Introduction
Cleft palate is among the most common congenital
birth defects in humans (1–3), and is caused by a failure in secondary
palate formation (4,5). From embryonic days (E) 12.5 to E15.5,
the secondary palate is initiated to extend from the maxillary
processes to form the palatal shelves (6). At approximately E13.5, the bilateral
palatal shelves elongate and elevate to form the midline edge seam
(7), while the size of the palatal
shelves is determined by the proliferation and apoptosis of mouse
embryonic palatal mesenchymal (MEPM) cells (8). During this period, disturbances in the
normal viability or extracellular matrix secretion of MEPM cells
can cause cleft palate (9). These
two processes are controlled by complex signaling cascades that are
induced by a number of growth factors, including members of the
wingless-related MMTV integration site gene family (Wnt) (8,10,11).
The Wnt signaling glycoproteins serve critical roles
in cell viability, differentiation, migration, polarity, and death,
and thereby regulate embryonic development (12–18). On
the basis of their downstream signal transduction, Wnts have been
divided into ‘β-catenin-dependent’ and ‘β-catenin-independent’
signaling proteins. β-catenin-dependent signaling is defined by the
stabilization of β-catenin, which enters the nucleus and forms a
complex with lymphoid-enhancer-binding factor/T cell factor (TCF)
DNA-binding proteins to activate transcription of Wnt target genes
(12–16). Meanwhile, β-catenin-independent
signaling performed without β-catenin includes a number of less
well characterized intracellular pathways (18–20). In
previous studies, the Wnt signaling pathways have been suggested to
serve important roles in craniofacial development (21,22) and
palate formation (8,21,23–25).
Wnt family member 6 (Wnt6), as a member of the Wnt
signaling family, has been reported to be essential for the
viability of macrophages (26),
myoblasts (27) and stromal cells
(28), and to be expressed in mouse
embryonic palatal tissue from E12.5 to E14.5 (10). Furthermore, the WNT6-WNT10A
cluster has been identified to exhibit linkage and disequilibrium
in cleft palate (29). These
previous data indicate that Wnt6 participates in embryonic
development of the palate. However, the exact role of Wnt6 in
palate development remains unclear.
The purpose of the present study was to investigate
the effect of Wnt6 in MEPM cells using the MTT assay, flow
cytometry, western blot analysis and reporter gene assay. The
results suggest that Wnt6 may regulate the viability of palatal
mesenchymal cells through the β-catenin pathway.
Materials and methods
Ethics statement
This study was carried out in strict accordance with
the recommendations in the Guide for the Care and Use of Laboratory
Animals of the National Institutes of Health (Bethesda, MD, USA)
(6). The protocol was approved by
the Committee for the Ethics of Animal Experiments of Xiamen
University, Xiamen, China (permit no. SCXK2013-0001). All surgical
procedures were performed under urethane (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) anesthesia (1.0 g/kg via intraperitoneal
injection), and all efforts were made to minimize suffering.
MEPM cell culture
A total of 60 female and 20 male wild-type CD1 mice
(Charles River Laboratories, Inc., Wilmington, MA, USA) of the same
strain were housed at an ambient temperature of 22°C with 12-h
light/dark cycle and had access to food and water ad
libitum. Mature male and female mice (6 weeks old, weighing
17–23 g) were mated overnight, and the presence of a vaginal plug
the following morning was designated as E0.5. Primary MEPM cell
cultures were established from secondary palatal tissue
microdissected from E13.5 mouse embryos (11). The dissected palate shelves were
treated with 1 U/ml dispase II (Roche Diagnostics, Indianapolis,
IN, USA) at 37°C for 15 min (8,30).
Following removal of the desquamated epithelia, the tissue was
digested with 0.25% trypsin/0.05% EDTA, and then the dissociated
cells were cultured for 48 h in Dulbecco's modified Eagle's
medium/nutrient mixture F-12 (DMEM/F12; Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA) with 10% fetal bovine serum (FBS;
Hyclone; GE Healthcare Life Sciences) and 1%
penicillin/streptomycin at 37°C in a humidified atmosphere of 5%
CO2 in air. Cells were passaged and seeded at
4×105 cells/25 cm2 in DMEM/F12 without FBS
and incubated at 37°C for 40 min; in such conditions, the
epithelial cells adhered more slowly than the MEPM cells. After
culturing for 40 min at 37°C, the medium without FBS was replaced
with fresh DMEM/F12 including FBS and antibiotics to acquire MEPM
cells of high purity. Cells at passage 3 cultured at 37°C for 8 h
were used for subsequent assays. Experimental cell groups were
established, in which cells were always incubated with additional
100 ng/ml recombinant Wnt6 (Abgent, Inc., San Diego, CA, USA) with
or without additional 100 ng/ml recombinant dickkopf WNT signaling
pathway inhibitor 1 (DKK1; R&D Systems, Inc., Minneapolis, MN,
USA) in DMEM/F12 containing 10% FBS and 1% penicillin/streptomycin
at 37°C. In the control cell group, MEPM cells were only cultured
with 10% FBS and 1% penicillin/streptomycin in DMEM/F12.
MTT assay
The MEPM cells were transferred to 24-well plates
(Corning Incorporated, Corning, NY, USA) at a density of
1.6×104 cells/well and cultured at 37°C in DMEM/F12 with
10% FBS for 1–4 days. MTT solution (5 mg/ml) was added to each well
and the plates were incubated at 37°C for 4 h. Subsequently, the
supernatant was removed and dimethyl sulfoxide 0.5 mg/ml was added
to each well to dissolve the formazan crystals. The optical density
at 490 nm was quantified using the PowerWave XS2 package at daily
intervals over the 4-day assay period (BioTek Instruments, Inc.,
Winooski, VT, USA). Three independent replicates were performed for
each cell group.
Flow cytometry
Following treatment with or without Wnt6 or Wnt6 +
DKK1 for 48 h as stated above, the MEPM cells were harvested and
fixed in cold 75% ethanol at −4°C overnight, cells were pelleted
and washed twice in 1× PBS at 1,000 × g for 5 min at 4°C.
Subsequently, cells were incubated with 50 µg/ml propidium iodide
and 5 µg/ml RNAase at room temperature for 30 min. Following
supplementation with 50 mg/l propidium iodide and incubation in the
dark for 1 h at room temperature, cell cycle analysis was performed
using a BD FACS Calibur flow cytometer (BD Biosciences, Franklin
Lakes, NJ, USA) and CellQuest Pro 5.1 software (BD Biosciences)
with a minimum of 20,000 events for each cell group.
Reporter gene assay
After 48 h of treatment with or without 100 ng/ml
recombinant Wnt6 as stated above, the MEPM cells were seeded at
1.6×105 cells/well onto 12-well plates, and transient
transfections for 5 min at room temperature were performed using
Lipofectamine 2,000 (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). Cells were transfected with 1.6 µg of TCF
luciferase reporter constructs (Promega Corporation, Madison, WI,
USA) (TOPflash or FOPflash, respectively) and 0.08 µg of Renilla
reniformis gene vectors (Promega Corporation) for
normalization. The TOPflash TCF reporter plasmid contains two sets
of three copies of the binding site upstream of the thymidine
kinase minimal promoter and luciferase open reading frame, while
FOPflash contains mutated TCF binding sites and was used as a
negative control (31–34). A subset of the cells lacking Wnt6
treatment were also cotransfected with β-catenin pcDNA (1.6 µg;
Biocytogen LLC, Beijing, China) as a positive control. Following 48
h of incubation, the luciferase assay was performed using a Dual
Luciferase Assay System kit (Promega Corporation) according to the
manufacturer's protocol. Relative luciferase activity was reported
as the ratio of firefly/Renilla luciferase activity.
Western blot analysis
Total protein was isolated from cultured MEPM cells
after 48 h of treatment with or without Wnt6 ± DKK1 using RIPA
lysis and extraction buffer (Beyotime Institute of Biotechnology,
Haimen, China). Protein quantification was performed with a Bio-Rad
DC Protein Assay kit (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). The intracellular protein expression levels of β-catenin and
β-actin and the Wnt6 protein levels in the cell culture supernatant
were assessed. To obtain Wnt6 protein from the supernatant,
conditioned culture media were collected and concentrated with
Amicon® Ultra-4 Centrifugal Filter Units (10,000 NMWL;
EMD Millipore, Billerica, MA, USA), then extracted with a Protein
Extraction kit II (Applygen Technologies, Inc., Beijing, China), as
described previously (22). Equal
amounts of protein (60 µg per lane) were separated on 10%
SDS-polyacrylamide gels and transferred onto
polyvinylidenedifluoride membranes (Roche Diagnostics). The
membrane was blocked in a 6% non-fat milk solution in Tris-buffered
saline with 0.5% Tween-20 (TBST) (Roche Diagnostics) at room
temperature for 1 h, and then incubated with rabbit anti-Wnt6
monoclonal antibody (Abcam, Cambridge, UK; cat. no. ab154144;
dilution 1:200), rabbit anti-β-catenin monoclonal antibody (Abcam;
cat. no. ab32572; dilution 1:500) or rabbit anti-β-actin monoclonal
antibody (Wuhan Antgene Biotechnology Co., Ltd, Wuhan, China; cat.
no. ANT009; dilution 1:800) overnight at 4°C. After rinsing with
TBST for three times, the goat anti-rabbit HRP-conjugated secondary
antibody (Wuhan Antgene Biotechnology Co., Ltd., cat. no. ANT020;
dilution 1:5,000) was applied to the membranes at room temperature
for 1 h. The blot was visualized using SuperSignal West Pico
Chemiluminesent Substrate (Thermo Fisher Scientific, Inc.) and the
protein bands were analyzed with ImageJ 1.48 software (National
Institutes of Health).
Statistical analysis
All quantitative data were presented as the mean ±
standard deviation. Statistical analysis of differences was
performed with SPSS 19.0 software (SPSS, Inc., Chicago, IL, USA)
P<0.05 was considered to indicate statistical significance. The
significance of data was determined by one-way analysis of variance
followed by a Bonferroni post-hoc test.
Results
Wnt6 promotes the viability of MEPM
cells
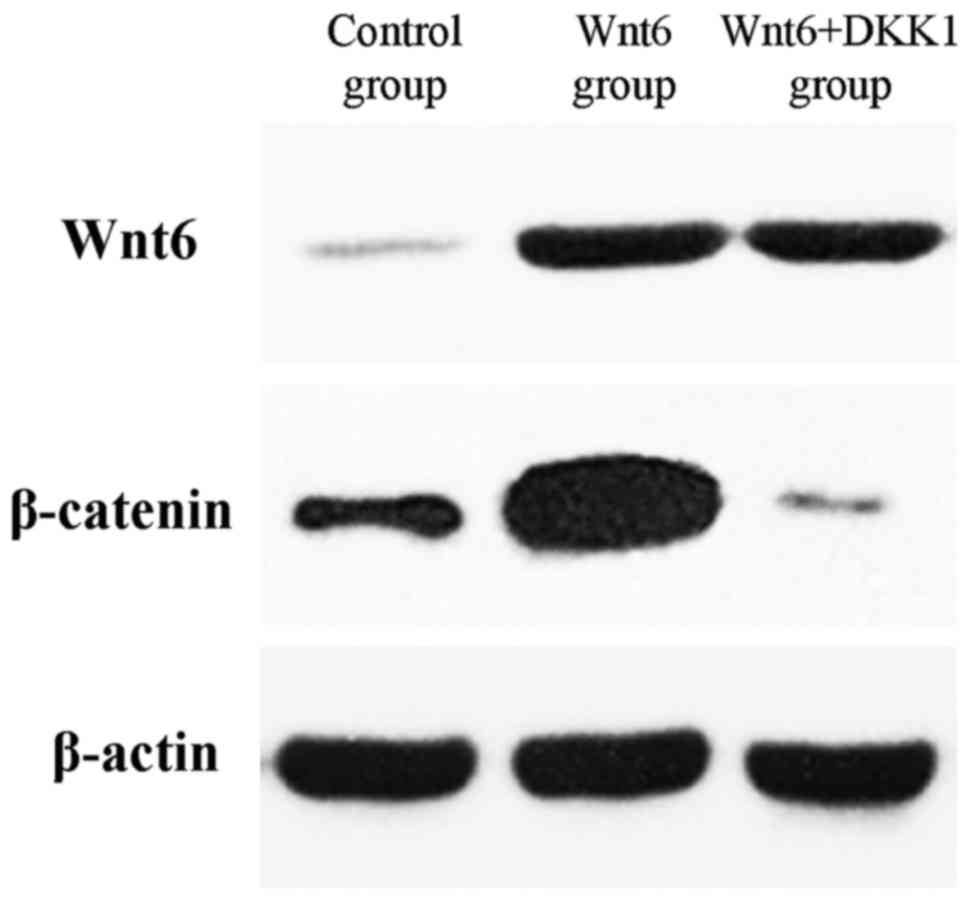
The expression of Wnt6 was identified in primary
(control) MEPM cells at E13.5 (Fig.
1). To investigate the effect of Wnt6 on MEPM cell viability,
recombinant Wnt6 was added to the culture medium, and cell
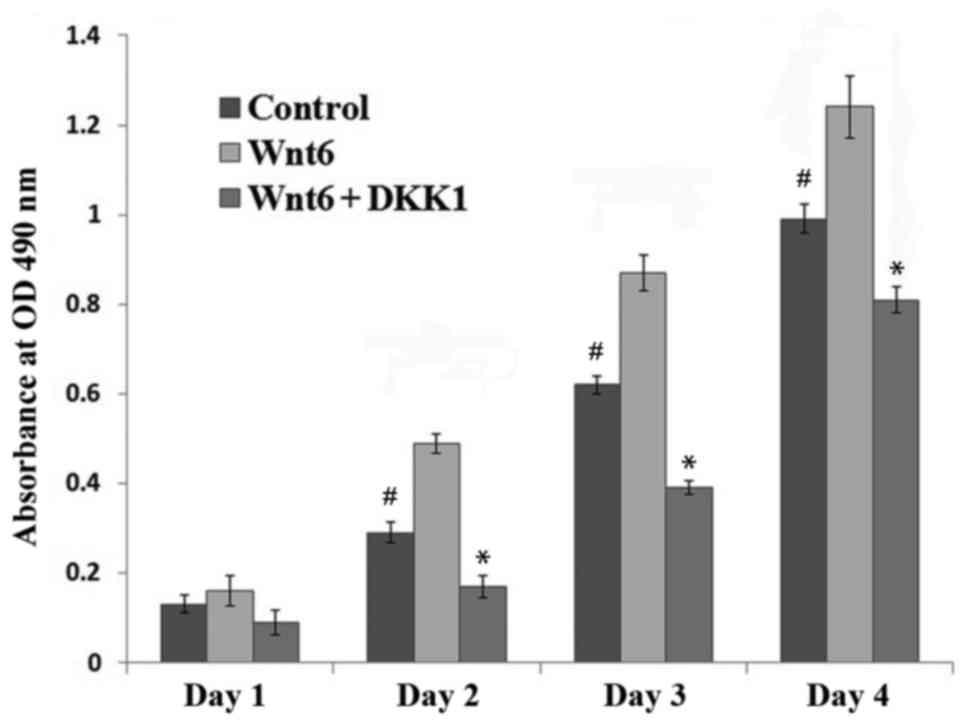
viability was assessed with an MTT assay. It was observed that the
viability of MEPM cells treated with Wnt6 was significantly higher
than that of control cells between 2 and 4 days after treatment
(P<0.01; Fig. 2).
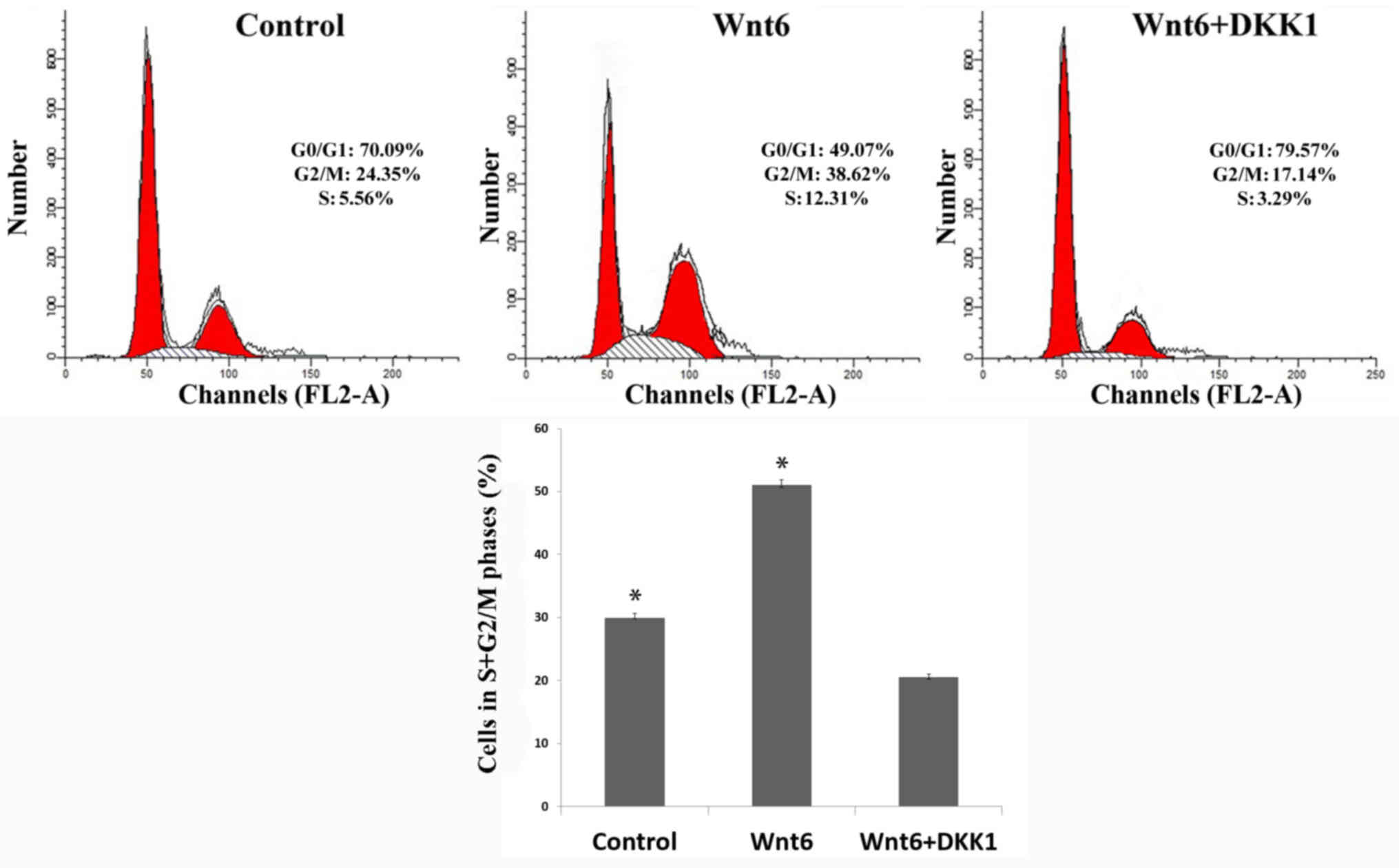
In addition, the results of flow cytometric analysis
indicated that the proportion of primary MEPM cells in S + G2/M
phases (29.91±0.72%) was significantly increased by treatment with
Wnt6 (50.93±0.89%; P<0.01; Fig.
3). These findings suggested that Wnt6 promoted the viability
of MEPM cells and increased the population of S + G2/M-phase
cells.
Wnt6 activates the β-catenin/TCF
signaling pathway
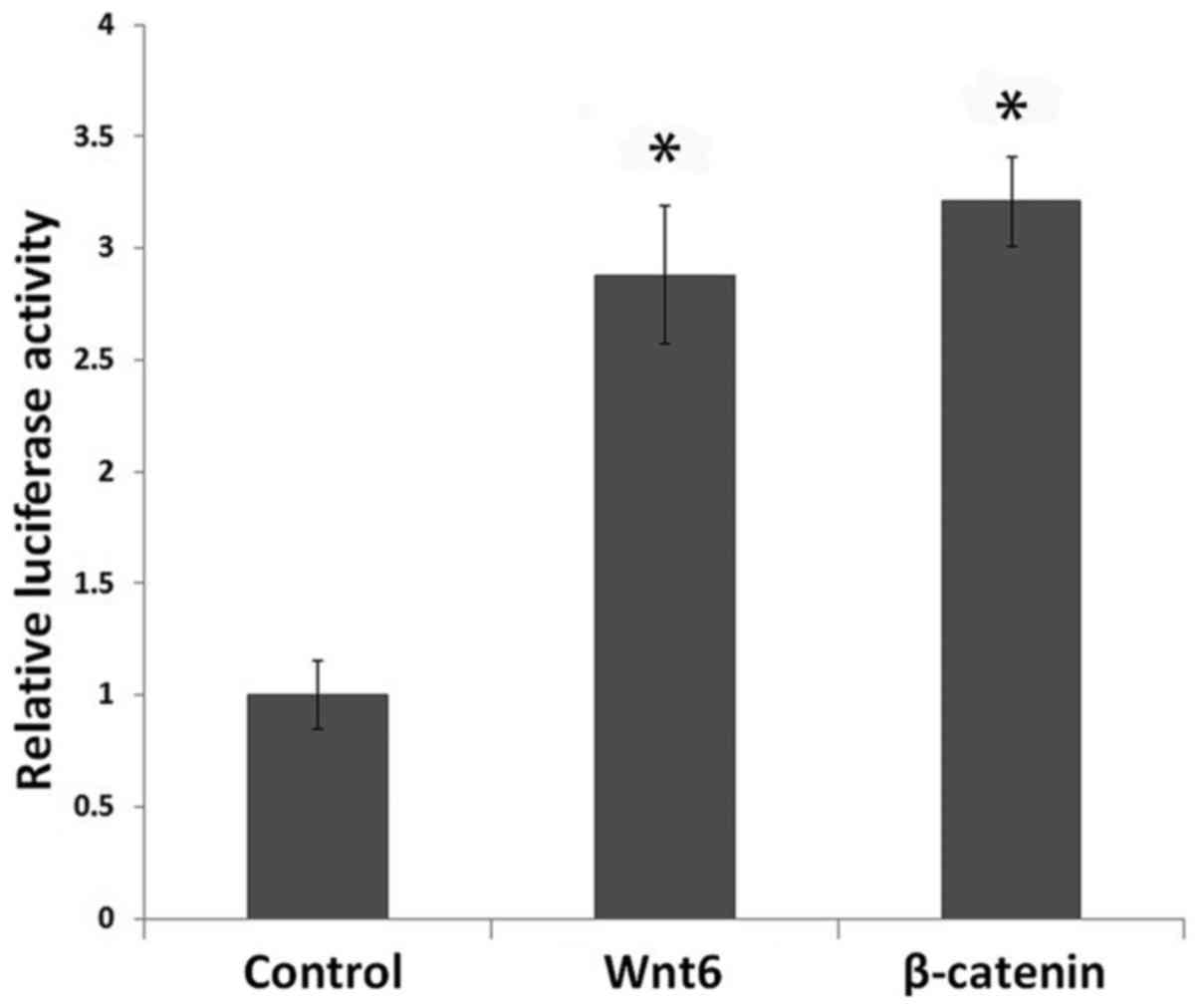
A TOP flash luciferase reporter assay was performed
to investigate whether Wnt6 activates Wnt/β-catenin signaling in
MEPM cells. Cells treated with Wnt6 or pcDNA-β-catenin exhibited
significantly higher baselines of β-catenin/TCF-mediated
transcriptional activity when compared with the control cells
(P<0.01). In addition, the TOPflash reporter activity did not
differ significantly between the Wnt6 and β-catenin groups
(FOPflash data not shown) (Fig. 4).
Furthermore, the total amount of β-catenin was markedly increased
in the Wnt6-treated cells when compared with the control cells
(Fig. 1).
Blockade of the WNT/β-catenin pathway
reduces the cytoactivity of Wnt6
DKK1 binds to low density lipoprotein
receptor-related protein 5/6 and is considered to be a general
canonical Wnt signaling inhibitor (35). In the present study, DKK1 was used to
confirm the effect of Wnt6⁄β-catenin signaling on MEPM cell
viability. Cells treated with Wnt6 were incubated with or without
DKK1. As depicted in Fig. 1, the
additional treatment with DKK1 suppressed the expression of
β-catenin. In addition, the results in Fig. 2 demonstrated that DKK1 significantly
reduced the Wnt6-mediated increase in cell viability from day 2
post-treatment (P<0.01). Furthermore, when the Wnt6/β-catenin
pathway was blocked, the viability of MEPM cells was significantly
lower than that of control cells (P<0.01). A notable decrease in
the S + G2/M cell proportion was also observed in the DKK1 + Wnt6
group (20.43±0.59%; Fig. 3) when
compared with the control and Wnt6 groups (P<0.01), and cell
cycle arrest in G0/G1 phase was indicated in the DKK1 + Wnt6
group.
Discussion
Embryonic development of the palate is a
temporospatially coordinated process. The secondary palate is
initiated to extend from the maxillary processes to form the
palatal shelves between E12.5 and E15.5 (6). At approximately E13.5, the bilateral
palatal shelves, composed of a core of mesenchyme cells, elongate
and elevate toward each other (7).
In the present study, to investigate the role of Wnt6 during the
stages of palatal shelf elongation and elevation, MEPM cells were
cultured from palatal shelves dissected from mouse fetuses at
E13.5. The results of MTT assay and flow cytometry analysis
indicated that the viability and S + G2/M population of the MEPM
cells was increased by Wnt6. Meanwhile, a dual luciferase assay and
analysis with DKK1 identified a pivotal role of β-catenin signaling
in the development of MEPM cells. Collectively, the results
indicated that Wnt6 promoted the viability of the MEPM cells by
increasing the population of S + G2/M cells. The involvement of
Wnt6 in the β-catenin pathway during palatal shelf elongation and
elevation was also implicated.
Former studies have already demonstrated that Wnt6
is a necessary cytoactive factor in macrophages (26), myoblasts (27) and stromal cells (28). Wnt6 has also been reported to be
expressed in the mouse embryonic palatal tissue from E12.5 to E14.5
(10), and the WNT6-WNT10A
cluster exhibits linkage and disequilibrium in cleft palate
(29). In the present study, the
effect of Wnt6 on MEPM cell viability during embryonic palate
development was elucidated in vitro. Further studies into
the effects of Wnt6 knockout on palatogenesis in mice should now be
performed to identify potentially associated malformations and
regulatory mechanisms.
Numerous different factors are involved in the
complex transduction of Wnt signals, though particular cells only
respond to specific Wnt factors (36). It has been reported that Wnt6
activates β-catenin signaling in F9 embryonal carcinoma cells and
serves an important role in endoderm specification (37,38) and
formation (39). Furthermore, Wnt6
restricted heart muscle development in embryonic mesenchymal cells
through the β-catenin pathway (40),
and the osteoblastogenesis of mesenchymal stem cells was regulated
by Wnt6⁄β-catenin signaling (41).
In the current study, Wnt6 was indicated to stimulate the
transcriptional activity of β-catenin, suggesting that it may
activate Wnt/β-catenin signaling to enhance the viability of MEPM
cells. Treatment with a recombinant version of the Wnt signaling
antagonist DKK1 was also performed to verify the relationship
between Wnt6 and β-catenin signaling. The results demonstrated that
the increased viability of Wnt6-treated MEPM cells was inhibited by
DKK1. This data confirmed the stimulatory effect of Wnt6 on the
viability of MEPM cells. However, as DKK1 is not a specific
inhibitor of Wnt6, further studies are required to confirm the
exact signal transduction mechanism of the Wnt6/β-catenin pathway
in MEPM cells.
In addition, the present study identified that
compromised Wnt6 activity and β-catenin signaling in MEPM cells
reduced the cytoactivity of Wnt6 and caused cell cycle arrest in
G0/G1 phase when compared with control cells. Thus, conditional
inhibition of the β-catenin pathway may disturb the normal
cytoactivity of MEPM cells, which may ultimately lead to the
development of cleft palate.
It is established that reduced Wnt/β-catenin
signaling is involved in the development of cleft palate (21,23–25).
Furthermore, the Wnt/β-catenin signaling pathway has previously
been associated with alterations in the viability and apoptosis of
MEPM cells (8). These previous
findings and the present data suggest that Wnt/β-catenin pathway is
among the most important signaling cascades for palatal
development. However, the exact mechanism regarding the involvement
of β-catenin in MEPM cell activity requires further
investigation.
In conclusion, to the best of our knowledge, the
present results are the first to indicate that Wnt6/β-catenin
signaling is involved in the viability of MEPM cells during the
stages of palatal shelf elongation and elevation in vitro,
thus suggesting that Wnt6/β-catenin signaling is involved in the
process of palatogenesis.
Acknowledgements
The present study was supported by the Science and
Technology Program of Xiamen City, China (grant no.
3502Z20154074).
References
|
1
|
Mossey PA, Little J, Munger RG, Dixon MJ
and Shaw WC: Cleft lip and palate. Lancet. 374:1773–1785. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fraser FC: The genetics of cleft lip and
cleft palate. Am J Hum Genet. 22:336–352. 1970.PubMed/NCBI
|
|
3
|
Schutte BC and Murray JC: The many faces
and factors of orofacial clefts. Hum Mol Genet. 8:1853–1859. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Meng T, Shi JY, Wu M, Wang Y, Li L, Liu Y,
Zheng Q, Huang L and Shi B: Overexpression of mouse TTF-2 gene
causes cleft palate. J Cell Mol Med. 16:2362–2368. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ferguson MW: Palate development.
Development. 103 Suppl:S41–S60. 1988.
|
|
6
|
Wu C, Endo M, Yang BH, Radecki MA, Davis
PF, Zoltick PW, Spivak RM, Flake AW, Kirschner RE and Nah HD:
Intra-amniotic transient transduction of the periderm with a viral
vector encoding TGFβ3 prevents cleft palate in Tgfβ3(−/-) mouse
embryos. Mol Ther. 21:8–17. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Meng L, Bian Z, Torensma R and Von den
Hoff JW: Biological mechanisms in palatogenesis and cleft palate. J
Dent Res. 88:22–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Feng C, Xu Z, Li Z, Zhang D, Liu Q and Lu
L: Down-regulation of Wnt10a by RNA interference inhibits
proliferation and promotes apoptosis in mouse embryonic palatal
mesenchymal cells through Wnt/β-catenin signaling pathway. J
Physiol Biochem. 69:855–863. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Li L, Shi JY, Zhu GQ and Shi B: MiR-17-92
cluster regulates cell proliferation and collagen synthesis by
targeting TGFB pathway in mouse palatal mesenchymal cells. J Cell
Biochem. 113:1235–1244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Warner DR, Smith HS, Webb CL, Greene RM
and Pisano MM: Expression of Wnts in the developing murine
secondary palate. Int J Dev Biol. 53:1105–1112. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Warner DR, Mukhopadhyay P, Brock GN, Pihur
V, Pisano MM and Greene RM: TGFβ-1 and Wnt-3a interact to induce
unique gene expression profiles in murine embryonic palate
mesenchymal cells. Reprod Toxicol. 31:128–133. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miller JR: The Wnts. Genome Biol.
3:REVIEWS30012002.PubMed/NCBI
|
|
13
|
Lee HY, Kléber M, Hari L, Brault V, Suter
U, Taketo MM, Kemler R and Sommer L: Instructive role of
Wnt/beta-catenin of in sensory fate specification in neural crest
stem cells. Science. 303:1020–1023. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miki T, Yasuda SY and Kahn M:
Wnt/β-catenin signaling in embryonic stem cell self-renewal and
somatic cell reprogramming. Stem Cell Rev. 7:836–846. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kühl SJ and Kühl M: On the role of
Wnt/β-catenin signaling in stem cells. Biochim Biophys Acta.
1830:2297–2306. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Reya T and Clevers H: Wnt signalling in
stem cells and cancer. Nature. 434:843–850. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Staal FJ and Luis TC: Wnt signaling in
hematopoiesis: Crucial factors for self-renewal, proliferation and
cell fate decisions. J Cell Biochem. 109:844–849. 2010.PubMed/NCBI
|
|
18
|
Nusse R: Wnt signaling and stem cell
control. Cell Res. 18:523–527. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moon RT, Bowerman B, Boutros M and
Perrimon N: The promise and perils of Wnt signaling through
beta-catenin. Science. 296:1644–1646. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Angers S and Moon RT: Proximal events in
Wnt signal transduction. Nat Rev Mol Cell Biol. 10:468–477.
2009.PubMed/NCBI
|
|
21
|
Jin YR, Han XH, Taketo MM and Yoon JK:
Wnt9b-dependent FGF signaling is crucial for outgrowth of the nasal
and maxillary processes during upper jaw and lip development.
Development. 139:1821–1830. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang Z, Von den Hoff JW, Torensma R, Meng
L and Bian Z: Wnt16 is involved in intramembranous ossification and
suppresses osteoblast differentiation through the Wnt/β-catenin
pathway. J Cell Physiol. 229:384–392. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hu X, Gao J, Liao Y, Tang S and Lu F:
Retinoic acid alters the proliferation and survival of the
epithelium and mesenchyme and suppresses Wnt/β-catenin signaling in
developing cleft palate. Cell Death Dis. 4:e8982013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu X, Gao JH, Liao YJ, Tang SJ and Lu F:
Dexamethasone alters epithelium proliferation and survival and
suppresses Wnt/β-catenin signaling in developing cleft palate. Food
Chem Toxicol. 56:67–74. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mostowska A, Hozyasz KK, Wójcicki P,
Lasota A, Dunin-Wilczyńska I and Jagodziński PP: Association of
DVL2 and AXIN2 gene polymorphisms with cleft lip with or without
cleft palate in a Polish population. Birth Defects Res A Clin Mol
Teratol. 94:943–950. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Schaale K, Brandenburg J, Kispert A,
Leitges M, Ehlers S and Reiling N: Wnt6 is expressed in
granulomatous lesions of Mycobacterium tuberculosis-infected mice
and is involved in macrophage differentiation and proliferation. J
Immunol. 191:5182–5195. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hitchins L, Fletcher F, Allen S and Dhoot
GK: Role of Sulf1A in Wnt1- and Wnt6-induced growth regulation and
myoblast hyper-elongation. FEBS Open Bio. 3:30–34. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang Q, Lu J, Zhang S, Wang S, Wang W,
Wang B, Wang F, Chen Q, Duan E, Leitges M, et al: Wnt6 is essential
for stromal cell proliferation during decidualization in mice. Biol
Reprod. 88:52013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Beaty TH, Hetmanski JB, Fallin MD, Park
JW, Sull JW, McIntosh I, Liang KY, Vanderkolk CA, Redett RJ,
Boyadjiev SA, et al: Analysis of candidate genes on chromosome 2 in
oral cleft case-parent trios from three populations. Hum Genet.
120:501–518. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Matsumura K, Taketomi T, Yoshizaki K, Arai
S, Sanui T, Yoshiga D, Yoshimura A and Nakamura S: Sprouty2
controls proliferation of palate mesenchymal cells via fibroblast
growth factor signaling. Biochem Biophys Res Commun. 404:1076–1082.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Thompson MD, Dar MJ and Monga SP:
Pegylated interferon alpha targets Wnt signaling by inducing
nuclear export of β-catenin. J Hepatol. 54:506–512. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kang YJ, Park HJ, Chung HJ, Min HY, Park
EJ, Lee MA, Shin Y and Lee SK: Wnt/β-catenin signaling mediates the
antitumor activity of magnolol in colorectal cancer cells. Mol
Pharmacol. 82:168–177. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
An JH, Yang JY, Ahn BY, Cho SW, Jung JY,
Cho HY, Cho YM, Kim SW, Park KS, Kim SY, et al: Enhanced
mitochondrial biogenesis contributes to Wnt induced osteoblastic
differentiation of C3H10T1/2 cells. Bone. 47:140–150. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim DY, Park YG, Quan HY, Kim SJ, Jung MS
and Chung SH: Ginsenoside Rd stimulates the differentiation and
mineralization of osteoblastic MC3T3-E1 cells by activating
AMP-activated protein kinase via the BMP-2 signaling pathway.
Fitoterapia. 83:215–222. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mao B, Wu W, Li Y, Hoppe D, Stannek P,
Glinka A and Niehrs C: LDL-receptor-related protein 6 is a receptor
for Dickkopf proteins. Nature. 411:321–325. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gordon MD and Nusse R: Wnt signaling:
Multiple pathways, multiple receptors, and multiple transcription
factors. J Biol Chem. 281:22429–22433. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lhomond G, McClay DR, Gache C and Croce
JC: Frizzled1/2/7 signaling directs β-catenin nuclearisation and
initiates endoderm specification in macromeres during sea urchin
embryogenesis. Development. 139:816–825. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Krawetz R and Kelly GM: Wnt6 induces the
specification and epithelialization of F9 embryonal carcinoma cells
to primitive endoderm. Cell Signal. 20:506–517. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hwang JT and Kelly GM: GATA6 and FOXA2
regulate Wnt6 expression during extraembryonic endoderm formation.
Stem Cells Dev. 21:3220–3232. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lavery DL, Martin J, Turnbull YD and
Hoppler S: Wnt6 signaling regulates heart muscle development during
organogenesis. Dev Biol. 323:177–188. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cawthorn WP, Bree AJ, Yao Y, Du B, Hemati
N, Martinez-Santibañez G and MacDougald OA: Wnt6, Wnt10a and Wnt10b
inhibit adipogenesis and stimulate osteoblastogenesis through a
β-catenin-dependent mechanism. Bone. 50:477–489. 2012. View Article : Google Scholar : PubMed/NCBI
|