Introduction
Ionizing radiation has positive applications in
agricultural production, medicine and health, scientific research
and national defense (1). However,
it also damages many aspects of human physiology, including the
peripheral blood cells, bone marrow DNA (2), immune-related organs (3) and antioxidant enzymes (4,5). The
United States Army Research Institute developed the first
anti-radiation drug, amifostine, which was approved by the United
States Food and Drug Administration (6). Amifostine is able to significantly
reduce the death of normal cells following radiotherapy; however,
side effects including hypotension, nausea, vomiting and other
adverse reactions have restricted its use (7). Therefore, studies are warranted to
identify an effective, natural non-toxic medicine that protects
against radiation and reduces radiation damage. Previous studies
have documented that plant proteins (8,9) and
non-heme iron-binding proteins (10–12)
exert protective effects against radiation. In addition, arginine,
glutamine, glycine, mycosporine-like amino acids and essential
amino acids may promote weight recovery, improve protein
nutritional conditions and exert anti-oxidant effects in rats
exposed to X-ray irradiation (13–15).
A previous study from the authors revealed that
Yak-activated proteins are extracted from the healthy tissue of
yaks from the Qinghai-Tibetan Plateau, and have been found to
contain a large number of small peptides (not published).
Yak-activated protein was initially identified following treatment
of isolated yak tissues with a combination of radiotherapy and
chemotherapy, whereby the mature white blood cell count was found
to be normal, while the activity of T cells, natural killer cells,
monocytes and neutrophils was unusually high. Isolated yak tissue
is typically prepared using extraction, separation and purification
methods with applied modern biotechnology and biological
engineering technologies, allowing it to be absorbed directly
without digestion in the intestinal tract. There are many sources
of yak-activated protein in the Qinghai-Tibetan Plateau.
Yak-activated protein provides rich nutrition without toxicity,
does not accumulate in the body and acts as a multifunctional
factor (16). Furthermore, it is
able to inhibit tumor growth, increase the number of white blood
cells and regulate the immune system (17).
The aim of the present study was to evaluate the
effect of yak-activated protein on peripheral blood cells, immune
function, bone marrow DNA content, antioxidant enzyme activity and
the expression of apoptosis-related proteins in radiation-induced
injury in mice. The underlying mechanisms regarding the potential
protective effects of yak-activated protein were also investigated.
Results of the current study may indicate novel methods of studying
radiation-protective agents and the clinical applications of
yak-activated protein.
Materials and methods
Materials and reagents
Yak-activated protein was purchased from Tibet
Buzhengtang Bio-tech Engineering Co. Ltd., (Lhasa, China). High
performance liquid chromatography (HPLC)-grade acetonitrile was
obtained from Shandong Yuwang Industrial Co., Ltd. (Yucheng,
China). Amifostine (cat. no. 130306) was purchased from Tianjin
Zhongrui Pharmaceutical Co., Ltd. (Tianjin, China). ELISA kits for
measurements of mouse B cell lymphoma 2 (Bcl-2; cat. no.
20141227.60284M), mouse Bcl-2-associated X protein (Bax; cat. no.
20141227.60283M), mouse interleukin 2 (IL-2; cat. no.
20141227.60019M), and mouse IL-6 (cat. no. 20141227.60023M) were
purchased from Beijing RigorBio Science Development Co., Ltd.
(Beijing, China). Blood cell hemolysis reagent (cat. no.
2013111101), class III probe cleaning fluid (cat. no. 2013112101)
and dilution buffer for blood cell analysis (M-23D; cat. no.
2013110701) were purchased from Shenzhen Mindray Bio-Medical
Electronics Co., Ltd. (Shenzhen, China). All other standard
laboratory reagents were chemically pure and the water used was
purified. HPLC was performed using a Waters 515 High Performance
Liquid Chromatograph (Waters Corporation, Milford, MA, USA), a
medical electronic linear accelerator (23EX; Varian Medical
Systems, Inc., Palo Alto, CA, USA) and a UV-2550 ultraviolet
spectrophotometer (Labtech International, Ltd., Uckfield, UK). A
TGL-16 B high speed-freezing centrifuge was obtained from the
Shanghai Anting Scientific Instrument Factory (Shanghai, China). An
RT-2100C enzyme-labeled meter was sourced from Rayto Life and
Analytical Sciences Co., Ltd. (Shenzhen, China) and an XW-80A
Vortex mixer was purchased from Shanghai Medical Instruments Ltd.,
Corp. (Shanghai, China). A BC-2300quasi automatic
three-classification blood cell analyzer was purchased from
Shenzhen Mindray Bio-Medical Electronics C. Ltd.
Sample hydrolysis
Yak-activated protein (33.5 mg) was placed in a
10-ml ampere bottle and 6 ml HCl (6 mol/l) was added. The vial was
sealed and incubated for 24 h at 110°C for sample hydrolysis. The
sample was then cooled at room temperature for 40 min, filtered
(pore size, 0.5 µm) and the filter liquor was added to a 15-ml
tube. The tube was vacuum dried at 90°C in a Multivapor evaporator.
Following drying, the residue was dissolved in water and the
aforementioned step was repeated. Finally, the evaporated residue
was dissolved in 5 ml HCl (20 mmol). The sample had undegone
hydrolysis when the vial was sealed and incubated for 24 h at
110°C. Following filtration to remove impurities, the sample was
contained in the filter liquor, and then HCl (20 mmol) was added to
dissolve the sample.
Sample derivatization
Samples (0.4 ml) were added to 1 ml sodium
bicarbonate (0.5 mol/l) and 0.4 ml fluorobenzene acetonitrile
solution (1%) in a volumetric flask (10 ml). The flask was
incubated at 60°C for 1 h and a potassium dihydrogen phosphate
buffer solution (0.1 mol/l, pH 7) was added. The mixture was
filtered through a microporous membrane (pores 0.22 µm), then
immediately subjected to high-performance liquid chromatography, as
follows.
Chromatographic detection
conditions
The following chromatographic conditions were used:
Column, Phenomenex Gemini 5 µ C18 (250×4.6 mm; Phenomenex, Inc.,
Torrance, CA, USA); detection wavelength, 360 nm; column
temperature, 37°C; flow rate, 1 ml/min, sample load, 8 µl; and a
gradient elution of mobile phase A (0.05 mol/l sodium acetate; pH
6.4) and mobile phase B (acetonitrile: water=1:1; Table I).
 | Table I.Amino acid samples separated by
gradient elution chromatography. |
Table I.
Amino acid samples separated by
gradient elution chromatography.
| Time, min | Solvent A (0.05
mol/l sodium acetate; pH 6.4), % |
|---|
| 0–5 | 74–65 |
| 5–15 | 65–60 |
| 15–20 | 60–45 |
| 20–25 | 45–30 |
| 25–30 | 30–20 |
| 30–35 | 20–2 |
| 35–40 | 2–2 |
| 40–42 | 2–74 |
Animals groups and dose regimens
A total of 180 male Kunming mice (6–8 weeks old,
22–25 g) were purchased from the Experimental Animal Center of
Gansu University of Traditional Chinese Medicine (Lanzhou, China).
The animals were adapted for a week at 23±2°C with a constant
humidity of 55±5% under a 12-h light-dark cycle and with ad
libitum access to water and food pellets. There were 10 animals
housed per cage. A total of 60 mice were randomly divided into
normal control, irradiated control, positive control (amifostine,
150 mg/kg) and high, medium and low dose yak-activated protein
groups (10, 5 and 2.5 mg/kg, respectively; n=10). The other 120
mice were used for subsequent experiments on days 7 and 14 after
radiation, respectively, in order to assess the changes in various
indicators at different time points. The normal control and
irradiated control groups received normal saline orally, and all
other treatment groups were administered yak-activated protein (10,
5 or 2.5 mg/kg) orally for 14 days. The mice in the positive
control group were treated with an intraperitoneal injection of
amifostine (150 mg/kg) 30 min prior to irradiation. Mice were
anesthetized with 50 mg/kg enterocoelia injection of 1% sodium
pentobarbital (Propbs Bio-tech Co. Ltd, Beijing, China) prior to
experiments.
Radiation damage model
The QingHai University Affiliated Hospital was used
for the irradiation experiment. All mice, with the exception of the
control group, were restrained in special boxes and exposed to 5.0
Gy total-body X-radiation at a does rate of 300 cGy/min once. The
source-to-animal distance was 100 cm. Radiation time: 100 sec
(18,19). Following exposure to radiation, 180
mice were used on days 3, 7 and 14.
Sample collection
Mice were anesthetized with enterocoelia injection
of 50 mg/kg 1% sodium pentobarbital prior to experiments. Ocular
blood was harvested from the mice in all treatment groups 7 days
after irradiation. Each sample was mixed with EDTA-2Na (Mingyuan
Industry Co., Ltd, Zhengzhou, China) to prevent coagulation, and
the remainder was coagulated to separate the serum by
centrifugation at 1,000 × g for 10 min at 4°C. All mice were
sacrificed with enterocoelia injection of 50 mg/kg 5% sodium
pentobarbital at 3, 7 and 14 days following irradiation (n=60 for
all groups). After sacrifice, the thymus and spleen (without fat)
were harvested, rinsed with saline to remove blood, dried using
filter paper and weighed.
Cell counts and organ indices
Blood cell diluents (catalogue no. M-23D, lot
2013110701; Mindray Bio-Medical Electronics Co., Ltd, Shenzhen,
China) were added in to 20 µl ocular blood following the
manufacturers protocol. Blood cell count (leucocytes-WBC,
erythrocytes-RBC, hemoglobin-HGB and thrombocytes-PLT) was
determined using BC-2300 blood cell analyzer (Mindray Bio-Medical
Electronics Co., Ltd.). The organ indices were calculated using the
following formula: Organ index (%) = Organ weight (g)/animal weight
(g) × 100 (20).
DNA content of bone marrow
Mice were sacrificed following the harvest of ocular
blood. The right femur was isolated and muscle tissue and blood
were removed. One side of the femoral head was cut and 10 ml
CaCl2 (0.005 mol/l) was used to flush the bone marrow
into a centrifuge tube. The bone marrow was placed in a
refrigerator at 4°C for 30 min, then centrifuged at 693 × g for 15
min at 4°C. The supernatant was discarded and 5 ml 0.2 mol/l
HClO4 was used to acidify the precipitate. The
precipitate was then agitated, heated to 90°C for 15 min, cooled,
centrifuged at 1,350 × g for 10 min at 4°C and filtered (pores 50
µm). The absorbance (A) of the supernatant was determined using a
UV spectrophotometer at 268 nm. DNA content was calculated
according to the following formula: DNA (µg)=40×50×A (21).
IL-2 and IL-6 content and Bcl-2 and Bax expression.
ELISA kits (Bcl-2; cat. no. 20141227.60284M; Bax; cat. no.
20141227.60283M; IL-2; cat. no. 20141227.60019M; IL-6; cat. no.
20141227.60023M; Beijing RigorBio Science Development Co., Ltd.)
were used to measure the levels of IL-2, IL-6, Bcl-2 and Bax in the
serum, according to the manufacturer's protocol.
Statistical analysis
All quantitative data are expressed as the mean ±
standard deviation. The data were analyzed using one-way analysis
of variance with SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA),
and the differences between the means of two groups were compared
using a least significant difference test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Amino acid content of yak-activated
protein
Amino acid content of yak-activated protein: ASP
(1.5%), Glu (2.04%), Ser (0.73%), Arg (1.30%), Thr (1.03%), Pro
(1.06%), Ala (0.98%), Val (0.52%), Met (0.06%), Cys (0.09%), Ile
(0.36%), Leu (0.57%), Phe (0.72%), His (0.34%), Lys (1.61%) and Tyr
(0.15%) as presented in Fig. 1. The
methods are accurate, convenient, reliable and may be replicated,
for the determination of amino acid content of yak-activated
protein. The amino acid content of yak-activated protein is
presented in Table II, and the
chromatogram is displayed in Fig.
1.
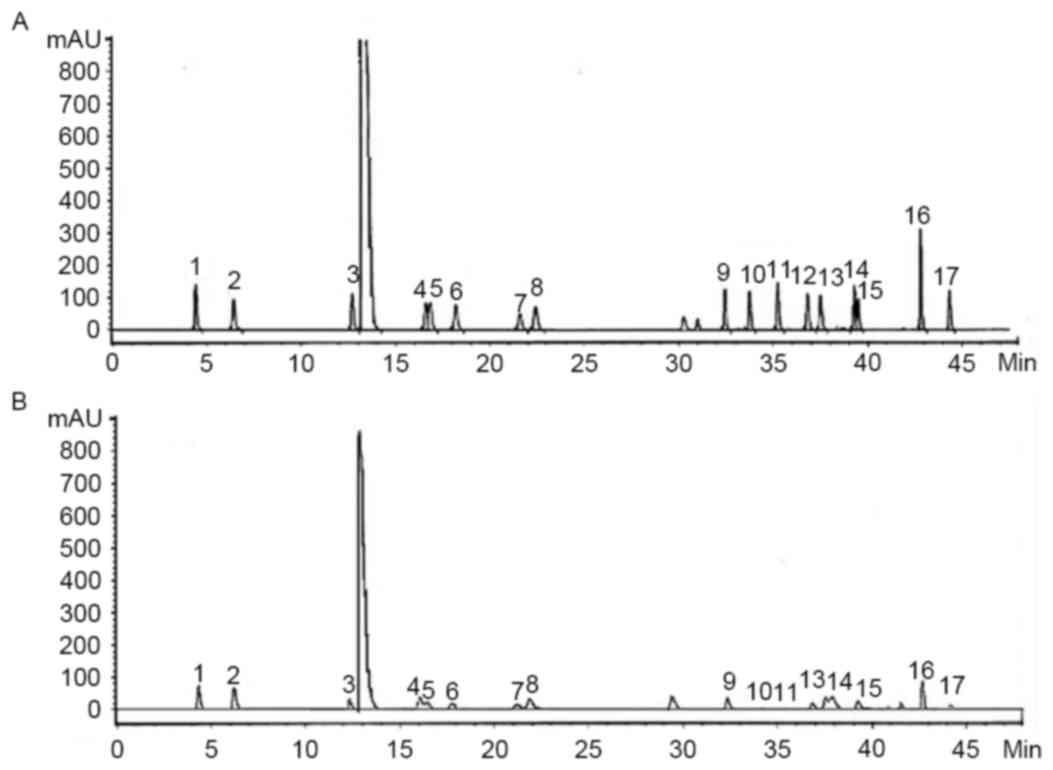 | Figure 1.High performance liquid chromatogram
of the (A) standards and (B) samples. 1, Asp; 2, Ser; 3, Glu; 4,
Gly; 5, His; 6, Arg; 7, Thr; 8, Ala; 9, Pro; 10, Cys; 11, Tyr; 12,
Val; 13, Met; 14, Lys; 15, Ile; 16, Leu; 17, Phe. |
 | Table II.Amino acid content of yak-activated
protein. |
Table II.
Amino acid content of yak-activated
protein.
| Amino acid | Content (%) |
|---|
| Asp | 1.50 |
| Glu | 2.04 |
| Ser | 0.73 |
| Gly | 0.73 |
| Arg | 1.30 |
| Thr | 1.03 |
| Pro | 1.06 |
| Ala | 0.98 |
| Val | 0.52 |
| Met | 0.06 |
| Cys | 0.09 |
| Ile | 0.36 |
| Leu | 0.57 |
| Phe | 0.72 |
| His | 0.34 |
| Lys | 1.61 |
| Tyr | 0.15 |
Effect of yak-activated protein on the
peripheral hemograms of mice following irradiation
The peripheral hemograms of irradiated mice are
presented in Fig. 2. In the
radiation model group, it was observed that WBC and PLT declined
significantly on days 3 and 7, respectively, compared with the
normal control group (P<0.05; Fig.
2). Furthermore, RBC count and HGB levels declined
significantly and reached a minimum 7 days after irradiation
(P<0.05 vs. normal control group). These results suggest that
the radiation damage model was successfully established. The WBC,
RBC, HGB and PLT count no significant different was observed
between the positive control group and yak-activated protein groups
(low, medium and high dose) 3 or 7 days after irradiation
(P>0.05; Fig. 2). On day 14 after
irradiation, the WBC of the positive group was significantly higher
than yak-activated protein groups (low, medium and high dose
groups; P<0.05; Fig. 2); The RBC
of the positive group was significantly higher than the medium dose
of yak-activated protein (P<0.05; Fig. 2). No significant difference was
observed between the positive group and yak-activated protein
groups (low, medium and high dose groups) in HBG (P>0.05;
Fig. 2); The PLT of the positive
group was significantly higher than yak-activated protein low dose
group (P<0.05; Fig. 2). Fig. 2 indicates that, compared with the WBC
count of the control group the WBC counts of mice in the model
group on days 3, 7 and 14 after irradiation were significantly
reduced (P<0.05; Fig. 2). The WBC
count of yak-activated protein groups were higher than model group
on day 14 after irradiation, but no significant difference was
observed (P>0.05; Fig. 2). The
HGB of the positive and yak-activated protein groups was
significantly higher than the model group on day 7 after
irradiation (P<0.05; Fig. 2). On
the 7th day after irradiation, the RBC count of mice in positive
group and yak-activated protein groups (low, medium and high)
compared with model was significantly increased (P<0.05;
Fig. 2). The RBC count of mice in
positive, high and low dose yak-activated protein groups compared
with model was significantly increased on day 14 (P<0.05;
Fig. 2). On day 7 after irradiation,
the PLT count of mice in high dose yak-activated protein groups
compared with model was significantly increased (P<0.05;
Fig. 2). The PLT count of mice in
positive, high and medium dose yak-activated protein groups
compared with model was significantly increased (P<0.05;
Fig. 2) on day 14. These results
suggest that Yak-activated protein significantly improved the
hematopoietic system in radiation-injured mice.
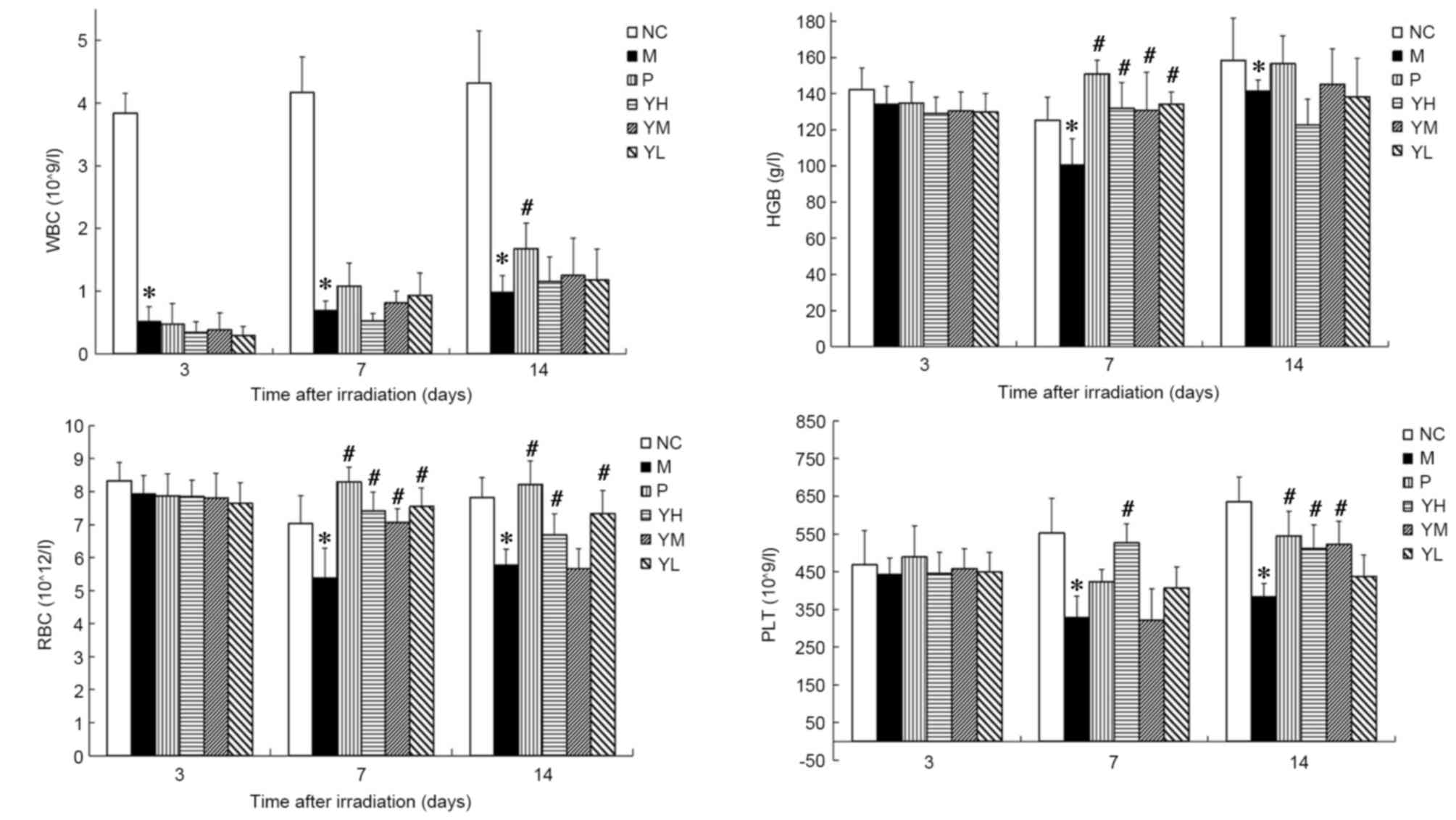 | Figure 2.Effect of yak-activated protein on the
peripheral hemograms of irradiated mice. Data are expressed as the
mean + standard error of the mean. n=10 per group. *P<0.05 vs.
normal control group, #P<0.05 vs. model group. NC,
normal control; M, radiation model; P, positive control; YH, high
dose (10 mg/kg) yak-activated protein; YM, medium-dose (5 mg/kg)
yak-activated protein; YL, low-dose (2.5 mg/kg) yak-activated
protein; WBC, white blood cell; HGB, hemoglobin; RBC, red blood
cell; PLT, platelet. |
Effect of yak-activated protein on the
thymus and spleen indices of irradiated mice
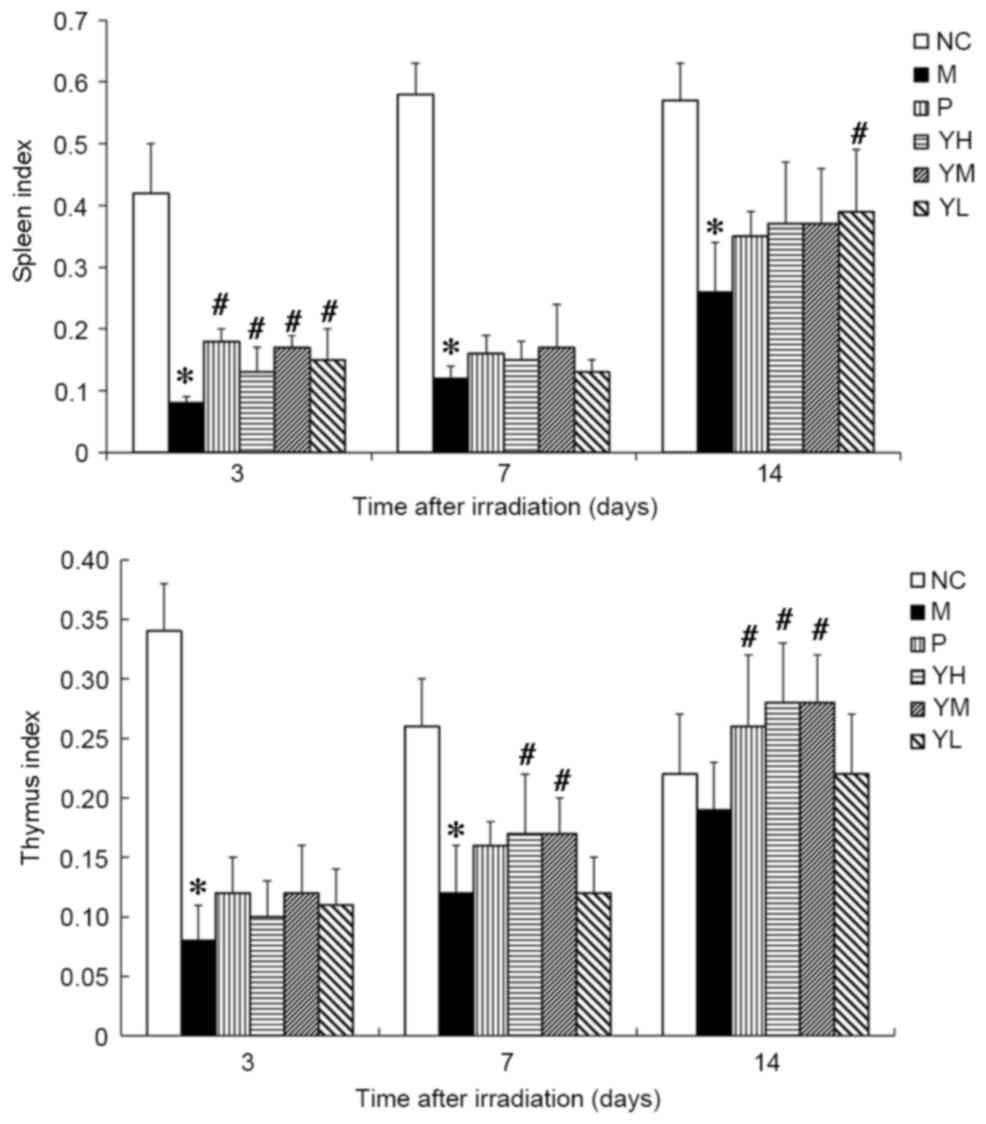
The thymus and spleen indices of irradiated mice are
presented in Fig. 3. Significant
immune organ atrophy was observed in irradiated model mice compared
with normal control mice at all time points (all P<0.05;
Fig. 3), thus indicating that the
radiation damage model was successfully established. The spleen
indices of the positive group and yak-activated protein groups were
significantly higher than model group on day 3 after irradiation
(P<0.05; Fig. 3). On day 7 after
irradiation, no significant difference was observed between the
spleen indices in the positive or yak-activated protein with the
model groups (P>0.05; Fig. 3). On
day 14 after irradiation, the spleen indices of mice in the low
dose yak-activated protein groups compared with model were
significantly increased (P<0.05; Fig.
3). On day 7 after irradiation, the thymus indices of mice in
high and medium dose yak-activated protein groups compared with
model was significantly increased (P<0.05; Fig. 3). On day 14 after irradiation, the
thymus indices of mice in the positive, high and medium dose
yak-activated protein groups compared with model was significantly
increased (P<0.05; Fig. 3). These
data indicate that yak-activated protein may promote immune organ
recovery.
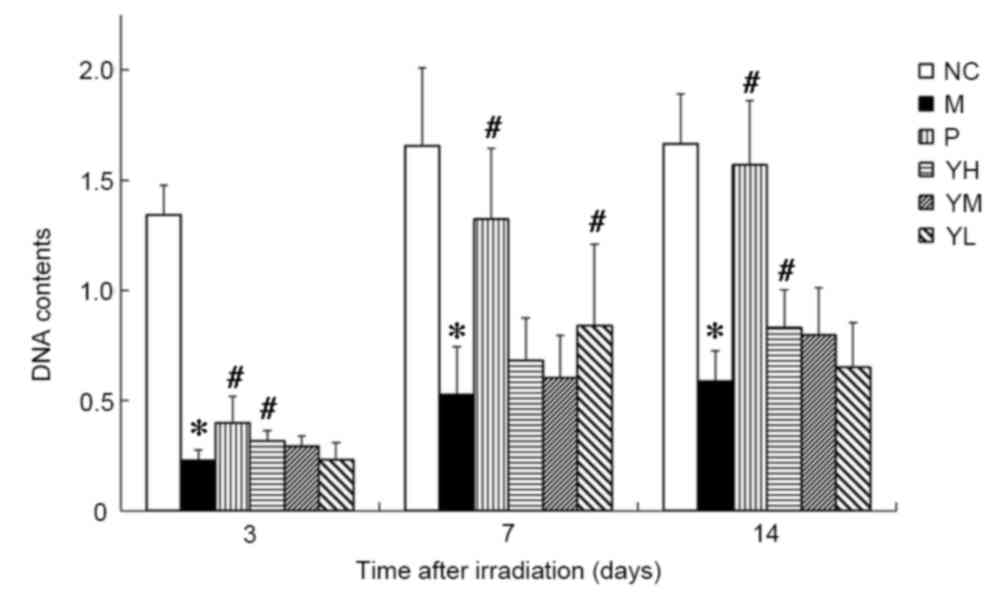
Effect of yak-activated protein on the
bone marrow DNA content of irradiated mice
The DNA content in the bone marrow of irradiated
mice is presented in Fig. 4. It was
observed that the DNA content in the drug and radiation model
groups was significantly decreased compared to that in the normal
control group (P<0.05; Fig. 4).
The DNA content of all groups reached a minimum on day 3
post-irradiation. On day 3 after irradiation, the DNA content of
mice in the positive and high dose yak-activated protein groups
compared with model was significantly increased (P<0.05;
Fig. 4). The DNA content in the bone
marrow of mice in the low yak-activated protein and positive
control groups was increased significantly compared with the
irradiation control group on day 7 after irradiation (P<0.05;
Fig. 4). On day 14 after
irradiation, the DNA content of mice in positive control and high
dose yak-activated protein groups compared with model was
significantly increased (P<0.05; Fig.
4). This suggests that yak-activated protein and the positive
control agent (amifostine) increased the bone marrow DNA content
and improved the hematopoietic system following radiation.
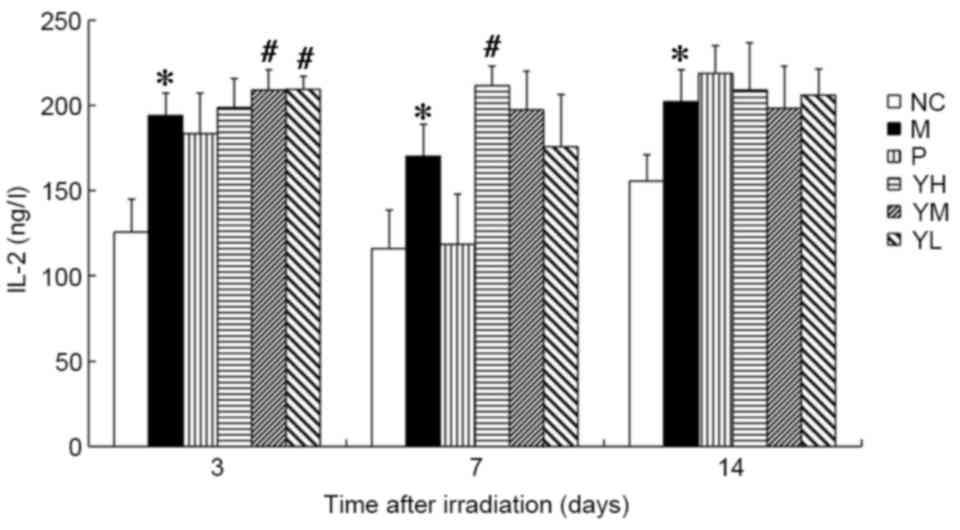
IL-2 and IL-6 content
The IL-2 content was significantly higher in the
radiation model group compared with the normal control group at all
time points following irradiation (P<0.05; Fig. 5). On day 3, the IL-2 content was
significantly increased in the low and medium dose yak-activated
protein groups (both P<0.05; Fig.
5). By contrast, IL-2 content was significantly increased in
only the high-dose yak-activated protein group on day 7 (P<0.05;
Fig. 5). These data suggest that
yak-activated protein may promote the expression and secretion of
IL-2 in X-ray irradiated mice. IL-2 content in the medium and low
dose groups trended toward recovery on day 7, and a similar trend
was observed in all yak-activated protein groups by day 14. These
data suggest that yak-activated protein may regulate IL-2 in
radiation injury.
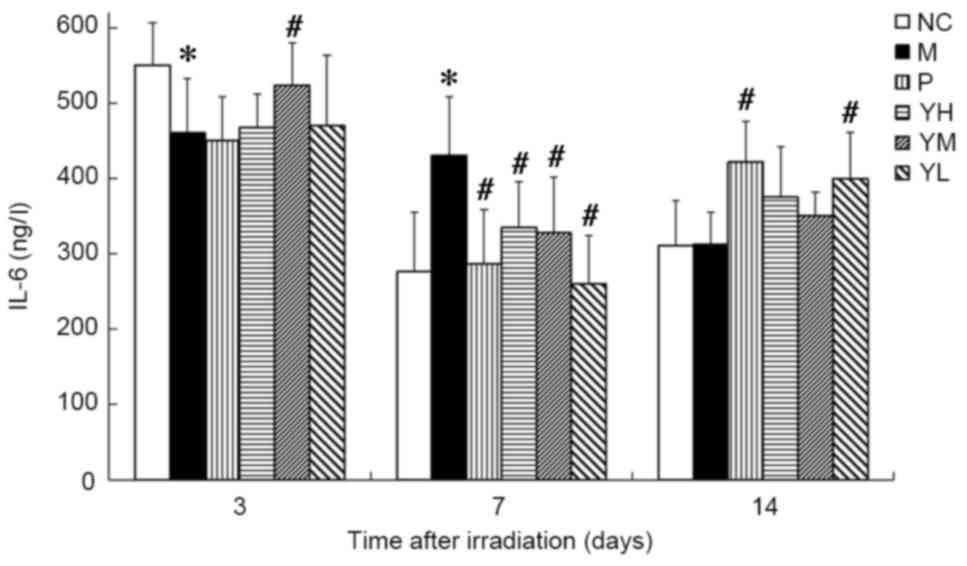
On day 3 post-irradiation, IL-6 content was
significantly lower in the radiation model group compared with the
normal control group (P<0.05; Fig.
6), while on day 7, it was significantly higher compared with
the normal control group (P<0.05; Fig. 6). Levels of IL-6 did not differ
significantly between the normal control and radiation model groups
on day 14. On day 3, the IL-6 content was significantly increased
in the medium dose yak-activated protein group compared with the
radiation model group (P<0.05; Fig.
6), suggesting that yak-activated protein promoted the
expression and secretion of IL-6 in irradiated mice. On day 7, IL-6
levels were decreased significantly in all yak-activated
protein-treated groups and in the positive control group (all
P<0.05 vs. model group; Fig. 6).
On day 14, IL-6 content was significantly increased in the low dose
yak-activated protein and positive control groups compared with the
model group (both P<0.05; Fig.
6).
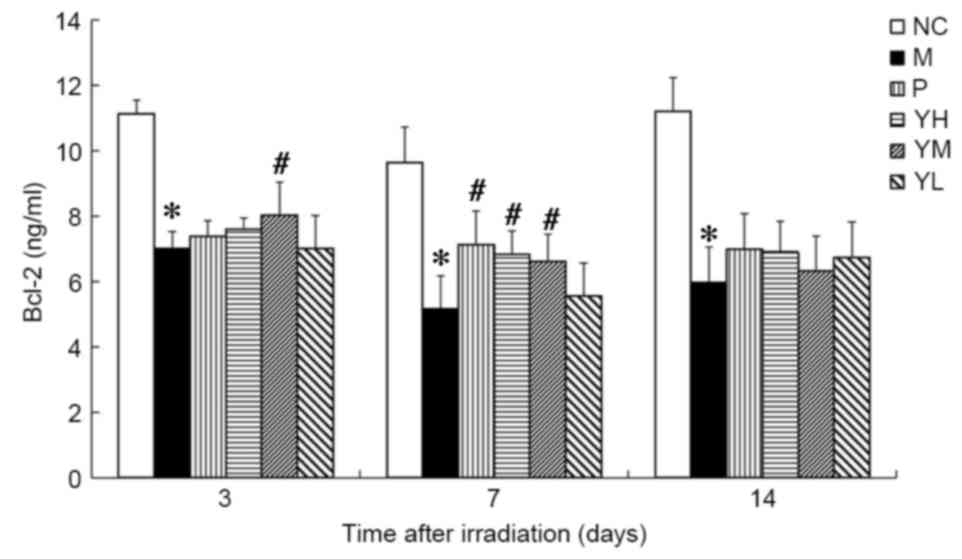
Bcl-2 and Bax expression
The expression of Bcl-2 was significantly reduced in
the irradiation model group compared with the normal group at all
time points (P<0.05; Fig. 7). On
day 3, the expression of Bcl-2 was significantly higher in the
medium dose yak-activated protein group compared with the model
group (P<0.05; Fig. 7), and on
day 7, levels of Bcl-2 were significantly increased in the positive
control and medium and high dose yak-activated protein groups (all
P<0.05 vs. model group; Fig. 7).
On day 14 following irradiation, Bcl-2 expression in the positive
control and yak-activated protein groups did not differ
significantly to that observed in the model group.
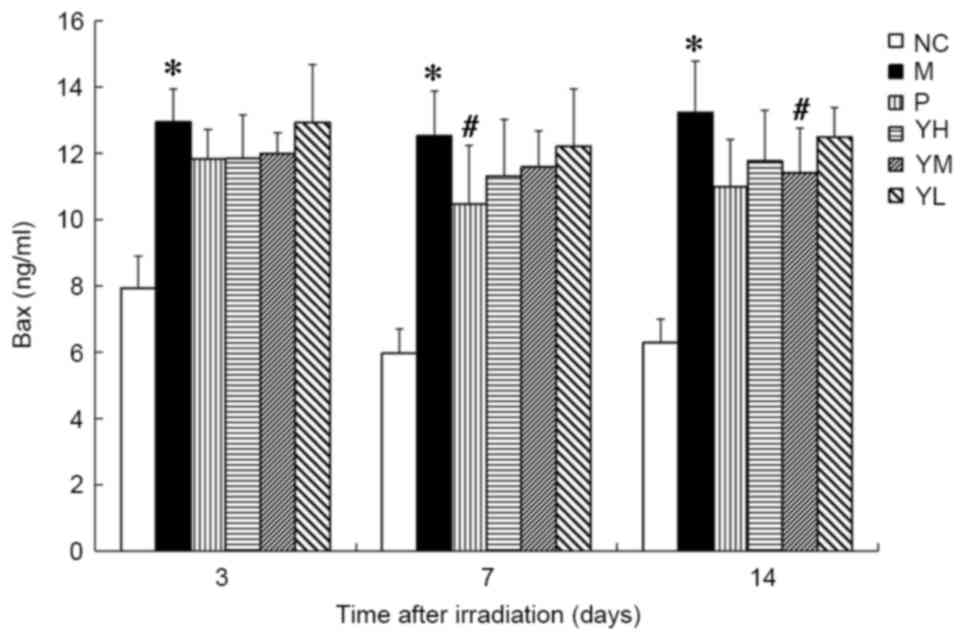
The expression of Bax was significantly increased in
the irradiation group compared with the normal group at all time
points (P<0.05; Fig. 8). Relative
to the radiation model group, Bax expression was significantly
decreased in the positive control group on day 7 (P<0.05) and
the medium-dose yak-activated protein group on day 14 (P<0.05;
Fig. 8). Marked decreases in Bax
were also observed in the other treatment groups compared to the
radiation model group following irradiation.
Discussion
The thymus and spleen are the most sensitive organs
to ionizing radiation, and indices of the thymus and spleen may be
used as indicators of immune system function (22). The results of the present study
indicated that the spleen and thymus were significantly reduced in
the radiation model group. Specifically, they were smaller in size
and weight, and their indices were decreased compared with the
normal control group, which is consistent with previous reports
(23,24). These results suggest that the
radiation injury mouse model was successfully established. The
administration of yak-activated protein lead to significant
increases in the thymus and spleen indices, although they did not
return to normal levels in the relatively short time assessed. It
should be result of radiation, which leads to a long-term weakened
immune system (25–27).
In the present study, levels of bone marrow DNA were
measured to assess whether yak-activated protein protected mice
from radiation. X-ray radiation affects DNA, biological membranes
and water molecules in the body, and produces free radicals that
interact with unsaturated fatty acids in the lipid bilayers of
biological membranes (28). This
leads to the destruction of macromolecular structures and single or
double strand breaks in DNA, which blocks DNA synthesis and
promotes gene mutations and apoptosis (29). In turn, these disruptions result in a
decreased content of DNA and slower cell division, particularly for
stem cells in the bone marrow (30).
The results of the present study demonstrated that yak-activated
protein significantly increased the content of DNA following
irradiation, particularly in the high dose group on days 3 and 14
and the low dose group on day 7. This suggests that yak-activated
protein may protect the DNA content in the bone marrow of
irradiated mice, potentially through the prevention of DNA damage
and stimulation of DNA synthesis.
Peripheral hemograms are considered to be key
indicators of normal body function following radiation damage
(31). Blood cells are the terminal
cells of the blood system; therefore, changes in hematopoietic
tissues reflect the degree of damage to the body, and X-ray
irradiation typically decreases the number of blood cells in the
body (32). In the present study,
the numbers of WBCs, RBCs, HGBs and PLTs were assessed to evaluate
the effects of yak-activated protein on the peripheral hemograms of
radiation-injured mice. WBC, RBC, HGB and PLT counts declined
following X-ray radiation; however, yak-activated protein inhibited
this decrease and increased levels of RBCs, HGBs, and PLTs
significantly. This suggests that yak-activated protein serves a
key role in protecting the hematopoietic system against
radiation-induced injury in mice.
IL-2 is a non-specific cytokine that is produced by
helper T cells (33). It affects the
activity and promotes the proliferation of activated T cells,
stimulates B cells to produce antibodies, and significantly
upregulates numerous factors, including monocytes and macrophages,
associated with the immune system (33). Results of the present study
demonstrated that X-ray radiation reduced the self-regulatory
capacity of the immune system, as indicated by reductions in IL-2
expression. However, yak-activated protein increased levels of IL-2
and potentially enhanced the humoral immune response following
irradiation. The effects of yak-activated protein were most evident
in the low and middle dose groups on day 3, and in the high dose
group on day 7. As indices of the thymus and spleen were also
improved by yak-activated protein, these results suggest that
yak-activated protein may stimulate T lymphocytes to produce IL-2
and facilitate recovery of the thymus and spleen. IL-6 is a
positive regulatory factor that exerts many biological effects,
including stimulatory effects on cell proliferation and inhibitory
effects on apoptosis (34). IL-6 is
primarily associated with the regulation of hematopoiesis,
inflammation and cellular immune responses in the body (34). Results of the present study
demonstrated that levels of IL-6 were decreased in the model group
compared with the normal group on day 3 following irradiation,
suggesting that ionizing radiation inhibited cell proliferation,
accelerated cell apoptosis and reduced the secretion and expression
of IL-6. However, similar to previous results (35), IL-6 content was significantly higher
in the model group compared with the normal control group days 7
and 14 after radiation. IL-6 content in each of the drug groups was
higher than in the control and model groups. Levels of IL-6 were
also significantly higher in the low-dose yak-activated protein
group compared with the model group on days 7 and 14, suggesting
that yak-activated protein may serve both positive and negative
roles in the regulation of IL-6 following X-ray irradiation.
Apoptosis is a primary pathway by which
radiation-induced cell death occurs (36). Cellular apoptosis is regulated by
complex and specific signal transduction pathways (36). The Bcl-2 family serves a key role in
apoptosis, and the expression of Bcl-2 inhibits apoptosis and
potentially promotes survival signaling to prolong cell longevity
(37). Upon receipt of a death
signal, the expression of Bax is upregulated in cells, leading to
the formation of Bax multimers, altered membrane permeability and
the release of pro-apoptotic factors into the cytoplasm to initiate
apoptosis (30). Results of the
present study indicated that X-ray irradiation significantly
increased and decreased the expression of Bax and Bcl-2,
respectively. However, treatment with yak-activated protein lead to
an upregulation in Bcl-2 and downregulation in Bax, suggesting that
yak-activated protein may scavenge free radicals and inhibit
increases in membrane permeability, thus reducing apoptosis.
Therefore, yak-activated protein may exert radioprotective effects
through the regulation of Bcl-2 and Bax.
In conclusion, results of the present study
indicated that yak-activated protein may reduce damage induced by
X-ray irradiation to peripheral blood cell counts, spleen and
thymus indices, and bone marrow DNA content. Yak-activated protein
may also improve the immune response and regulate the expression of
anti- and pro-apoptotic proteins. Yak-activated protein is a
high-efficiency and low-toxicity agent, which appears to protect
against radiation. These results may serve as an experimental basis
for further studies into radiation protection and the clinical
applications of yak-activated protein.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81460568), the
Program for New Century Excellent Talents in University of China
(grant no. NCET-10-0917), the Natural Science Foundation of Qinghai
Province of China (grant no. 2013-Z-909) and the Science Foundation
of Qinghai University of China (grant no. 2014-QYT-2).
References
|
1
|
Wicki A, Witzigmann D, Balasubramanian V
and Huwyler J: Nanomedicine in cancer therapy: Challenges,
opportunities, and clinical applications. J Control Release.
200:138–157. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chen L, Liu Y, Dong L and Chu X: Edaravone
protects human peripheral blood lymphocytes from
γ-irradiation-induced Apoptosis and DNA Damage. Cell Stress
Chaperones. 20:289–295. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Staples JA, Ponsonby AL, Lim LL and
McMichael AJ: Ecologic analysis of some immune-related disorders,
including type 1 diabetes, in Australia: Latitude, regional
ultraviolet radiation, and disease prevalence. Environ Health
Perspect. 111:518–523. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rouleau M, Patel A, Hendzel MJ, Kaufmann
SH and Poirier GG: PARP inhibition: PARP1 and beyond. Nat Rev
Cancer. 10:293–301. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Choi HN, Chung MJ, Park JK and Yong IP:
Neuroprotective effects of N-Acetylglucosamine against hydrogen
peroxide-induced apoptosis in human neuronal SK-N-SH cells by
inhibiting the activation of caspase-3, PARP, and p38. Food Sci
Biotechnol. 22:853–858. 2013. View Article : Google Scholar
|
|
6
|
Koukourakis MI: Amifostine in clinical
oncology: Current use and future applications. Anticancer Drugs.
13:181–209. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hospers GA, Eisenhauer EA and de Vries EG:
The sulfhydryl containing compounds WR-2721 and glutathione as
radio-and chemoprotective agents. A review, indications for use and
prospects. Br J Cancer. 80:629–638. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li HY, Zhao Y, Sun XD, Bai XZ and Zhao DQ:
Effect of ginseng protein against radiation injury in mice. Shizhen
Guo Yi Guo Yao. 21:2143–2144. 2010.(In Chinese).
|
|
9
|
Deng Q, Chen C, Duan H, Wang L and Xie B:
Protection effect of ginkgo albumin extract on γ-ray irradiated
mice. J Radiat Res Radiat Process. 23:360–365. 2005.
|
|
10
|
Wei C: The effects of lactoferrin against
injuries induced by radiation (unpublished masters thesis). Jinan
University. 2013.(In Chinese).
|
|
11
|
Guinan EC, Barbon CM, Kalish LA, Parmar K,
Kutok J, Mancuso CJ, Stoler-Barak L, Suter EE, Russell JD, Palmer
CD, et al: Bactericidal/permeability-increasing protein (rBPI21)
and fluoroquinolone mitigate radiation-induced bone marrow aplasia
and death. Sci Transl Med. 3:110ra1182011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wen J, Zhao J, Bi X, Jin ZL, Jin RY and Ru
BG: The experimental study on anti-radiation effect of
metallothionein. Ying Yang Xue Bao. 23:44–47. 2001.(In
Chinese).
|
|
13
|
Seed TM, Inal CE and Singh VK:
Radioprotection of hematopoietic progenitors by low dose amifostine
prophylaxis. Int J Radiat Biol. 90:594–604. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ren GP, Li YY and Gu WY: Advancement in
physiological function of gln - A conditionally essential amino
acid and its use in nutrition. Anjisuan He Shengwu Ziyuan.
23:39–43. 2001.(In Chinese).
|
|
15
|
Suh SS, Hwang J, Park M, Seo HH, Kim HS,
Lee JH, Moh SH and Lee TK: Anti-inflammation activities of
mycosporine-like amino acids (MAAs) in response to UV radiation
suggest potential anti-skin aging activity. Mar Drugs.
12:5174–5187. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wakabayashi H, Yamauchi K and Takase M:
Lactoferrin research, technology, and applications. Int Dairy J.
16:1241–1251. 2006. View Article : Google Scholar
|
|
17
|
Xu B, Yao X, Tie J, Lu D, Yuan M, Li Y,
Zhu J and Li X: The immunoregulation effect of yak activated
protein on murine immune system. J Qing hai Med College. 34:54–56.
2013.
|
|
18
|
Marusyk A, Casás-Selves M, Henry CJ,
Zaberezhnyy V, Klawitter J, Christians U and DeGregori J:
Irradiation alters selection for oncogenic mutations in
hematopoietic progenitors. Cancer Res. 69:7262–7269. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song JY, Yang HO, Shim JY, Ji-Yeon-Ahn,
Han YS, Jung IS and Yun YS: Radiation protective effect of an
extract from Chelidonium majus. Int J Hematol. 78:226–232. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang Y, Wu H and Guan XJ: Effect of
prophylactic administration with angelica polysaccharides on
expression of adhesion molecules and cell cycle of bone marrow
mononuclear cells of radiation injured mice. Chin J Biologicals.
23:627–631. 2010.
|
|
21
|
Xie XD, Su G, Nian XF, Ma GR and Jia ZP:
Protective effect of chondroitin sulfate-A on X-ray irradiated
mice. J Northwest Normal University (Natural Science). 49:86–90.
2013.
|
|
22
|
Fang JJ, Zhu ZY, Dong H, Zheng GQ, Teng AG
and Liu AJ: Effect of spleen lymphocytes on the splenomegaly in
hepatocellular carcinoma-bearing mice. Biomed Environ Sci.
27:17–26. 2014.PubMed/NCBI
|
|
23
|
Fan ZL, Wang ZY, Zuo LL and Tian SQ:
Protective effect of anthocyanins from lingonberry on
radiation-induced damages. Int J Environ Res Public Health.
9:4732–4743. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tajima G, Delisle AJ, Hoang K, O'Leary FM,
Ikeda K, Hanschen M, Stoecklein VM and Lederer JA: Immune system
phenotyping of radiation and radiation combined injury in outbred
mice. Radiat Res. 179:101–112. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Choi HN, Chung MJ, Park JK and Park Y:
Neuroprotective effects of N-Acetylglucosamine against hydrogen
peroxide-induced apoptosis in human neuronal SK-N-SH cells by
inhibiting the activation of caspase-3, PARP and p38. Food Sci
Biotechnol. 22:853–858. 2013. View Article : Google Scholar
|
|
26
|
Wei S, Egenti MU, Teitz-Tennenbaum S, Zou
W and Chang AE: Effects of tumor irradi-ation on host T-regulatory
cells and systemic immunity in the context of adoptive T-cell
therapy in mice. J Immunother. 36:124–132. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xie P, Qin ZY and Tang MJ: Research
developments in anti-radiation effects of polysaccharide. Zhongguo
Fu She Wei Sheng. 19:507–509. 2010.(In Chinese).
|
|
28
|
Bhilwade HN, Jayakumar S and Chaubey RC:
Age-dependent changes in spontaneous frequency of micronucleated
erythrocytes in bone marrow and DNA damage in peripheral blood of
Swiss mice. Mutat Res Genet Toxicol Environ Mutagen. 770:80–84.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yao XC and Li XY: Research advance about
anti-radiation effect of protein. Shandong Yi Yao. 54:88–90.
2014.(In Chinese).
|
|
30
|
Johnke RM, Sattler JA and Allison RR:
Radioprotective agents for radiation therapy: Future trends. Future
Oncol. 10:2345–2357. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin Z, Nei M and Ma H: The origins and
early evolution of DNA mismatch repair genes-multiple horizontal
gene transfers and co-evolution. Nucleic Acids Resr. 35:7591–7603.
2007. View Article : Google Scholar
|
|
32
|
Widel M, Jedrus S, Lukaszczyk B,
Raczek-Zwierzycka K and Swierniak A: Radiation-induced micronucleus
frequency in peripheral blood lymphocytes is correlated with normal
tissue damage in patients with cervical carcinoma undergoing
radiotherapy. Radiat Res. 159:713–721. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
He Y, Fang J, Peng X, Cui H, Zuo Z, Deng
J, Chen Z, Lai W, Shu G and Tang L: Effects of Sodium Selenite on
Aflatoxin B1-induced decrease of ileac T cell and the mRNA Contents
of IL-2, IL-6, and TNF-α in Broilers. Biol Trace Elem Res.
159:167–173. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Schulte-Herbrüggen O, Nassenstein C,
Lommatzsch M, Quarcoo D, Renz H and Braun A: Tumor necrosis
factor-alpha and interleukin-6 regulate secretion of brain-derived
neurotrophic factor in human monocytes. J Neuroimmunol.
160:204–209. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang YH, Jiang LZ, Li Q and Zhong XH:
Effect of high power microwave radiation on liver injury and serum
cytokine in rats. Acadedemic J Second Military Medical University.
35:672–675. 2014. View Article : Google Scholar
|
|
36
|
Raj PV, Nitesh K, Prateek J, Sankhe MN,
Rao JV, Rao CM and Udupa N: Effect of Lecithin on D-galactosamine
induced hepatotoxicity through mitochondrial pathway involving
Bcl-2 and Bax. Ind J Clin Biochem. 26:378–384. 2011. View Article : Google Scholar
|
|
37
|
Chipuk JE, Kuwana T, Bouchier-Hayes L,
Droin NM, Newmeyer DD, Schuler M and Green DR: Direct activation of
Bax by p53 mediates mitochondrial membrane permeabilization and
apoptosis. Science. 303:1010–1014. 2004. View Article : Google Scholar : PubMed/NCBI
|