Introduction
Renal ischemia/reperfusion (I/R) injury is a primary
cause of acute renal failure, which is commonly observed in a
number of clinical settings, including shock and renal
transplantation (1,2). In addition, renal I/R injury is a
leading contributor to the morbidity and mortality of the aging
population, and results from the process of recovering the blood or
oxygen supply following ischemia in the kidney. This process may
then lead to cellular damage in renal tissues (3–5). Under
these conditions, the tissue structure and renal function
deteriorate, and tubular necrosis, medullary hemorrhage and
congestion are observed. Patients with I/R injury have a poor
prognosis and there is currently no available effective therapy to
mitigate this injury (6).
Apigenin is a plant flavone that exist in a variety
of fruits and vegetables, such as celery, parsley and wheat sprouts
(7,8). This compound has been proven to possess
a number of biological properties, including anti-inflammatory,
antioxidant and antitumor effects (9), as well as a protective effect on
several organs. Apigenin can protect cells against apoptosis and
necrosis by inhibiting oxidative stress. Furthermore, it has been
demonstrated that apigenin has a potent therapeutic effect on liver
in rats (10). However, the effects
of apigenin on renal I/R injury remain unclear.
In the kidney, the cellular death receptors Fas and
Fas ligand (FasL) are major mediators of the apoptotic pathway. Fas
belongs to the tumor necrosis factor (TNF) receptor superfamily of
cell surface death receptors, and FasL is a member of the TNF
family that induces apoptosis by cross-linking its Fas receptor
(11,12). Several studies revealed that Fas/FasL
are involved in multifarious forms of renal injury, including I/R
injury and tubular injury in glomerulonephritis (13,14).
Furthermore, the role of B-cell lymphoma 2 (Bcl-2) in the
development of apoptotic cell death has been widely investigated
(15,16). The Bcl-2 family of proteins regulates
cell apoptosis and cell necrosis (17). The Fas/FasL and Bcl-2 genes are two
of the key factors influencing apoptosis and regulating the
intrinsic apoptosis pathway in renal tubular epithelial cells
(18,19). In addition, the intrinsic apoptosis
pathway is activated by oxidative stress, which is mediated by
increased mitochondrial membrane permeability (20). Previous studies have suggested that
oxidative stress is involved in the apoptotic mechanism mediated by
the Fas/FasL signaling pathway (21–23).
Therefore, it is important to verify whether the renoprotective
effect of apigenin are associated with the modulation of the
Fas/FasL and Bcl-2 pathway.
The aim of the present study was to investigate the
protective role of apigenin against renal I/R injury in rats and
the underlying mechanism of its action. The study examined whether
the protective effect of apigenin on renal function was through the
modulation of the Fas/FasL pathway and improvement of the
expression of Bcl-2.
Materials and methods
Ethical approval
The present study was approved by the Local Ethical
Committee of the Renmin Hospital of Wuhan University (Wuhan,
China), and the experimental procedures were performed in
accordance with the principles of the Declaration of Helsinki.
Animals and in vivo experimental
protocol
A total of 36 Sprague-Dawley rats (male; weight,
220±20 g; Hubei Provincial Academy of Preventive Medicine, Wuhan,
China) were randomly separated into six groups (n=6 per group)
based on the randomized block design method, as follows: i) Sham
surgery; ii) I/R injury only; iii) apigenin (50 mg/kg) + sham; iv)
I/R injury + 2 mg/kg apigenin treatment; v) I/R injury + 10 mg/kg
apigenin treatment; and vi) I/R injury + 10 mg/kg apigenin
treatment. In the three I/R + apigenin groups, apigenin was
intravenously injected at 10 min prior to the induction of
ischemia. Apigenin (purity, >98%) was purchased from Shanghai
Aladdin Bio-Chem Technology Co., Ltd. (Shanghai, China).
All the rats were anesthetized with chloral hydrate
intraperitoneally (350 mg/kg). After intravenous injection of
heparin (1,000 UI/kg) and maintaining the body temperature at 37°C,
a midline laparotomy was performed. In the I/R groups, a right
nephrectomy was performed, followed by isolation of the left renal
pedicles (artery, vein and nerve). The left kidney was then
subjected to 45 min of ischemia followed by reperfusion subsequent
to right nephrectomy. During ischemia, the color of the kidneys
changed to a purple shade, which was altered to a blush color
during reperfusion. In the sham and apigenin + sham groups, rats
were subjected to the same surgical procedures as the I/R groups
without left renal clamping. At 24 h after I/R injury, all rats
were sacrificed. Blood samples (1 ml) were collected from the heart
for the measurement of serum creatinine (Cr) and blood urea
nitrogen (BUN) levels. The left kidney was removed and fixed in 4%
paraformaldehyde or immediately frozen, and stored at −80°C for
routine paraffin embedding and further examinations.
Serum assays
Blood samples were centrifuged at 15,000 × g for 10
min at 20°C and the serum was stored at −20°C until further
analyses. Serum was analyzed according to the protocols of the
Creatinine and Urea Assay kits (C013-1 and C011-2; Nanjing
Jiancheng Bioengineering Institute, Nanjing, China). The absorbance
was measured using a spectrophotometer (UV-1700; Shimadzu
Corporation, Tokyo, Japan) at 520 nm. Then, the concentrations of
BUN and Cr were calculated.
Measurement of malondialdehyde (MDA),
superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) in
renal tissues
The renal tissues were homogenized and centrifuged
at 3,000 × g for 10 min at 4°C. Then, the supernatants were
collected for analysis of the MDA, SOD and GSH-Px activity using
commercial SOD, MDA and GSH-Px assay kits (A001-1-1, A003-1 and
A005; Nanjing Jiancheng Bioengineering Institute) according to the
manufacturer's protocols.
Histological examination of renal
tissues
Half of each kidney was removed from the rats and
fixed in 4% paraformaldehyde, followed by routine paraffin
embedding. According to standard procedures, the tissues were cut
into 4-µm sections and stained with hematoxylin and eosin (H&E)
for histological grading. The sections were assessed by an
experienced renal pathologist to determine the grading scores by
Jablonski's standard (24) for the
histopathological assessment of renal I/R injury.
Immunohistochemical analysis of renal
tissues
The expression levels of Fas/FasL and Bcl-2 in renal
tissues were examined by immunohistochemical staining. The
endogenous peroxidase activity was blocked with 3% hydrogen
peroxide at 37°C for 10 min. Next, the tissue sections were treated
with 1:50 normal horse serum in Tris-buffered saline (TBS) for 30
min at 37°C. Subsequently, rabbit anti-Bcl-2 (1:1,000 dilution;
A2212; ABclonal, Woburn, MA, USA) or rabbit anti-Fas/FasL (1:500
dilution; A2639/A0234; ABclonal) antibodies were added to the
tissues and incubated overnight at 4°C. Phosphate-buffered saline
(PBS) was then used for washing these sections three times.
Subsequent to incubating with the anti-rabbit (1:100; sc-3753;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA) secondary antibody
for 30 min at 20°C, the sections were treated with the DAB
chromogen for visualization. The average optical density (AOD) was
calculated from five random fields-of-view per slide using
Image-Pro Plus software, version 5.0 (Media Cybernetics, Inc.,
Shanghai, China), and the AOD was presented as the mean value of
three detections for each sample.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
The extraction of total RNA from rat kidney tissues
was performed using TRIzol RNA Reagent kit (Takara Bio, Inc., Otsu,
Japan). RNA concentration was obtained by spectrophotometry.
Single-stranded cDNA was synthesized using the cDNA synthesis kit
(Takara Bio, Inc.) according to the manufacturer's protocol.
Subsequently, qPCR was conducted with the Applied Biosystems
SYBR-Green Mix kit (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The PCR reaction mixture contained: 2 µl cDNA, 12.5 µl 2X
SYBR-Green mix, 1 µl forward primer, 1 µl reverse primer and 8.5 µl
ddH 2 O, in a final volume of 25 µl. The primers used were as
follows: Bcl-2 forward, 5′-TTTGATTTCTCCTGGCTGTCT-3′ and reverse,
5′-CTGATTTGACCATTTGCCTG-3′; Fas forward,
5′-CAAGGGACTGATAGCATCTTTGAGG-3′ and reverse,
5′-GTCCTTAACTTTTCGTTCACCCAGG-3′; FasL forward,
5′-TCCACCACCACCTCCATCAC-3′ and reverse, 5′-CCAACCTTACCCCAATCCTT-3′;
GAPDH forward, 5-GGTCATCAACGGGAAACCC-3′ and reverse,
5′-TCTGAGTGGCAGTGATGGCA-3′. Analysis of the relative gene
expression levels was performed using GAPDH as an endogenous
reference gene. Bcl-2, Fas and FasL transcript levels were
normalized to GAPDH transcript levels, with the mean values
reported for each group.
Western blot assay
The kidney tissues were dissociated using a Total
Protein Extraction kit (Wuhan Goodbio Technology Co., Ltd., Wuhan,
China) according to the specifications of the kit. Total proteins
extracted were then examined via western blot analysis. Briefly, 40
µg protein from each sample was separated by 10% SDS-PAGE and
transferred to a nitrocellulose membrane. The membranes were then
blocked by 5% non-fat milk in TBS/Tween-20 (TBST) buffer and
incubated with polyclonal primary antibodies of anti-Bcl-2 (1:500
dilution; A2212; ABclonal), anti-Fas/FasL (1:500 dilution;
A2639/A0234; ABclonal) and anti-GAPDH (1:500 dilution; A10868;
ABclonal) at 4°C overnight. Subsequent to washing three times with
TBST for ~15 min, the membranes were incubated with the horseradish
peroxidase (HRP)-conjugated goat anti-rabbit secondary antibodies
(1:200 dilution; sc-3753; Santa Cruz Biotechnology, Inc.).
Membranes were then washed three times with TBST, and specific
bands were visualized using an enhanced chemiluminescence detection
kit (Immobilon Western Chemiluminescence HRP Substrate; Merck KGaA,
Darmstadt, Germany). The band intensity was detected using the
Quantity One software (Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Cell culture for in vitro
experiments
Rat renal tubular epithelial NRK-52E cells were
purchased from the Shanghai Institutes for Biological Sciences
(Shanghai, China). The cells were cultured in Dulbecco's modified
Eagle's medium (DMEM; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% FBS (Thermo Fisher Scientific, Inc.),
0.1 mg/ml streptomycin and 100 U/ml penicillin. The medium was
replaced every 24 h.
In vitro experimental groups and
treatments
The NRK-52E cells were randomly divided into six
groups, as follows: i) Normal; ii) I/R only; iii) normal + 1,000 nM
apigenin; iv) I/R + 10 nM apigenin; v) I/R + 100 nM apigenin; and
vi) I/R + 1,000 nM apigenin. Prior to the experiment, all the
groups were cultured for 24 h simultaneously. The normal group was
incubated with a control medium (24.0 mM NaHCO3, 0.8 mM
Na2HPO4, 0.2 mM
NaH2PO4, 86.5 mM NaCl, 5.4 mM KCl, 1.2 mM
CaCl2, 0.8 mM MgCl2, 20 mM HEPES and 5 mM
glucose; pH 7.4). The I/R group was cultured with an anoxia medium
(4.5 mM NaHCO3, 0.8 mM Na2HPO4,
0.2 mM NaH2PO4, 106.0 mM NaCl, 5.4 mM KCl,
1.2 mM CaCl2, 0.8 mM MgCl2 and 20 mM
morpholinoethanesulfonic acid; pH 6.6), and then exposed to hypoxia
(0.5% O2, 5% CO2 and 94.5% N2) at
37°C for 3 h, followed by reoxygenation (21% O2, 5%
CO2 and 74% N2) at 37°C for 24 h. Similarly,
the I/R + apigenin (10, 100 and 1,000 nM) groups were subjected to
the same procedure as the I/R group, but cells were pretreated for
24 h with apigenin prior to the procedure. In the normal + apigenin
group, the same procedures were performed as for the normal group,
along with pretreatment for 24 h with apigenin (1,000 nM).
Cell counting kit-8 (CCK8) assay
The NRK-52E cells were inoculated in a 96-well plate
at a cell density of 105 cells/well. After 24 h of
culture at 37°C, the cells were treated with CCK8 solution, and the
plate was incubated in an incubator for 4 h. The absorbance of
cells at wavelength of 450 nm in terms of the OD was measured using
a microplate reader. The cell survival rate was calculated
according to the following formula: Survival (%) = (treatment group
OD-blank control OD)/(normal group OD-blank control OD) ×100%.
Annexin V-FITC/propidium iodide (PI)
detection of apoptosis in NRK-52E cells
An Annexin V-FITC/PI assay was conducted using the
Annexin V-FITC Apoptosis Detection kit (70-AP101-100; Liankebio,
Hangzhou, China) according to the manufacturer's protocol, followed
by flow cytometric analysis. Briefly, NRK-52E cells were collected,
washed twice with cold PBS and then resuspended in binding buffer.
The cells were subsequently incubated with 10 µl Annexin V-FITC and
5 µl PI for 5 min at room temperature in the dark, followed by
analysis by flow cytometry.
Immunohistochemical analysis in
NRK-52E cells
The NRK-52E cells were inoculated in a 96-well plate
at a cell density of 106 cells/well. The NRK-52E cells
were fixed with 4% paraformaldehyde and incubated with rabbit
anti-Bcl-2 (1:1,000 dilution; A2212; ABclonal) or rabbit anti-Fas
(1:500 dilution; A2639; ABclonal) antibodies overnight at 4°C.
Next, cells were washed three times with PBS, incubated with the
anti-rabbit secondary antibody (1:100; sc-3753; Santa Cruz
Biotechnology, Inc.) for 30 min at 20°C and then visualized by
addition of DAB. The AOD was calculated in five random fields per
slide using Image-Pro Plus software (version 5.0) and presented as
the mean of three independent measurements.
Western blot assay in NRK-52E
cells
The cells were washed three times with PBS,
trypsinized, suspended in DMEM and then centrifuged at 3,000 × g
for 5 min at 4°C to remove the supernatant. The total proteins
extracted were examined via western blot analysis. Briefly, 40 µg
protein from each sample was separated by 10% SDS-PAGE and
transferred to a nitrocellulose membrane. Next, the membrane was
blocked with 5% non-fat milk in TBST buffer and incubated at 4°C
overnight with the following primary polyclonal antibodies:
Anti-Bcl-2, anti-Fas/FasL and anti-GAPDH (dilution, all 1:500).
Following three washed with TBST for ~15 min, the membrane was
incubated with a secondary antibody conjugated with horseradish
peroxidase (dilution, 1:2,000), followed by further washing with
TBST for three times. Specific bands were visualized using an
enhanced chemiluminescence detection kit, and the band intensity
was detected using the Quantity One software.
Statistical analyses
All data are expressed as the mean ± standard
deviation. Statistically significant differences between groups
were tested by analysis of variance, and all statistical analyses
were processed by SPSS version 11.0 statistical software (SPSS,
Inc., Chicago, IL, USA). P<0.05 was regarded as an indicator of
statistically significant differences.
Results
Serum BUN and Cr Levels
The levels of the renal function parameters BUN and
serum Cr in rats of the various groups are shown in Fig. 1A and B. Compared with the sham
surgery rats, the I/R group demonstrated a significant increase in
BUN and Cr levels (P<0.01). However, the renal function of rats
subjected to I/R was improved by treatment with apigenin as
observed by the significantly reduced levels of BUN and Cr in the
I/R + apigenin groups compared with I/R alone groups (P<0.05).
In addition, the difference in the BUN and Cr levels between the
apigenin + sham and the sham-operated group was not statistically
significant. Furthermore, the BUN and Cr levels of the I/R + api (2
mg/kg) group was higher than in other apigenin treatment groups (10
and 50 mg/kg), which indicated that the concentration of 10 and 50
mg/kg apigenin were the most effective at reducing I/R injury
(Fig. 1).
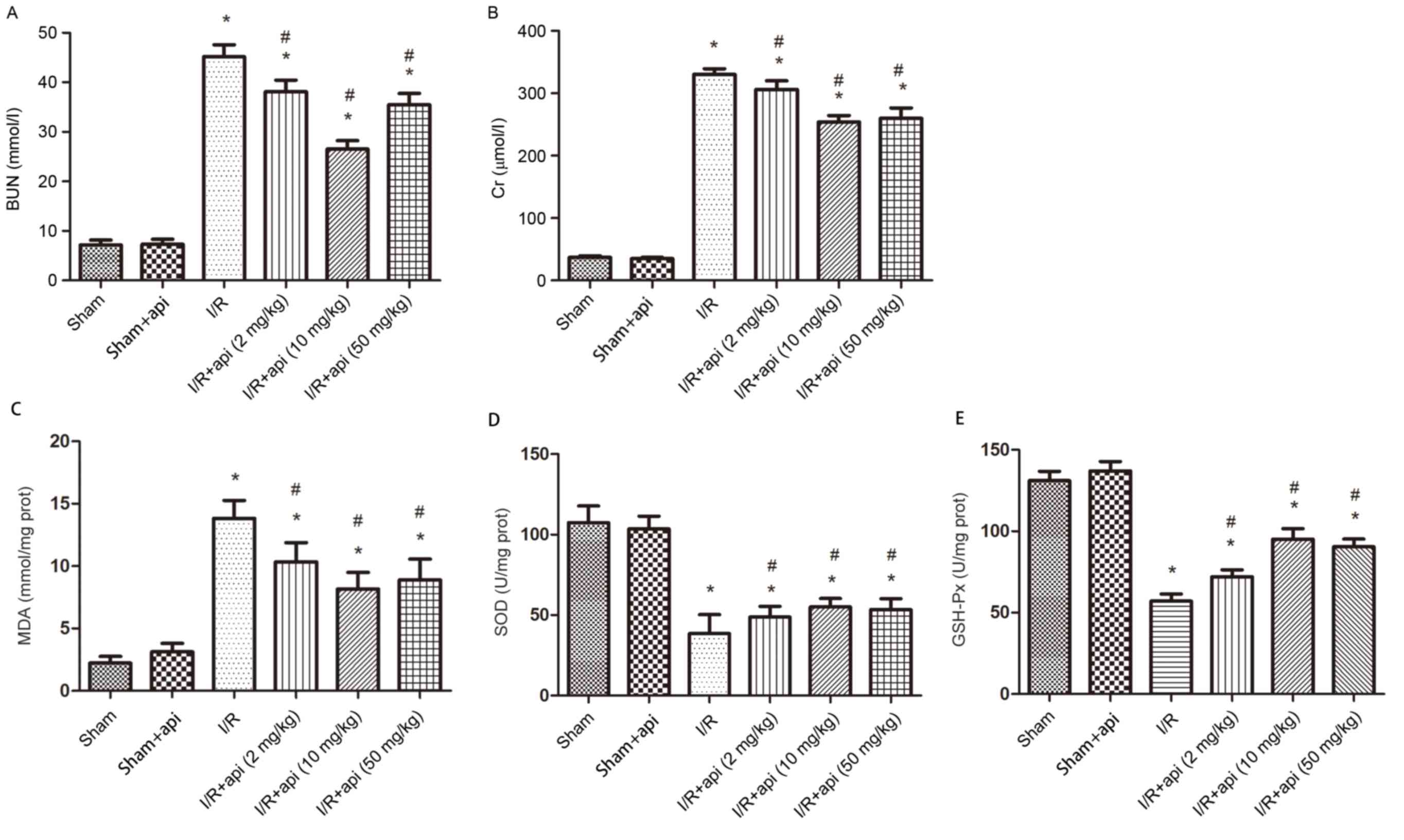 | Figure 1.Effect of apigenin on the renal
function and anti-oxidative stress of rats. Effect of apigenin on
the (A) BUN, (B) Cr, (C) MDA, (D) SOD and (E) GSH-Px levels after
45 min of ischemia. Bars represent the means ± standard deviation
(n=6). *P<0.01 vs. sham group; #P<0.05 vs. I/R
group. I/R, ischemia-reperfusion; api, apigenin treatment; BUN,
blood urea nitrogen; Cr, creatinine; MDA, malondialdehyde; SOD,
superoxide dismutase; GSH-Px, glutathione peroxidase. |
Measurement of MDA, SOD and
GSH-Px
As shown in Fig.
1C-E, the I/R group exhibited a significant increase in MDA
(P<0.01), a marker of lipid peroxidation, as well as significant
decreases in the SOD and GSH-Px levels compared with the sham group
(P<0.01). By contrast, apigenin pretreatment in I/R rats
significantly inhibited the decrease in the SOD and GSH-Px levels
compared with the I/R only group (P<0.05). Furthermore, no
significant difference was identified between the sham and apigenin
+ sham groups (P>0.05; Fig.
1C-E). Similarly, high doses of apigenin exerted a marked
increase in renoprotection against renal I/R injury compared with
lower doses of apigenin.
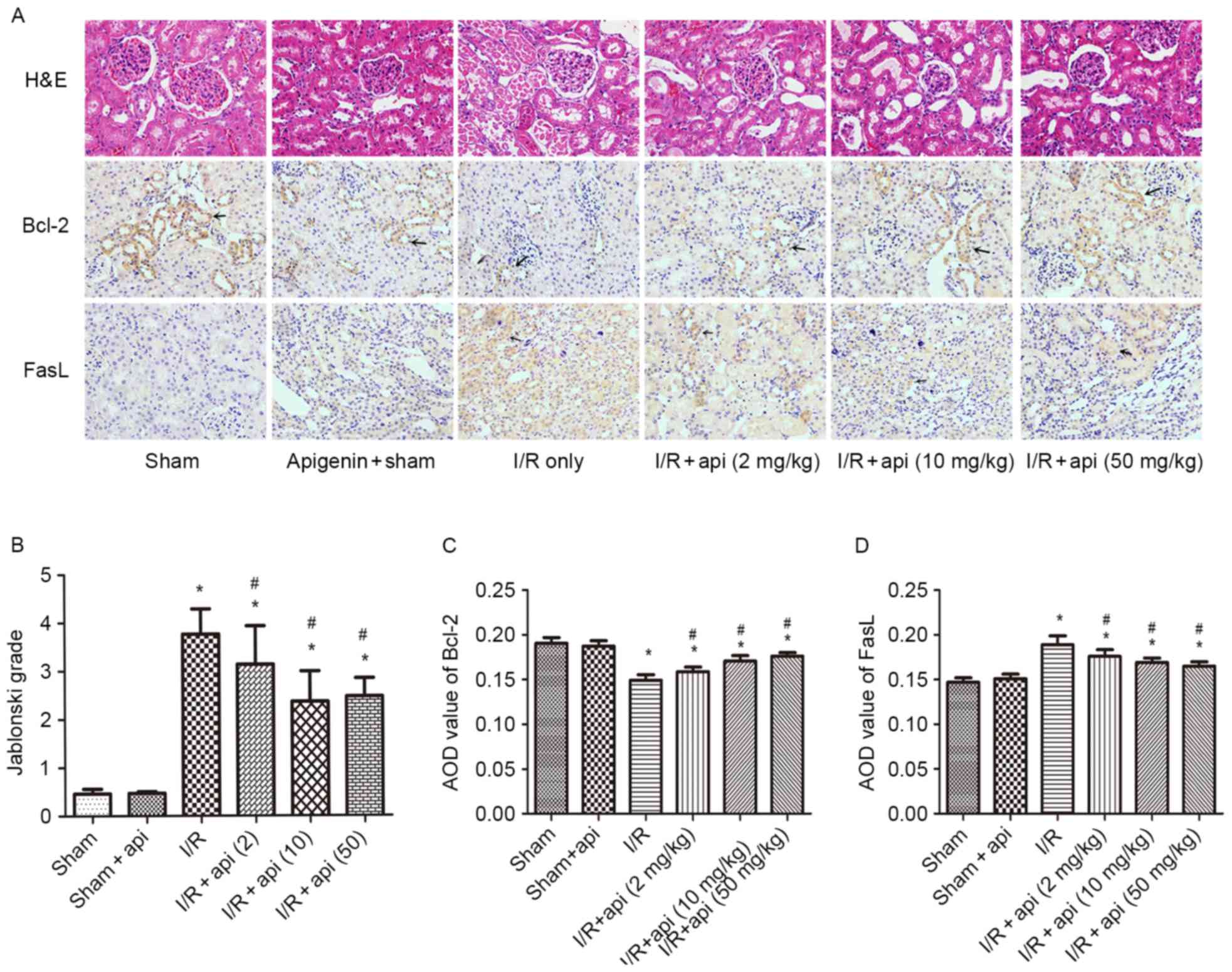
Morphological features of kidneys and
immunohistochemical staining
In the I/R group, various morphological
abnormalities were identified by H&E staining, including
tubular cell necrosis, cytoplasmic vacuolization and tubular lumen
obstruction and impairments. However, apigenin pretreatment
relieved the severe renal damage caused by I/R injury. Quantitative
analysis indicated that 45 min of renal ischemia followed by 24 h
of reperfusion resulted in severe acute tubular necrosis, but the
Jablonski histological scores in the I/R + apigenin groups were
significantly lower than those in the I/R group, which indicated
that this renal damage was attenuated by apigenin (P<0.05;
Fig. 2A and B).
Immunohistological staining for Bcl-2, Fas and FasL
was also conducted in the kidney tissues (Fig. 2A, C and D). The results indicated
that Fas/FasL expression significantly increased in the I/R group
compared with the sham group (P<0.01), and this tendency was
suppressed by apigenin treatment (P<0.05). The
immunohistochemical results of Fas gene were almost identical with
those for FasL, thus only the immunohistochemical results for FasL
are illustrated in Fig. 2. In
contrast to the Fas/FasL results, Bcl-2 expression significantly
decreased in the I/R group compared with the sham group
(P<0.01); however, apigenin pretreatment significantly improved
this expression (P<0.05).
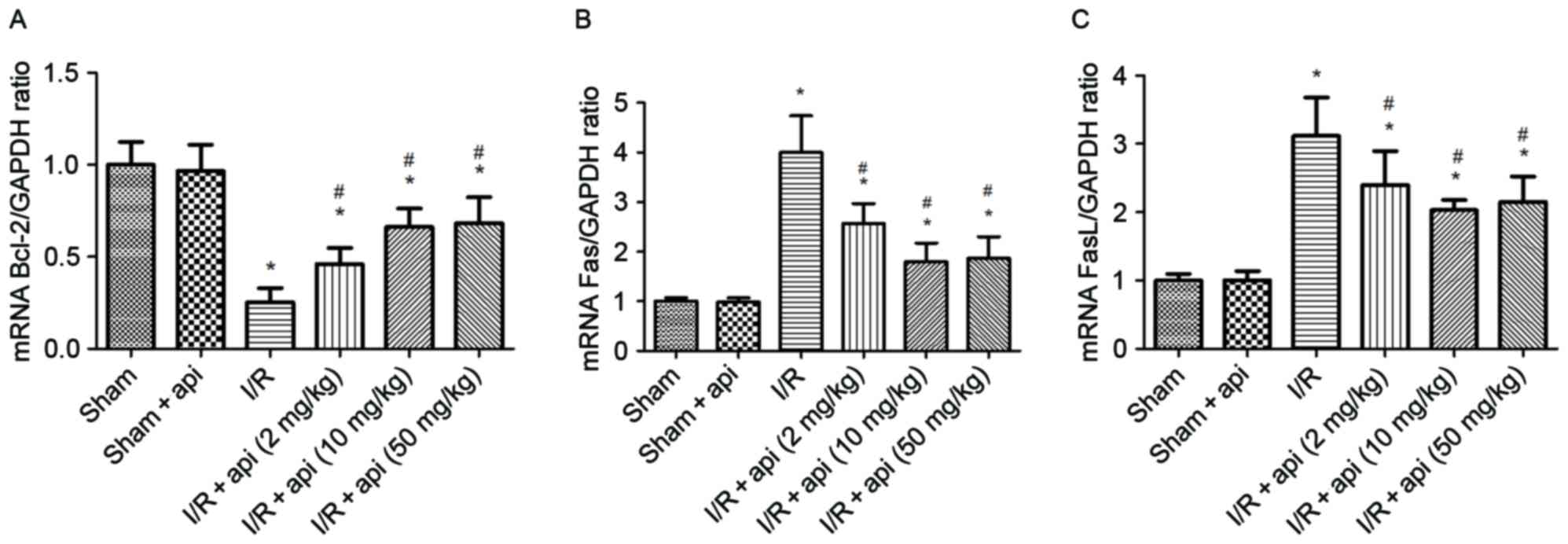
Bcl-2, Fas and FasL mRNA expression
levels in the kidneys
RT-qPCR was used to investigate the differences in
the mRNA expression levels of Bcl-2, Fas and FasL. As shown in
Fig. 3A, the expression of Bcl-2 in
kidney tissues at the mRNA level demonstrated a significant
decrease in the I/R group compared with that in the sham group
(P<0.01). However, apigenin evidently improved the mRNA
expression of Bcl-2 in the I/R + apigenin groups, since an
increased level was observed compared with the I/R group
(P<0.05). On the contrary, the mRNA levels of Fas and FasL were
significantly higher in I/R group in comparison with the sham group
(P<0.01), while apigenin pretreatment inhibited the mRNA
expression of Fas and FasL in the I/R + apigenin group compared
with the I/R only group (P<0.05; Fig.
3B and C).
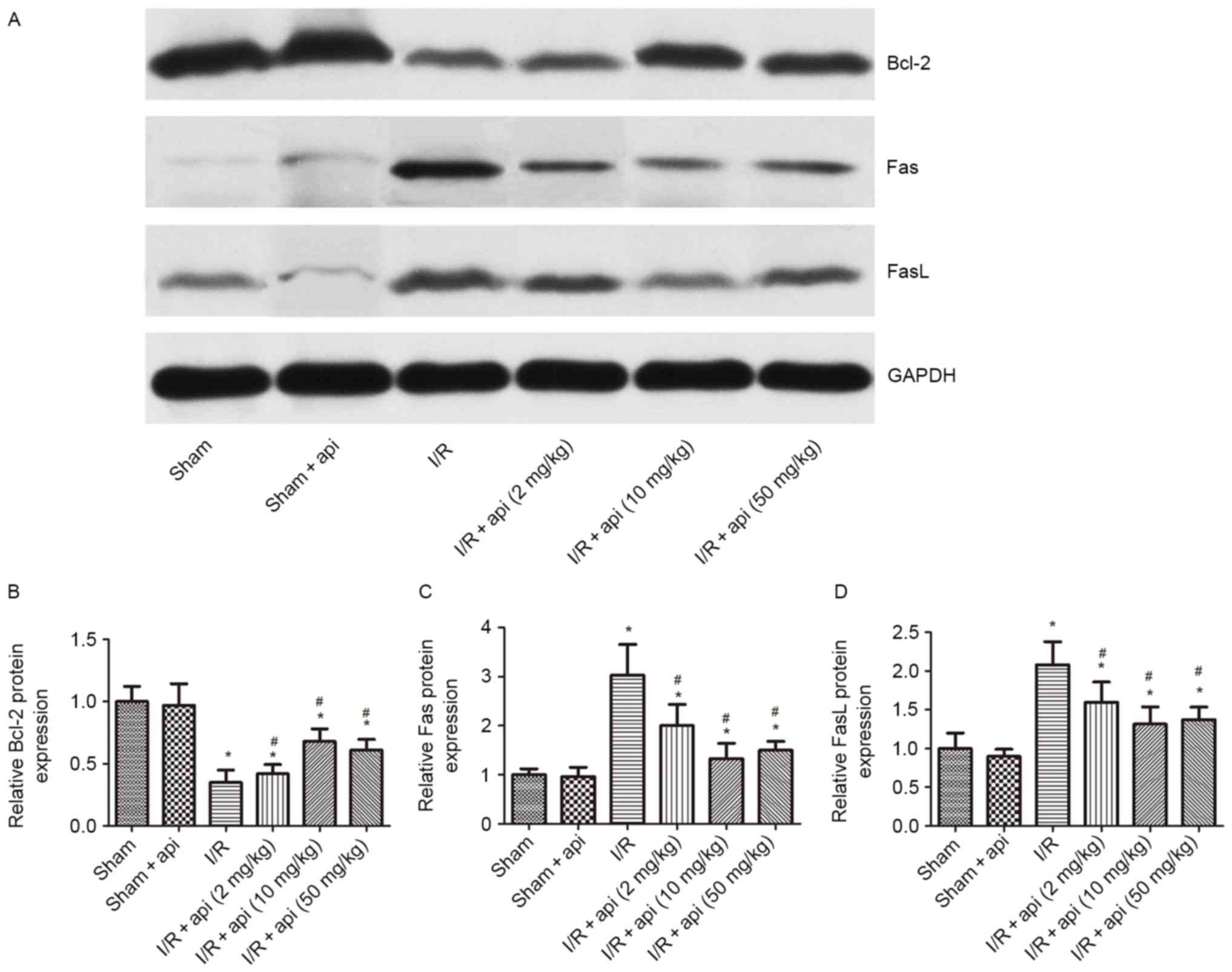
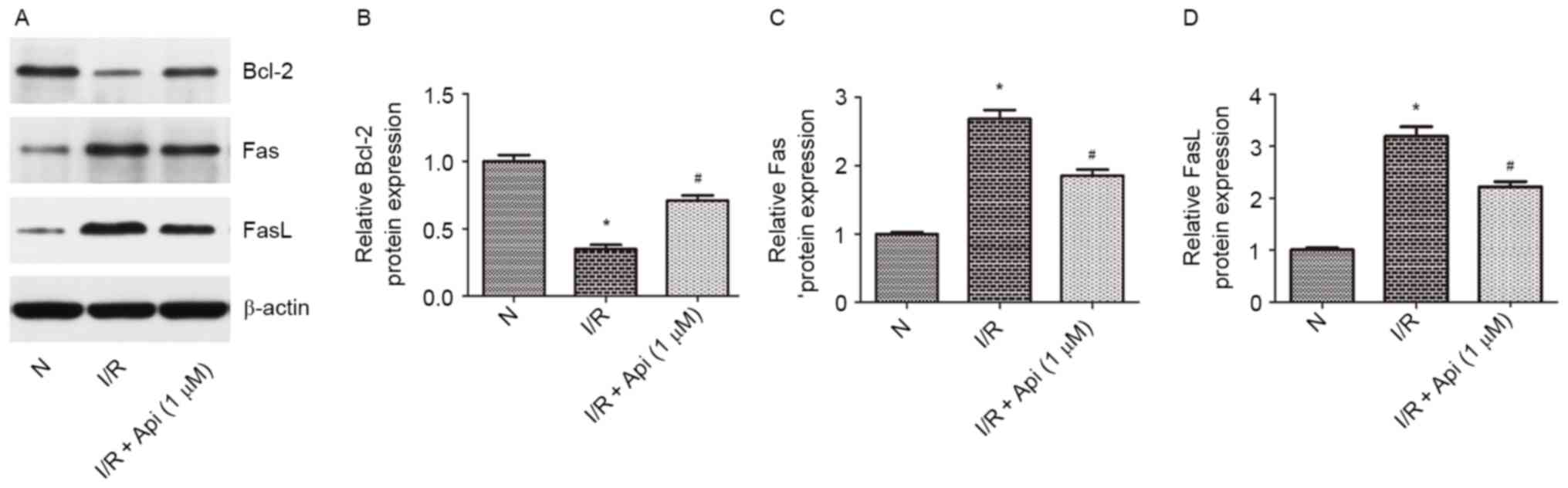
Similarly, western blot analysis revealed a
significant increase in Fas and FasL protein expression levels in
the I/R group compared with the sham group (P<0.01), and these
levels were decreased by apigenin pretreatment in the I/R +
apigenin groups (P<0.05). Furthermore, as compared with the
sham-operated group, the I/R group induced a significant decrease
in Bcl-2 protein expression (P<0.01), whereas these effects were
antagonized in the I/R + apigenin groups (P<0.05; Fig. 4). The western blot analysis results
were consistent with the immunohistochemical findings on Bcl-2 and
Fas/FasL expression levels. Furthermore, apigenin was indicated to
increase Bcl-2 expression and decrease Fas/FasL expression
significantly in a dose-dependent manner. The Bcl-2 expression was
increased to the highest at a final concentration of 10 and 50
mg/kg, which indicated that apigenin protected kidneys against I/R
injury.
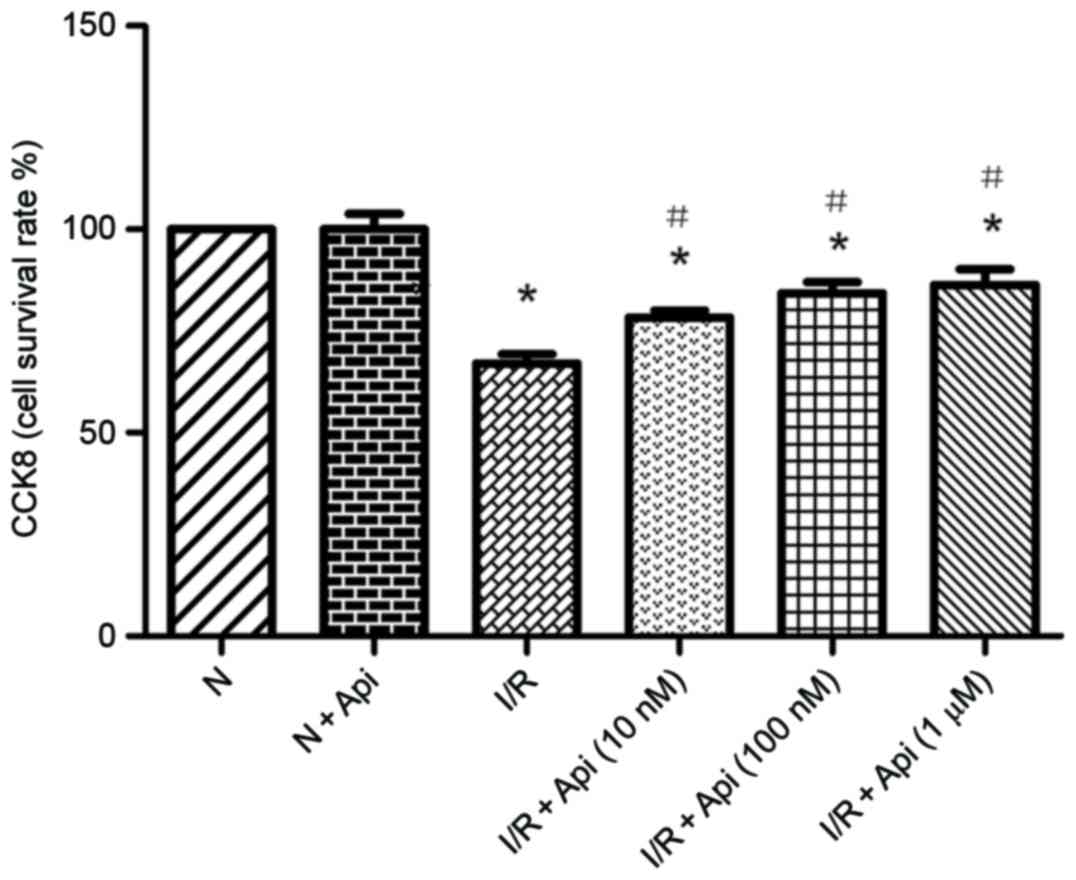
Effects of apigenin on NRK-52E cell
viability
In order to further investigate the effects of
apigenin, in vitro experiments were also conducted in an I/R
cell model established by anoxia/reoxygenation. The effects of
apigenin on the viability of NRK-52E cells induced by I/R were
determined quantitatively with a CCK8 assay. I/R in cells
significantly decreased the cell survival rate as compared with the
normal group. However, apigenin pretreatment prior to I/R
significantly increased the viability of NRK-52E cells (Fig. 5). In addition, the effect of apigenin
was dose dependent, since the viability of NRK-52E cells was the
highest at the final concentration of 1,000 nM apigenin, although
the difference among the 1,000 and 100 nM doses was not
statistically significant (Fig. 5).
Thus, the concentration of 1 µM apigenin was used in subsequent
experiments. In addition, there was no evident difference in cell
viability between the normal and normal + apigenin groups,
therefore, only the normal group was used in subsequent
experiments.
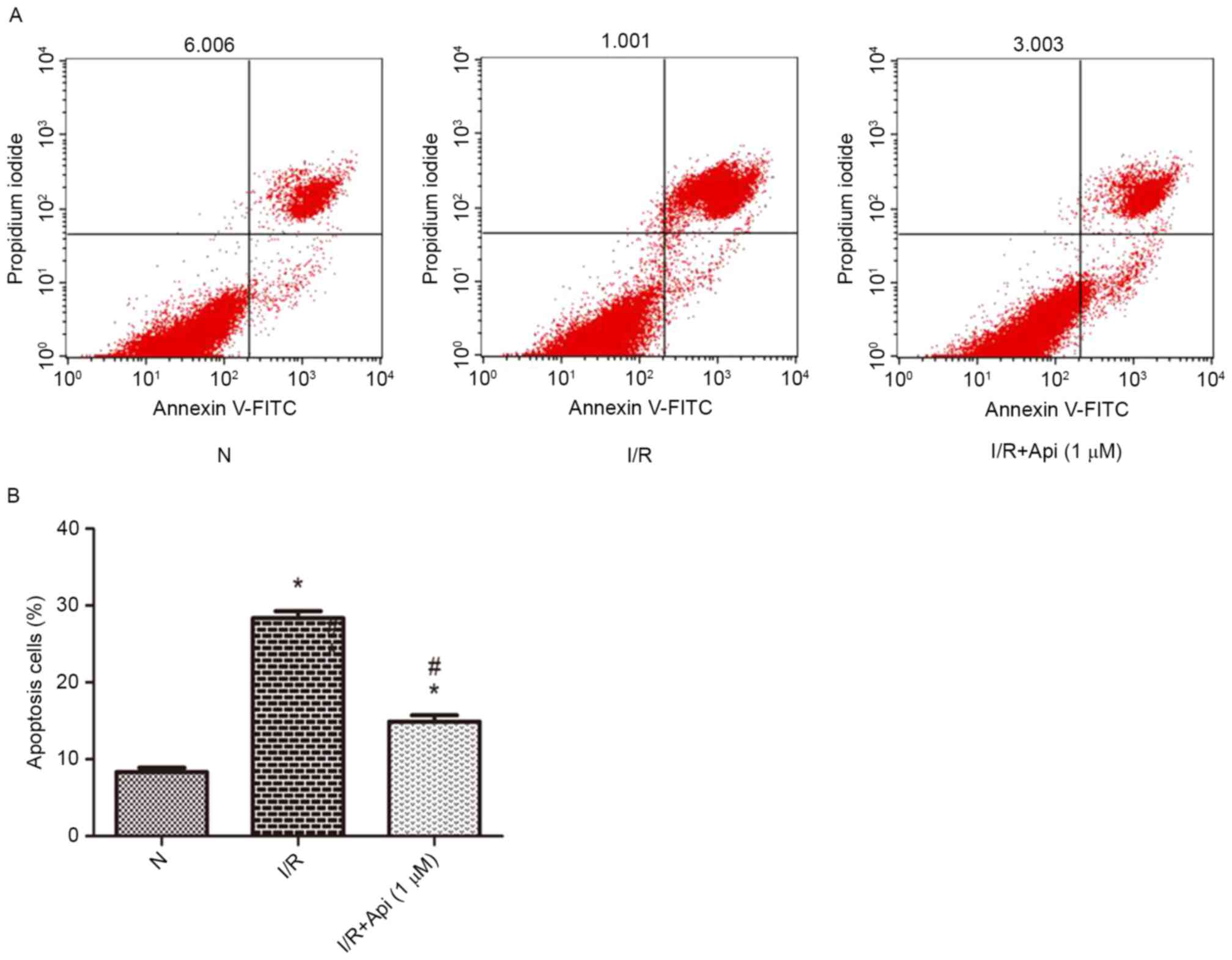
Effects of apigenin on apoptosis in
NRK-52E cells
Flow cytometry was conducted to determine whether
apigenin was able to inhibit the apoptosis of NRK-52E cells.
Compared with the normal group, the I/R group demonstrated
significantly increased cell apoptosis. However, the cell apoptosis
induced by I/R was reduced upon pretreatment with apigenin
(Fig. 6).
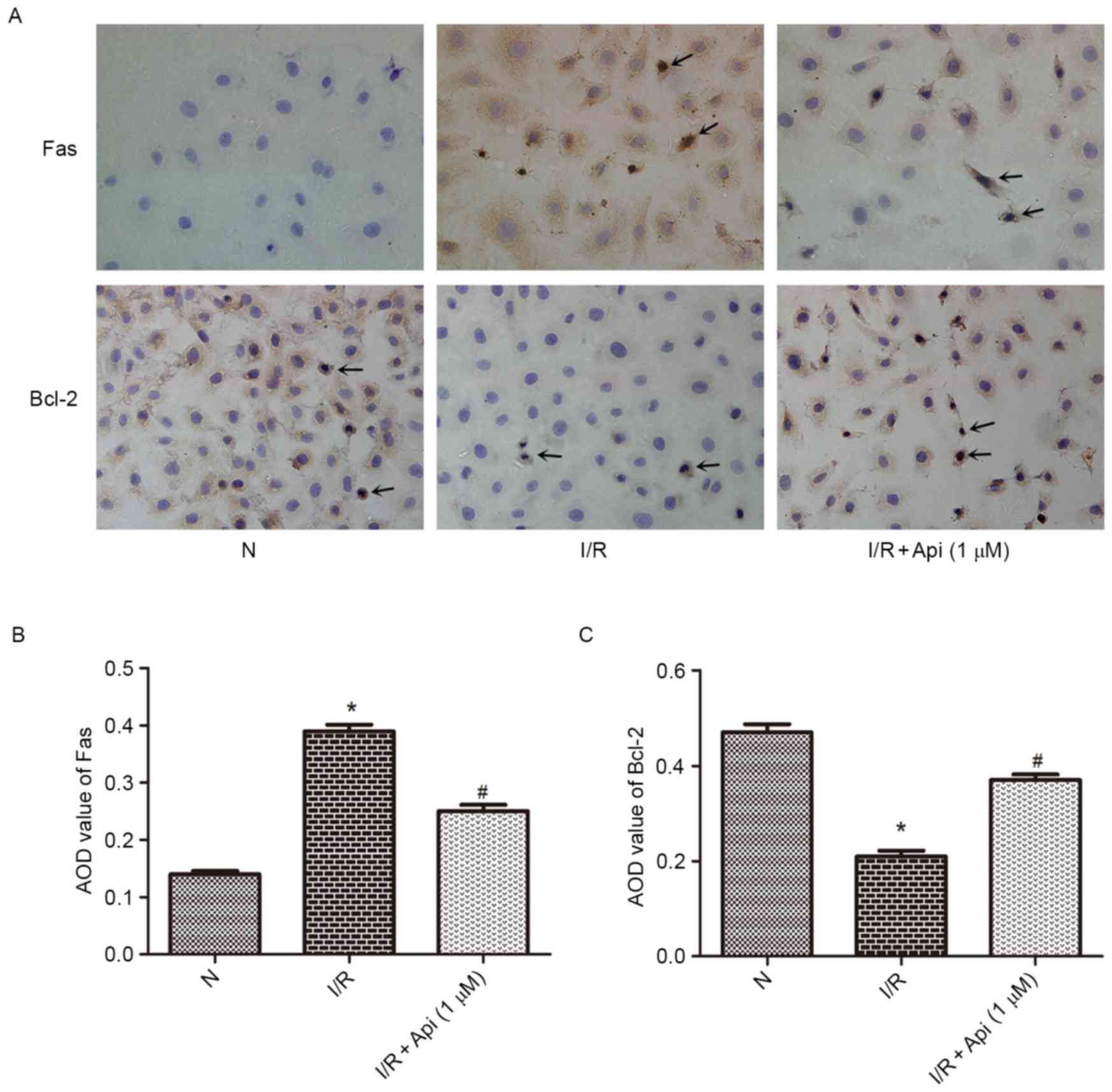
Bcl-2, Fas and FasL protein expression
levels in NRK-52E cells
To determine the effect of Bcl-2 and Fas/FasL in
NRK-52E cells, immunohistochemical analysis was performed. As shown
in Fig. 7, the I/R group displayed
low protein expression of Bcl-2 and strong protein expression of
Fas compared with the normal group. By contrast, pretreatment with
apigenin at a concentration of 1,000 nM significantly increased the
Bcl-2 protein levels and decreased the Fas protein levels in the
I/R + apigenin (1,000 nM) group when compared with the I/R group
(Fig. 7). Furthermore, western blot
analysis was conducted to analyze the expression levels of Bcl-2
and Fas/FasL. The results indicated that the protein expression
levels of Fas and FasL were significantly increased, while the
Bcl-2 level was significantly decreased, as compared with the
normal group. In addition, pretreatment with apigenin at a
concentration of 1,000 nM attenuated the increase in Fas/FasL and
the decrease in Bcl-2 expression levels (Fig. 8).
Discussion
Renal I/R injury associated with kidney
transplantation and nephrectomy are unresolved problems in clinical
practice (25). Acute renal failure
can be induced by the renal I/R injury, and it has been suggested
that cell apoptosis and necrosis are the main features of this
disease (26). Cell apoptosis serves
an important role in the process of cell development and the
internal environment homeostasis (27). Dysfunction of the apoptosis
regulatory mechanism results in a variety of clinical diseases,
including neurodegenerative diseases, autoimmune diseases,
hematopoietic dysfunction, infertility and cancer (28–32). A
large number of animal experiments have demonstrated that renal
tubular cell apoptosis can be observed subsequent to kidney damage
caused by ureteral obstructive diseases, renal artery stenosis,
biological toxin exposure and trauma (33–36).
Furthermore, renal I/R injury can induce renal tubular epithelial
cell apoptosis and necrosis (37).
These two modes exist in kidney pathology as interdependent
phenomena resulting from the activation of shared pathways and
signals (38).
The present study demonstrated that apigenin, a
common edible plant flavonoid and a well-characterized antioxidant
(39), had a renoprotective effect
by inhibiting apoptosis in renal I/R injury. However, the
mechanisms underlying cell apoptosis are complicated. To clarify
the possible mechanisms mediating the anti-apoptotic effect of
apigenin, the expression levels of Bcl-2 and Fas/FasL, as well as
the levels of MDA, SOD and GSH-Px, were investigated in the current
study. The results revealed that the expression of Bcl-2 was
decreased by I/R injury and improved by apigenin treatment, while
the opposite expression alterations were observed for Fas/FasL.
Furthermore, apigenin exhibited protective effects by reducing the
oxidative stress through reduced production of free radical
derivatives and increased production of antioxidants, as evidenced
by the decreased MDA level and the increased levels of SOD and
GSH-Px. These results suggest that apigenin inhibits renal cell
apoptosis and protects against the oxidation of renal cellular
membrane damage by enhancing the activation of the Bcl-2 pathway
and blocking the activation of the Fas/FasL pathway in renal I/R
injury. Furthermore, the current data indicated that the 10 and 50
mg/kg treatment subgroups exhibited a marked renoprotective effect
as compared with the 2 mg/kg treatment.
In order to examine the toxicity of apigenin, the
renal function and protein levels of Bcl-2, Fas and FasL in the
sham surgery and apigenin + sham groups were first compared. No
significant differences were observed between these two groups in
terms of the BUN, serum Cr, Bcl-2, Fas and FasL protein levels
(P>0.1), indicating that apigenin was non-toxic for normal renal
tissues.
The Bcl-2 family is one of the primary regulatory
factors of cell apoptosis (40), and
has received the most attention among a variety of relevant
proteins functioning in apoptosis regulation (41). Bcl-2 is one of the most important
anti-apoptotic genes, and is expressed at low levels in renal
tissues following I/R (42). When
I/R injury was induced in the present study, the amount of
apoptotic cells in distal convoluted tubules increased when
compared with the sham surgery group. However, compared with the
I/R group, Bcl-2 was highly expressed at the distal convoluted
tubules of the I/R + apigenin groups. High expression of Bcl-2
serves an important role in the anti-apoptosis mechanism of renal
tissues treated with I/R. Thus, the present study suggested that
apigenin regulated the expression of the apoptosis-inhibitory Bcl-2
gene in I/R injury. The anti-apoptosis mechanism of Bcl-2 may be
associated with several factors: i) Bcl-2 functions as an
anti-apoptotic protein causing endoplasmic reticulum
Ca2+ depletion and helping to keep the luminal
Ca2+ concentration at physiological levels, decreasing
the cellular Ca2+ concentration by inhibiting
Ca2+ release (43); ii)
cell apoptosis gene signaling and apoptosis-associated gene
products are blocked by Bcl-2; iii) oxidative stress triggers
multiple signaling pathways, including the pathways involving the
Bcl-2 proteins. A previous study suggested that the upregulated
expression of Bcl-2 can lead to the decrease of oxidative stress
(44). Bcl-2 gene is a terminal part
of apoptotic regulation, and high expression of Bcl-2 protein may
reduce the formation of lipid peroxide and oxygen free radicals.
Thus, Bcl-2 is an important survival factor, which is sensitive to
oxidative stress and an increase in Bcl-2 suppress cell
apoptosis.
Fas, a transmembrane protein that belongs to the TNF
superfamily, is one of the death receptors in the cell membrane
(45). Death receptors and the
apoptotic cascade are activated upon engagement with the
corresponding ligand, which is FasL in the case of Fas (46). Fas/FasL are considered as cell
apoptosis genes, and the combination of Fas and its ligand result
in Fas-associated cell apoptosis (47). In addition, FasL, expressed in renal
tubular epithelial cells of normal rat kidney, may accelerate the
apoptosis of lymphocytes (48). The
apoptosis mechanism of Fas/FasL may be associated with the
overexpression of the immediate early gene, immunological
dysfunction and the effect of certain inflammatory cytokines. In
the present experimental study, the expression of the Fas protein
was significantly increased following I/R injury, in comparison
with the sham surgery group. Similar results were observed for the
expression of FasL protein. Thus, Fas/FasL induced cell apoptosis
in the kidney following the induction of I/R injury. Furthermore,
the data demonstrated that there was a significant difference
between the I/R and I/R + apigenin groups. It was observed that
apigenin was able to block the interaction between Fas and FasL,
strongly inhibiting renal injury after I/R. The Fas system may be
involved in the triggering of renal tissue cell apoptosis by
oxidative stress. Thus, the present study also validated the effect
of oxidative stress on membrane Fas/FasL expression in renal
tubular epithelial cells. Nevertheless, the mechanism of Fas/FasL
expression induced by oxidative stress remains unclear. A possible
mechanism of oxidative stress-induced Fas/FasL expression is the
tyrosine phosphorylation of signaling molecules, including p38
mitogen-activated protein kinase and Jun-N-terminal kinase, which
have been implicated in Fas/FasL expression (49).
In conclusion, the results of the present study
revealed that apigenin increased the expression of Bcl-2 and
reduced Fas/FasL expression in renal I/R injury, providing marked
protection against this injury in rats. The present study also
suggested that the upregulation of Bcl-2 and downregulation of
Fas/FasL protein expression levels are involved in anti-apoptosis
and antioxidation, and may be one of the mechanisms underlying the
protective effect of apigenin on renal I/R injury.
Acknowledgements
The current study was supported by grants from the
Application and Basic Research Project Of Wuhan City (no.
2015060101010049), Hubei Province Health and Family Planning
Scientific Research Project (nos. WJ2017M025 and WJ2017Z005) and
the Bureau of Public Health of Hubei Province (no. JX6B62).
References
|
1
|
Jang HR, Ko GJ, Wasowska BA and Rabb H:
The interaction between ischemia-reperfusion and immune responses
in the kidney. J Mol Med (Berl). 87:859–864. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sementilli A and Franco M: Renal acute
cellular rejection: Correlation between the immunophenotype and
cytokine expression of the inflammatory cells in acute
glomerulitis, arterial intimitis, and tubulointerstitial nephritis.
Transplant Proc. 42:1671–1676. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Di Nardo M, Ficarella A, Ricci Z, Luciano
R, Stoppa F, Picardo S, Picca S, Muraca M and Cogo P: Impact of
severe sepsis on serum and urinary biomarkers of acute kidney
injury in critically ill children: An observational study. Blood
Purif. 35:172–176. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Levy EM, Viscoli CM and Horwitz RI: The
effect of acute renal failure on mortality. A cohort analysis.
JAMA. 275:1489–1494. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Santos WJ, Zanetta DM, Pires AC, Lobo SM,
Lima EQ and Burdmann EA: Patients with ischaemic, mixed and
nephrotoxic acute tubular necrosis in the intensive care unit-a
homogeneous population? Crit Care. 10:R682006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jia Y, Zhao J, Liu M, Li B, Song Y, Li Y,
Wen A and Shi L: Brazilin exerts protective effects against renal
ischemia-reperfusion injury by inhibiting the NF-κB signaling
pathway. Int J Mol Med. 38:210–216. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Birt DF, Hendrich S and Wang W: Dietary
agents in cancer prevention: Flavonoids and isoflavonoids.
Pharmacol Ther. 90:157–177. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Patel D, Shukla S and Gupta S: Apigenin
and cancer chemoprevention: Progress, potential and promise
(review). Int J Oncol. 30:233–245. 2007.PubMed/NCBI
|
|
9
|
Nicholas C, Batra S, Vargo MA, Voss OH,
Gavrilin MA, Wewers MD, Guttridge DC, Grotewold E and Doseff AI:
Apigenin blocks lipopolysaccharide-induced lethality in vivo and
proinflammatory cytokines expression by inactivating NF-kappaB
through the suppression of p65 phosphorylation. J Immunol.
179:7121–7127. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsalkidou EG, Tsaroucha AK, Chatzaki E,
Lambropoulou M, Papachristou F, Trypsianis G, Pitiakoudis M, Vaos G
and Simopoulos C: The effects of apigenin on the expression of
Fas/FasL apoptotic pathway in warm liver ischemia-reperfusion
injury in rats. Biomed Res Int. 2014:1572162014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Brunner T, Mogil RJ, LaFace D, Yoo NJ,
Mahboubi A, Echeverri F, Martin SJ, Force WR, Lynch DH, Ware CF, et
al: Cell-autonomous Fas (CD95)/Fas-ligand interaction mediates
activation-induced apoptosis in T-cell hybridomas. Nature.
373:441–444. 1995. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao J, Duan S, Zhou J, Sun R, Zhang L and
Wang D: Mild hypothermia reduces expression of Fas/FasL and MMP-3
after cerebral ischemia-reperfusion in rats. Iran J Basic Med Sci.
17:454–459. 2014.PubMed/NCBI
|
|
13
|
Del RM, Imam A, DeLeon M, Gomez G, Mishra
J, Ma Q, Parikh S and Devarajan P: The death domain of kidney
ankyrin interacts with Fas and promotes Fas-mediated cell death in
renal epithelia. J Am Soc Nephrol. 15:41–51. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Erkan E, Garcia CD, Patterson LT, Mishra
J, Mitsnefes MM, Kaskel FJ and Devarajan P: Induction of renal
tubular cell apoptosis in focal segmental glomerulosclerosis: Roles
of proteinuria and Fas-dependent pathways. J Am Soc Nephrol.
16:398–407. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gahl RF, Dwivedi P and Tjandra N: Bcl-2
proteins bid and bax form a network to permeabilize the
mitochondria at the onset of apoptosis. Cell Death Dis.
7:e24242016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang Y, Wang W and Qiu E: Protection of
oxidative stress induced apoptosis in osteosarcoma cells by
dihydromyricetin through down-regulation of caspase activation and
up-regulation of BcL-2. Saudi J Biol Sci. 24:837–842. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsujimoto Y and Shimizu S: Bcl-2 family:
Life-or-death switch. FEBS Lett. 466:6–10. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Furuichi K, Kokubo S, Hara A, Imamura R,
Wang Q, Kitajima S, Toyama T, Okumura T, Matsushima K, Suda T, et
al: Fas ligand has a greater impact than TNF-α on apoptosis and
inflammation in ischemic acute kidney injury. Nephron Extra.
2:27–38. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu MX, Ran B, Feng ZQ and Pan QW: Effects
of Rb1 and Rg1 on the expression of Bcl-2, Bax in apoptosis of HK-2
cells induced by the serum of kidney ischemia/reperfusion. Zhongguo
Ying Yong Sheng Li Xue Za Zhi. 25:496–499. 2009.(In Chinese).
PubMed/NCBI
|
|
20
|
Wang J, Su B, Zhu H, Chen C and Zhao G:
Protective effect of geraniol inhibits inflammatory response,
oxidative stress and apoptosis in traumatic injury of the spinal
cord through modulation of NF-κB and p38 MAPK. Exp Ther Med.
12:3607–3613. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vogt M, Bauer MK, Ferrari D and
Schulze-Osthoff K: Oxidative stress and hypoxia/reoxygenation
trigger CD95 (APO-1/Fas) ligand expression in microglial cells.
FEBS Lett. 429:67–72. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chang WT, Hsieh BS, Cheng HL, Lee KT and
Chang KL: Progesterone augments epirubicin-induced apoptosis in
HA22T/VGH cells by increasing oxidative stress and upregulating
Fas/FasL. J Surg Res. 188:432–441. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bussuan LA, Fagundes DJ, Marks G, Bussuan
PM and Teruya R: The role of Fas ligand protein in the oxidative
stress induced by azoxymethane on crypt colon of rats. Acta Cir
Bras. 25:501–506. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jablonski P, Howden BO, Rae DA, Birrell
CS, Marshall VC and Tange J: An experimental model for assessment
of renal recovery from warm ischemia. Transplantation. 35:198–204.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gianello P, Squifflet JP, Carlier M,
Lambotte L, Ketelslegers JM and Alexandre GP: Atrial natriuretic
factor: A protective role after acute renal ischemia? Is there room
for it in kidney transplantation? Transpl Int. 3:41–46. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Gobé G, Willgoss D, Hogg N, Schoch E and
Endre Z: Cell survival or death in renal tubular epithelium after
ischemia-reperfusion injury. Kidney Int. 56:1299–1304. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ameisen JC: On the origin, evolution, and
nature of programmed cell death: A timeline of four billion years.
Cell Death Differ. 9:367–393. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hanlon MG, Gacis ML, Kakakios AM and
Kilham H: Investigation of suspected deficient Fas-mediated
apoptosis in a father and son. Cytometry. 43:195–198. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kiechle FL and Zhang X: The postgenomic
era: Implications for the clinical laboratory. Arch Pathol Lab Med.
126:255–262. 2002.PubMed/NCBI
|
|
30
|
Markström E, Svensson ECh, Shao R,
Svanberg B and Billig H: Survival factors regulating ovarian
apoptosis-dependence on follicle differentiation. Reproduction.
123:23–30. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Müllauer L, Gruber P, Sebinger D, Buch J,
Wohlfart S and Chott A: Mutations in apoptosis genes: A
pathogenetic factor for human disease. Mutat Res. 488:211–231.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yuan J and Yankner BA: Apoptosis in the
nervous system. Nature. 407:802–809. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hashemi M: The study of pentoxifylline
drug effects on renal apoptosis and BCL-2 gene expression changes
following ischemic reperfusion injury in rat. Iran J Pharm Res.
13:181–189. 2014.PubMed/NCBI
|
|
34
|
Nilsson L, Madsen K, Krag S, Frøkiær J,
Jensen BL and Nørregaard R: Disruption of cyclooxygenase type 2
exacerbates apoptosis and renal damage during obstructive
nephropathy. Am J Physiol Renal Physiol. 309:F1035–F1048. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li H, Sun JJ, Chen GY, Wang WW, Xie ZT,
Tang GF and Wei SD: Carnosic acid nanoparticles suppress liver
ischemia/reperfusion injury by inhibition of ROS, Caspases and
NF-κB signaling pathway in mice. Biomed Pharmacother. 82:237–246.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu A, Huang L, Fan H, Fang H, Yang Y, Liu
S, Hu J, Hu Q, Dirsch O and Dahmen U: Baicalein pretreatment
protects against liver ischemia/reperfusion injury via inhibition
of NF-κB pathway in mice. Int Immunopharmacol. 24:72–79. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zweier JL and Talukder MA: The role of
oxidants and free radicals in reperfusion injury. Cardiovasc Res.
70:181–190. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lemasters JJ: V. Necrapoptosis and the
mitochondrial permeability transition: Shared pathways to necrosis
and apoptosis. Am J Physiol. 276:G1–G6. 1999.PubMed/NCBI
|
|
39
|
Bruno A, Siena L, Gerbino S, Ferraro M,
Chanez P, Giammanco M, Gjomarkaj M and Pace E: Apigenin affects
leptin/leptin receptor pathway and induces cell apoptosis in lung
adenocarcinoma cell line. Eur J Cancer. 47:2042–2051. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen C, He H, Luo Y, Zhou M, Yin D and He
M: Involvement of Bcl-2 signal pathway in the protective effects of
apigenin on Anoxia/Reoxygenation-induced myocardium injury. J
Cardiovasc Pharmacol. 67:152–163. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Walls KC, Ghosh AP, Ballestas ME, Klocke
BJ and Roth KA: bcl-2/Adenovirus E1B 19-kd interacting protein 3
(BNIP3) regulates hypoxia-induced neural precursor cell death. J
Neuropathol Exp Neurol. 68:1326–1338. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Meier P, Finch A and Evan G: Apoptosis in
development. Nature. 407:796–801. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lam M, Dubyak G, Chen L, Nuñez G, Miesfeld
RL and Distelhorst CW: Evidence that BCL-2 represses apoptosis by
regulating endoplasmic reticulum-associated Ca2+ fluxes. Proc Natl
Acad Sci USA. 91:pp. 6569–6573. 1994, View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Jin GF, Hurst JS and Godley BF: Hydrogen
peroxide stimulates apoptosis in cultured human retinal pigment
epithelial cells. Curr Eye Res. 22:165–173. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tsalkidou EG, Tsaroucha AK, Chatzaki E,
Lambropoulou M, Papachristou F, Trypsianis G, Pitiakoudis M, Vaos G
and Simopoulos C: The effects of apigenin on the expression of
Fas/FasL apoptotic pathway in warm liver ischemia-reperfusion
injury in rats. Biomed Res Int. 2014:1572162014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Malhi H, Gores GJ and Lemasters JJ:
Apoptosis and necrosis in the liver: A tale of two deaths?
Hepatology. 43 2 Suppl 1:S31–S44. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mor G, Straszewski S and Kamsteeg M: Role
of the Fas/Fas ligand system in female reproductive organs:
Survival and apoptosis. Biochem Pharmacol. 64:1305–1315. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ortiz A, Lorz C and Egido J: The Fas
ligand/Fas system in renal injury. Nephrol Dial Transplant.
14:1831–1834. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Hsu SC, Gavrilin MA, Tsai MH, Han J and
Lai MZ: p38 mitogen-activated protein kinase is involved in Fas
ligand expression. J Biol Chem. 274:25769–25776. 1999. View Article : Google Scholar : PubMed/NCBI
|