5). Low birth weight has also been
demonstrated to be associated with adult cardiovascular disease and
diabetes (6–10). The mechanism behind these
associations is still unclear. One proposal is the fetal
programming hypothesis: A lack of intrauterine nutrients causes a
permanent metabolic shift towards insulin resistance to support
brain glucose supply. After birth, the nutrient supply increases,
which may lead to obesity and insulin resistance (5,11–15). An
alternative proposal is the fetal insulin hypothesis, which
suggests that common genetic variants decrease insulin secretion
and cause low birth weight (16).
The relationship between high birth weight and T2DM is not
consistent. Some studies have demonstrated that birth weight is
negatively associated with T2DM and high birth weight (>4,000 g)
decreases the risk of T2DM (17–19).
Other studies have reported that both high and low birth weight
increase the risk of T2DM, and high birth weight is also a risk
factor for T2DM (20–23).
In the present study, a meta-analysis was conducted
in order to provide a comprehensive overview of the association
between birth weight and T2DM.
Materials and methods
Search strategy
A literature search was conducted on PubMed
(https://www.ncbi.nlm.nih.gov/pubmed),
ScienceDirect (http://www.sciencedirect.com/), SpringerLink
(https://link.springer.com/advanced-search), China
National Knowledge Infrastructure (http://www.wanfangdata.com.cn/) and Chinese Biomedical
Literature Database (http://www.sinomed.ac.cn/zh/) from January 1990 to
June 2016 for relevant papers using the following terms: ‘birth
weight’, ‘type 2 diabetes’, ‘non-insulin-dependent’, ‘NIDDM’ and
‘risk factor’. The articles were restricted to those written in
English or Chinese. The reference lists of the retrieved articles
were also manually reviewed to identify publications on the same
topic.
Study selection
To qualify for the present meta-analysis, studies
were required to meet the following criteria: i) Unrelated cohort
study; ii) recruited sufficient dichotomous data on T2DM and low
birth weight; and iii) presented relative risk (RR) and 95%
confidence intervals (CI), or data with which to calculate them,
for T2DM in at least two strata of birth weight. Birth weight was
required to be expressed in a specific range, such as <2,500 and
>2,500 g, or <4,000 and >4,000 g. Alternatively, an RR and
95% CI for the change in T2DM risk per unit change in birth weight
could have been reported. Studies were considered irrespective of
the definition of T2DM (definitions used included those of the
World Health Organization (24), the
National Diabetes Data Group (25)
and the America Diabetes Association (26).
Data extraction
A standard extraction form was used to collect the
following information from each study: First author name, year of
publication, country in which the study was conducted, year of
patient birth, patient age, trend declared by the study's authors,
final cohort size and number of cases with T2DM. Data were
extracted independently by two investigators. Discrepancies, if
any, were resolved by discussion and consultation with a third
reviewer.
Quality assessment
Two investigators performed a quality assessment
using the Newcastle-Ottawa scale (27) for included studies. This scale
allocates a maximum of nine stars for the highest quality of
selection, comparability and ascertainment of exposure to risks.
The four criteria in evaluating the selection were as follows: i)
Representativeness of the low birth weight; ii) selection of the
non-low birth weight; iii) ascertainment of low birth weight; and
iv) demonstration that T2DM was not present at the start of the
study. A maximum of two stars was awarded for comparability: i)
Study controls for age; and ii) study controls for any additional
factors. The three criteria in evaluating the outcomes were as
follows: i) Assessment of T2DM; ii) follow-up was long enough for
T2DM to occur (>10 years); and iii) adequacy of follow-up of
cohort (>80%). The two authors discussed the implementation of
this assessment tool and agreed on a method of implementation prior
to their independent assessment of studies.
Statistical analysis
Meta-analysis was conducted using Review Manager
(version 5.1; The Nordic Cochrane Centre, Copenhagen, Denmark).
Measurement data were presented as the weighted mean difference and
95% CI. Enumerated data were presented as the odds ratio (OR) and
95% CI. Cochran's Q statistic and I2 statistic were used
to assess heterogeneity. If significant heterogeneity was observed,
the random-effects model was used. Otherwise, the fixed-effects
model was used (28,29). P<0.05 was considered to indicate a
statistically significant result.
Publication bias was assessed by inspection of a
funnel plot and formal testing for funnel plot asymmetry was
performed using Begg's test and Egger's test. Sensitivity analysis
was performed by excluding one study at a time to identify the
influence of individual data sets on the pooled RR.
Results
Preliminary screening of
literature
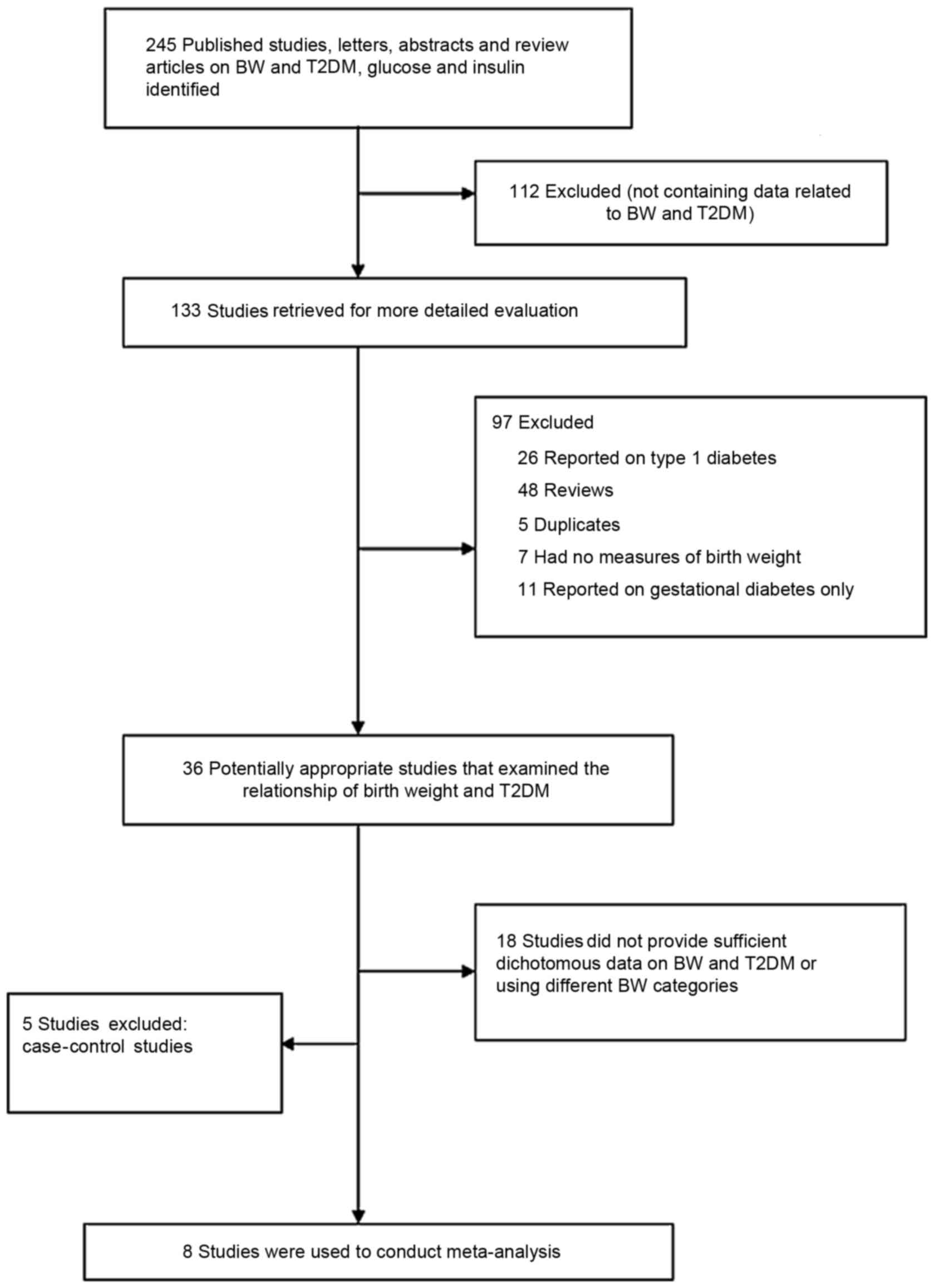
A total of 245 studies related to birth weight and
T2DM were identified during the literature search. However, 237
studies were excluded for the following reasons: i) Did not contain
birth weight and T2DM data (n=112); ii) reported on type 1 diabetes
(n=26); iii) review articles (n=48); iv) duplicates (n=5); v) did
not contain birth weight-related data (n=7); vi) reported on
gestational diabetes (n=11); vii) did not present binary data about
birth weight and T2DM and did not use the indicated birth weight
range (n=18); viii) case-control studies (n=5) (23,30–33). The
remaining eight studies were selected in order to conduct the
meta-analysis (Fig. 1) (21,34–40).
The eight included studies are presented in Table I. A total of 108,369 individuals were
included in these studies and 3,892 were diagnosed with T2DM. All
eight studies indicated the OR value of T2DM through comparing low
birth weight (<2,500 g) and normal birth weight (2,500–4,000 g).
Seven studies indicated the OR value of T2DM through comparing
normal birth weight and high birth weight (>4,000 g). These
seven studies also indicated the OR value through comparing low
birth weight and high birth weight. In one study, there was a
U-shaped curve relationship between birth weight and T2DM.
Furthermore, one study reported that birth weight was positively
associated with T2DM. There was a linear inverse trend in 6
studies. According to the Newcastle-Ottawa scale, the mean score
for the selection, comparability and exposure for the included
studies was 6.5 stars (Table
II).
 | Table I.Characteristics of eight studies
included in the meta-analysis. |
Table I.
Characteristics of eight studies
included in the meta-analysis.
| First author,
year | Country | Year of birth | Age, years | Trend declared by
the study's authors | Birth weight
reference category for adjusted estimate, g | Final cohort size,
n | Cases with type 2
diabetes, n | (Refs.) |
|---|
| Curhan et
al, 1996 | USA | 1911–1946 | 40–75 | Linear inverse | 3,180–3,810 | 22,846 | 424 | (34) |
| McCance et
al, 1994 | USA | 1940–1972 | 20–39 | U-shaped | 2,500–4,499 | 1,179 | 210 | (21) |
| Rich-Edwards et
al, 1999 | USA | 1921–1946 | 60 | Linear inverse | 3,260–3,820 | 69,526 | 2,123 | (35) |
| Hales et al,
1991 | England | 1920–1930 | 59–70 | Linear inverse | – | 370 | 27 | (36) |
| Fall et al,
1998 | India | 1934–1953 | 39–60 | Linear
positive | – | 501 | 75 | (37) |
| Forsén et
al, 2000 | Finland | 1924–1933 | 64–73 | Linear inverse | – | 7,044 | 471 | (38) |
| Eriksson et
al, 2004 | Sweden | 1913–1963 | 50 | Linear inverse | 3,000–4,250 | 478 | 54 | (39) |
| Kaijser et
al, 2009 | Sweden | 1925–1949 | 37–62 | Linear inverse | 1,500–4,000 | 6,425 | 508 | (40) |
 | Table II.Assessment of study quality based on
the Newcastle-Ottawa scale. |
Table II.
Assessment of study quality based on
the Newcastle-Ottawa scale.
| First author,
year | Selection (stars
out of 4) | Comparability
(stars out of 2) | Exposure (stars out
of 3) | (Refs.) |
|---|
| Curhan et
al, 1996 | ⋆⋆⋆ | ⋆ | ⋆⋆ | (34) |
| McCance et
al, 1994 | ⋆⋆⋆ | ⋆⋆ | ⋆⋆ | (21) |
| Rich-Edwards et
al, 1999 | ⋆⋆ | ⋆⋆ | ⋆⋆ | (35) |
| Hales et al,
1991 | ⋆⋆⋆ | ⋆ | ⋆⋆ | (36) |
| Fall et al,
1998 | ⋆⋆⋆ | ⋆ | ⋆⋆ | (37) |
| Forsen et
al, 2000 | ⋆⋆⋆ | ⋆ | ⋆⋆ | (38) |
| Eriksson et
al, 2004 | ⋆⋆⋆⋆ | ⋆⋆ | ⋆⋆⋆ | (39) |
| Kaijser et
al, 2009 | ⋆⋆⋆ | ⋆ | ⋆⋆ | (40) |
Meta-analysis results
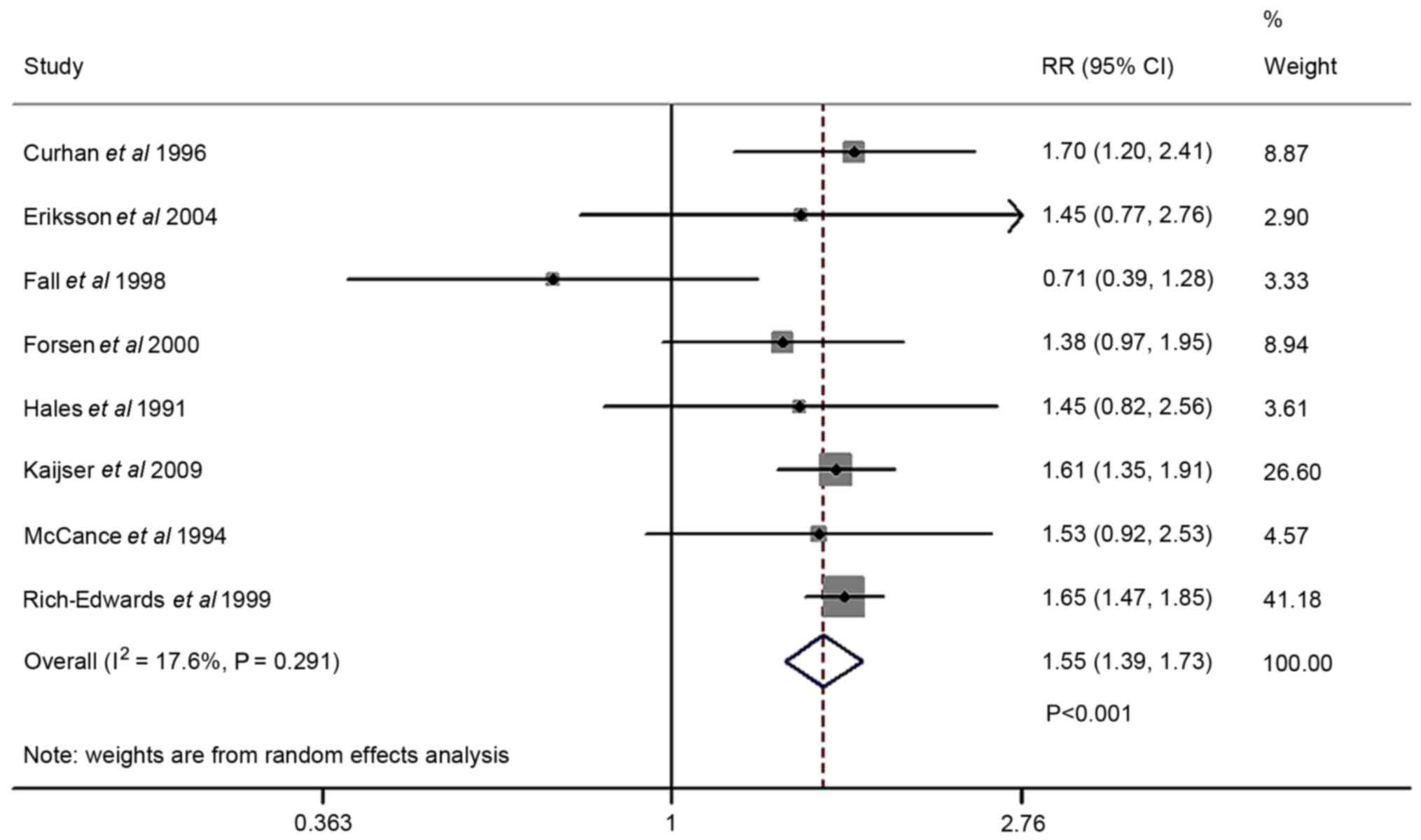
The forest plot comparing T2DM risk in cases of low
birth weight and normal birth weight is presented in Fig. 2. The T2DM risk analysis was included
in eight studies. Random-effects model assessment
(Q2=8.49; P=0.291; I2=17.6%) indicated that
low birth weight increased the risk of T2DM compared with normal
birth weight (OR=1.55; 95% CI, 1.39–1.73; P<0.001). Sensitivity
analysis indicated that the pooled ORs were no statistically
significant no matter what study was excluded from analysis,
suggesting the robustness of results. This analysis also revealed
that one study, by Fall et al (37), was the largest source of
heterogeneity (Table III). The
I2 measure for low birth weight markedly declined from
17.6 to 0.0% when this study was omitted. Homogeneity was achieved
after excluding Fall et al (37) [Q=1.28; degrees of freedom (df)=6;
P=0.973; I2=0.00) and an RR of 1.62 was obtained (95%CI,
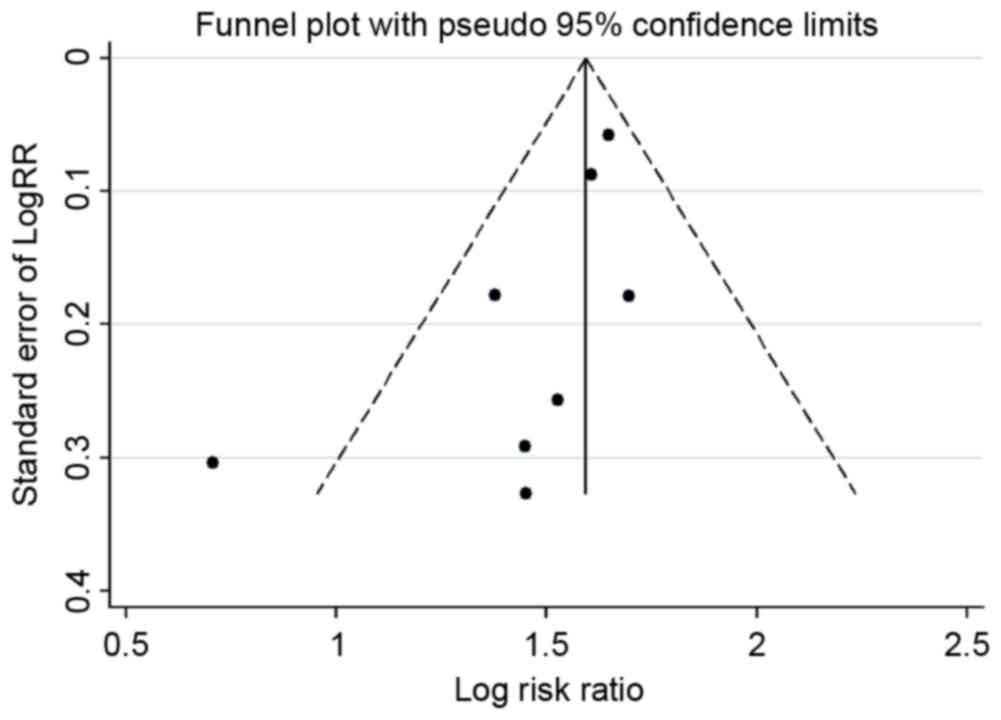
1.478–1.754; fixed-effects; P<0.001; data not shown). A funnel
plot (Fig. 3) and Begg's and Egger's
tests were conducted to assess the publication bias of the included
studies. Evidence of publication bias was also not seen with the
Egger's or Begg's tests (Egger's, P=0.103; Begg's, P=0.083; data
not shown).
 | Table III.Result of leave-one-out sensitivity
analysis; low birth weight vs. normal birth weight). |
Table III.
Result of leave-one-out sensitivity
analysis; low birth weight vs. normal birth weight).
| First author,
year | I2
(%) | P-value | (Refs.) |
|---|
| Curhan et
al, 1996 | 28.0 | 0.215 | (34) |
| McCance et
al, 1994 | 29.2 | 0.206 | (21) |
| Rich-Edwards et
al, 1999 | 19.8 | 0.278 | (35) |
| Hales et al,
1991 | 28.5 | 0.211 | (36) |
| Fall et al,
1998 | 0.0 | 0.973 | (37) |
| Forsen et
al, 2000 | 23.5 | 0.250 | (38) |
| Eriksson et
al, 2004 | 28.7 | 0.209 | (39) |
| Kaijser et
al, 2009 | 29.0 | 0.207 | (40) |
The forest plot of T2DM risk comparing high birth
weight and normal birth weight is presented in Fig. 4. The risk analysis was conducted in 7
studies. Random-effects model assessment (Q2=18.38;
P=0.005; I2=67.4%) indicated that there was no
significant association between high birth weight and T2DM
(OR=0.98; 95% CI, 0.79–1.22; P=0.872). Sensitivity analysis
revealed that one study by Hales et al (36) was the largest source of heterogeneity
(Table IV). The I2
measure for high birth weight markedly declined from 67.4 to 56.3%
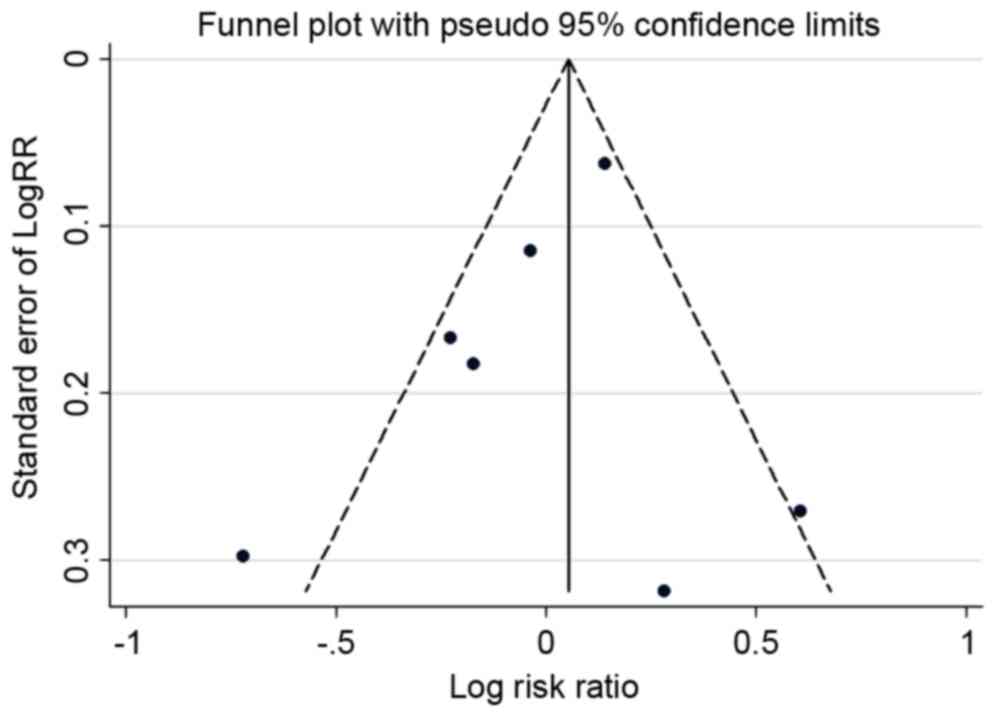
when this study was omitted. A funnel plot (Fig. 5) and Begg's and Egger's tests were
conducted to assess the publication bias of the included studies.
No evidence of publication bias was observed. Evidence of
publication bias was also not observed with the Egger's or Begg's
tests (Egger's, P=0.167; Begg's, P=0.024; data not shown).
 | Table IV.Result of leave-one-out sensitivity
analysis; birth weight vs. normal birth weight). |
Table IV.
Result of leave-one-out sensitivity
analysis; birth weight vs. normal birth weight).
| First author,
year | I2
(%) | P-value | (Refs.) |
|---|
| Curhan et
al, 1996 | 71.6 | 0.003 | (34) |
| McCance et
al, 1994 | 64.5 | 0.015 | (21) |
| Rich-Edwards et
al, 1999 | 63.0 | 0.019 | (35) |
| Hales et al,
1991 | 56.3 | 0.043 | (36) |
| Forsen et
al, 2000 | 67.4 | 0.009 | (38) |
| Eriksson et
al, 2004 | 72.0 | 0.003 | (39) |
| Kaijser et
al, 2009 | 70.2 | 0.005 | (40) |
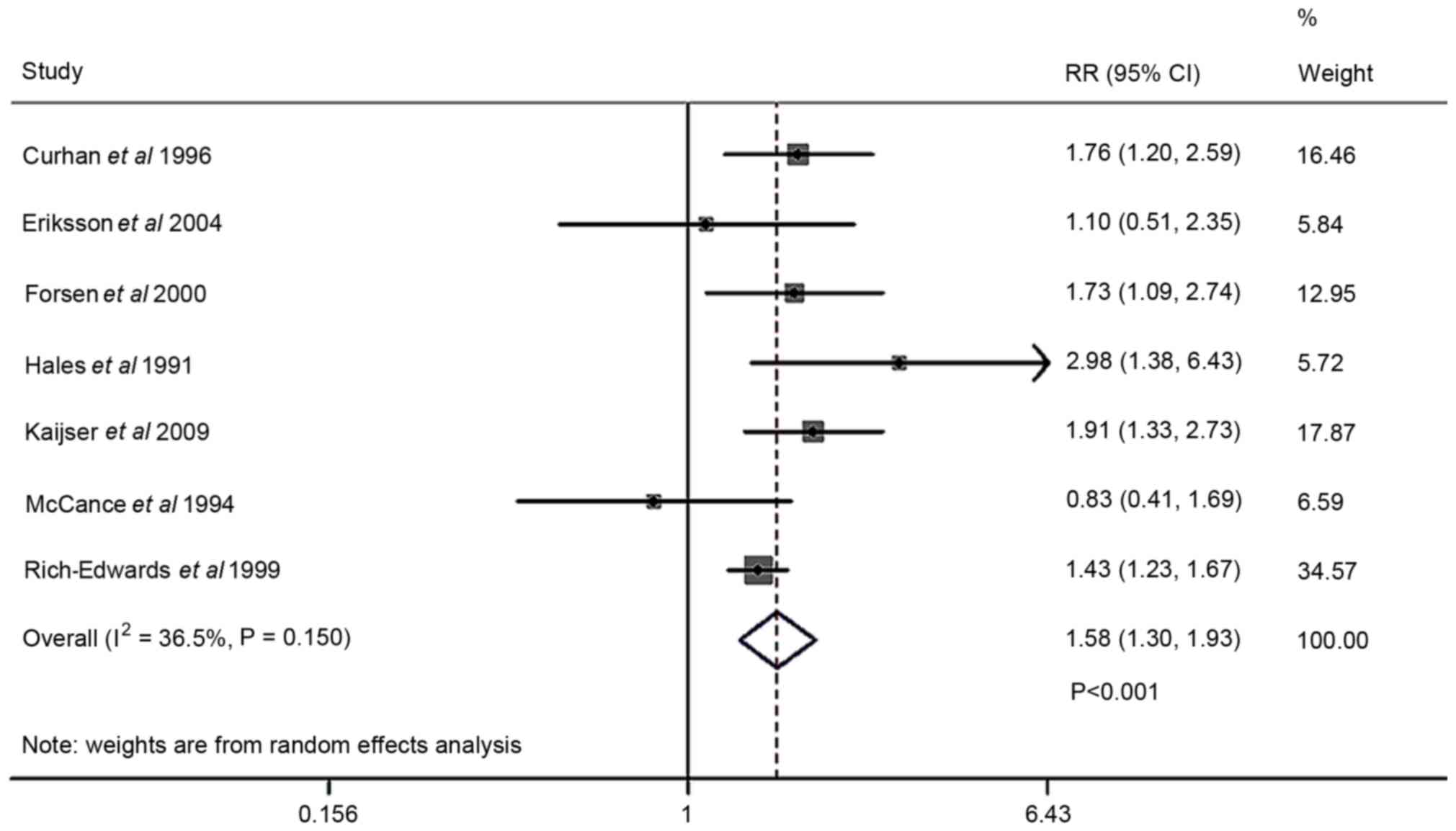
The forest plot comparing T2DM risk in cases of low
birth weight and high birth weight is presented in Fig. 6. The risk analysis was conducted in 7
studies. Random-effects model assessment (Q2=9.45;
P=0.150; I2=36.5%) indicated that low birth rate was
associated with a higher risk of T2DM compared with high birth
weight (RR, 1.58; 95% CI 1.30–1.93; P<0.001). Sensitivity
analysis revealed that one study, by McCance et al (21), was the largest source of
heterogeneity (Table V). The
I2 measure for low birth weight markedly declined from
36.5 to 24.2%. Homogeneity was achieved after excluding a study
(Q=6.59; df=5; P=0.253; I2=24.2%), and an OR of 1.63 was
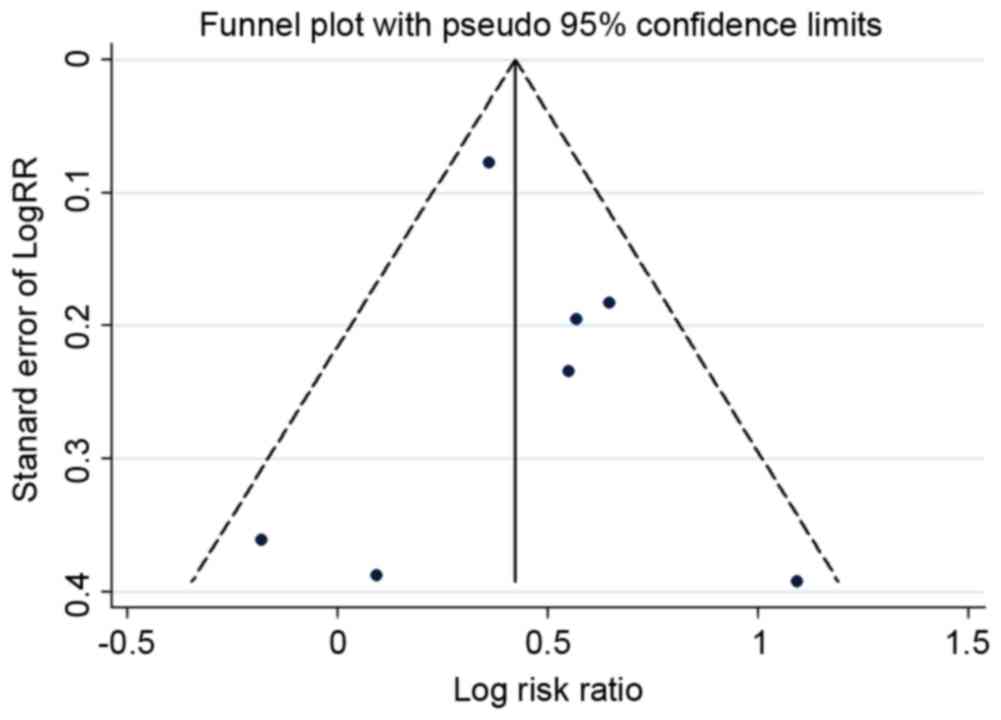
obtained (95% CI, 1.367–1.937; fixed-effects; P<0.001). A funnel
plot (Fig. 7) and Begg's and Egger's
tests were conducted to assess the publication bias of the included
studies. Evidence of publication bias was also not observed with
the Egger's or Begg's tests (Egger's, P=0.663; Begg's. P=0.881;
data not shown).
 | Table V.Result of leave-one-out sensitivity
analysis; low birth weight vs. high birth weight. |
Table V.
Result of leave-one-out sensitivity
analysis; low birth weight vs. high birth weight.
| First author,
year | I2
(%) | P-value | (Refs.) |
|---|
| Curhan et
al, 1996 | 43.4 | 0.116 | (34) |
| Eriksson et
al, 2004 | 42.7 | 0.121 | (39) |
| Forsen et
al, 2000 | 45.3 | 0.104 | (38) |
| Hales et al,
1991 | 22.5 | 0.265 | (36) |
| Kaijser et
al, 2009 | 35.4 | 0.171 | (40) |
| McCance et
al, 1994 | 24.2 | 0.253 | (21) |
| Rich-Edwards et
al, 1999 | 34.7 | 0.176 | (35) |
Discussion
Previous studies have demonstrated that birth weight
is associated with chronic diseases, including obesity (41), cardiovascular disease (42) and hypertension (43). However, the association between birth
weight and T2DM is still unclear. Some studies have demonstrated a
U-shaped curve relationship between them (20–22),
while other studies have indicated a negative linear association
(18,44–46). In
the present study, a meta-analysis of the published literature was
conducted, which studied the association between birth weight and
T2DM. A total of eight studies were selected for analysis. Forest
plots were constructed comparing T2DM risk in cases of low and
normal birth weight, high and normal birth weight, and low and high
birth weight, respectively. The analysis indicated that low birth
weight increased the risk of T2DM and high birth weight had no
notable influence on the risk of T2DM.
The studies indicated that low birth weight was
related to T2DM; however, the mechanism by which low birth weight
increases the risk of T2DM remains unclear (47–52).
Some research has suggested that it may be a compensatory
adaptation to an adverse intrauterine environment during fetal
development. The smaller fetus and structural and functional change
of important organs leads to insulin resistance and abnormal islet
development, which could cause diabetes in adults (53). A lack of nutrients may have a
permanent influence on the fetal metabolism, increasing the risk of
obesity and T2DM in adults (54).
Some studies employed 31P magnetic
resonance spectroscopy and identified that glycolysis was decreased
in cases of low birth weight. The adipose tissue in muscles also
was decreased in these cases (55,56).
Indirect calorimetry or carbohydrate metabolism efficiency using
13C indicated that the oxidation ability of postprandial
glucose was decreased in cases of low birth weight (57). These studies are instrumental to
understanding a possible mechanism between low birth weight and
T2DM.
Poor intrauterine nutrition leads to low birth
weight (58). For a fetus with low
birth weight, leptin level was increased during childhood and
adiponectin level was also positively related to birth weight,
which increased the incidence rate of T2DM (59). This suggests that low birth weight
may be a clinical marker of poor intrauterine environment and a
potential risk factor for T2DM.
The present study has several limitations that
require further consideration. Due to limited data, it was not
possible to perform further stratification analyses of other
potential influencing factors, including gender.
In conclusion, the current meta-analysis
demonstrated that low birth weight increases the risk of T2DM. Low
birth weight may be a potential risk factor and marker for T2DM.
Further research is required to elucidate the etiopathogenic
mechanisms behind this association.
Acknowledgements
The present study was funded by the Beijing Talents
Fund (grant no. 2015000021469G220), the National Natural Science
Foundation of China (grant no. 8160105) and the Beijing Municipal
Administration of Hospitals' Youth Programme (grant no.
QML20160501).
References
|
1
|
Wild S, Roglic G, Green A, Sicree R and
King H: Global prevalence of diabetes: Estimates for the year 2000
and projections for 2030. Diabetes care. 27:1047–1053. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zimmet P: Globalization, coca-colonization
and the chronic disease epidemic: Can the Doomsday scenario be
averted? J Intern Med. 247:301–310. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Beaumont RN, Horikoshi M, McCarthy MI and
Freathy RM: How can genetic studies help us to understand links
between birth weight and Type 2 diabetes? Curr Diab Rep. 17:222017.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Andersen MK, Pedersen CE, Moltke I, Hansen
T, Albrechtsen A and Grarup N: Genetics of Type 2 diabetes: The
power of isolated populations. Curr Diab Rep. 16:652016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vejrazkova D, Lukasova P, Vankova M,
Bradnova O, Vacinova G, Vcelak J, Cirmanova V, Andelova K, Krejci H
and Bendlova B: Gestational diabetes-metabolic risks of adult women
with respect to birth weight. Physiol Res. 64 Suppl 2:S135–S145.
2015.PubMed/NCBI
|
|
6
|
Stansfield BK, Fain ME, Bhatia J, Gutin B,
Nguyen JT and Pollock NK: Nonlinear relationship between birth
weight and visceral fat in adolescents. J Pediatr. 174:185–192.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barker DJ, Hales CN, Fall CH, Osmond C,
Phipps K and Clark PM: Type 2 (non-insulin-dependent) diabetes
mellitus, hypertension and hyperlipidaemia (syndrome X): Relation
to reduced fetal growth. Diabetologia. 36:62–67. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Brons C, Jacobsen S, Hiscock N, White A,
Nilsson E, Dunger D, Astrup A, Quistorff B and Vaag A: Effects of
high-fat overfeeding on mitochondrial function, glucose and fat
metabolism, and adipokine levels in low-birth-weight subjects. Am J
Physiol Endocrinol Metab. 302:E43–E51. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kahn HS, Graff M, Stein AD and Lumey LH: A
fingerprint marker from early gestation associated with diabetes in
middle age: The Dutch hunger winter families study. Int J
Epidemiol. 38:101–109. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Norris SA, Osmond C, Gigante D, Kuzawa CW,
Ramakrishnan L, Lee NR, Ramirez-Zea M, Richter LM, Stein AD, Tandon
N, et al: Size at birth, weight gain in infancy and childhood, and
adult diabetes risk in five low- or middle-income country birth
cohorts. Diabetes care. 35:72–79. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ong KK and Dunger DB: Thrifty genotypes
and phenotypes in the pathogenesis of type 2 diabetes mellitus. J
Pediatr Endocrinol Metab. 13 Suppl 6:S1419–S1424. 2000. View Article : Google Scholar
|
|
12
|
Ong KK, Ahmed ML, Emmett PM, Preece MA and
Dunger DB: Association between postnatal catch-up growth and
obesity in childhood: Prospective cohort study. BMJ (Clinical
research ed). 320:967–971. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yajnik C: Interactions of perturbations in
intrauterine growth and growth during childhood on the risk of
adult-onset disease. Proc Nutr Soc. 59:pp. 257–265. 2000,
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Stettler N, Bovet P, Shamlaye H, Zemel BS,
Stallings VA and Paccaud F: Prevalence and risk factors for
overweight and obesity in children from Seychelles, a country in
rapid transition: The importance of early growth. Int J Obes Relat
Metab Disord. 26:214–219. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Durmus B, Mook-Kanamori DO, Holzhauer S,
Hofman A, van der Beek EM, Boehm G, Steegers EA and Jaddoe VW:
Growth in foetal life and infancy is associated with abdominal
adiposity at the age of 2 years: The generation R study. Clin
Endocrinol (Oxf). 72:633–640. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Fowden AL and Forhead AJ: Endocrine
interactions in the control of fetal growth. Nestle Nutr Inst
Workshop Ser. 74:pp. 91–102. 2013, View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hales CN: Non-insulin-dependent diabetes
mellitus. Br Med Bull. 53:109–122. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Godfrey KM and Barker DJ: Fetal nutrition
and adult disease. Am J Clin Nutr. 71 5 Suppl:1344S–1352S.
2000.PubMed/NCBI
|
|
19
|
Whincup PH, Kaye SJ, Owen CG, Huxley R,
Cook DG, Anazawa S, Barrett-Connor E, Bhargava SK, Birgisdottir BE,
Carlsson S, et al: Birth weight and risk of type 2 diabetes: A
systematic review. Jama. 300:2886–2897. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dyck RF, Klomp H and Tan L: From ‘thrifty
genotype’ to ‘hefty fetal phenotype’: The relationship between high
birth weight and diabetes in Saskatchewan registered Indians. Can J
Public Health. 92:340–344. 2001.PubMed/NCBI
|
|
21
|
McCance DR, Pettitt DJ, Hanson RL,
Jacobsson LT, Knowler WC and Bennett PH: Birth weight and
non-insulin dependent diabetes: Thrifty genotype, thrifty
phenotype, or surviving small baby genotype? BMJ (Clinical research
ed). 308:942–945. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wei JN, Sung FC, Li CY, Chang CH, Lin RS,
Lin CC, Chiang CC and Chuang LM: Low birth weight and high birth
weight infants are both at an increased risk to have type 2
diabetes among school children in taiwan. Diabetes care.
26:343–348. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Harder T, Rodekamp E, Schellong K,
Dudenhausen JW and Plagemann A: Birth weight and subsequent risk of
type 2 diabetes: A meta-analysis. Am J Epidemiol. 165:849–857.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Alberti KG and Zimmet PZ: Definition,
diagnosis and classification of diabetes mellitus and its
complications. Part 1: Diagnosis and classification of diabetes
mellitus provisional report of a WHO consultation. Diabet Med.
15:539–553. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Classification and diagnosis of diabetes
mellitus and other categories of glucose intolerance. National
diabetes data group. Diabetes. 28:1039–1057. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
American Diabetes Association, . Diagnosis
and classification of diabetes mellitus. Diabetes Care. 35 Suppl
1:S64–S71. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wells GA, Shea BJ, O'Connell D, Peterson
J, Welch V, Losos M and Tugwell P: The Newcastle-Ottawa Scale (NOS)
for assessing the quality of non-randomized studies in
meta-analysis. Ottawa Hospital Research Institute; Ottawa, ON, USA:
2009, http://www.ohri.ca/programs/clinical_epidemiology/oxford.aspFebruary
1–2009
|
|
28
|
Higgins JP, Thompson SG, Deeks JJ and
Altman DG: Measuring inconsistency in meta-analyses. BMJ (Clinical
research ed). 327:557–560. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Begg CB and Mazumdar M: Operating
characteristics of a rank correlation test for publication bias.
Biometrics. 50:1088–1101. 1994. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Eriksson JG, Forsen T, Tuomilehto J,
Osmond C and Barker DJ: Early adiposity rebound in childhood and
risk of Type 2 diabetes in adult life. Diabetologia. 46:190–194.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Barker DJ, Eriksson JG, Forsen T and
Osmond C: Fetal origins of adult disease: Strength of effects and
biological basis. Int J Epidemiol. 31:1235–1239. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Young TK, Martens PJ, Taback SP, Sellers
EA, Dean HJ, Cheang M and Flett B: Type 2 diabetes mellitus in
children: Prenatal and early infancy risk factors among native
canadians. Arch Pediatr Adolesc Med. 156:651–655. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jornayvaz FR, Vollenweider P, Bochud M,
Mooser V, Waeber G and Marques-Vidal P: Low birth weight leads to
obesity, diabetes and increased leptin levels in adults: The CoLaus
study. Cardiovasc Diabetol. 15:732016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Curhan GC, Willett WC, Rimm EB, Spiegelman
D, Ascherio AL and Stampfer MJ: Birth weight and adult
hypertension, diabetes mellitus, and obesity in US men.
Circulation. 94:3246–3250. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rich-Edwards JW, Colditz GA, Stampfer MJ,
Willett WC, Gillman MW, Hennekens CH, Speizer FE and Manson JE:
Birth weight and the risk for type 2 diabetes mellitus in adult
women. Ann Intern Med. 130:278–284. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hales CN, Barker DJ, Clark PM, Cox LJ,
Fall C, Osmond C and Winter PD: Fetal and infant growth and
impaired glucose tolerance at age 64. BMJ (Clinical research ed).
303:1019–1022. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fall CH, Stein CE, Kumaran K, Cox V,
Osmond C, Barker DJ and Hales CN: Size at birth, maternal weight,
and type 2 diabetes in South India. Diabet Med. 15:220–227. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Forsen T, Eriksson J, Tuomilehto J,
Reunanen A, Osmond C and Barker D: The fetal and childhood growth
of persons who develop type 2 diabetes. Ann Intern Med.
133:176–182. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Eriksson M, Wallander MA, Krakau I, Wedel
H and Svardsudd K: Birth weight and cardiovascular risk factors in
a cohort followed until 80 years of age: The study of men born in
1913. J Intern Med. 255:236–246. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kaijser M, Bonamy AK, Akre O, Cnattingius
S, Granath F, Norman M and Ekbom A: Perinatal risk factors for
diabetes in later life. Diabetes. 58:523–526. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bernardi JR, Goldani MZ, Pinheiro TV,
Guimaraes LSP, Bettiol H, da Silva AAM and Barbieri MA: Gender and
social mobility modify the effect of birth weight on total and
central obesity. Nutr J. 16:382017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Yeung Au SL, Lin SL, Li AM and Schooling
CM: Birth weight and risk of ischemic heart disease: A Mendelian
randomization study. Sci Rep. 6:384202016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Dong YH, Zou ZY, Yang ZP, Wang ZH, Jing J,
Luo JY, Zhang X, Luo CY, Wang H, Zhao HP, et al: Association
between high birth weight and hypertension in children and
adolescents: A cross-sectional study in China. J Hum Hypertens.
2017. View Article : Google Scholar
|
|
44
|
Lithell HO, McKeigue PM, Berglund L,
Mohsen R, Lithell UB and Leon DA: Relation of size at birth to
non-insulin dependent diabetes and insulin concentrations in men
aged 50–60 years. BMJ (Clinical research ed). 312:406–410. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Milovanovic I, Njuieyon F, Deghmoun S,
Chevenne D, Levy-Marchal C and Beltrand J: SGA children with
moderate catch-up growth are showing the impaired insulin secretion
at the age of 4. PLoS One. 9:e1003372014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
James-Todd TM, Karumanchi SA, Hibert EL,
Mason SM, Vadnais MA, Hu FB and Rich-Edwards JW: Gestational age,
infant birth weight, and subsequent risk of type 2 diabetes in
mothers: Nurses' health study II. Prev Chronic Dis. 10:E1562013.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Lawlor DA, Smith Davey G and Ebrahim S:
Birth weight of offspring and insulin resistance in late adulthood:
Cross sectional survey. BMJ (Clinical research ed). 325:3592002.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yajnik CS: Early life origins of insulin
resistance and type 2 diabetes in India and other Asian countries.
J Nutr. 134:205–210. 2004.PubMed/NCBI
|
|
49
|
Mi J, Law C, Zhang KL, Osmond C, Stein C
and Barker D: Effects of infant birth weight and maternal body mass
index in pregnancy on components of the insulin resistance syndrome
in China. Ann Intern Med. 132:253–260. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Osmond C and Barker DJ: Fetal, infant, and
childhood growth are predictors of coronary heart disease,
diabetes, and hypertension in adult men and women. Environ Health
Perspect. 108 Suppl 3:S545–S553. 2000. View Article : Google Scholar
|
|
51
|
Eriksson JG, Forsen TJ, Osmond C and
Barker DJ: Pathways of infant and childhood growth that lead to
type 2 diabetes. Diabetes Care. 26:3006–3010. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kajantie E, Osmond C, Barker DJ and
Eriksson JG: Preterm birth-a risk factor for type 2 diabetes? The
Helsinki birth cohort study. Diabetes care. 33:2623–2625. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Phillips DI, Hirst S, Clark PM, Hales CN
and Osmond C: Fetal growth and insulin secretion in adult life.
Diabetologia. 37:592–596. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mortensen B, Hingst JR, Frederiksen N,
Hansen RW, Christiansen CS, Iversen N, Friedrichsen M, Birk JB,
Pilegaard H, Hellsten Y, et al: Effect of birth weight and 12 weeks
of exercise training on exercise-induced AMPK signaling in human
skeletal muscle. Am J Physiol Endocrinol Metab. 304:E1379–E1390.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Taylor DJ, Thompson CH, Kemp GJ, Barnes
PR, Sanderson AL, Radda GK and Phillips DI: A relationship between
impaired fetal growth and reduced muscle glycolysis revealed by 31P
magnetic resonance spectroscopy. Diabetologia. 38:1205–1212. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Ivorra C, Garcia-Vicent C, Chaves FJ,
Monleon D, Morales JM and Lurbe E: Metabolomic profiling in blood
from umbilical cords of low birth weight newborns. J Transl Med.
10:1422012. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
von Bonsdorff MB, Muller M, Aspelund T,
Garcia M, Eiriksdottir G, Rantanen T, Gunnarsdottir I, Birgisdottir
BE, Thorsdottir I, Sigurdsson G, et al: Persistence of the effect
of birth size on dysglycaemia and type 2 diabetes in old age:
AGES-Reykjavik study. Age (Dordr). 35:1401–1409. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Mooorthi MMS, Nadesan B, Ramalingam E and
Thirumalaikumarasamy S: A study of maternal factors influencing
very low birth weight babies. Int J Contemp Pediatr. 4:1173–1178.
2017. View Article : Google Scholar
|
|
59
|
Stocker C, O'Dowd J, Morton NM, Wargent E,
Sennitt MV, Hislop D, Glund S, Seckl JR, Arch JR and Cawthorne MA:
Modulation of susceptibility to weight gain and insulin resistance
in low birth weight rats by treatment of their mothers with leptin
during pregnancy and lactation. Int J Obes Relat Metab Disord.
28:129–136. 2004. View Article : Google Scholar : PubMed/NCBI
|