Introduction
The increase in sporting activity over since 2000 in
the USA has led to an increase in the incidence of knee joint
injuries, with knee ligament injuries now the most prevalent sports
injury (1). Injured anterior and
posterior cruciate ligaments (ACL and PCL, respectively) heal
poorly compared with medial and lateral collateral ligaments (MCL
and LCL, respectively), and thus result in the onset of secondary
complications, including meniscus tears, osteoarthritis and
degenerative joint disease (2). In
terms of treatment, cruciate ligament reconstitution through
autografts, allografts and synthetic materials is considered to be
the most effective treatment method, although difficulties remain
in reproducing the biomechanics of ligaments following surgery
(3,4).
It has been demonstrated that the poor healing
ability of cruciate ligaments may be due to a restricted vascular
supply and limited vascular bed in the surrounding microenvironment
(5). A number of previous studies
suggest that the intrinsic cellular properties of the ACL and MCL,
including proliferation, migration, extracellular matrix (ECM)
synthesis and ECM remodeling, may be key contributors to the
diminished healing potential of the ligaments (6–9). During
tissue remodeling, older ECM molecules are subject to gradual
degradation by proteolytic enzymes, while nascent ECM molecules
undergo aggregation and cross-linking to form fibers (10). Normally, an equilibrium in the
synthesis and degradation of ECM molecules exists and a disruption
in this equilibrium may delay wound healing.
Matrix metalloproteinases (MMPs) are a family of
zinc-dependent proteolytic enzymes that are involved in normal and
pathological tissue remodeling processes, including tissue repair,
embryonic development, rheumatoid arthritis and tumor invasion.
This is through the proteolysis of selective ECM components,
including collagens, elastin and glycoproteins (11,12).
Lysyl oxidases (LOXs) are a group of copper-dependent amine
oxidases that initiate formation of the covalent cross-links
between ECM proteins, thus providing the mechanical properties of
the ECM, as well as proteinase resistance, including resistance to
MMPs (13). Previous results have
demonstrated that the presence of 0.1 Schiff-base cross-links per
collagen molecule results in a 2–3-fold increase in resistance of
the molecule to human collagenase, relative to non-cross-linked
controls or samples (14,15). Therefore, the balance between ECM
synthesis and degradation during tissue remodeling is maintained by
MMPs and LOXs.
During mechanical injury, a number of studies have
documented differential expression of LOXs and MMPs in ACL
fibroblasts relative to MCL fibroblasts, with ACL cells exhibiting
decreased expression of LOXs and increased expression of MMPs. This
may disrupt the equilibrium of the ECM remodeling process,
potentially contributing to the differential healing abilities of
the two ligaments (9,16,17).
Furthermore, in an in vivo rat model of ACL trauma, it was
observed that articular tissues contributed to an elevation in
MMP-2 in synovial fluids, thus inhibiting the remodeling process
within injured cruciate ligaments. The synovium also exhibited a
capacity to release MMP-2 into synovial fluids and convert inactive
pro-MMP-2 into its active form among the articular tissues, thus
indicating that synovium may be a key regulator of the joint cavity
microenvironment following tissue injury within synovial joints
(18). Furthermore, a previous
mechanical compression study in vitro demonstrated that the
differential expression and activity of MMP-2 in synovial
fibroblasts may regulate the joint cavity microenvironment
following articular tissue rupture (19). Similarly, a previous mechanical
stretching study in vitro by our group demonstrated that
during ligament remodeling, insufficient healing of cruciate
ligaments is linked to an imbalance in the production of MMPs and
LOXs in synovial fibroblasts, further indicating a regulatory
effect of synovial fibroblasts on the joint cavity microenvironment
(20).
Levels of the inflammatory cytokines tumor necrosis
factor (TNF)-α, interleukin (IL)-1β and IL-6 are increased in knee
joint fluid during the acute inflammatory phase of ACL trauma
(21). These cytokines are
considered to be important chemical mediators in the acute
inflammatory phase of wound healing (22). Wang et al (19) observed that the regulatory activity
of synovial fibroblasts regarding the joint cavity microenvironment
was sensitive to inflammatory cytokines. In our previous mechanical
stretching study, it was observed that TNF-α suppressed the
expression of LOXs, while stimulating the expression and activity
of MMPs in synovial fibroblasts, thus leading to a disruption in
the ECM remodeling equilibrium and inhibition of articular tissue
healing following trauma (20).
Similar to TNF-α, synovial fibroblasts may be sensitive to IL-1β in
their regulation of the joint cavity microenvironment following
articular tissue rupture (23).
Therefore, the present in vitro study investigated the
effects of IL-1β alone or in combination with TNF-α on the
expression of LOXs and MMPs-1, −2 and −3 in human synovial
fibroblasts, principally by evaluating the expression of LOXs and
MMPs and the activity of MMP-2 by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR) and
zymography, respectively, in the presence of IL-1β and TNF-α.
Materials and methods
Cell culture
Human synovial fibroblasts were isolated from donor
synovial tissues of four patients undergoing limb amputation (age
range 30–60 years, mean 47.5±11.5 years, two male and two female
subjects) at the First Affiliated Hospital of Chongqing Medical
University (Chongqing, China). These patients were enrolled in the
study between September 2009 and March 2013. Donors with a
pre-existing inflammatory reaction in the knee joint due to
rheumatoid arthritis (RA), osteoarthritis (OA) or long-term knee
joint pathological changes were excluded from the present study.
The donor synovial tissue was obtained from patients following
amputation surgery. The synovial tissues were immediately washed
with 1× PBS with penicillin/streptomycin and cut into small 2
mm3 sections. Sections were suspended in high-glucose
DMEM (Gibco; Thermo Fisher Scientific Inc., Waltham, MA, USA)
supplemented with 10% fetal bovine serum (FBS; HyClone; Thermo
Fisher Scientific Inc., Logan, UT, USA) and 100 U/ml
penicillin/streptomycin and incubated at 37°C in a humidified
atmosphere of 5% CO2. After the fibroblasts had migrated
out from small tissues and attached to the bottom, the tissues were
transferred to another flask and the adherent cells were grown
until confluent. Some of the cells were frozen in 10% dimethyl
sulfoxide with FBS in liquid nitrogen until use. The remaining
cells were cultured and maintained in 10% FBS-DMEM at 37°C in a
humidified atmosphere of 5% CO2. Cells isolated from
different donors were kept as separate samples. All experiments
were carried out on cells from passages 1 to 5. Ethical approval
from the Institutional Review Board (Chongqing University,
Chongqing, China) was obtained prior to the study. All procedures
were followed according to the ethical principles and protocols
approved by Chongqing University and Chongqing Medical University.
All patients provided informed consent for the use of their tissues
in the study.
Cytokine treatment
For each experiment, fibroblasts of the control
groups and the treatment groups were seeded at a density of
5×105 cells/25 cm2 flask (Corning
Incorporated, Corning, NY, USA). Cells were given 48 h to seed and
equilibrate at 37°C. Culture medium was then removed and replaced
by 2% (FBS) HG-DMEM for a 16 h starvation period (a pilot study
revealed that cells were more vulnerable to death in a FBS-free
HG-DMEM). Medium was subsequently removed and replaced with 1% FBS
HG-DMEM containing TNF-α (1, 5, 10 and 20 ng/ml) or IL-1β (1, 5, 10
and 20 ng/ml) (both Peprotech, Rocky Hill, NJ, USA) and incubated
at 37°C for 3 h. Based on the results of these experiments,
separate groups of fibroblasts were cultured for 1, 2, 3 and 6 h in
the presence or absence (control cells) of 10 ng/ml TNF-α and/or 10
ng/ml IL-1β. Total RNA samples were extracted at 1, 2, 3 and 6 h
and at 0 h, as a control, prior to RT-qPCR. Conditioned medium was
also collected after 12, 24, 48, and 72 h of cell culture for
zymography analysis of MMP-2 activity (23).
Cell viability assay
Cell viability was determined by the trypan blue dye
exclusion test. A total of 2×105 cells/well were seeded
in in 6-well plates with different concentrations of TNF-α (1, 5,
10 and 20 ng/ml) and IL-1β (1, 5, 10 and 20 ng/ml) for 24 h in a
humidified incubator (5% CO2 at 37°C). Cells were
trypsinized and resuspended in equal volumes of culture medium and
trypan blue at 37°C for 3 min. Viable (unstained) and nonviable
(blue-stained) cells were counted using a Neubauer chamber (LO;
Laboroptik GmbH, Bad Homburg, Germany) to calculate the total
number of viable cells.
RT-qPCR
RT-qPCR was used to compare the levels of steady
state mRNA expression for a number of genes in conditioned and
control cultures of human knee synovial fibroblasts. Total RNA was
isolated from synovial fibroblasts using an RNeasy Plus Mini kit
(Qiagen GmbH, Hilden, Germany), according to the manufacturer's
protocol. RNA samples were quantified using a Nanodrop®
spectrophotometer at 230/260/280 nm (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) and stored at −80°C. RNA samples were then
treated with DNase I (Fermentas; Thermo Fisher Scientific, Inc.).
RT-qPCR was then performed as described previously (16). Briefly, 20 µl cDNA was synthesized
from 1 µg total RNA using a RevertAid First Strand cDNA Synthesis
kit (Fermentas; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. qPCR was performed with a Quanti-Tect SYBR
Green PCR kit (Qiagen GmbH) using an iCycler iQ Real Time Detection
System (Bio-Rad Laboratories, Inc.). The reaction was initiated by
activating the polymerase with a 15 min pre-incubation at 95°C
Amplification was achieved with 45 cycles of 15 sec denaturation at
94°C, 20–30 sec annealing at 65°C and 10 sec extension at 72°C. The
program was concluded by a melting curve analysis. All experiments
were performed in triplicates. The copy numbers of each gene were
determined using the 2−∆∆Cq method (24). GAPDH was used as an internal control.
The Basic Local Alignment Search Tool database (https://blast.ncbi.nlm.nih.gov/Blast.cgi) was used to
verify gene specificity of all primer sequences. The primers used
were for MMP-1, MMP-2, MMP-3, LOX, LOX- like homolog 1 (LOXL-1),
LOXL-2, LOXL-3, LOXL-4 and GAPDH, as an internal control, and are
presented in Table I.
 | Table I.Primer sequences used in reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences used in reverse
transcription-quantitative polymerase chain reaction.
| Gene | Forward primer
sequence (5′-3′) | Reverse primer
sequence (5′-3′) |
|---|
| GAPDH |
GCACCGTCAAGGCTGAGAAC |
TGGTGAAGACGCCAGTGGA |
| LOX |
GCATACAGGGCAGATGTCAGA |
TTGGCATCAAGCAGGTCATAG |
| LOXL-1 |
TGCCACCAGCATTACCACAG |
GAGGTTGCCGAAGTCACAGG |
| LOXL-2 |
CTGCAAGTTCAATGCCGAGT |
TCTCCACCAGCACCTCCACTC |
| LOXL-3 |
CAACAGGAGGTTTGAACGCTAC |
GCTGACATGGGTTTCTTGGTAA |
| LOXL-4 |
TTCACCCACTACGACCTCCTCA |
CAGCAGCCTACAGTCACTCCCT |
| MMP-1 |
GGCTGAAAGTGACTGGGAAACC |
TGCTCTTGGCAAATCTGGCGTG |
| MMP-2 |
ACCGGGATAAGAAGTATGGATT |
GTCATCATCGTAGTTGGTTGTG |
| MMP-3 |
GACAAAGGATACAACAGGGAC |
TGAGTGAGTGATAGAGTGGG |
Zymography
MMP-2 activity in culture media samples was assayed
using a 0.05% gelatin zymography gel, as described previously
(5). Briefly, 10 µl of each sample
was mixed with an equal amount of Laemmli sample buffer (62.5 mM
Tris-hydrogen chloride, 25% glycerol, 2% SDS and 0.01% bromophenol
blue, pH 6.8) and separated on a 10% SDS-PAGE gel copolymerized
with 0.05% gelatin. Enzyme activity was regained by removing the
SDS, whereby gels were washed three times for 1.5 h in total in
2.5% Triton X-100 at room temperature following electrophoresis.
Washed gels were then bathed in proteolysis buffer (50 mM calcium
chloride, 0.5 M sodium chloride and 50 mM Tris, pH 7.8) and
incubated at 37°C for 15 h. Following incubation, gels were rinsed
in a 2.5% Triton X-100 solution and stained at room temperature
with Coomassie blue (45% methanol, 44.75% H2O, 10%
acetic acid and 0.25% Coomassie blue R-250) for 1 h on a rotator.
Gels were then destained with a 40% methanol, 7.5% acetic acid and
52.5% H2O solution until white bands were clearly
visible against the Coomassie blue background. Bands were scanned
with a densitometer (GS-800; Bio-Rad Laboratories, Inc.) and
quantification was performed using Quantity One 4.6.3 software
(Bio-Rad Laboratories, Inc.). The experiment was repeated three
times, and the relative density values were subjected to
statistical analysis.
Statistical analysis
Data are expressed as the mean ± standard deviation.
Data were statistically analyzed by one-way analysis of variance
and a post hoc Fisher's LSD test. Statistical analysis was
performed using a SPSS software, version 13.0 (SPSS, Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Cell viability
No cytotoxic effects of the exogenous inflammatory
factors TNF-α and IL-1β on human synovial fibroblasts were observed
by Trypan blue staining (data not shown). Furthermore, cell
viability did not significantly differ with increasing doses of
TNF-α and IL-1β.
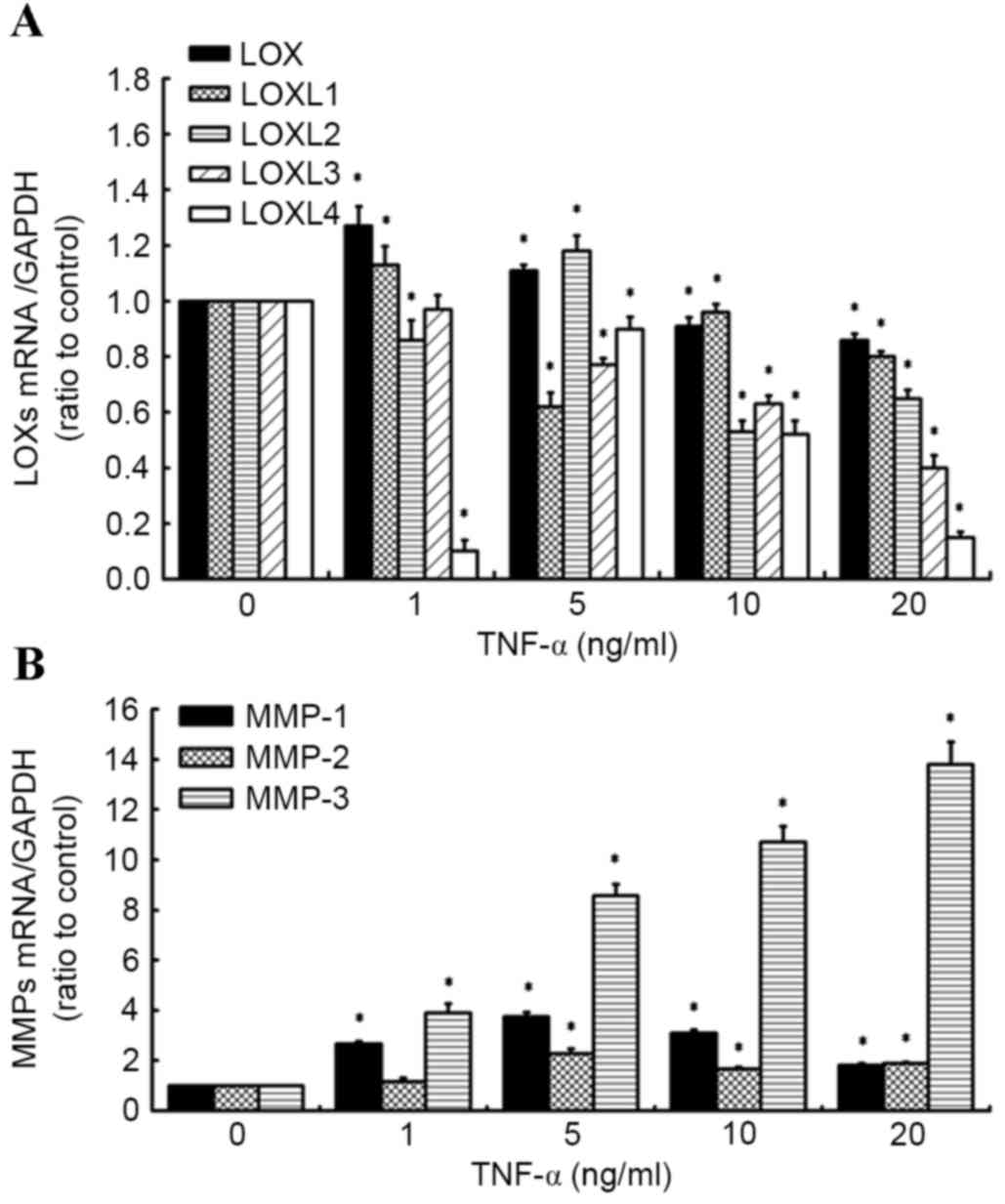
TNF-α has differential effects on the
expression of LOXs and MMPs in human knee synovial fibroblasts
RT-qPCR was used to evaluate the effects of TNF-α on
the expression of LOX and MMP mRNA (Fig.
1A and B). It was observed that addition of TNF-α (0–20 ng/ml)
led to a decrease in LOX expression. The inhibitory effect of LOX
and LOXL-3 by TNF-α was concentration-dependent. The inhibitory
effect reached a maximum at 5 ng/ml TNF-a for LOXL-1. The
expressions of LOXL-2 and LOXL-4 reached a maximum at 5 ng/ml TNF-α
and subsequently declined below the control values in response to
increasing TNF-α concentration (Fig.
1A), while significantly increasing the level of MMP expression
(MMP-1, MMP-2, MMP-3; Fig. 1B) in
synovial fibroblasts, relative to untreated control cells. These
results are similar to those of our previous study into the effects
of TNF-α (1 to 20 ng/ml) on the expression of LOX and MMP family
members (20).
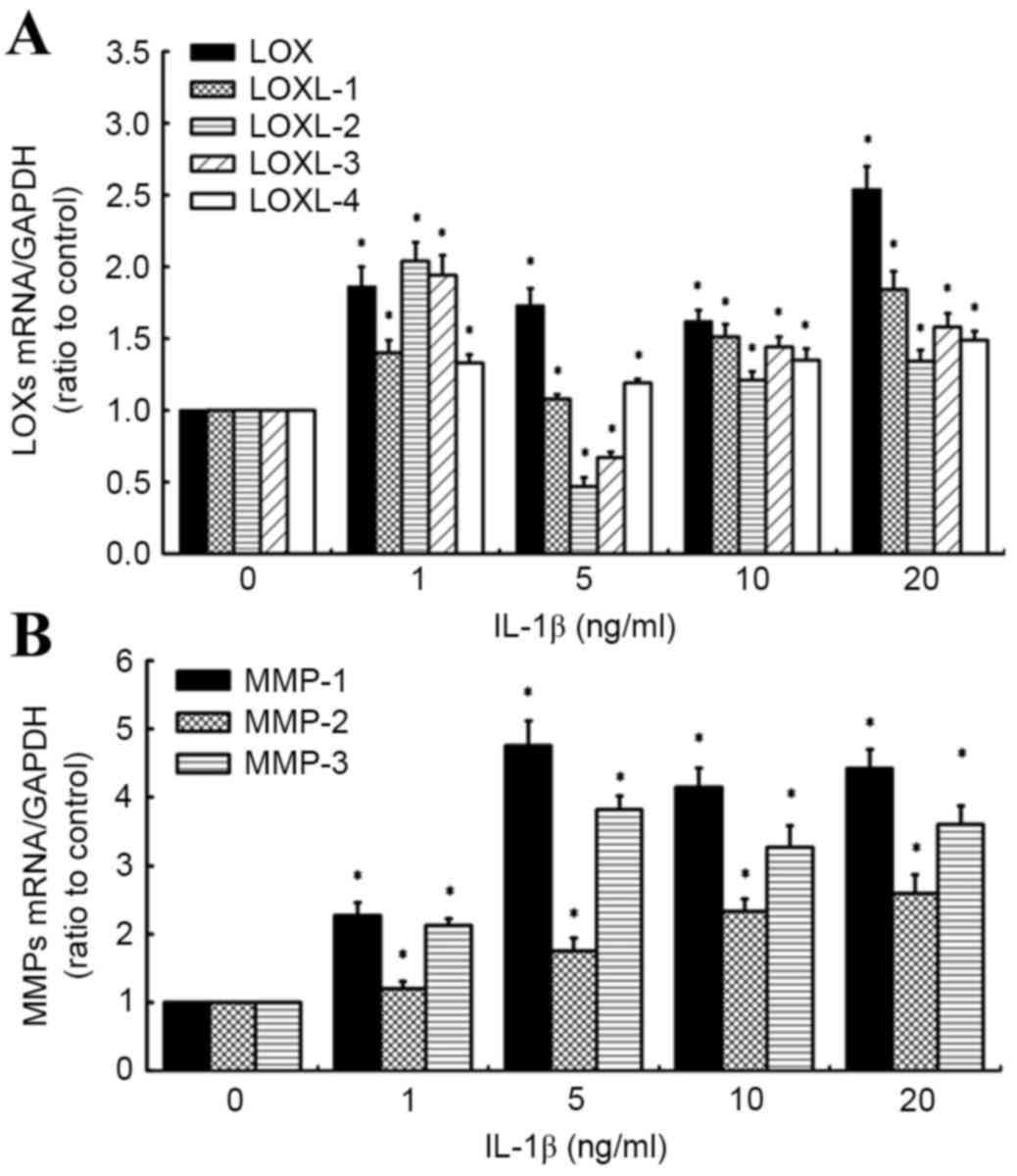
IL-1β has differential effects on the
expression of LOXs and MMPs in human knee synovial fibroblasts
RT-qPCR was also used to evaluate the effects of
IL-1β on the expression of LOX and MMP mRNA (Fig. 2A and B). It was observed that all
doses of IL-1β (1, 5, 10 and 20 ng/ml) significantly increased
levels of LOX, LOXL-1 and −4 expression in synovial fibroblasts,
relative to untreated control cells (all P<0.05). Specifically,
levels of LOXL-1 and −4 mRNA increased in a concentration-dependent
manner between 5 and 20 ng/ml IL-1β. By contrast, a significant
increase in LOXL-2 and −3 expression at 1 ng/ml IL-1β (both
P<0.05) was followed by a significant decrease in LOXL-2 and −3
expression at 5 ng/ml IL-1β (both P<0.05), relative to control
cells. However, significant increases in LOXL-2 and −3 mRNA were
observed in a concentration-dependent manner between 5 and 20 ng/ml
IL-1β, relative to control cells (both P<0.05; Fig. 2A).
Analogous to the effects of TNF-α, it was observed
that all concentrations of IL-1β significantly increased the levels
of MMP-1, 2 and 3 mRNA, relative to control cells (all P<0.05;
Fig. 2B). Specifically, maximum
increases in MMP-1 and −3 mRNA were observed in cells treated with
5 ng/ml IL-1β, relative to control cells (4.76- and 3.82-fold
increases, respectively), while MMP-2 expression increased in a
concentration-dependent manner, with a maximum 2.6-fold increase
observed at the highest IL-1β concentration (20 ng/ml). Based on
these results, doses of 10 ng/ml TNF-α and 10 ng/ml IL-1β were used
in further experiments.
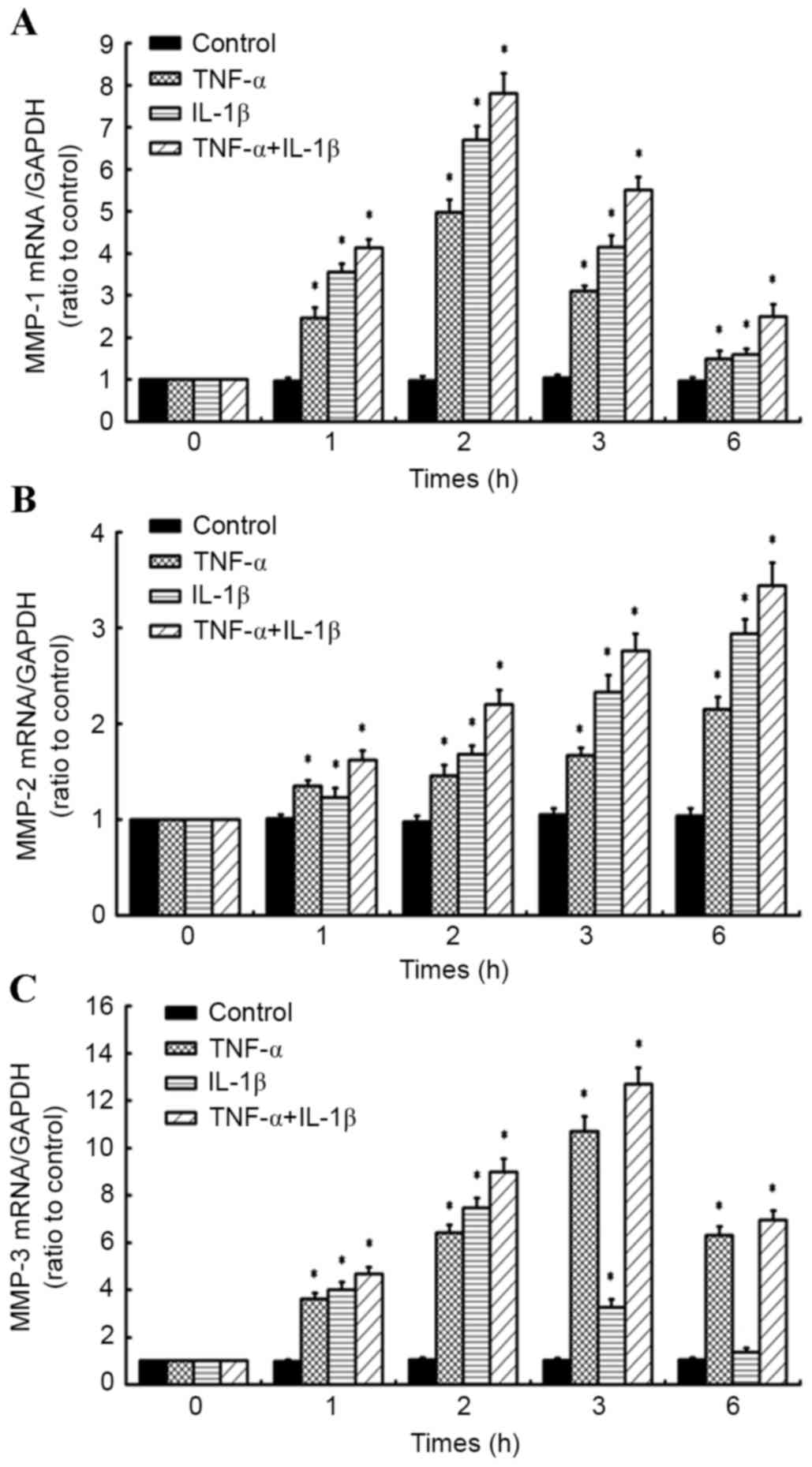
A time course of TNF-α in combination
with IL-1β alters the expression of LOXs in human knee synovial
fibroblasts
RT-qPCR was performed on fibroblasts administered a
time course of 10 ng/ml TNF-α and/or 10 ng/ml IL-1β (for 1, 2, 3
and 6 h) to determine the effects on the expression of LOXs (LOX,
LOXL-1, −2, −3 and −4; Fig. 3A-D).
Relative to control cells at corresponding time points, it was
observed that levels of LOX mRNA in synovial fibroblasts treated
with 10 ng/ml IL-1β significantly increased at all time points (1,
2, 3 and 6 h; all P<0.05) in a time-dependent manner, reaching a
maximum at 6 h post-treatment (Fig.
3A). By contrast, 10 ng/ml TNF-α significantly inhibited
expression of LOX at all time points relative to control cells
(P<0.05; Fig. 3A). In fibroblasts
treated with a combination of the inflammatory factors (10 ng/ml
TNF-α + 10 ng/ml IL-1β), LOX expression was significantly inhibited
at 1 h (P<0.05) and 6 h (P<0.05) post-cytokine treatment, all
relative to control cells at the corresponding time points
(Fig. 3A).
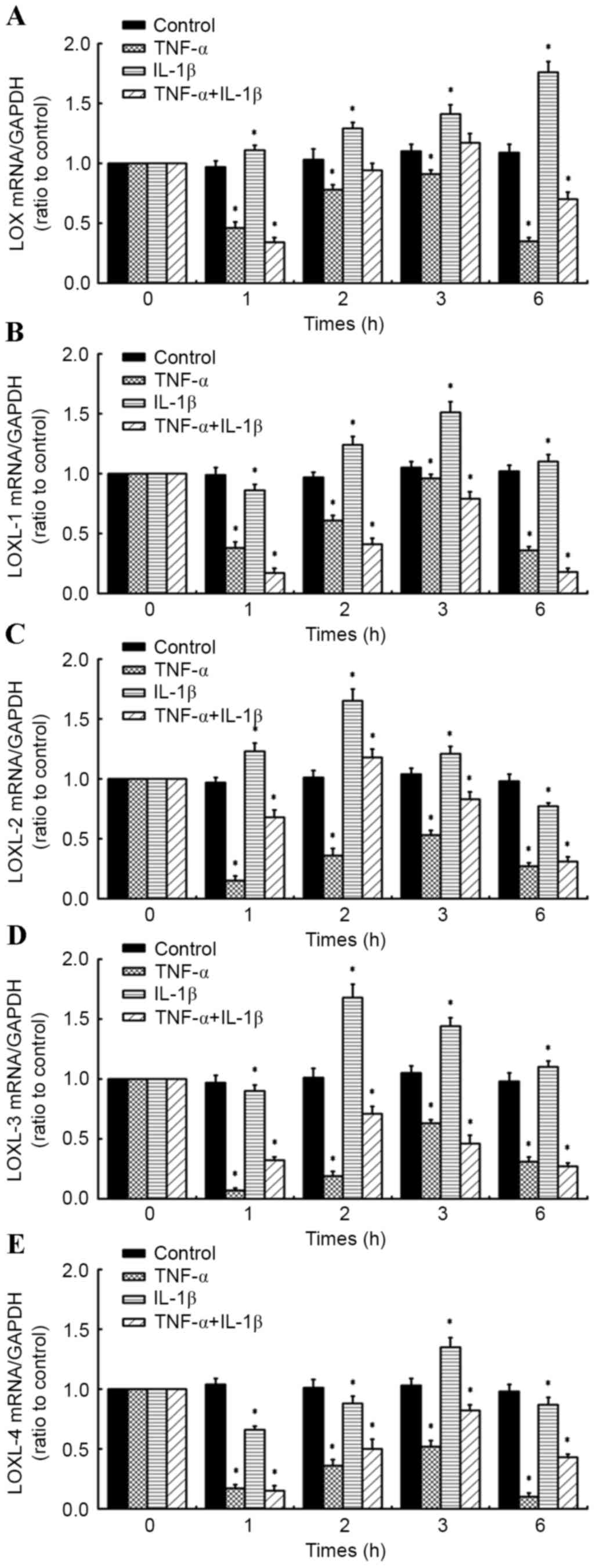 | Figure 3.Effects of inflammatory cytokine time
course on LOX family member expression in human knee synovial
fibroblasts. Reverse transcription-quantitative polymerase chain
reaction was performed on fibroblasts administered a time course of
10 ng/ml TNF-α and/or 10 ng/ml IL-1β (for 1, 2, 3 and 6 h) to
determine the effects on the expression of (A) LOX, (B) LOXL-1, (C)
LOXL-2, (D) LOXL-3 and (E) LOXL-4 expression. Data are presented as
the mean ± standard deviation, n=4. *P<0.05 vs. untreated
control cells at corresponding time points. TNF-α, tumor necrosis
factor-α; IL-1β, interleukin-1β; LOX, lysyl oxidase; LOXL, LOX-like
homolog 1. |
In addition, it was observed that LOXL-1 expression
was significantly inhibited in synovial fibroblasts treated with 10
ng/ml TNF-α at all time points (all P<0.05). LOXL-1 was also
significantly inhibited by 10 ng/ml IL-1β at 1 h post-treatment,
relative to control cells (P<0.05); however was significantly
increased at 2, 3 and 6 h post-treatment (all P<0.05), reaching
a maximum at 3 h. In fibroblasts treated with a combination of the
inflammatory factors, there was a significant inhibitory effect on
LOXL-1 expression at all time points (P<0.05), with more marked
inhibition of LOXL-1 observed compared to that in cells treated
with TNF-α or IL-1β alone, relative to their respective control
cells (Fig. 3B).
At all time points, TNF-α inhibited the expression
of LOXL-2 in synovial fibroblasts, relative to control cells at
corresponding time points (all P<0.05; Fig. 3C). By contrast, LOXL-2 was
significantly upregulated following treatment with IL-1β for 1, 2
and 3 h, reaching a maximum at 2 h post-treatment, relative to
control cells (all P<0.05). However, LOXL-2 expression
significantly decreased at 6 h post-treatment (P<0.05). In
fibroblasts treated with a combination of the inflammatory factors,
LOXL-2 expression significantly decreased at 1, 3 and 6 h
post-treatment (all P<0.05). However, LOXL-2 mRNA significantly
increased at 2 h post-treatment (P<0.05), though this was less
marked than for fibroblasts treated with IL-1β alone, relative to
their respective control cells (Fig.
3C).
Furthermore, LOXL-3 expression was significantly
inhibited in synovial fibroblasts treated with 10 ng/ml TNF-α at
all time points (all P<0.05; Fig.
3D). By contrast, exposure of synovial fibroblasts to 10 ng/ml
IL-1β caused a significant increase in LOXL-3 expression at 2, 3
and 6 h post-treatment (each P<0.05), though significant
downregulation in LOXL-3 mRNA was initially observed at 1 h
post-treatment (P<0.05; Fig. 3D).
The combination of TNF-α plus IL-1β significantly inhibited LOXL-3
expression at all time points, relative to control cells at
corresponding time points (Fig.
3D).
Analogous to LOX, LOXL-1, 2 and 3, LOXL-4 expression
was significantly inhibited by 10 ng/ml TNF-α at all time points
(all P<0.05; Fig. 3E). Similarly,
exposure of synovial fibroblasts to 10 ng/ml IL-1β caused a
significant downregulation in LOXL-4 expression at 1, 2 and 6 h
post-treatment (all P<0.05), though a significant upregulation
in LOXL-4 mRNA was observed at 3 h post-treatment (P<0.05;
Fig. 3E). In fibroblasts exposed to
a combination of TNF-α and IL-1β, a significant decrease in LOXL-4
expression was observed at all time points (all P<0.05), with
more marked inhibition of LOXL-4 observed compared to that in cells
treated with IL-1β alone at 1, 2 and 6 h post-treatment, relative
to their respective control cells (Fig.
3E).
TNF-α in combination with IL-1β
induces MMP-1, 2 and 3 expression in human knee synovial
fibroblasts
RT-qPCR was also used to determine the effects of 10
ng/ml TNF-α and/or 10 ng/ml IL-1β on the expression of MMP-1, 2 and
3 in synovial fibroblasts (Fig.
4A-C). It was observed that MMP-1 mRNA was significantly
upregulated by TNF-α or IL-1β treatment alone at all time points,
relative to untreated control cells at corresponding time points
(all P<0.05; Fig. 4A). In
addition, combined treatment with TNF-α and IL-1β caused a
significant upregulation in MMP-1 expression at all time points
(all P<0.05), with more marked upregulation of MMP-1 observed
compared to that in cells treated with TNF-α or IL-1β alone
(Fig. 4A).
Individual treatment with TNF-α or IL-1β also caused
a significant upregulation in MMP-2 expression in a time-dependent
manner (P<0.05; Fig. 4B). It was
also observed that the combined treatment with TNF-α and IL-1β
significantly increased MMP-2 expression at all time points (all
P<0.05), with more marked upregulation of MMP-2 observed
compared to that in cells treated with TNF-α or IL-1β alone
(Fig. 4B).
In fibroblasts exposed to TNF-α alone, significant
upregulation of MMP-3 mRNA was observed at all times points (all
P<0.05), while individual IL-1β treatment induced significant
upregulation of MMP-3 at 1, 2 and 3 h post-treatment (each
P<0.05). This upregulation in MMP-3 peaked at 2 and 3 h in
IL-1β- and TNF-α-treated cells, respectively. The combined
treatment of TNF-α and IL-1β also significantly increased MMP-3
expression at all time points (all P<0.05), with more marked
upregulation of MMP-3 observed compared to that of cells treated
with TNF-α or IL-1β alone (Fig. 4C).
Collectively, these data suggest that TNF-α and IL-1β have
synergistic effects on the expression of MMPs.
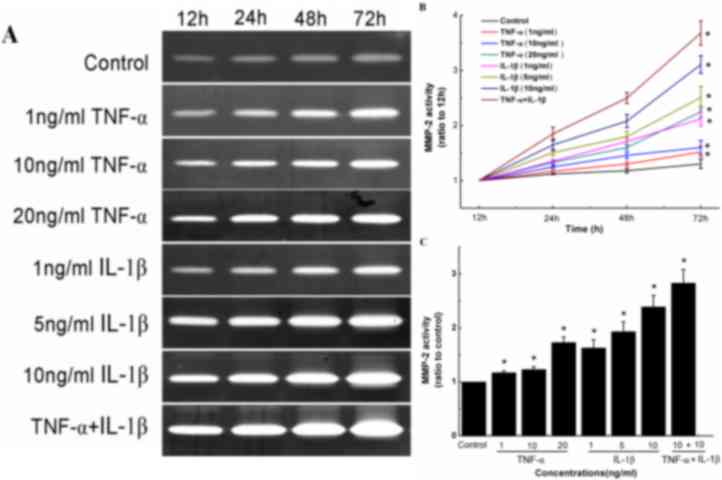
TNF-α in combination with IL-1β
induces MMP-2 expression and activity in human knee synovial
fibroblasts
Gelatin zymography was used to determine the effects
of TNF-α and/or IL-1β on the activity of MMP-2 in synovial
fibroblasts by measuring the increase in band intensity for the
active form of MMP-2 (62 kDa; Fig.
5A). Two forms (62 and 72 kDa) carry out the same enzymatic
reaction; however, the 72 kDa MMP-2 has ~10% the activity of the 62
kDa MMP-2 (9). It was observed that
TNF-α or IL-1β treatment alone significantly stimulated the
conversion of inactive pro-MMP-2 (72 kDa) into active MMP-2 (62
kDa) at all concentrations of each cytokine and when treated with a
combination of both cytokines, in a time-dependent manner (Fig. 5B and C). Specifically, relative to
control cells, synovial fibroblasts treated with 1, 10 and 20 ng/ml
TNF-α for 72 h exhibited 1.32-, 1.38- and 1.62-fold increases in
MMP-2 activity, respectively (Fig.
5C). Similarly, synovial fibroblasts treated with 1, 5 and 10
ng/ml IL-1β for 72 h exhibited 1.54-, 1.86- and 2.3-fold increases
in MMP-2 activity, respectively (Fig.
5C). The combined treatment of TNF-α and IL-1β (each 10 ng/ml)
also significantly increased MMP-2 activity (P<0.05), with a
more marked increase in MMP-2 activity observed compared to that in
cells treated with TNF-α or IL-1β alone (Fig. 5C).
Discussion
The current study principally observed that synovial
fibroblasts participate in the healing process of injured cruciate
ligaments by increasing the expression of MMPs and decreasing the
expression of LOXs within the inflammatory microenvironment of
damaged ligament tissue.
Previous studies have documented that the time
course of inflammatory cytokine levels, including TNF-α and IL-1β,
observed in joint fluid surrounding acute ACL injuries is similar
to that in wound fluid during ordinary wound healing processes.
This suggests that the ordinary wound healing mechanism, which
involves three overlapping phases of inflammation, matrix formation
and remodeling, also occurs in ACL injuries (21,25).
Thus, disruption in any phase of the healing sequence may result in
a non-healing wound.
Wound healing is a complex process dependent on
reactions and interactions between distinct tissues, cells and
mediators (26). A number of these
mediators are necessary, particularly TNF-α and IL-1β, which are
secreted by resident fibroblasts and inflammatory cells (including
macrophages and monocytes) (27).
Although TNF-α and IL-1β are structurally unrelated and bind to
distinct receptors, they operate in a similar biological manner and
commonly work synergistically (28).
The cytokines influence a number of processes at wound sites,
including fibroblast proliferation, chemotaxis, synthesis and
breakdown of ECM proteins, and regulation of the immune response
(29). However, excessive amounts of
inflammatory cytokines may have a negative effect on wound healing,
with previous results in animal models indicating differing roles
of cytokines depending on dosage (30,31).
Following cruciate ligament injury, increased levels
of TNF-α in synovial fluid may stimulate synovial fibroblasts to
produce IL-1, with IL-1 itself increasing TNF-α activity (32). In addition, TNF-α and IL-1 may
stimulate the production of IL-6 from synovial fibroblasts and
chondrocytes, which in turn promotes the immune response by
stimulating lymphocyte differentiation (33,34).
Mutual promotion among proinflammatory factors may also increase
levels of the factors in synovial fluids. Furthermore, the knee
joint cavity is a relatively isolated fluid-filled space enclosed
by synovial membrane, which facilitates the accumulation of
inflammatory cytokines in synovial fluid. High concentrations of
inflammatory cytokines in synovial fluid may inhibit ACL healing by
a number of mechanisms, including inhibition of ACL fibroblast
migration (35), suppression of type
I collagen synthesis (36) and
stimulation of ACL fibroblast apoptosis (37). It has previously been demonstrated
that the impaired healing ability of the ACL is associated with a
high level of expression and/or activation of MMPs in ACL
fibroblasts induced by inflammatory cytokines (38). Wang et al (19) and previous studies by our group
(20,39) have also indicated that synovial
fibroblasts are sensitive to TNF-α, due to its regulatory effects
on the production and activity of MMPs and LOXs. These data suggest
that synovial fibroblasts serve a key role in the regulation of the
joint cavity microenvironment.
The current study demonstrated that TNF-α, IL-1β and
a combination of both cytokines upregulated the expression of
MMP-1, −2 and −3 in synovial fibroblasts. In addition, a
combination of the inflammatory cytokines exhibited synergistic
effects in the induction of MMP-2 expression. Gelatin zymography
also indicated that TNF-α and IL-1β together increased the
production and activity of MMP-2 in synovial fibroblasts in a dose-
and time-dependent manner, although IL-1β was more efficient at
this than TNF-α. Pro-MMP-2 is visible at ~72 kDa and active MMP-2
is visible at ~62 kDa. These two forms carry out the same enzymatic
reaction, however pro-MMP-2 has ~10% the activity of active MMP-2
(9). It was observed that a
combination of TNF-α and IL-1β enhanced MMP-2 activity to a greater
extent than TNF-α and IL-1β alone. These results suggest that the
release of MMPs from synovial fibroblasts is sensitive to
inflammatory cytokines, and that TNF-α and IL-1β are involved in
the healing process of knee joint tissues. In addition, findings
that TNF-α and IL-1β may directly stimulate the synthesis of MMP-1,
−2 and −3 in synovial cells are similar to those of previous
studies on a number of other cells types, including tendon
fibroblasts (40), periodontal
ligament cells (41,42), chondrocytes (43,44),
vascular smooth muscle cells (45)
and gingival fibroblasts (46).
Increased levels of MMPs in synovial fibroblasts,
induced by TNF-α and IL-1β, may be secreted into synovial fluid,
where they potentially activate other members of the MMP family. A
previous study has demonstrated that MMP-1 activates latent MMP-2,
MMP-2 activates latent MMP-13 and MMP-3 activates latent MMP-1, 9
and 13 (47). Therefore, the mutual
activation of MMPs creates a complex network of proteases in the
synovial fluids. In addition, it has been previously documented
that MMPs are inflammatory cytokine-converting enzymes, with
findings that TNF-α and IL-1β may be proteolytically activated by
different MMPs, including MMP-2, −3, −7, −9, −12, −14 and −17
(48). Thus, the formation of a
positive feedback loop may increase the level of MMPs and
inflammatory cytokines within the sealed joint cavity. In turn,
high levels of MMP expression and activity within the joint fluid
may impede ACL healing by altering the balance between the
degradative and biosynthetic arms of the ligament tissue remodeling
process.
LOX staining surrounding areas of inflammation has
been documented in immunohistochemical studies in a number of
pathologies, including gingival hyperplasia and liver fibrosis,
suggesting that the expression of LOXs may also be regulated by
inflammatory factors, such as TNF-α and IL-1β (49,50).
Similar to the effects of TNF-α, the current study observed that
IL-1β downregulated LOXL-2 and −4 expression in synovial
fibroblasts. By contrast, IL-1β also upregulated LOX, LOXL-1 and −3
expression. The ability of IL-1β to promote the expression of LOXs
in synovial cells also exists in other cell types, including human
lung (51) and adult skin
fibroblasts (52). When TNF-α and
IL-1β cytokines were combined, the expression of LOXs was
downregulated to below that in control cells. In synovial fluid,
this inhibitory effect of TNF-α and IL-1β may decrease the
concentration of LOXs in synovial fluid and result in a reduced
degree of cross-linking between ECM proteins, thus weakening the
mechanical properties of ECM and increasing its susceptibility to
degradation by MMPs. These effects would again alter the balance
between the degradative and biosynthetic arms of the ligament
remodeling process and offer an additional explanation for the poor
healing ability of cruciate ligaments.
A limitation of the current study is that ACL injury
was only partially mimicked. This does not represent an in
vivo situation, in which levels of IL-1α TGF-β and other
cytokines are elevated in knee joint fluid following cruciate
ligament injury. This effect may additionally modulate the levels
of LOXs and MMP-1, 2 and 3 produced by synovial cells and other
intra-articular tissues. Thus, studies investigating the effects of
a global cytokine profile on ACL injury are warranted.
In conclusion, the present study observed that TNF-α
and IL-1β synergistically regulate the expression of the majority
of LOXs and MMPs investigated, and the activity of MMP-2, in
synovial fibroblasts. This indicates that the synovium is sensitive
to inflammatory factors and serves a key regulatory role in the
knee joint cavity microenvironment during the healing process.
These findings may aid in the development of therapeutic methods
for the replacement and/or regeneration of ligament tissue in
patients with cruciate ligament trauma.
Acknowledgements
The current study was supported by the National
Natural Science Foundation of China (grant no. 11702093), the
General Project of Hunan Provincial Education Department (grant no.
14C0452), the Natural Science Foundation of Hunan Province (grant
no. 2015JJ6042), the Innovation and Attracting Talents Program for
Colleges and Universities, China (grant no. B06023) and the
National Institutes of Health, USA (grant no. AR45635).
References
|
1
|
Woo SL, Abramowitch SD, Kilger R and Liang
R: Biomechanics of knee ligaments: Injury, healing, and repair. J
Biomech. 39:1–20. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hill CL, Seo GS, Gale D, Totterman S, Gale
ME and Felson DT: Cruciate ligament integrity in osteoarthritis of
the knee. Arthritis Rheum. 52:794–799. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sun K, Zhang J, Wang Y, Xia C, Zhang C, Yu
T and Tian S: Arthroscopic reconstruction of the anterior cruciate
ligament with hamstring tendon autograft and fresh-frozen
allograft: A prospective, randomized controlled study. Am J Sports
Med. 39:1430–1438. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim HS, Seon JK and Jo AR: Current trends
in anterior cruciate ligament reconstruction. Knee Surg Relat Res.
25:165–173. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bray RC, Leonard CA and Salo PT:
Correlation of healing capacity with vascular response in the
anterior cruciate and medial collateral ligaments of the rabbit. J
Orthop Res. 21:1118–1123. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nagineni CN, Amiel D, Green MH, Berchuck M
and Akeson WH: Characterization of the intrinsic properties of the
anterior cruciate and medial collateral ligament cells: An in vitro
cell culture study. J Orthop Res. 10:465–475. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sung KL, Yang L, Whittemore DE, Shi Y, Jin
G, Hsieh AH, Akeson WH and Sung LA: The differential adhesion
forces of anterior cruciate and medial collateral ligament
fibroblasts: Effects of tropomodulin, talin, vinculin, and
alpha-actinin. Proc Nati Acad Sci USA. 93:pp. 9182–9187. 1996,
View Article : Google Scholar
|
|
8
|
Wiig ME, Amiel D, Ivarsson M, Naqineni CN,
Wallace CD and Arfors KE: Type I procollagen gene expression in
normal and early healing of the medial collateral and anterior
cruciate ligaments in rabbits: An in situ hybridization study. J
Orthop Res. 9:374–382. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou D, Lee HS, Villarreal F, Teng A, Lu
E, Reynolds S, Qin C, Smith J and Sung KL: Differential MMP-2
activity of ligament cells under mechanical stretch injury: An in
vitro study on human ACL and MCL fibroblasts. J Orthop Res.
23:949–957. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chithra P, Sajithlal GB and Chandrakasan
G: Influence of Aloe vera on collagen characteristics in healing
dermal wounds in rats. Mol Cell Biochem. 181:71–76. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Garqiulo S, Gamba P, Poli G and
Leonarduzzi G: Metalloproteinases and metalloproteinase inhibitors
in age-related diseases. Curr Pharm Des. 20:2993–3018. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fan SH, Wang YY, Lu J, Zheng YL, Wu DM,
Zhang ZF, Shan Q, Hu B, Li MQ and Cheng W: CERS2 suppresses tumor
cell invasion and is associated with decreased V-ATPase and
MMP-2/MMP-9 activities in breast cancer. J Cell Biochem.
116:502–513. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vater CA, Harris ED Jr and Siegel RC:
Native cross-links in collagen fibrils induce resistance to human
synovial collagenase. Biochem J. 181:639–645. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Avery NC and Bailey AJ: Enzymic and
non-enzymic cross-linking mechanisms in relation to turnover of
collagen: Relevance to aging and exercise. Scand J Med Sci Sports.
15:231–240. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang Y, Tang Z, Xue R, Singh GK, Lv Y, Shi
K, Cai K, Deng L and Yang L: TGF-β1 promoted MMP-2 mediated wound
healing of anterior cruciate ligament fibroblasts through NF-κB.
Connect Tissue Res. 52:218–225. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xie J, Jiang J, Huang W, Zhang Y, Xu C,
Wang C, Yin L, Chen PC and Sung KL: TNF-α induced down-regulation
of lysyl oxidase family in anterior cruciate ligament and medial
collateral ligament fibroblasts. Knee. 21:47–53. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tang Z, Yang L, Xue R, Zhang J, Wang Y,
Chen PC and Sung KL: Differential expression of matrix
metalloproteinases and tissue inhibitors of metalloproteinases in
anterior cruciate ligament and medial collateral ligament
fibroblasts after a mechanical injury: Involvement of the p65
subunit of NF-kappaB. Wound Repair Regen. 17:709–716. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tang Z, Yang L, Wang Y, Xue R, Zhang J,
Huang W, Chen PC and Sung KL: Contributions of different
intraarticular tissues to the acute phase elevation of synovial
fluid MMP-2 following rat ACL rupture. J Orthop Res. 27:243–248.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Yang L, Zhang J, Xue R, Tang Z,
Huang W, Jiang D, Tang X, Chen P and Sung KL: Differential MMP-2
activity induced by mechanical compression and inflammatory factors
in human synoviocytes. Mol Cell Biomech. 7:105–114. 2010.PubMed/NCBI
|
|
20
|
Zhang Y, Huang W, Jiang J, Xie J, Xu C,
Wang C, Yin L, Yang L, Zhou K, Chen P and Sung KP: Influence of
TNF-α and biomechanical stress on matrix metalloproteinases and
lysyl oxidases expressions in human knee synovial fibroblasts. Knee
Surg Sports Traumatol Arthrosc. 22:1997–2006. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Irie K, Uchiyama E and Iwaso H:
Intraarticular inflammatory cytokines in acute anterior cruciate
ligament injured knee. Knee. 10:93–96. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Behm B, Babilas P, Landthaler M and
Schreml S: Cytokines, chemokines and growth factors in wound
healing. J Acad Dermatol Venereol. 26:812–820. 2012. View Article : Google Scholar
|
|
23
|
Xie J, Wang C, Yin L, Xu C, Zhang Y and
Sung KL: Interleukin-1 beta influences on lysyl oxidases and matrix
metalloproteinases profile of injured anterior cruciate ligament
and medial collateral ligament fibroblasts. Int Orthop. 37:495–505.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sinno H and Prakash S: Complements and the
wound healing cascade: An updated review. Plast Surg Int.
2013:1467642013.PubMed/NCBI
|
|
26
|
Barrientos S, Brem H, Stojadinovic O and
Tomic-Canic M: Clinical application of growth factors and cytokines
in wound healing. Wound Repair Regen. 22:569–578. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Migita K, Eguchi K, Kawabe Y, Ichinose Y,
Tsukada T, Aoyagi T, Nakamura H and Nagataki S: TNF-alpha-mediated
expression of membrane-type matrix metalloproteinase in rheumatoid
synovial fibroblasts. Immunology. 89:553–557. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Soller JT, Murua-Escobar H, Willenbrock S,
Janssen M, Eberle N, Bullerdiek J and Nolte I: Comparison of the
human and canine cytokines IL-1(alpha/beta) and TNF-alpha to
orthologous other mammalians. J Hered. 98:485–490. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Shona MI, Shaun G and David MT:
Differential regulation of metalloproteinase production,
proliferation and chemotaxis of human lung fibroblasts by PDGF,
interleukin-1beta and TNF-alpha. Mediators Inflamm. 9:155–160.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Guirao X and Lowry SF: Biologic control of
injury and inflammation: Much more than too little or too late.
World J Surg. 20:437–446. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Menke NB, Ward KR, Witten TM, Bonchev DG
and Diegelmann RF: Impaired wound healing. Clin Dermatol. 25:19–25.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Di Giovine FS, Nuki G and Duff GW: Tumour
necrosis factor in synovial exudates. Ann Rheum Dis. 47:768–772.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cameron ML, Fu FH, Paessler HH, Schneider
M and Evans CH: Synovial fluid cytokine concentrations as possible
prognostic indicators in the ACL-deficient knee. Knee Surg Sports
Traumatol Arthrosc. 2:38–44. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Choi HM, Oh DH, Bang JS, Yang HI, Yoo MC
and Kim KS: Differential effect of IL-1β and TNFα on the production
of IL-6, IL-8 and PGE2 in fibroblast-like synoviocytes and THP-1
macrophages. Rheumatol Int. 30:1025–1033. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Witkowski J, Yang L, Wood DJ and Sung KL:
Migration and healing of ligament cells under inflammatory
conditions. J Orthop Res. 15:269–277. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Greenwel P, Tanaka S, Penkov D, Zhang W,
Olive M, Moll J, Vinson C, Di Liberto M and Ramirez F: Tumor
necrosis factor alpha inhibits type I collagen synthesis through
repressive CCAAT/enhancer-binding proteins. Mol Cell Biol.
20:912–918. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Murakami H, Shinomiya N, Kikuchi T,
Yoshihara Y and Nemoto K: Upregulated expression of inducible
nitric oxide synthase plays a key role in early apoptosis after
anterior cruciate ligament injury. J Orthop Res. 24:1521–1534.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang Y, Tang Z, Xue R, Singh GK, Shi K, Lv
Y and Yang L: Combined effects of TNF-α, IL-1β, and HIF-1α on MMP-2
production in ACL fibroblasts under mechanical stretch: An in vitro
study. J Orthop Res. 29:1008–1014. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fu C, Xie J, Chen R, Wang C, Xu C, Chen C,
Wang Z, Lin L, Huang W, Liang X and Sung KL: Effect of titanium
particles and TNF-alpha on the gene expression and activity of
MMP-1, 2, 3 in human knee joint synovial cells. Sheng Wu Yi Xue
Gong Cheng Xue Za Zhi. 30:1022–1026. 2013.(In Chinese). PubMed/NCBI
|
|
40
|
Schulze-Tanzil G, Al-Sadi O, Wiegand E,
Ertel W, Busch C, Kohl B and Pufe T: The role of pro-inflammatory
and immunoregulatory cytokines in tendon healing and rupture: New
insights. Scand J Med Sci Sports. 21:337–351. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nakaya H, Oates TW, Hoang AM, Kamoi K and
Cochran DL: Effects of interleukin-1beta on matrix
metalloproteinase-3 levels in human periodontal ligament cells. J
Periodontol. 68:517–523. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ahn SJ, Rhim EM, Kim JY, Kim KH, Lee HW,
Kim EC and Park SH: Tumor necrosis factor-α induces matrix
metalloproteinases-3, −10 and −13 in human periodontal ligament
cells. J Periodontol. 85:490–497. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Raymond L, Eck S, Hays E, Tomek I, Kantor
S and Vincenti M: RelA is required for IL-1beta stimulation of
matrix metalloproteinase-1 expression in chondrocytes.
Osteoarthritis Cartilage. 15:431–441. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Dunn SL, Wilkinson JM, Crawford A, Le
Maitre CL and Bunning RA: Cannabinoid WIN-55,212-2 mesylate
inhibits interleukin-1β induced matrix metalloproteinase and tissue
inhibitor of matrix metalloproteinase expression in human
chondrocytes. Osteoarthritis Cartilage. 22:133–144. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhong Y, Yu W, Feng J, Fan J and Li J:
Curcumin suppresses tumor necrosis factor-α-induced matrix
metalloproteinase-2 expression and activity in rat vascular smooth
muscle cells via the NF-κB pathway. Exp Ther Med. 7:1653–1658.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Gotoh K, Nemoto E, Kanaya S and Shimauchi
H: Extracellular β-NAD(+) inhibits interleukin-1-induced matrix
metalloproteinase-1 and −3 expression on human gingival
fibroblasts. Connect Tissue Res. 54:204–209. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kerrigan JJ, Mansell JP and Sandy JR:
Matrix turnover. J Orthod. 27:227–233. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Rodríguez D, Morrison C and Overall CM:
Matrix metalloproteinases: What do they not do? New substrates and
biological roles identified by murine models and proteomics.
Biochim Biophys Acta. 1803:39–54. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Trackman PC, Graham RJ, Bittner HK, Carnes
DL, Gilles JA and Graves DT: Inflammation-associated lysyl oxidase
protein expression in vivo, and modulation by FGF-2 plus IGF-1.
Histochem Cell Biol. 110:9–14. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Desmoulière A, Darby I, Costa AM, Raccurt
M, Tuchweber B, Sommer P and Gabbiani G: Extracellular matrix
deposition, lysyl oxidase expression, and myofibroblastic
differentiation during the initial stages of cholestatic fibrosis
in the rat. Lab Invest. 76:765–778. 1997.PubMed/NCBI
|
|
51
|
Roy R, Polgar P, Wang Y, Goldstein RH,
Taylor L and Kagan HM: Regulation of lysyl oxidase and
cyclooxygenase expression in human lung fibroblast: Interaction
among TGF-beta, IL-1 beta, and prostaglandin E. J Cell Biochem.
62:411–417. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Cenizo V, André V, Reymermier C, Sommer P,
Damour O and Perrier E: LOXL as a target to increase the elastin
content in adult skin: A dill extract induces the LOXL gene
expression. Exp Dermatol. 15:574–581. 2006. View Article : Google Scholar : PubMed/NCBI
|