Introduction
Ischemic heart disease (IHD) is a serious health
problem worldwide, as reported by the World Health Organization in
2016 (1). Several risk factors are
associated with IHD, which include smoking, lack of exercise,
diabetes mellitus and elevated cholesterol levels. The pathogenesis
of IHD starts with an inflammatory reaction that leads to scar
formation, interstitial fibrosis and vascular remodeling,
ultimately causing dysfunction of the heart muscles. This
myocardial dysfunction is due to oxidative stress under hypoxic
conditions, which is influenced by the presence of reactive oxygen
species (ROS). ROS, which are recognized as toxic cellular
radicals, affect the viability of cardiomyocytes through the
activation of apoptotic pathways (2), induction of mitochondrial damage
(3) and inhibition of
cardioprotective processes (4).
The conventional medical and surgical treatments for
IHD aim to promote blood circulation to the myocardium in order to
restore cardiomyocyte functions. However, dead myocardial tissue
cannot be rescued. Thus, treatments that effectively recover
damaged tissue are important for sustaining the heart function. At
present, stem cell therapy is considered to be a primary option for
salvaging damaged cells. The treatment of IHD using various types
of stem cells, such as hematopoietic stem cells, cardiac stem cells
and mesenchymal stem cells (MSCs), has been extensively studied in
numerous clinical trials (5).
MSCs are multipotent stem cells that may be isolated
from various tissue types, including perinatal tissues and
Wharton's jelly. Wharton's jelly-derived MSCs (WJ-MSCs) are
regarded as a promising tool for cell therapy due to their
expression of numerous embryonic markers, such as (sex-determining
region Y) box 2, NANOG, LIN28, stage-specific embryonic antigen
(SSEA) 1, SSEA3, SSEA4, Kelch-like factor 4, c-MYC, Criptic family
1 and REX1 (6). Elevated expression
levels of embryonic markers are strongly associated with
pluripotency, anti-inflammatory properties (7), deceleration of senescence (8), low immunogenicity (9) and shorter doubling-time compared with
bone marrow-derived MSCs (BM-MSCs) (10).
The utility of MSCs in cell therapy is due to their
secretion of several survival factors. MSC secretory factors, such
as transforming growth factor β, platelet-derived growth factor and
fibroblast growth factor, have been reported to promote MSC
proliferation and differentiation (11). In addition, these factors may also
function as growth-stimulatory factors for other cell types
(12). Furthermore, proteins
secreted from MSCs have been demonstrated to be involved in the
migration of primitive stem cells from bone marrow to local sites
of injury, the reduction of scar formation and fibrosis, the
enhancement of cardiac function via formation of new blood vessels
and the differentiation of stem cells into cardiomyocytes (13). However, numerous studies have
demonstrated poor survival of MSCs following transplantation, which
is thought to be due to their response to the noxious
microenvironment; oxidative stress present in the damaged tissues
may cause the death of transplanted cells through apoptotic
pathways and upregulation of inflammatory mediators in local injury
(14,15). In an attempt to counteract this,
anti-oxidant enzymes may be expressed at a high level in MSCs to
antagonize the environmental oxidative damage (16). However, the underlying mechanisms and
effects of oxidative environments on MSC function remain to be
fully established.
In the present study, WJ-MSCs were used as an
alternative cell source and their response to treatment with
H2O2 was studied. In particular, the cell
number, viability and morphology as well as the expression of
cardiac-specific genes and proteins were observed in order to
determine the possible effects of oxidative stress on MSC
function.
Materials and methods
Chemicals and reagents
Alizarin Red S (cat. no. 130-22-3), Oil Red O (cat.
no. O0625), dexamethasone (cat. no. D4902), ascorbic acid (cat. no.
508107), β-glycerophosphate (cat. no. 35675) and MTT reagent (cat.
no. M6494) were purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). All antibodies for flow cytometry were
supplied by BD PharmingenÔ (San Jose, CA, USA). Cell culture
reagents, including Dulbecco's modified Eagle's medium (DMEM) (cat.
no. 31600-034), 0.25% trypsin-EDTA (cat. no. 25200-056), PenStrep
(cat. no. 15140-122) and GlutaMAX (cat. no. 35050-061), were
purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA,
USA).
Isolation and expansion
MSCs derived from Wharton's jelly were collected
from the umbilical cords of mothers after a full-term, healthy
delivery (n=6; age of mother, 25–35 years). This collection step
and the informed consent process were approved by the Mahidol
University Institutional Review Board (protocol no. 147.1311).
Written informed consent was obtained from all participants. The
umbilical cords were washed several times with PBS prior to cutting
them into small pieces (length, 3 cm). Each piece of Wharton's
jelly was longitudinally cut for removal of all blood vessels. The
matrix was scraped and minced into small pieces prior to digestion
with 3 mg/ml collagenase type II (cat. no. 44S51434A; Worthington
Biochemical Corp., Lakewood, NJ, USA) in DMEM for 1 h at 37°C,
following washing by centrifugation at 25°C, 2,000 × g for 5 min
and incubation with 0.25% trypsin-EDTA for 20 min at 37°C.
Following the trypsin digestion step, all samples were collected
and washed twice by centrifugation at 25°C, 2,000 × g for 5 min
each. The pellet was re-suspended in complete medium consisting of
low-glucose DMEM (DMEM-LG) supplemented with 10% fetal bovine serum
(FBS; cat no. ES-009-V Merck KGaA, Darmstadt, Germany), 1% PenStrep
and 1% GlutaMAX. The harvested cells were cultured at 37°C in 5%
CO2 and 95% humidified air. Non-adherent cells were
removed after 3 days of culture and medium replacement was
performed twice per week.
BM-MSCs were purchased from Merck KGaA (cat. no.,
SCC034 and lot no, 2460401). BM-MSCs were cultured in complete
Dulbecco's modified Eagle's medium-low glucose (DMEM-LG)
supplemented with 10% fetal bovine serum (FBS), 1% penicillin
streptomycin and 1% GlutaMAX. After reaching 80% cell confluence,
sub-passaging was performed using 0.25% trypsin-EDTA. WJ-MSCs and
BM-MSCs at passage 4–7 were utilized in this study.
MSC characterization
WJ-MSCs and BM-MSCs were characterized following the
minimal criteria for defining MSCs as outlined by the International
Society for Cellular Therapy (17).
The differentiation of WJ-MSCs and BM-MSCs into
adipocytes was performed according to the adipogenic
differentiation protocol provided by the manufacturer. WJ-MSCs were
seeded into 35-mm dishes (3×104 cells per dish) with
adipogenic differentiation medium (cat. no. 05403; Stem Cell
Technologies, Vancouver, Canada) and cultured for 35 days.
Differentiated cells were stained with Oil Red O. Briefly, Oil Red
O staining was performed as follows: differentiated MSCs were
rinsed with 1X phosphate buffered saline (PBS) twice and fixed with
formalin vapour for 10 min at room temperature. MSCs were stained
with 0.3% Oil Red O for 20 min at room temperature. Cells were
washed twice and observed using light microscopy.
For osteogenic differentiation, WJ-MSCs and BM-MSCs
were plated into 35-mm dishes (3×104 cells per dish)
with osteogenic differentiation medium (complete DMEM-LG, 0.1 µM
dexamethasone, 50 µg/ml ascorbic acid and 10 mM β-glycerophosphate)
and cultured for 35 days. Differentiated cells were stained with
Alizarin Red S. In brief, the culture medium was discarded from the
culture flask, and rinsed twice with 1X PBS. The differentiated
cells were fixed with 10% formaldehyde for 15 min at room
temperature. Cells were washed twice with distilled water (DW)
followed by DW removal. A total of 40 mM Alizarin red S (pH 4.1)
was applied for 20 min with gentle agitation. All staining steps
were performed at room temperature. The excess dye was removed and
washed with DW three times. The samples were examined by inverted
microscopy.
For flow cytometric studies of MSCs, WJ-MSCs and
BM-MSCs were trypsinized for 5 min at 37°C to produce single-cell
suspensions. The cell suspensions were collected and washed twice
prior to resuspension in PBS containing 2% FBS and 1 mM EDTA. Cells
were adjusted to 1×106 in 100 µl cells suspension. For
cell surface labelling, cells suspensions were incubated at 4°C for
30 min with 5 µl of antibodies (dilution, 1:20) against
MSC-specific surface markers [phycoerythrin (PE)-conjugated mouse
anti-human CD105 (cat. no. 560839), PE-Cy7-conjugated mouse
anti-human CD73 (cat. no. 561258) and allophycocyanine-conjugated
mouse anti-human CD90 (cat. no. 559869)] and hematopoietic stem
cell (HSC)-specific markers [(PE-conjugated mouse anti-human CD34
(cat. no. 343606) and peridinin chlorophyll-conjugated mouse
anti-human CD45 (cat. no. 304026)]. All antibodies were supplied by
BD PharmingenTM (San Jose, CA, USA). Cell surface marker analysis
was performed by use of a BD FACSCanto™ II Flow
Cytometer and FACSDIVA software version 6.1.3 (BD Biosciences, San
Jose, CA, USA).
Cell viability assay
According to previous studies (18,19),
treatment with 100–2,000 µM H2O2 was able to
influence the cellular senescence of MSCs. In a preliminary study
performed by our group, MSCs were treated with 50, 100, 200, 500,
700 and 1,000 µM H2O2. There was no
difference in the MTT results among the groups treated with 50, 100
and 200 µM H2O2. Thus, 200 µM was selected as
the minimal dose of H2O2 in the present
study. WJ-MSCs and BM-MSCs were seeded in 96-well plates at a
density of 1×104 cells/well, and allowed to attach to
the plastic surface. Wells were inoculated with various
concentrations (200, 500 and 1,000 µM) of
H2O2 (cat. no., 107209; Merck KGaA) in
culture medium. All treatments for each time-point were performed
in triplicate cell samples. After incubation for 24, 48 or 72 h, 50
µl MTT reagent was applied. MTT was reduced by succinate
dehydrogenase enzyme into an insoluble formazan crystal product.
The intracellular crystals were solubilized by dimethyl sulfoxide
(cat. no., 2743C104; Amresco, Solon, OH, USA) and the absorbance
was measured at 570 nm. The absorbance values were then recorded
and compared with those of the control cells.
Analysis of the effect of H2O2
on cardiogenic differentiation of WJ-MSCs. To study the effect of
H2O2 on the cardiogenic differentiation of
MSCs, they were treated with 5-azacytidine followed by various
doses of H2O2 (20). Cardiomyocyte differentiation was
initiated by treatment with 10 µM 5-azacytidine for 24 h followed
by different doses of H2O2 for 24 h. The
short-term effect on cardiogenic markers was observed at days 3 and
7 after treatment with H2O2. These optimized
time-points were selected because the properties of MSCs
dedifferentiate after day 7 (data not shown). WJ-MSCs and BM-MSCs
were plated in culture vessels and incubated overnight. Complete
DMEM supplemented with 10 µM 5-azacytidine was applied to adherent
cells for 24 h. The medium containing 5-azacytidine was then
removed, and the cells were washed twice with DMEM-LG. Complete
DMEM supplemented with 200, 500 or 1,000 µM
H2O2 was applied for 24 h prior to washing
twice and replacement with fresh complete medium. Treated WJ-MSCs
and BM-MSCs were maintained in culture for further detection of
cardiogenic marker expression on days 3 and 7.
Reverse-transcription quantitative
polymerase chain reaction (RT-qPCR) analysis of cardiac-specific
genes
RNA was collected using TRIzol® reagent
(cat. no., 15596026; Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) and isolated using Direct-zol columns (cat. no.,
R2050; Zymo Research, Irvine, CA, USA), following the
manufacturer's instructions. Quantification of RNA was performed
with a Nanodrop 2000 ultraviolet-vis spectrophotometer (Thermo
Fisher Scientific, Inc.). RNA (2 µg/sample) was converted to
complementary DNA using Sensiscript Reverse Transcription kits
(cat. no., 205211; Qiagen, Valencia, CA, USA). To study cardiogenic
genes, qPCR was performed with SYBR Green Supermix (cat. no.,
170-8880; Bio-Rad Laboratories, Inc., Hercules, CA, USA), according
to the manufacturer's instructions. The PCR mixture was composed of
12.5 µl of iQ™ SYBR® Green Supermix, 0.5 µl of 10 µM
forward primer, 0.5 µl of 10 µM reverse primer 5.5 ml of
nuclease-free water and 1 µl of cDNA (100 ng/µl). The detection was
performed with a CFX96 Real-Time PCR platform (Bio-Rad
Laboratories, Inc.). The PCRs were performed in duplicate and
conditions were as follows: 95°C for 3 min; followed by 40 cycles
of 95°C for 3 sec (denaturation), 60°C for 30 sec (annealing
temperature) and 72°C for 45 sec (elongation); and 72°C for 5 min.
The primers were as follows: Nkx2.5 forward,
5′-CTGCCGCCGCCAACAAC-3′ and reverse, 5′-CGCGGGTCCCTTCCCTACCA-3′;
cardiac troponin T (cTnT) forward, 5′-AGAGCGGAAAAGTGGGAAGA-3′ and
reverse, 5′-CTGGTTATCGTTGATCCTGT-3′; and cardiac α-actin forward,
5′-TCTATGAGGGCTACGCTTTG-3′ and reverse, 5′-GCCAATAGTGATGACTTGGC-3′;
GAPDH forward, 5′-CAACTACATGGTTTACATGTTCCAA-3′ and reverse,
5′-CAGCCTTCTCCATGGTGGT-3′. The levels of the cardiac-specific genes
(Nkx2.5, cTnT and cardiac α-actin) were analyzed using Bio-Rad CFX
Manager version 3.1 (Bio-Rad Laboratories, Inc.) and normalized to
the level of GAPDH (21).
Immunofluorescence staining
Treated WJ-MSCs and BM-MSCs were fixed with 4%
paraformaldehyde prior to being washed twice with PBS.
Permeabilization was performed using 0.3% Triton X (cat. no.
9002931; Merck KGaA). Prior to staining with primary antibodies,
cells were non-specifically blocked with 3% bovine serum albumin
(cat. no. A7906; Invitrogen; Thermo Fisher Scientific, Inc.).
Subsequently, cells were incubated overnight at 4°C with anti-GATA4
(cat. no. sc-25310; Santa Cruz Biotechnologies, Inc., Danvers, TX,
CA, USA) and anti-cTnT (cat. no. MAB1693; Merck KGaA) antibodies
(1:100 dilution). Cells were washed with PBS prior to incubation
with Alexa Fluor® 568-conjugated goat anti-mouse
immunoglobulin antibodies (cat. no. A-11004; Invitrogen; Thermo
Fisher Scientific, Inc.) and fluorescein isothiocyanate
(FITC)-conjugated rabbit anti-mouse immunoglobulin antibodies (cat.
no. F0232; Dako Cytomation, Tokyo, Japan) for 45 min at room
temperature. Cells were washed twice with PBS prior to mounting
with ProLong® Gold Antifade Mountant with DAPI solution
(cat no. P36941; Invitrogen; Thermo Fisher Scientific, Inc.,). The
fluorescent micrographs were captured using a confocal laser
scanning microscope and analyzed with FluoView FV1000 Software
version 3.01 (Olympus Corp., Tokyo, Japan). To evaluate
fluorescence intensity, the micrographs captured at a magnification
of ×40 were equally divided into 9 areas, and 5 counting areas (4
corner squares and 1 center square) were selected as regions of
interest (ROIs) for subsequent study. Only positively stained cells
in the ROIs were quantified for fluorescence intensity measurements
(22,23).
Statistical analysis
WJ-MSCs from 6 donors were performed independently
(n=6) all values were expressed as the mean ± standard error of the
mean. Statistical analysis was performed using PASW Statistics
version 18 (IBM Corp., Armonk, NY, USA). The Mann-Whitney U test
was used to analyze differences between groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
Characterization of MSCs
WJ-MSCs were isolated from Wharton's jelly; the
cells usually grew from the cell cluster after 3 days of culture.
WJ-MSCs and BM-MSCs exhibited a fibroblast-like morphology and a
high level of adherence to the plastic culture flasks (Fig. 1A). Characterization of the MSCs was
subsequently performed.
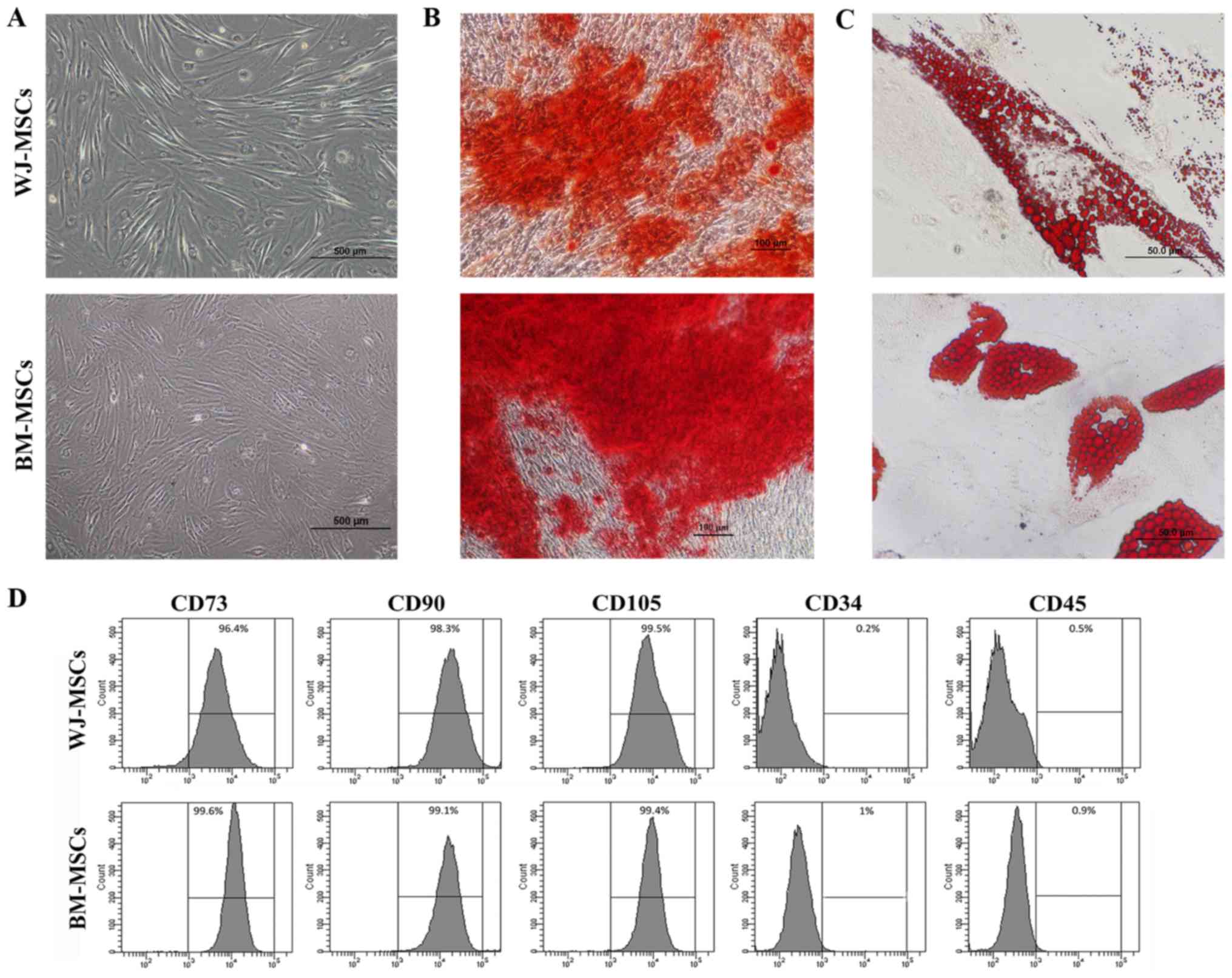 | Figure 1.(A) Images of enzymatically
disaggregated Wharton's jelly-derived cells in primary culture
displaying their fibroblastoid shape, similar to that of BM-MSCs
(scale bars, 500 µm). (B and C) Mesodermal differentiation of MSCs
into osteoblasts and adipocytes was performed. (B) BM-MSCs and
WJ-MSCs cultured in osteogenic differentiation medium for 35 days
displayed positive Alizarin Red S staining, indicating osteoblastic
differentiation (scale bars, 100 µm). (C) BM-MSCs and WJ-MSCs
cultured in adipogenic differentiation medium for 35 days were
positive for Oil Red O staining, indicating adipogenic
differentiation (scale bars, 50 µm). (D) Flow cytometric analysis
of MSC surface markers indicated reactivity for CD73, CD90 and
CD105, and no reactivity for CD34 and CD45. BM-MSCs, bone
marrow-derived mesenchymal stem cells; WJ-MSCs, Wharton's
jelly-derived MSCs. |
For osteogenic and adipogenic differentiation, MSCs
were cultured with osteogenic and adipogenic differentiation
medium, respectively, for 5 weeks. Under osteogenic differentiation
conditions, the morphology of the MSCs became osteoblast-like with
matrix accumulation (Fig. 1B). In
MSCs cultured in adipogenic differentiation medium, fat
droplet-containing cells were observed (Fig. 1C). Cytochemical staining with
Alizarin Red S and Oil Red O indicated differentiation into
osteoblast-like and adipocyte-like cells, respectively.
The analysis of specific cell surface markers
revealed that MSCs at passage 4 exhibited high rates of positive
staining for CD73 (99.3±0.4%), CD90 (96.9±1.1%) and CD105
(99.9±0.4%), but were mostly negative for the hematopoietic markers
CD34 (1±0.3%) and CD45 (1.3±0.6%) (Fig.
1D).
Viability of MSCs after treatment with
H2O2
The effect of H2O2 on the
viability of MSCs was investigated. MSCs treated with
H2O2 alone and compared with the control (no
H2O2 treatment using an MTT assay. The
absorbance was measured at 24, 48 and 72 h. The absorbance ratios
of control vs. treated cells were used for the calculation of the
percent viability. The viability of WJ-MSCs after treatment with
H2O2 was decreased in a dose- and
time-dependent manner (Fig. 2A). The
viability of BM-MSCs was only slightly decreased after treatment
with 200 and 500 µM H2O2, but significantly
decreased after treatment with 1,000 µM H2O2
(Fig. 2B).
Effect of H2O2
on MSC morphology and cardiac-specific marker expression
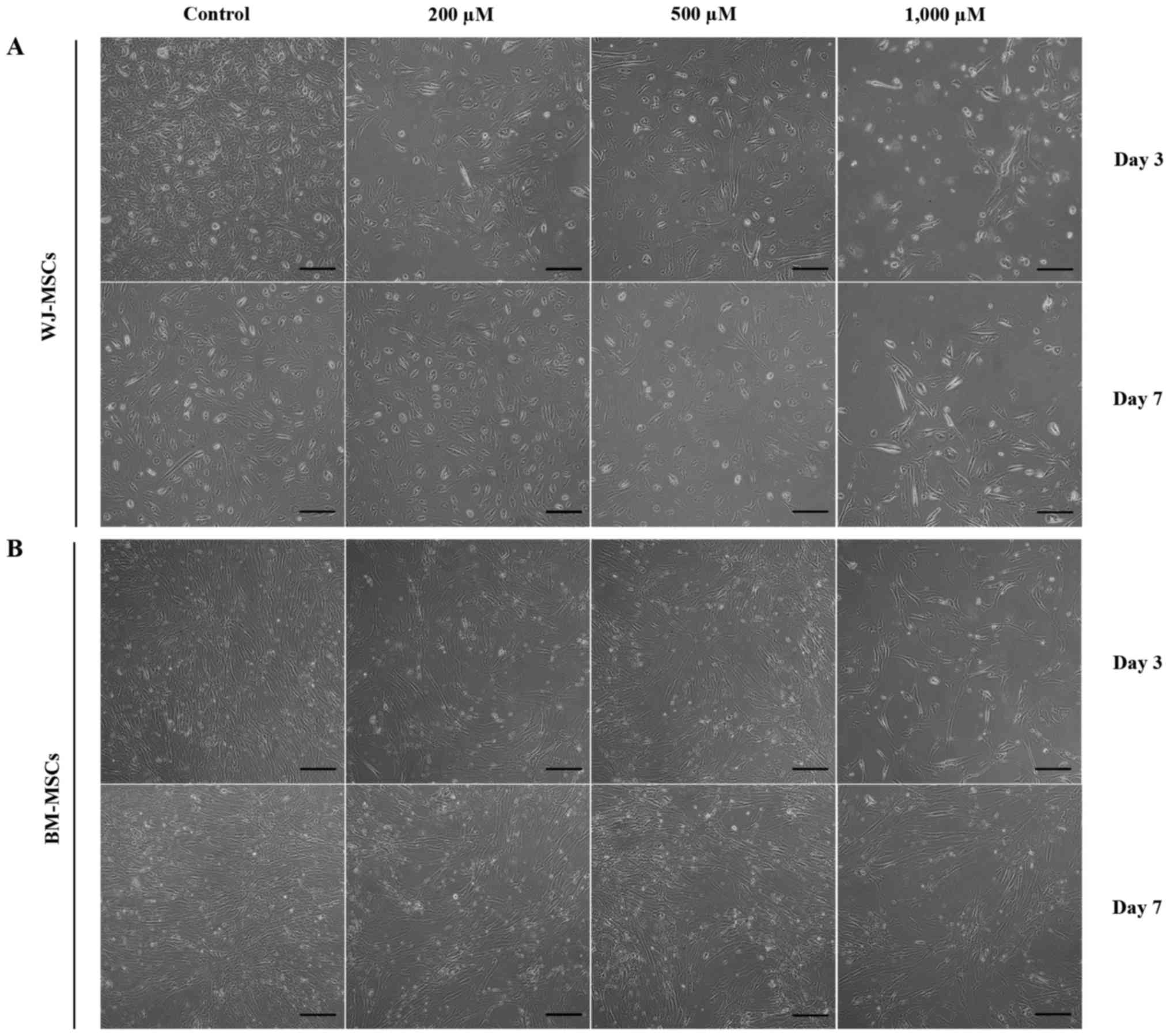
The morphology and cardiomyocyte differentiation of
MSCs were observed after treatment with 5-azacytidine with and
without H2O2 (200, 500 and 1,000 µM). Cells
in all treatment groups appeared similar, with a fibroblastoid
shape. However, the cell density was attenuated by treatment with
increasing H2O2 concentrations (Fig. 3).
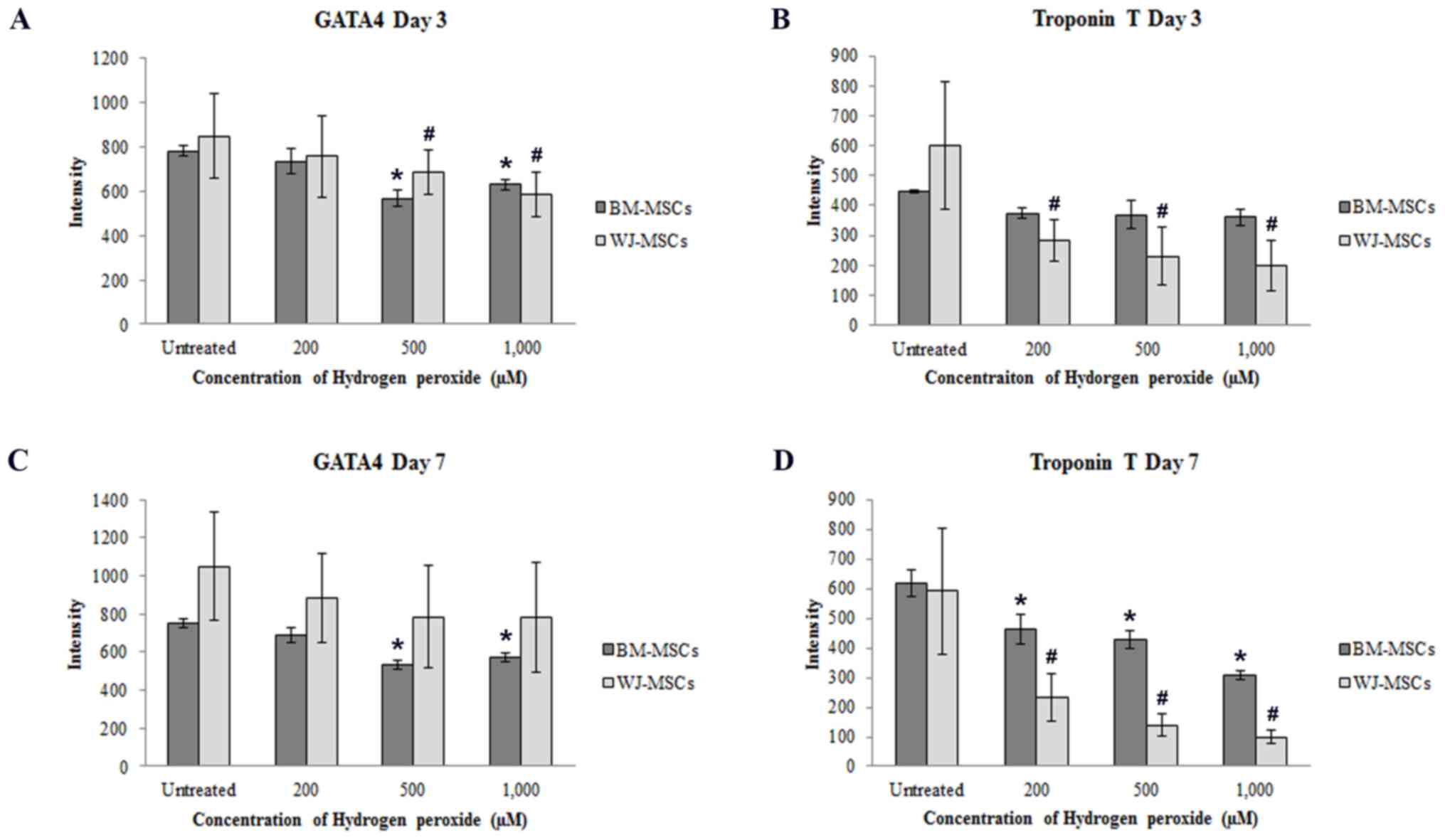
Quantitative analysis of the mRNA expression levels
of cardiac-specific genes (Nkx2.5, cTnT and α-cardiac actin) was
performed by RT-qPCR. After culture for 3 days as illustrated in
Fig. 4A, the expression of Nkx2.5,
cTnT and cardiac α-actin in 5-azacytidine-treated MSCs was reduced
in a dose-dependent manner following treatment with
H2O2 (200, 500 or 1,000 µM). The gene
expression levels then recovered and returned almost to the
expression levels of the untreated cells. The recovery of gene
expression was observed in treated cells after culture at day 7
(Fig. 4B and C).
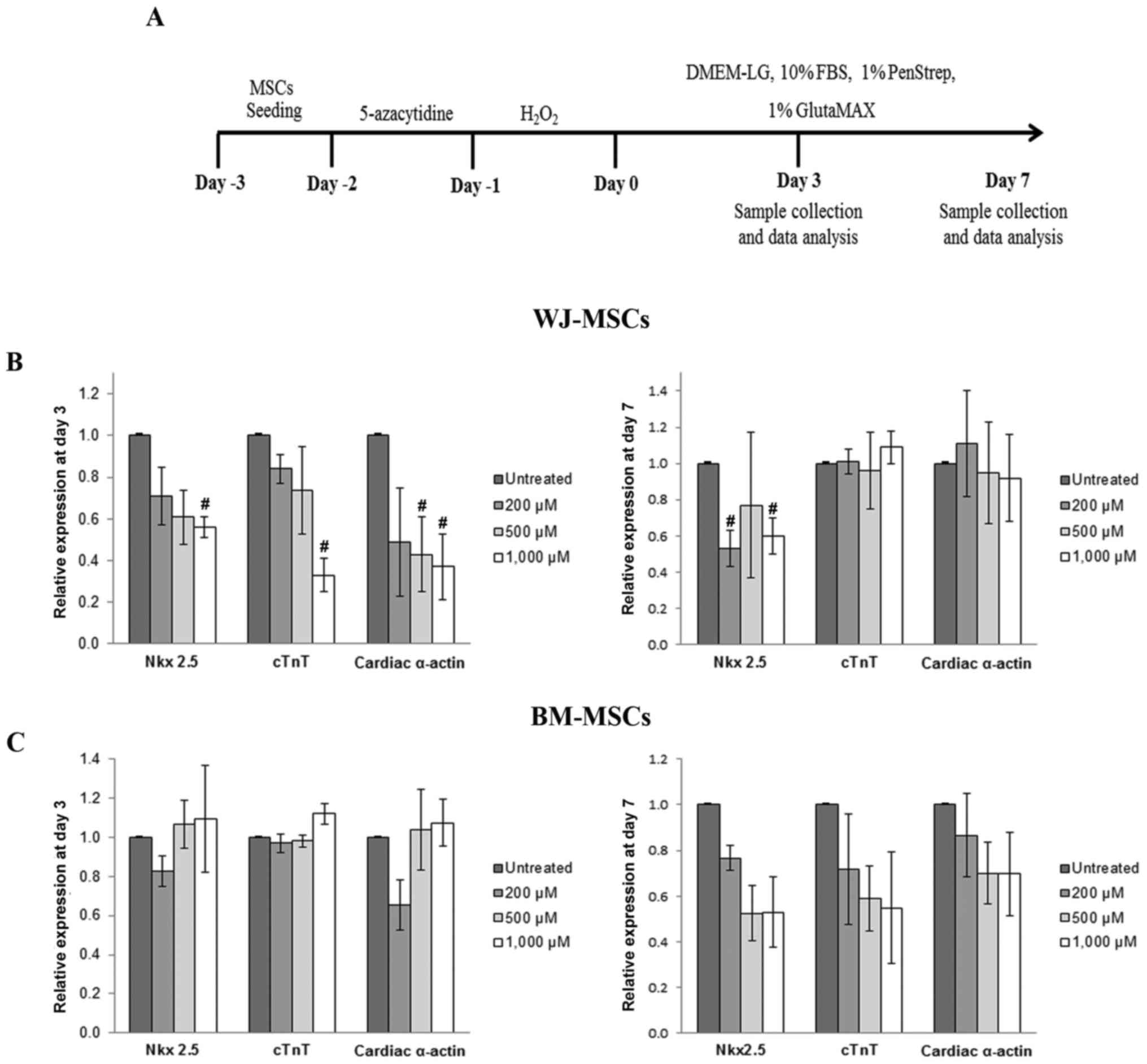 | Figure 4.(A) Schematic diagram representing
the process used to study the cardiomyocyte differentiation of MSCs
using 5-azacytidine and hydrogen peroxide. (B) Cardiac-specific
gene expression in WJ-MSCs after treatment for 3 and 7 days. The
levels of Nkx2.5, cTnT and cardiac α-actin were significantly
reduced after treatment for 3 days compared with untreated cells
(MSCs treated with 5-azacytidine to make them differentiate into
cardiomyocytes, but not with H2O2), and these
expression levels recovered after culture for 7 days. In BM-MSCs,
the relative expression levels of cardiac-specific markers after
treatment were decreased compared with those of untreated cells
only after culture for 7 days (C). Values are expressed as the mean
± standard error of the mean of six separate experiments performed
in duplicate in WJ-MSCs and three separate experiments performed in
duplicate in BM-MSCs. #P<0.05 vs. untreated cells for
WJ-MSCs or BM-MSCs, respectively. BM-MSCs, bone marrow-derived
mesenchymal stem cells; WJ-MSCs, Wharton's jelly-derived MSCs;
DMEM-LG, low-glucose Dulbecco's modified Eagle's medium; FBS, fetal
bovine serum; cTnT, cardiac troponin T. |
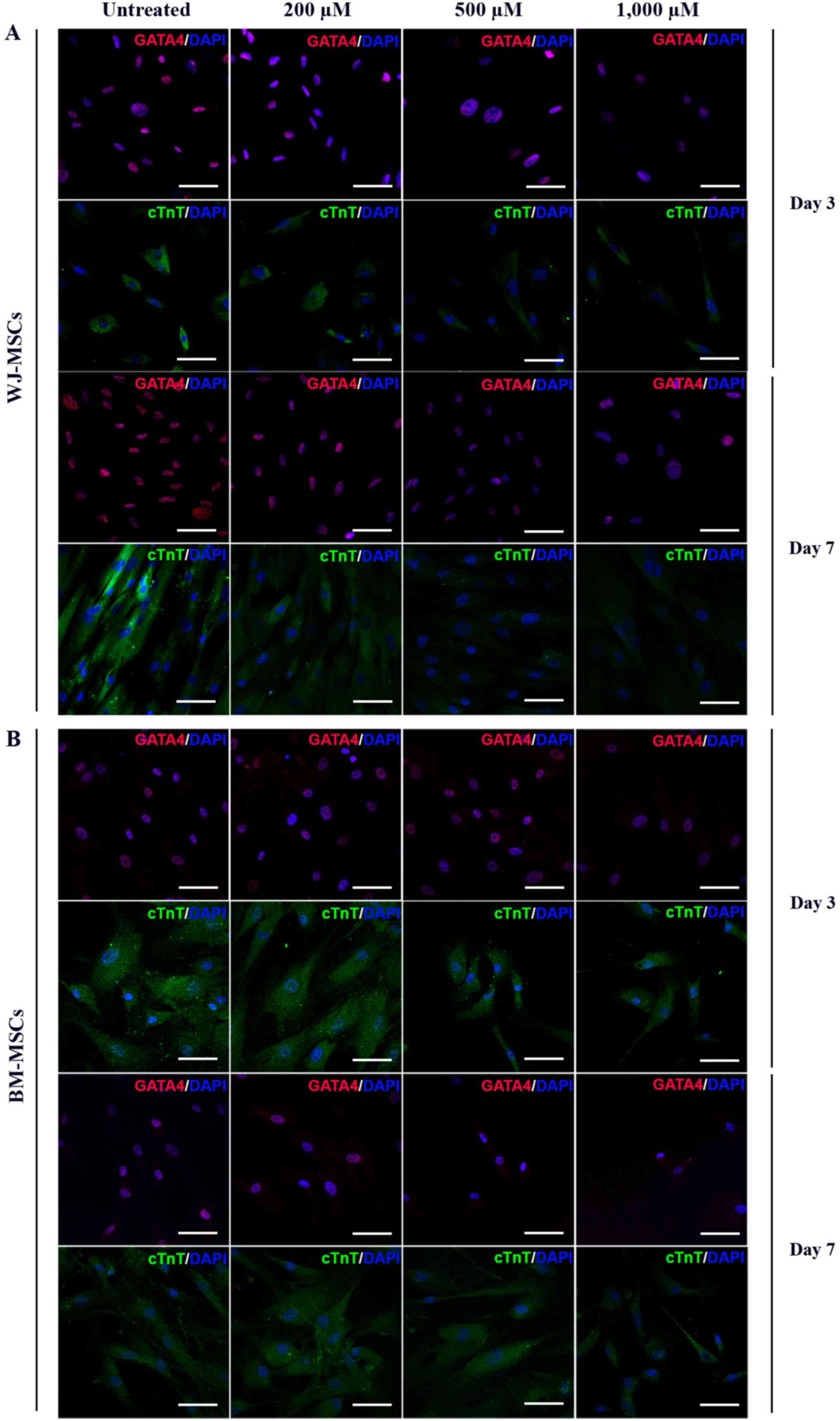
MSCs were exposed to 10 µM of 5-azacytidine and
treated with 200, 500 or 1,000 µM H2O2 for 24
h. The differentiated cells were stained with antibodies against
GATA4 (labelled with Alexa Fluor 568) and cTnT (labelled with
FITC). Immunofluorescent micrographs of WJ-MSCs (Fig. 5A) and BM-MSCs (Fig. 5B) were captured and analyzed to
assess the fluorescence intensity (using ROIs) at days 3 and 7
which was presented in Fig. 6.
Following treatment with H2O2,
the fluorescence intensity of GATA4 in BM-MSCs and WJ-MSCs was
dose-dependently decreased compared with that in untreated cells
(Fig. 6A and C). Similarly, the
fluorescence intensity of cTnT in
H2O2-treated WJ-MSCs was decreased after
culture for 3 and 7 days, and was significantly different from that
of untreated cells. In BM-MSCs, a significant decrease of cTnT
fluorescence intensity was only observed after treatment for 7 days
(Fig. 6B and D).
Discussion
Stem cell therapy is a novel and promising method
for treating degenerative disorders. One of the most interesting
therapeutic applications is MSC transplantation in IHD. Previous
studies have demonstrated that the IHD conditions trigger the death
of cardiomyocytes due to the presence of excessive ROS in the
infarct area. This results in premature senescence (24), apoptosis (14), impairment of immunomodulatory
function (25) and fibrous tissue
formation (26). Stem cell
transplantation approaches for the treatment of cardiac injury have
been investigated in several animal studies and clinical trials
(27,28). The efficacy of MSC transplantation
depends on the survival and differentiation ability of MSCs and
their role in the recovery of cardiac function (25,26). The
rate of successful transplantation outcomes remains low, likely due
to the effect of residual oxidative stress on the transplanted
cells in damaged tissues (15).
However, the underlying mechanisms of this inefficiency have
remained to be fully elucidated.
In the present study, the effect of ROS on one
particular function of MSCs, namely cardiomyocyte differentiation,
was evaluated. The characteristics of WJ-MSCs and BM-MSCs were
assessed, which revealed that MSCs from each of these two sources
exhibited the same characteristics, in that they were positive for
Oil Red O and Alizarin Red S staining after induction of adipogenic
and osteogenic differentiation, respectively. Mesenchymal surface
markers (CD90, CD73 and CD105) were present on MSCs from each of
the two sources (>95% of cells), whereas they were mostly
negative for HSC markers (<2% of cells), which was in accordance
with previous studies (29). WJ-MSCs
and BM-MSCs were cultured under stress conditions to evaluate their
survivability. The viability of only the WJ-MSCs was demonstrated
to be significantly reduced by H2O2 in a
dose- and time-dependent manner. This suggested that BM-MSCs may
have higher resistance to ROS compared with WJ-MSCs. Under stress
conditions, this property may be modulated in BM-MSCs via the
release of hydrogen peroxide-scavenging enzymes, such as superoxide
dismutase, catalase and glutathione peroxidase, providing
resistance to oxidative stress-induced cell death (30). However, studies on the effects of ROS
on MSCs of different origin have yielded controversial and
inconclusive results. Certain studies suggested that WJ is an
optimal source of MSCs due WJ-MSCs having a higher potential for
proliferation (31) and
differentiation (32) than other
types of MSCs, such as BM-MSCs. This was supported by the fact that
WJ-MSCs had higher expression levels of several cell
cycle-associated genes, including cyclin (CCN)D2, cell division
cycle (CDC)25A, CCNA2, CCNB1, CDC28, cyclin-dependent kinases (CDK)
regulatory subunit 2, CDC25C, CDC20 and aurora B kinase, compared
with the levels in BM-MSCs. These genes promote the cell cycle
transition from S to G2/M phase. By contrast, compared with
WJ-MSCs, BM-MSCs exhibit increased expression levels of B-cell
lymphoma 2 and CDK inhibitor 1B, which mediate delayed transition
into S phase (33). These findings
implied that endogenous differences between MSC sources may lead to
different responses between BM-MSCs and WJ-MSCs. Therefore, further
studies should be performed to prove that the different properties
of MSCs depend on their origin.
MSCs originating outside of the bone marrow appear
to manage oxidative stress differently; they may resist oxidative
insults to a greater extent than normal somatic cells, such as
fibroblasts (34). For instance,
Ertaş et al (35) identified
that BM-MSCs and adipose-derived MSCs possessed higher resistance
to H2O2-induced apoptosis, but had a greater
tolerance for H2O2 compared with BM-MSCs. The
underlying mechanisms of these anti-oxidant properties are largely
unknown. Release of extracellular vesicles from WJ-MSCs may be an
important mechanism, as reported by Zhang et al (36), who demonstrated that WJ-MSCs cultured
in conditioning medium may improve the function of kidney cells and
decrease apoptosis following acute renal injury. In that study, the
extracellular vesicles isolated from the conditioning medium
attenuated oxidative damage in injured kidney tissue, and this
antioxidant effect was demonstrated to result from nuclear
erythroid 2-related factor 2/antioxidant responsive element
activation. Pre-conditioning of WJ-MSCs with 200 µM
H2O2 in vitro promoted the expression
of interleukin-6, leading to increased migration, proliferation and
neovascularization properties of endothelial cells. A previous
animal study also revealed that the infusion of
H2O2-pre-conditioned WJ-MSCs in to the tail
vein significantly enhances myocardial contractility (37).
In the present study, the cardiogenic
differentiation of BM-MSCs and WJ-MSCs was evaluated under normal
conditions and in the presence of ROS. The RNA expression of
cardiogenesis-associated genes (Nkx2.5, cTnT and cardiac α-actin)
and proteins (GATA4 and cTnT), which contribute to cell fate in the
early development of cardiomyocytes (38), were identified to rapidly decrease in
WJ-MSCs following exposure to H2O2, and then
recover by day 7. In BM-MSCs, a reduction in GATA4 expression was
detected on day 3 and day 7; however, in cTnT expression, reduction
in expression was detected only on day 7. In vitro studies
of the effect of ROS on MSCs have frequently reported that ROS acts
to promote cell growth inhibition (39), apoptosis (40) and premature senescence (41). The effect of ROS on differentiation
capacity has also been reported in other cell types, such as
osteoblasts (42). In a previous
study, ROS had a different impact on BM-MSCs and WJ-MSCs, but the
underlying mechanisms remained elusive (43). WJ-MSCs had certain advantages as a
cell therapy for myocardial injury over adult or fetal BM-MSCs.
Furthermore, the in vivo transplantation of WJ-MSCs was
demonstrated to have a high potential for homing to infarcted
myocardium and decreasing tissue damage (43). Thus, the type of MSCs used for
transplantation must be selected with awareness of the specific
aim; MSCs from different origins have similar but varying
characteristics, and these distinctions must be considered to
optimize the outcome of transplantation.
Transplantation efficiency is currently under
extensive study for the purpose of identifying strategies for
improvement. The concept of cell death prevention has been widely
explored based on several concepts. Overexpression of antioxidants
was considered to be highly promising, but the results are
controversial; although high ROS levels cause cellular damage and
dysfunction, it is thought that a low basal level of ROS is
necessary and advantageous for maintaining cellular proliferation,
differentiation and survival. ROS also has an important role in the
post-infarction healing process by augmenting inflammation and
tissue repair through the release of proteolytic enzymes, cytokines
and growth factors (44,45). Therefore, understanding the effects
of ROS on stem cell behaviour is important and requires further
study to allow for improvement of stem cell transplantation
outcomes.
In conclusion, the present study suggested that the
differentiation potency of MSCs of different origin may be helpful
for selecting suitable cell sources to obtain better outcomes.
Improvement of the selection of cells used for transplantation may
be achieved through enhancing the current understanding of the
underlying mechanisms of how MSCs respond to ROS. Comparing
different abilities of WJ-MSCs and BM-MSCs in in vitro
studies will help in selecting the optimal cell source for clinical
trials.
Acknowledgements
The present study was supported by a research grant
from Mahidol University (2556-2559 B.E.).
References
|
1
|
McAloon CJ, Boylan LM, Hamborg T, Stallard
N, Osman F, Lim PB and Hayat SA: The changing face of
cardiovascular disease 2000–2012: An analysis of the world health
organisation global health estimates data. Int J Cardiol.
224:256–264. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
von Harsdorf R, Li PF and Dietz R:
Signaling pathways in reactive oxygen species-induced cardiomyocyte
apoptosis. Circulation. 99:2934–2941. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu Z, Park SS, Mueller RA, Bagnell RC,
Patterson C and Boysen PG: Adenosine produces nitric oxide and
prevents mitochondrial oxidant damage in rat cardiomyocytes.
Cardiovasc Res. 65:803–812. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tao L, Gao E, Jiao X, Yuan Y, Li S,
Christopher TA, Lopez BL, Koch W, Chan L, Goldstein BJ and Ma XL:
Adiponectin cardioprotection after myocardial ischemia/reperfusion
involves the reduction of oxidative/nitrative stress. Circulation.
115:1408–1416. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bernstein HS and Srivastava D: Stem cell
therapy for cardiac disease. Pediatr Res. 71:491–499. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gao LR, Zhang NK, Ding QA, Chen HY, Hu X,
Jiang S, Li TC, Chen Y, Wang ZG, Ye Y and Zhu ZM: Common expression
of stemness molecular markers and early cardiac transcription
factors in human Wharton's jelly-derived mesenchymal stem cells and
embryonic stem cells. Cell Transplant. 22:1883–1900. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li Q, Han SM, Song WJ, Park SC, Ryu MO and
Youn HY: Anti-inflammatory effects of Oct4/Sox2-overexpressing
human adipose tissue-derived mesenchymal stem cells. In Vivo.
31:349–356. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shahini A, Mistriotis P, Asmani M, Zhao R
and Andreadis ST: NANOG restores contractility of mesenchymal stem
cell-based senescent microtissues. Tissue Eng Part A. 23:535–545.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou C, Yang B, Tian Y, Jiao H, Zheng W,
Wang J and Guan F: Immunomodulatory effect of human umbilical cord
Wharton's jelly-derived mesenchymal stem cells on lymphocytes. Cell
Immunol. 272:33–38. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Baksh D, Yao R and Tuan RS: Comparison of
proliferative and multilineage differentiation potential of human
mesenchymal stem cells derived from umbilical cord and bone marrow.
Stem Cells. 25:1384–1392. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ng F, Boucher S, Koh S, Sastry KS, Chase
L, Lakshmipathy U, Choong C, Yang Z, Vemuri MC, Rao MS and Tanavde
V: PDGF, TGF-beta, and FGF signaling is important for
differentiation and growth of mesenchymal stem cells (MSCs):
Transcriptional profiling can identify markers and signaling
pathways important in differentiation of MSCs into adipogenic,
chondrogenic, and osteogenic lineages. Blood. 112:295–307. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kyurkchiev D, Bochev I, Ivanova-Todorova
E, Mourdjeva M, Oreshkova T, Belemezova K and Kyurkchiev S:
Secretion of immunoregulatory cytokines by mesenchymal stem cells.
World J Stem Cells. 6:552–570. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xiang MX, He AN, Wang JA and Gui C:
Protective paracrine effect of mesenchymal stem cells on
cardiomyocytes. J Zhejiang Univ Sci B. 10:619–624. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rodrigues M, Turner O, Stolz D, Griffith
LG and Wells A: Production of reactive oxygen species by
multipotent stromal cells/mesenchymal stem cells upon exposure to
fas ligand. Cell Transplant. 21:2171–2187. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Elahi MM, Kong YX and Matata BM: Oxidative
stress as a mediator of cardiovascular disease. Oxid Med Cell
Longev. 2:259–269. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jin G, Qiu G, Wu D, Hu Y, Qiao P, Fan C
and Gao F: Allogeneic bone marrow-derived mesenchymal stem cells
attenuate hepatic ischemia-reperfusion injury by suppressing
oxidative stress and inhibiting apoptosis in rats. Int J Mol Med.
31:1395–1401. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dominici M, Le Blanc K, Mueller I,
Slaper-Cortenbach I, Marini F, Krause D, Deans R, Keating A,
Prockop Dj and Horwitz E: Minimal criteria for defining multipotent
mesenchymal stromal cells. The international society for cellular
therapy position statement. Cytotherapy. 8:315–317. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brandl A, Meyer M, Bechmann V, Nerlich M
and Angele P: Oxidative stress induces senescence in human
mesenchymal stem cells. Exp Cell Res. 317:1541–1547. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Burova E, Borodkina A, Shatrova A and
Nikolsky N: Sublethal oxidative stress induces the premature
senescence of human mesenchymal stem cells derived from
endometrium. Oxid Med Cell Longev. 2013:4749312013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Supokawej A, Kheolamai P, Nartprayut K,
U-Pratya Y, Manochantr S, Chayosumrit M and Issaragrisil S:
Cardiogenic and myogenic gene expression in mesenchymal stem cells
after 5-azacytidine treatment. Turk J Haematol. 30:115–121. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dunn KW, Kamocka MM and McDonald JH: A
practical guide to evaluating colocalization in biological
microscopy. Am J Physiol Cell Physiol. 300:C723–C742. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sheikhzadeh F, Ward RK, Carraro A, Chen
ZY, van Niekerk D, Miller D, Ehlen T, MacAulay CE, Follen M, Lane
PM and Guillaud M: Quantification of confocal fluorescence
microscopy for the detection of cervical intraepithelial neoplasia.
Biomed Eng Online. 14:962015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Benameur L, Charif N, Li Y, Stoltz JF and
de Isla N: Toward an understanding of mechanism of aging-induced
oxidative stress in human mesenchymal stem cells. Biomed Mater Eng.
25 Suppl 1:S41–S46. 2015.
|
|
25
|
Gan P, Gao Z, Zhao X and Qi G: Surfactin
inducing mitochondria-dependent ROS to activate MAPKs, NF-κB and
inflammasomes in macrophages for adjuvant activity. Sci Rep.
6:393032016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao W, Zhao T, Chen Y, Ahokas RA and Sun
Y: Oxidative stress mediates cardiac fibrosis by enhancing
transforming growth factor-beta1 in hypertensive rats. Mol Cell
Biochem. 317:43–50. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen S, Chen X, Wu X, Wei S, Han W, Lin J,
Kang M and Chen L: Hepatocyte growth factor-modified mesenchymal
stem cells improve ischemia/reperfusion-induced acute lung injury
in rats. Gene Ther. 24:3–11. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mureli S, Gans CP, Bare DJ, Geenen DL,
Kumar NM and Banach K: Mesenchymal stem cells improve cardiac
conduction by upregulation of connexin 43 through paracrine
signaling. Am J Physiol Heart Circ Physiol. 304:H600–H609. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Reppel L, Schiavi J, Charif N, Leger L, Yu
H, Pinzano A, Henrionnet C, Stoltz JF, Bensoussan D and Huselstein
C: Chondrogenic induction of mesenchymal stromal/stem cells from
Wharton's jelly embedded in alginate hydrogel and without added
growth factor: An alternative stem cell source for cartilage tissue
engineering. Stem Cell Res Ther. 6:2602015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Valle-Prieto A and Conget PA: Human
mesenchymal stem cells efficiently manage oxidative stress. Stem
Cells Dev. 19:1885–1893. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Balasubramanian S, Venugopal P, Sundarraj
S, Zakaria Z, Majumdar AS and Ta M: Comparison of chemokine and
receptor gene expression between Wharton's jelly and bone
marrow-derived mesenchymal stromal cells. Cytotherapy. 14:26–33.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pu L, Meng M, Wu J, Zhang J, Hou Z, Gao H,
Xu H, Liu B, Tang W, Jiang L and Li Y: Compared to the amniotic
membrane, Wharton's jelly may be a more suitable source of
mesenchymal stem cells for cardiovascular tissue engineering and
clinical regeneration. Stem Cell Res Ther. 8:722017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Batsali AK, Pontikoglou C, Koutroulakis D,
Pavlaki KI, Damianaki A, Mavroudi I, Alpantaki K, Kouvidi E,
Kontakis G and Papadaki HA: Differential expression of cell cycle
and WNT pathway-related genes accounts for differences in the
growth and differentiation potential of Wharton's jelly and bone
marrow-derived mesenchymal stem cells. Stem Cell Res Ther.
8:1022017. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vinoth KJ, Manikandan J, Sethu S,
Balakrishnan L, Heng A, Lu K, Poonepalli A, Hande MP and Cao T:
Differential resistance of human embryonic stem cells and somatic
cell types to hydrogen peroxide-induced genotoxicity may be
dependent on innate basal intracellular ROS levels. Folia Histochem
Cytobiol. 53:169–174. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ertaş G, Ural E, Ural D, Aksoy A, Kozdağ
G, Gacar G and Karaöz E: Comparative analysis of apoptotic
resistance of mesenchymal stem cells isolated from human bone
marrow and adipose tissue. ScientificWorldJournal. 2012:1056982012.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang G, Zou X, Huang Y, Wang F, Miao S,
Liu G, Chen M and Zhu Y: Mesenchymal stromal cell-derived
extracellular vesicles protect against acute kidney injury through
anti-oxidation by Enhancing Nrf2/ARE activation in rats. kidney
Blood Press Res. 41:119–128. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhang J, Chen GH, Wang YW, Zhao J, Duan
HF, Liao LM, Zhang XZ, Chen YD and Chen H: Hydrogen peroxide
preconditioning enhances the therapeutic efficacy of Wharton's
Jelly mesenchymal stem cells after myocardial infarction. Chin Med
J (Engl). 125:3472–3478. 2012.PubMed/NCBI
|
|
38
|
Zhang Y, Sivakumaran P, Newcomb AE,
Hernandez D, Harris N, Khanabdali R, Liu GS, Kelly DJ, Pébay A,
Hewitt AW, et al: Cardiac repair with a novel population of
mesenchymal stem cells resident in the Human Heart. Stem Cells.
33:3100–3113. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bai J, Hu Y, Wang YR, Liu LF, Chen J, Su
SP and Wang Y: Comparison of human amniotic fluid-derived and
umbilical cord Wharton's Jelly-derived mesenchymal stromal cells:
Characterization and myocardial differentiation capacity. J Geriatr
Cardiol. 9:166–171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang FW, Wang Z, Zhang YM, Du ZX, Zhang
XL, Liu Q, Guo YJ, Li XG and Hao AJ: Protective effect of melatonin
on bone marrow mesenchymal stem cells against hydrogen
peroxide-induced apoptosis in vitro. J Cell Biochem. 114:2346–2355.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Choo KB, Tai L, Hymavathee KS, Wong CY,
Nguyen PN, Huang CJ, Cheong SK and Kamarul T: Oxidative
stress-induced premature senescence in Wharton's jelly-derived
mesenchymal stem cells. Int J Med Sci. 11:1201–1207. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang Y, Yi XD and Li CD: Suppression of
mTOR signaling pathway promotes bone marrow mesenchymal stem cells
differentiation into osteoblast in degenerative scoliosis: In vivo
and in vitro. Mol Biol Rep. 44:129–137. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
López Y, Lutjemeier B, Seshareddy K,
Trevino EM, Hageman KS, Musch TI, Borgarelli M and Weiss ML:
Wharton's jelly or bone marrow mesenchymal stromal cells improve
cardiac function following myocardial infarction for more than 32
weeks in a rat model: A preliminary report. Curr Stem Cell Res
Ther. 8:46–59. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
D'Autréaux B and Toledano MB: ROS as
signalling molecules: Mechanisms that generate specificity in ROS
homeostasis. Nat Rev Mol Cell Biol. 8:813–824. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kobayashi CI and Suda T: Regulation of
reactive oxygen species in stem cells and cancer stem cells. J Cell
Physiol. 227:421–430. 2012. View Article : Google Scholar : PubMed/NCBI
|