Introduction
Heart valve disease is a common clinical heart
disease, which involves the aortic valve, mitral valve, tricuspid
valve and pulmonary valve; the main clinical manifestations are
valve stenosis and insufficiency (1). Traditional cardiopulmonary bypass (CPB)
is the main method of treating valvular heart disease. CPB
represents a stress on the body, and intubation and sternotomy
during surgery further enhances the stress response, resulting in
hemodynamic fluctuations in patients, making them prone to
myocardial ischemia and ischemia-reperfusion injury. This may lead
to systemic inflammatory response syndrome, and multiple organ
dysfunction, affecting the prognosis. Brain damage is one of the
most serious potential complications after heart valve replacement
surgery (2).
In the 1990s, non-CPB coronary artery bypass
grafting, that is, heart off pump bypass surgery began to be
applied clinically. This technique can help avoid damage caused by
CPB, and is more conducive to the protection of heart and brain
function and reducing postoperative complications (3). Heart-type fatty acid binding protein
(H-FABP), creatine kinase myocardial isoenzyme (CK-MB), troponin I
(cTnI) are markers that can reflect the degree of myocardial injury
(4). S100β protein and serum
neuron-specific enolase (NSE) are markers that can reflect the
degree of brain injury and neurological impairment (5).
Anesthesia can also induce a stress response and
inhibit immune function, while postoperative pain will prolong the
hospital stay, increasing the economic burden. Dexmedetomidine
(Dex) is a highly selective α2 adrenergic receptor agonist with
analgesic, sedative and anti-sympathetic tonic effects (6). In this study, we examined the effects
of performing Dex anesthesia on patients undergoing cardiac valve
replacement on H-FABP, CK-MB, cTnI and neurological function of
patients.
Materials and methods
Subjects
Ninety-six patients admitted to Shanxi Provincial
People's Hospital from January 2015 to December 2015 for heart
valve replacement surgery were selected. Inclusion criteria were
New York Heart Society heart function grade II–III; non-CPB heart
off-pump coronary artery bypass surgery opted for, with a certain
degree of preoperative myocardial injury, and complete patient
medical records; informed consent signed. Exclusion criteria were
severe renal insufficiency, heart failure history and coagulation
abnormalities; allergy to dextromethorphan. Patients were divided
into observation group and control group according to random number
table (both n=48). The studywas approved by the Ethics Committee of
Shanxi Provincial People's Hospital and informed consents were
signed by the patients and/or guardians.
Surgical treatment
Patients were fasted for 8 h before surgery, with
blood pressure, heart rate (HR), oxygen saturation (SpO2) and
Bipolar Spectrum Index (BIS) monitoring. All patients underwent
non-CPB off-pump coronary artery bypass surgery.
The control group was treated with remifentanil
anesthesia, using propofol (3 mg/kg) (Sichuan Guorui Pharmaceutical
Co., Ltd., approval no. H20040079), remifentanil (0.3 µg/kg;
Yichang Humanwell Pharmaceutical Co., Ltd., approval no. H20054171)
and vecuronium bromide (0.1 mg/kg) (Beijing Mengjin Medical
Technology Development Co., Ltd., approval no. H20063122) to induce
anesthesia; tracheal intubation was performed when BIS was less
than 55, then the patient was connected to the ventilator
(respiratory rate: 12–15 times/min, suction ratio: 1:2, tidal
volume: 8–9 ml/kg), and remifentanil was intermittently added to
maintain the BIS value at 40–45, with remifentanil dosage adjusted
according to BIS value, the floating range was ±30%.
The observation group were treated with Dex
anesthesia, and the anesthesia induction was the same as that of
the control group. Dex (0.5 µg/kg/h) was pumped after tracheal
intubation. Intravenous injection of remifentanil (0.3 µg/kg) was
performed every 3–5 min during surgery to maintain anesthesia, the
pumping of Dex was stopped 30 min before the end of surgery, and
tracheal extubation was performed when the patient's spontaneous
breathing tidal volume reached 5 ml/kg, heartrate 20 beats/min,
oxygen SpO2 ≥95% and maintained for more than 5 min.
Detection of related indicators
Immediately before induction (C1), 10 min after the
start of surgery (C2), at the end of surgery (C3), 6 h after the
end of surgery (C4), and 24 h after surgery (C5), 4 ml of central
venous blood was taken, centrifuged and the supernatant isolated
and stored at −70°C. The levels of H-FABP, CK-MB and cTnI were
detected by double antibody one step sandwich enzyme-linked
immunosorbent assay (ELISA) according to the manufacturer's
instructions (R&D Systems Inc., Minneapolis, MN, USA). OD value
was read at the wavelength of 450 nm with the microplate reader
(Jiangsu Potebio Biotechnology Co., Ltd.), and the concentration of
H-FABP, CK-MB and cTnI was calculated.
Venous blood samples (4 ml) were taken from the
jugular bulb immediately before and 24 h after anesthesia
induction. The sample was centrifuged at 4°C and the supernatant
stored at −70°C. The concentration of S100β and NSE was detected by
solid sandwich ELISA method according to the manufacturer's
instructions (reagents supplied by RB systems, USA). The OD value
was read at a wavelength of 450 nm using a microplate reader and
the concentration of S100β and NSE were calculated.
The hemodynamics of the two groups before anesthesia
induction (T1), 1 min after intubation (T2), after 10 min of
surgery (T3) and at the end of surgery (T4) were compared,
including HR, and mean arterial pressure (MAP).
Changes in the concentration of H-FABP, CK-MB and
cTnI were measured by enzyme-linked immunosorbent assay (ELISA)
before the induction of anesthesia (C1), after 10 min of surgery
(C2), immediately at the end of surgery (C3), 6 h after surgery
(C4), and 24 h after surgery (C5). The levels of S100β and NSE were
measured by ELISA before and 24 h after anesthesia induction.
One day before and 24 h after surgery, the cognitive
function was evaluated using the Montreal Cognitive Assessment
(MoCA) scale and the Simple Mental State Examination Scale (MMSE).
The MoCA scale was evaluated from the eight areas including space
and the ability of implementation, memory, attention, naming,
language, delayed memories, abstract thinking and orientation, with
a total score of 30 points. A total of <26 points was judged as
impaired cognitive function. MMSE criteria scored the orientation
force, language, memory, attention and computing power. Scores were
judged as mild cognitive dysfunction: 21–24 points, moderate
cognitive dysfunction 11–20 points, severe cognitive dysfunction
0–10 points.
Myocardial contractility score formula 24 h after
operation was (dopamine + dobutamine) × 1 + milrinone × 15 +
(adrenaline + norepinephrine + isoproterenol) × 100 µg/kg/min;
ventricular arrhythmia criteria was judged as 0 points: no
arrhythmia occurred, 1 point: atrial arrhythmia or <10
pre-ventricular contractions, 2 points: ≥10 times ventricular
contraction, 3 points: ventricular tachycardia attack 1–2 times, 4
points: ventricular tachycardia or ventricular tachycardia attack
≥3 times. Patients were followed up for 1 year, and the incidence
of adverse cardiovascular events was observed, including cardiac
arrest, arrhythmia, and heart functional failure.
Statistical analysis
Data were processed using SPSS 19.0 software (SPSS
Inc., Chicago, IL, USA), measurement data were expressed as mean ±
standard deviation, using t-test; enumeration data were expressed
as a percentage, using χ2 test, and P<0.05 indicated
statistical significance.
Results
Comparison of hemodynamics
There was no significant difference between the two
groups in terms of baseline characteristics (P>0.05) (Table I). There was no significant
difference in perioperative HR between the two groups (P>0.05).
The MAP of the observation group was lower than that of the control
group after anesthesia (P<0.05) (Table II).
 | Table I.Baseline characteristics. |
Table I.
Baseline characteristics.
| Item | Control group
n=48 | Observation group
n=48 |
t-value/χ2 | P-value |
|---|
| Sex (M/F) | 26/22 | 25/23 | 0.042 | 0.838 |
| Age (years) | 45–76 | 45–75 |
|
|
| Mean age (years) | 56.38±6.47 | 56.43±6.57 | 0.038 | 0.970 |
| BMI
(Kg/m2) | 22.23±3.15 | 22.56±3.18 | 0.511 | 0.611 |
| NYHA heart function
(n, %) |
|
|
|
|
| Grade
II | 18 (37.50) | 20 (41.67) | 0.174 | 0.676 |
| Grade
III | 30 (62.50) | 28 (58.33) |
|
|
| Anesthesia (min) | 243.56±81.24 | 243.73±80.34 | 0.010 | 0.992 |
| Operation time
(min) | 212.54±63.17 | 210.43±63.36 | 0.163 | 0.871 |
 | Table II.Comparison of hemodynamic indicators
at different time points. |
Table II.
Comparison of hemodynamic indicators
at different time points.
| Indicator | Group | Case no. | T1 | T2 | T3 | T4 |
|---|
| HR (times) | Observation | 48 | 84.23±7.18 | 74.37±6.38 | 68.43±5.25 | 64.73±4.48 |
|
| Control | 48 | 85.06±7.15 | 75.02±6.57 | 67.94±5.17 | 65.06±4.72 |
| MAP (mmHg) | Observation | 48 | 56.19±3.57 |
67.15±4.13a |
65.56±3.18a |
56.35±3.17a |
|
| Control | 48 | 57.02±3.68 | 79.32±4.24 | 75.18±3.24 | 59.68±3.45 |
Concentration changes of H-FABP,
CK-MB, cTnI
At the time points C1, C2, C3, C4, and C5, the
levels of H-FABP were 0.26±0.06, 0.48±0.13, 1.36±0.27, 1.94±0.23,
1.42±0.37 µg/ml in the observation group, and were 0.25±0.04,
0.65±0.14, 1.89±0.26, 2.34±0.25, 1.71±0.45 µg/ml in the control
group (Fig. 1A). The levels of CK-MB
in the observation group were 22.78±1.56, 60.68±2.53, 110.36±2.47,
130.64±2.13, 89.52±2.37 U/ml, and in the control group were
22.44±1.34, 69.63±2.74, 141.75±3.26, 156.64±3.25, 112.73±3.45 U/ml
(Fig. 1B). The levels of cTnI in the
observation group were 0.38±0.06, 0.63±0.13, 0.96±0.17, 1.34±0.13,
1.12±0.27 ng/ml, and in the control group 0.39±0.08, 0.79±0.14,
1.25±0.26, 1.78±0.25, 1.48±0.25 ng/ml (Fig. 1C). The levels of H-FABP, CK-MB and
cTnI at C2, C3, C4 and C5 were significantly higher than at C1 in
both groups (P<0.05), however, the levels in the observation
group at C2, C3, C4 and C5 were significantly lower than those in
the control group (P<0.05).
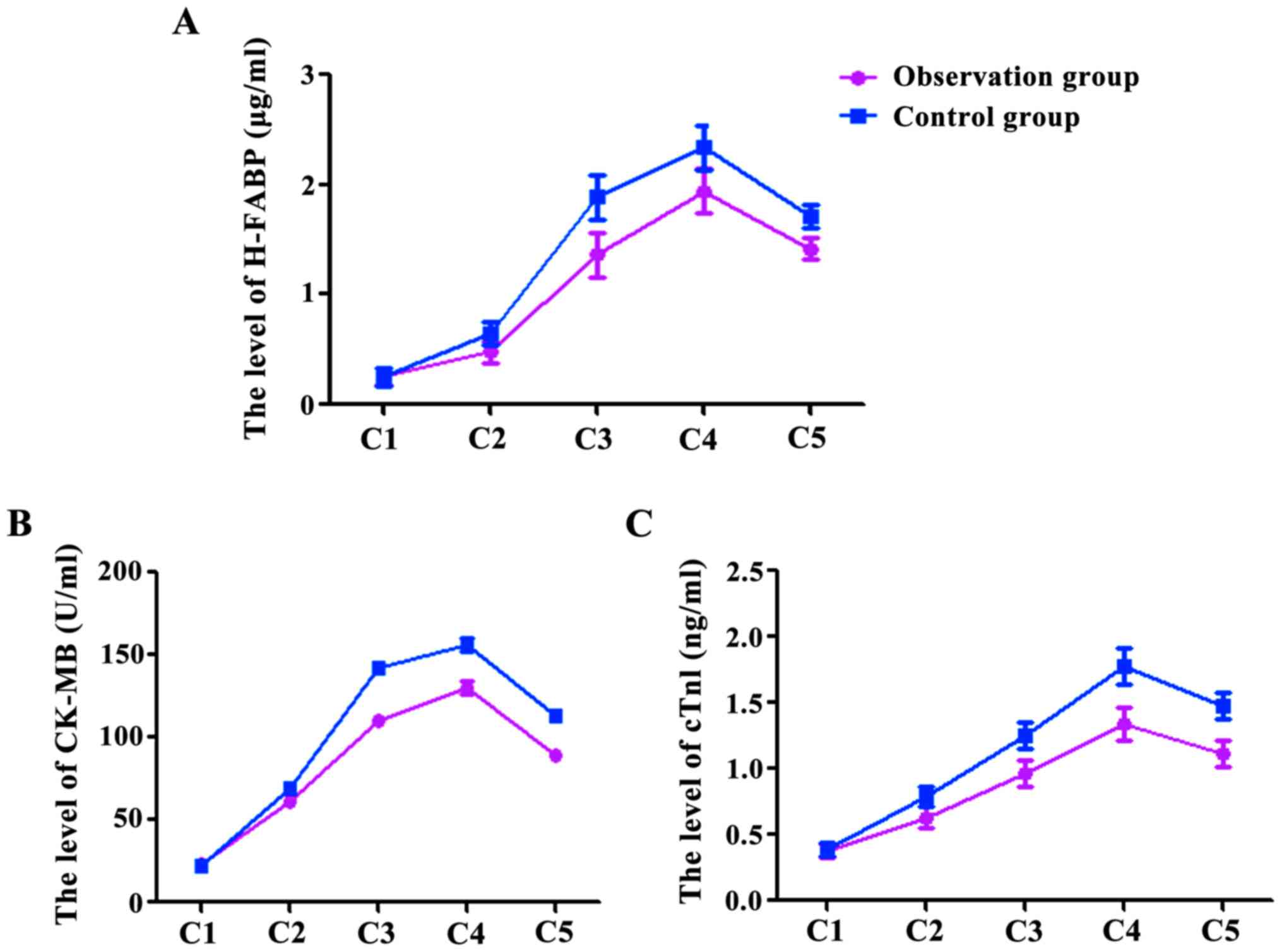 | Figure 1.The levels of H-FABP (A), CK-MB (B)
and cTnI (C) at C2, C3, C4 and C5 were significantly higher than at
C1, P<0.05, and there was no significant difference between the
two groups at C1, P>0.05; at C2, C3, C4 and C5, the observation
group was significantly lower than the control group,
P<0.05. |
The levels of S100β and NSE in the two
groups
The levels of S100β and NSE after surgery were
significantly higher than those before surgery (P<0.05). The
levels of S100β and NSE in the observation group were significantly
lower than those in the control group (P<0.05) (Table III).
 | Table III.Comparison of S100β and NSE
concentrations in both groups. |
Table III.
Comparison of S100β and NSE
concentrations in both groups.
|
| S100β (pg/ml) |
|
| NSE (ng/ml) |
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Group | 1 day before
surgery | 24 h after
surgery | t-value | P-value | 1 day before
surgery | 24 h after
surgery | t-value | P-value |
|---|
| Observation | 103.24±5.13 | 326.45±8.29 | 156.632 | <0.001 | 12.42±1.32 | 18.12±1.73 | 18.148 | <0.001 |
| Control | 104.73±6.24 | 375.27±12.38 | 135.199 | <0.001 | 12.78±1.23 | 23.36±1.84 | 33.119 | <0.001 |
| t-value | 1.280 | 22.701 |
|
| 1.382 | 14.374 |
|
|
| P-value | 0.204 | <0.001 |
|
| 0.170 | <0.001 |
|
|
The MoCA and MMSE scores of the two
groups
The MoCA and MMSE scored in the two groups after
surgery were lower than those before surgery, and the decrease in
scores in the observation group was lower than that of the control
group, P<0.05 (Table IV).
 | Table IV.Comparison of MoCA and MMSE scores in
both groups. |
Table IV.
Comparison of MoCA and MMSE scores in
both groups.
|
| MoCA |
|
| MMSE |
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Group | 1 day before
surgery | 24 h after
surgery | t-value | P-value | 1 day before
surgery | 24 h after
surgery | t-value | P-value |
|---|
| Observation | 28.24±1.33 | 25.15±1.39 | 11.128 | <0.001 | 30.62±3.12 | 23.12±2.43 | 13.139 | <0.001 |
| Control | 28.53±1.64 | 26.37±1.38 | 6.982 | <0.001 | 29.98±2.13 | 26.26±3.23 |
6.661 | <0.001 |
| t-value | 0.952 | 4.315 |
|
| 1.174 | 5.382 |
|
|
| P-value | 0.344 | <0.001 |
|
| 0.243 | <0.001 |
|
|
Cardiac parameters
The myocardial contractility and cardiac arrhythmias
scores in the observation group were significantly lower than those
of the control group 24 h after surgery (P<0.05); after 1-year
follow-up, the incidence of adverse events observed in the
observation group was significantly lower than in the control group
(P<0.05) (Table V).
 | Table V.Comparison of myocardial
contractility and arrhythmia scores and cardiovascular adverse
event incidence. |
Table V.
Comparison of myocardial
contractility and arrhythmia scores and cardiovascular adverse
event incidence.
| Group | N | Myocardial
contractility score | Arrhythmias
score | Cardiovascular
adverse event incidence (n, %) |
|---|
| Observation | 48 | 2.53±0.78 | 4.24±1.42 | 6
(12.50) |
| Control | 48 | 3.74±1.43 | 6.37±2.68 | 20 (41.67) |
|
t-value/χ2 |
| 5.147 | 4.866 | 10.338 |
| P-value |
| <0.001 | <0.001 |
0.001 |
Discussion
The incidence of neurological complications of heart
valve replacement surgery is higher than other surgeries, and the
degree of postoperative neurological impairment of patients is more
severe than other heart surgeries. Possible reasons for this are
the formation of brain micro-thrombi during surgery, the strong
stimulation of the operation, hypoxia due to low perfusion,
ischemia and systemic inflammatory responses (7). Related studies have shown that the
incidence of postoperative cognitive dysfunction (POCD) after heart
valve replacement can be as high as 69% (8). Heart valve replacement surgery can
cause damage to the myocardium, and different methods of anesthesia
can lead to certain stress responses during the perioperative
period, which may have some influence on the surgical outcomes. It
has recently become a clinical focus to define strategies to reduce
the neurological impairment and myocardial damage after heart valve
replacement, and how to preserve heart and brain function and
improve the success rates of surgery and prognosis (9).
Both tracheal intubation and the surgical procedure
in the perioperative period of heart valve replacement can result
in stress responses that cause HR acceleration and elevated blood
pressure (10). Studies have shown
that Dex activates the α2 receptor in the medullary dorsal motor
neuron complex, thereby reducing blood pressure (11). The results of this study showed that
there was no significant difference in perioperative HR between the
two groups (P>0.05). MAP in the observation group was lower than
that in the control group (P<0.05) after anesthesia, which was
consistent with the results of related studies. Various anesthetic
methods can bring varying degrees of stress response, resulting in
the increased release of glucagon, catecholamines and
norepinephrine and other secretions, therefore exciting the
sympathetic nervous system, causing neuroendocrine system changes
and hemodynamic instability (12).
Dex can inhibit a variety of stress responses, maintaining
hemodynamic stability. Dex has both analgesic and sedative effects,
inhibits the release of norepinephrine, reduces the tension of the
sympathetic nervous system, reduces the increased blood pressure
response during surgery, and regulates blood pressure in the
recovery period (13).
H-FABP is a low molecular weight cytosolic protein
present in cardiomyocytes, which is commonly used as an early
indicator of myocardial injury. Usually in early myocardial injury
H-FABP quickly leaks from the cardiomyocytes, and is released into
the peripheral blood, entering into the energy metabolism system by
binding with long-chain fatty acid, and providing energy for the
myocardium; its release is positively correlated with the level of
myocardial injury (14). CK-MB is
composed of the four isomers of creatine kinase, which is related
to muscle contraction and intracellular energy transport. It is
clinically used as an indicator of myocardial injury, and its
concentration level is positively correlated with the degree of
myocardial injury (15).
CTnI is an inhibitory protein in the
troponin-protoelin-modulating complex that regulates the
interaction between myofibrin and myosin and can inhibit muscle
contractions, and is rapidly released into the peripheral blood
during myocardial ischemia and reaches a peak in a few hours
(16). The results of this study
showed that H-FABP, CK-MB and cTnI were significantly increased in
the two groups at the beginning of surgery for 10 min, at the end
of surgery, 6 and 24 h after surgery, however, the increases
occurring in the observation group were significantly lower than
those in the control group (P<0.05). This may be due to the fact
that Dex can directly act on ischemic myocardium, reducing the
level of coronary norepinephrine, and reducing coronary blood flow
by activating the α2 receptor, triggering ischemic preconditioning
signals at all levels, and thereby playing a cardio-protective role
(17). In addition, Dex can inhibit
the release of catecholamines, thereby changing the balance of
myocardial oxygen supply and demand, resulting in protection of the
myocardium (17).
S100β is an acidic calcium binding protein that
exists in glial cells and is a glial cell marker (18). Under normal conditions S100β is
metabolized by the kidney without passing through the
blood-cerebrospinal fluid barrier, but when brain injury occurs,
the blood-brain barrier is compromised, and permeability increases.
Therefore, when S100β enters the blood circulation through the
blood-brain barrier, S100β concentration level can be used as a
diagnostic indicator of brain injury, and is directly related to
the recovery of neurological function in patients after surgery
(18). NSE is a dimeric enzyme often
present in the neuronal cytoplasm, and when neuronal damage occurs,
it is released into the extracellular fluid (19). The results of this study showed that
levels of S100β and NSE were higher after surgery before, but the
levels of S100β and NSE in the observation group were significantly
lower than in the control group (P<0.05), indicating that
different degrees of brain injury occurred in both groups. The
lower levels of S100β and NSE in the observation group is likely
due to the use of Dex anesthesia, which reduces cerebral oxygen
uptake rate, promoting cerebral oxygen supply and demand balance,
and thereby reducing oxidative stress injury (20); Dex anesthesia may also inhibit
neuronal discharge, resulting in anti-sympathetic nerve effect and
reduced neurotoxicity, reducing the damage to neurons, and thereby
playing a neuro-protective role (20).
MoCA and MMSE scores in the both groups 1 day after
surgery were significantly decreased, with the scores of the
control group significantly lower than those of the observation
group. Twenty-four hours after surgery, the myocardial
contractility and arrhythmia scores of the observation group were
significantly lower than those of the control group (P<0.05).
After one year of follow-up, the incidence of cardiovascular
adverse event in the observation group was significantly lower than
in the control group (P<0.05), and this may be due to patient
discomfort after surgery, excessive tension of the sympathetic
nervous system, and inflammatory reactions leading to POCD in the
control group. Dex has anti-inflammatory effects, which can inhibit
the production of inflammatory factors, thereby reducing the damage
to the nervous system, improving the level of acetylcholine,
reducing cognitive impairment and protecting the brain (20). Dex can also reduce the surgical
stress response, inhibit apoptosis, reduce myocardial ischemia and
reperfusion injury, therefore reducing intraoperative and
postoperative ventricular arrhythmia (21).
In conclusion, Dex can maintain perioperative
hemodynamic stability of patients undergoing heart valve
replacement surgery and reduce myocardial and brain damage, playing
a protective role on the heart and the brain, which is conducive to
a better prognosis.
References
|
1
|
Cavero I and Guillon JM: Safety
Pharmacology assessment of drugs with biased 5-HT(2B) receptor
agonism mediating cardiac valvulopathy. J Pharmacol Toxicol
Methods. 69:150–161. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nombela-Franco L, Eltchaninoff H, Zahn R,
Testa L, Leon MB, Trillo-Nouche R, D'Onofrio A, Smith CR, Webb J,
Bleiziffer S, et al: Clinical impact and evolution of mitral
regurgitation following transcatheter aortic valve replacement: A
meta-analysis. Heart. 101:1395–1405. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gurbuz O, Kumtepe G, Yolgosteren A, Ozkan
H, Karal IH, Ercan A and Ener S: A comparison of off- and on-pump
beating-heart coronary artery bypass surgery on long-term
cardiovascular events. Cardiovasc J Afr. 27:12016.PubMed/NCBI
|
|
4
|
Willemsen RT, Buntinx F, Winkens B, Glatz
JF and Dinant GJ: ‘RAPIDA’-study team: The value of signs, symptoms
and plasma heart-type fatty acid-binding protein (H-FABP) in
evaluating patients presenting with symptoms possibly matching
acute coronary syndrome: Background and methods of a diagnostic
study in primary care. BMC Fam Pract. 15:2032014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Patro N, Naik A and Patro IK: Differential
temporal expression of S100β in developing rat brain. Front Cell
Neurosci. 9:87–99. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tomasi R and Dossow-Hanfstingl VV:
Analgesie-, sedierungs- und delir- management auf der
intensivstation. Intensiv- und Notfallbehandlung. 39:167–172.
2015.(In German). View
Article : Google Scholar
|
|
7
|
Whitlock RP, Devereaux PJ, Teoh KH, Lamy
A, Vincent J, Pogue J, Paparella D, Sessler DI, Karthikeyan G,
Villar JC, et al: SIRS Investigators: Methylprednisolone in
patients undergoing cardiopulmonary bypass (SIRS): A randomised,
double-blind, placebo-controlled trial. Lancet. 386:1243–1253.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sigaut S, Tremey B, Ouattara A, Couturier
R, Taberlet C, Grassin-Delyle S, Dreyfus JF, Schlumberger S and
Fischler M: Comparison of two doses of tranexamic acid in adults
undergoing cardiac surgery with cardiopulmonary bypass.
Anesthesiology. 120:590–600. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Howard BT, Iles TL, Coles JA, Sigg DC and
Iaizzo PA: Reversible and irreversible damage of the myocardium:
Ischemia/reperfusion injury and cardioprotectionHandbook of Cardiac
Anatomy, Physiology, and Devices. 3rd edition. Springer
International Publishing; pp. 279–293. 2015, View Article : Google Scholar
|
|
10
|
Green JS and Tsui BC: Impact of anesthesia
for cancer surgery: Continuing Professional Development. Can J
Anaesth. 60:1248–1269. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kiliç K, Hanci V, Selek S, Sözmen M, Kiliç
N, Citil M, Yurtlu DA and Yurtlu BS: The effects of dexmedetomidine
on mesenteric arterial occlusion-associated gut ischemia and
reperfusion-induced gut and kidney injury in rabbits. J Surg Res.
178:223–232. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Jung SM and Cho CK: The effects of deep
and light propofol anesthesia on stress response in patients
undergoing open lung surgery: A randomized controlled trial. Korean
J Anesthesiol. 68:224–231. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bell MT, Agoston VA, Freeman KA, Puskas F,
Herson PS, Mares J, Fullerton DA and Reece TB: Interruption of
spinal cord microglial signaling by alpha-2 agonist dexmedetomidine
in a murine model of delayed paraplegia. J Vasc Surg. 59:1090–1097.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Glatz JF and Renneberg R: Added value of
H-FABP as plasma biomarker for the early evaluation of suspected
acute coronary syndrome. Clin Lipidol. 9:205–220. 2014. View Article : Google Scholar
|
|
15
|
Banning A, Musumeci F, Penny W and Tovey
JA: Reference intervals for cardiac troponin T, creatine kinase and
creatine kinase-MB isoenzyme following coronary bypass graft
surgery. Ann Clin Biochem. 33:561–562. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sandoval Y, Smith SW, Schulz KM, Murakami
MM, Love SA, Nicholson J and Apple FS: Diagnosis of type 1 and type
2 myocardial infarction using a high-sensitivity cardiac troponin I
assay with sex-specific 99th percentiles based on the third
universal definition of myocardial infarction classification
system. Clin Chem. 61:657–663. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Funai Y, Pickering AE, Uta D, Nishikawa K,
Mori T, Asada A, Imoto K and Furue H: Systemic dexmedetomidine
augments inhibitory synaptic transmission in the superficial dorsal
horn through activation of descending noradrenergic control: An in
vivo patch-clamp analysis of analgesic mechanisms. Pain.
155:617–628. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pfeifer R, Franz M and Figulla HR:
Hypothermia after cardiac arrest does not affect serum levels of
neuron-specific enolase and protein S-100b. Acta Anaesthesiol
Scand. 58:1093–1100. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Benedict C, Cedernaes J, Giedraitis V,
Nilsson EK, Hogenkamp PS, Vågesjö E, Massena S, Pettersson U,
Christoffersson G, Phillipson M, et al: Acute sleep deprivation
increases serum levels of neuron-specific enolase (NSE) and S100
calcium binding protein B (S-100B) in healthy young men. Sleep.
37:195–198. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun L, Guo R and Sun L: Dexmedetomidine
for preventing sevoflurane-related emergence agitation in children:
A meta-analysis of randomized controlled trials. Acta Anaesthesiol
Scand. 58:642–650. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen S, Hua F, Lu J, Jiang Y, Tang Y, Tao
L, Zou B and Wu Q: Effect of dexmedetomidine on myocardial
ischemia-reperfusion injury. Int J Clin Exp Med. 8:21166–21172.
2015.PubMed/NCBI
|















