Introduction
Crohn disease (CD) and ulcerative colitis (UC) are
chronic relapsing gastrointestinal disorders, two main components
of inflammatory bowel disease (IBD) (1). To date, the etiology of IBD is unknown;
however, various inflammatory reactions constantly stimulate the
mucosal and systemic immune systems and are involved in the
inflammatory cascade (2).
Approximately 25% of IBD cases occur before the age of 20 years,
and 4% occur in persons younger than 5 years of age (3). In the treatment of IBD, a variety of
pharmacological agents that target the inflammatory process are
effective in controlling disease in patients and in sustaining
symptomatic remission for prolonged periods, including tumor
necrosis factor blockers, probiotics and corticosteroids (4).
The complement system is widely considered to
function to protect the host against invading microorganisms
(5). However, previous studies
investigating complement system activation have demonstrated that
it may serve an injurious role in the pathogenesis of a number of
inflammatory and immunological diseases (6,7).
Complement activation products include complement component (C) 3a,
C4a, C5a and C5b-9 and the membrane attack complex. C5a is an
anaphylatoxin that exhibits chemotactic activities and promotes
oxidative bursts, phagocytosis and the release of granule enzymes
from neutrophils, monocytes and macrophages (7).
A study involving C5a knockout mice has demonstrated
that C5a serves a critical role in inflammatory diseases (8). Several inflammatory diseases have been
attributed to the ability of C5a to bind with high-affinity C5a
receptors (9,10), including the C5a anaphylatoxin
chemotactic receptor and C5L2, which is expressed on numerous
myeloid and non-myeloid cells (11).
Inhibition of C5a using a C5a antagonist has been considered as an
intervention that may protect against excessive C5a production and
the associated systemic inflammatory response impairment (12). Antibodies are the primary antagonists
that have been used to inhibit the biological activity of C5a
(12). However, the disadvantages of
using protein-based C5a inhibitors include an increased risk of
immunogenicity due to their chronic use, as well as the cost of
production, resulting in expensive therapies for patients (13). Similar to antibodies, aptamers can be
designed to bind to multiple targets. Aptamers exhibit a high
affinity for their targets, as their dissociation constants, which
are comparable to those of certain monoclonal antibodies, are
typically between the µM and low pM range (14,15).
Therefore, the present study aimed to investigate the effect of C5a
in a mouse model of acute 2,4,6-trinitrobenzene sulfonic acid
(TNBS)-induced experimental colitis. In addition, macroscopic and
histological parameters, and the diversification of inflammatory
cytokine expression were analyzed. The results suggest that C5a
serves a critical role in inflammation.
Materials and methods
Animals and grouping
A total of 24 adult female, 10-week-old BALB/c mice
(weight, 20–24 g; Laboratory Animal Center of Jilin University,
Jilin, China) were employed in the present study. All animals were
maintained in a temperature-controlled environment (temperature,
23°C, humidity, 50–55%), were group-housed (2 mice per cage) in a
pathogen-free facility with a 12 h light/dark cycles and were
provided mouse chow and water ad libitum. The animals were
handled in accordance with the Welfare and Ethics of Laboratory
Animals of China guidelines, and protocols were approved by
Military Veterinary Institute Animal Investigational Committee
(Changchun, China), and were performed in accordance with the Guide
for the Care and Use of Laboratory Animals published by the
Ministry of Health of China.
The mice were randomly and equally allocated to the
following three groups (8 mice/group): TNBS-induced colitis with no
treatment, TNBS-induced colitis with C5a aptamer treatment
(TNBS+C5a aptamer), and a negative control group. In the
experimental colitis groups, colitis was induced with a single dose
of intrarectal injections of 2.75 mg TNBS. A total of 2 days later,
8 mice from the TNBS-induced colitis group were intraperitoneally
injected with 10 mg/kg C5a aptamers. The C5a aptamers coupled with
40 kDa polyethylene glycol were obtained from Ms Man Chen (The
Institute of Military Veterinary, Academy of Military Medical
Sciences, Changchun, China). In the negative control group, 50%
ethanol was intrarectally instilled into the colon. The weight and
behavior changes were observed for 4 days after treatment.
TNBS-induced colitis
Following fasting for 24 h, the mice were
anesthetized with an intraperitoneal injection of pentobarbital
sodium (50 mg/kg, cat no. 76744; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany). Subsequent to anesthesia, the mice were
intrarectally administered with 70 µl TNBS (2.75 mg; Sigma-Aldrich;
Merck KGaA) dissolved in 50% ethanol. Mice in the negative control
group received 70 µl ethanol (50%) under the same conditions. A
total of 8 mice of the TNBS-induced colitis group were
intraperitoneally injected with C5a aptamers 48 h after induction
of colitis. The weight of all the mice was recorded every morning
for 6 days by electronic balance (0.01 accuracy). On the 7th
morning, blood samples from mice in each group were collected from
the retro-orbital plexus. Serum samples obtained from three groups
of mice blood were centrifuged at 1,000 × g for 3 min at 4°C.
Determination of C5a serum
concentration
The quantitative sandwich enzyme immunoassay
technique was performed using the Mouse Complement fragment 5a,
ELISA kit (cat no. CSB-E08514m; Cusabio Biotech Co., Ltd., Wuhan,
China) according to the manufacturer's protocol. The antibodies
specific to C5a were used to pre-coat wells of a microplate. A
total of 200 µl mouse serum samples and standards from the kit were
pipetted into the wells, and C5a bound to the immobilized
antibodies. Following the removal of all unbound substances, a
biotin-conjugated antibody directed against C5a was added to the
wells. Subsequent to washing the wells with wash buffer (25X) from
the Mouse Complement fragment 5a ELISA kit, avidin-conjugated
horseradish peroxidase was added to the wells. The plate was washed
to remove any unbound avidin-enzyme reagent, and a substrate
solution was added to the wells. The subsequent extent of color
change was proportional to the quantity of C5a present in the
sample. Color development was then inhibited and the microplate was
read at 450 nm within 5 min using a microplate reader.
Quantitative analysis of mouse
cytokine levels
To quantify the concentration of cytokines in serum,
the Quantibody® Mouse Inflammation Array Q1 kit (cat no.
QAM-INF-1-1; Raybiotech, Inc., Norcross, GA, USA) was used
according to the manufacturer's protocol. Similar to a traditional
sandwich-based ELISA, pairs of cytokine-specific antibodies were
used for detection. A variety of cytokine antibodies were coated on
glass slides, and following incubation with the sample, any target
cytokines present within the sample bound to antibodies in the
solid surface. A second biotin-labeled detection antibody was
subsequently added, which recognized a different isotope on the
target cytokine. The antibody-cytokine-antibody-biotin complex was
visualized through the addition of the streptavidin-labeled cyanine
3 equivalent dye using a laser scanner (535 nm). By comparing the
signals produced by unknown samples with the signal curve produced
by the standards, the cytokine concentration in the samples was
determined.
Histopathology
Colon specimens from mice in the three groups were
collected 7 days after the establishment of the model and fixed in
10% buffered formalin for 2 days at 23°C, embedded in paraffin and
then cut into 3-µm sections. The sections were stained with
hematoxylin for 5 min and eosin for 1 min at 23°C and observed at a
magnification of ×132 using a light microscope.
Immunohistochemistry
Inflammatory cells in colon tissue samples were
detected by assessing the level of the inflammatory marker,
myeloperoxidase (MPO), by immunohistochemical analysis. The method
of fixing and slicing tissue samples was the same as previously
described. The sections were then deparaffinized and rehydrated via
standard protocols according to the manufacturer's guidelines of
UltraSensitive™ SP IHC kit (MXR® Corporation, Fuzhou,
China) and 3% H2O2 was subsequently added to
the sections until they were covered. Bovine serum albumin (3%) was
then added to the sections to inhibit non-specific binding. Tissue
sections were incubated with a 500-fold dilution of primary
anti-MPO rabbit monoclonal antibody (cat no. RB-373-R7; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) at 37°C for 24 h. This
was followed by incubation with streptavidin peroxidase-conjugated
biotinylated goat anti-rabbit IgG secondary antibody
(UltraSensitive™ SP IHC kit). Tissue sections were subsequently
stained with a 3,3′-diaminobenzidine for 15 min at room temperature
(Sigma-Aldrich; Merck KGaA) and counterstained with Mayer's
hematoxylin for 5 min at 23°C. The result from stained tissue
sections observed at a magnification of ×132 using a light
microscope.
Statistical analysis
Statistical analyses were performed using GraphPad
Prism software (version 6.0; GraphPad Software, Inc., La Jolla, CA,
USA). The results were expressed as the mean ± standard deviation,
and were analyzed by a one-way analysis of variance followed by
Tukey's post hoc. P<0.0001 was considered to indicate a
statistically significant difference (Student's t-test). All
experiments were repeated three times.
Results
Aptamer treatment attenuates
TNBS-induced weight loss, lethargy and hoarding
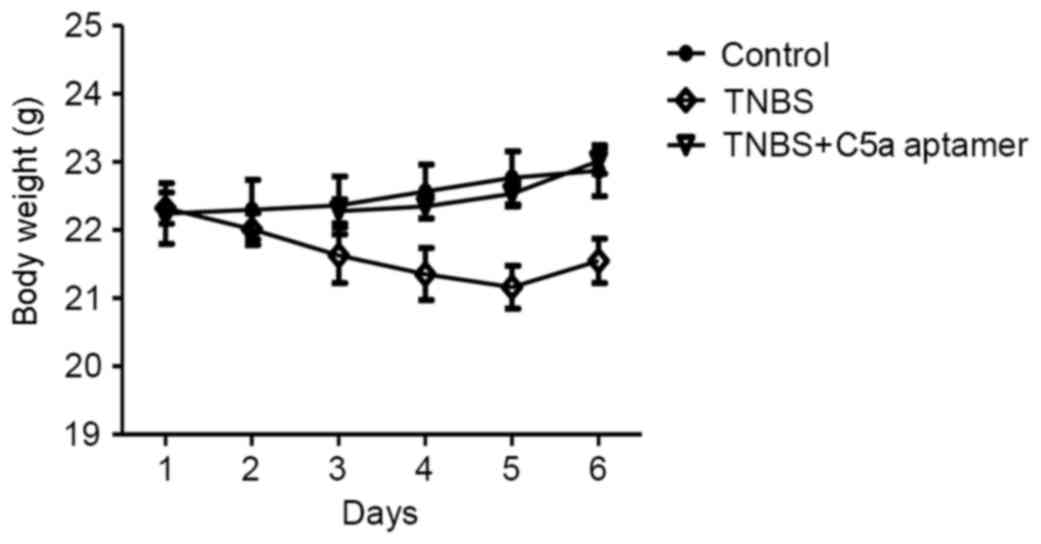
Mice in the TNBS group exhibited a marked reduction
in weight and appetite decline (weight was reduced from 22.32±0.23
to 21.55±0.32 g) when compared with the control (weight changed
from 22.24±0.45 to 22.87±0.37 g) and TNBS+C5a aptamer group (weight
changed from 22.28±0.18 to 23.02±0.19 g; Fig. 1). Mice in the TNBS group compared
with the control exhibited greater lethargy, were more crowded
together and half-closed their eyes or were more immobile (data not
shown).
C5a aptamer treatment significantly
attenuates the TNBS-induced increase in serum C5a levels
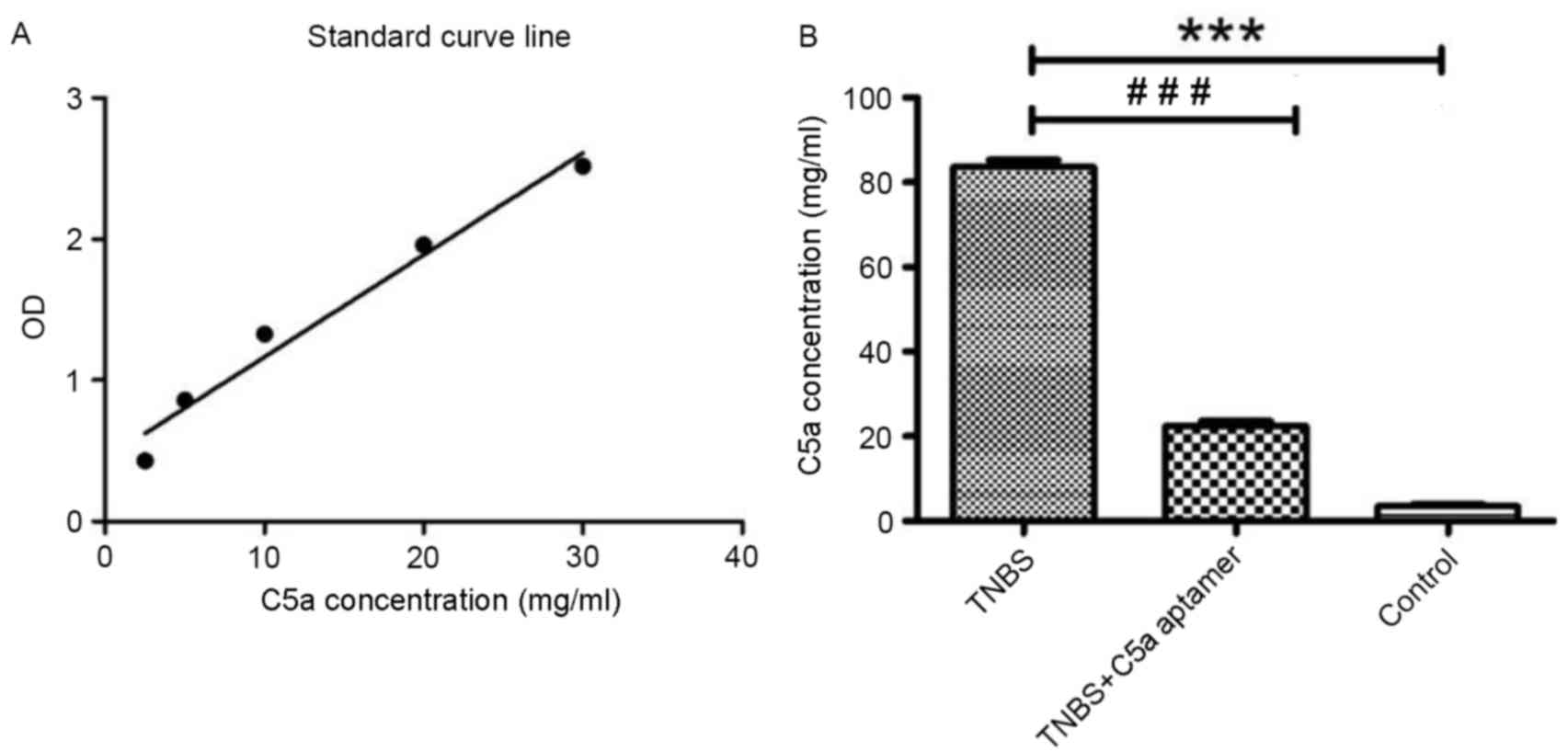
In order to determine the concentration of C5a in
mouse serum samples, a standard curve of C5a concentration vs.
absorbance was generated (r2=0.97; Fig. 2A). The concentration of C5a in the
serum of mice from the TNBS group (82.93±3.31 mg/ml) was
significantly higher when compared with those from the TNBS+C5a
aptamer (24.07±4.10 mg/ml) and control (2.94±0.90 mg/ml) groups
(P<0.0001; Fig. 2B).
Treatment with C5a aptamers reverses
the TNBS-induced damage to epithelial, submucosal and lamina
propria layers
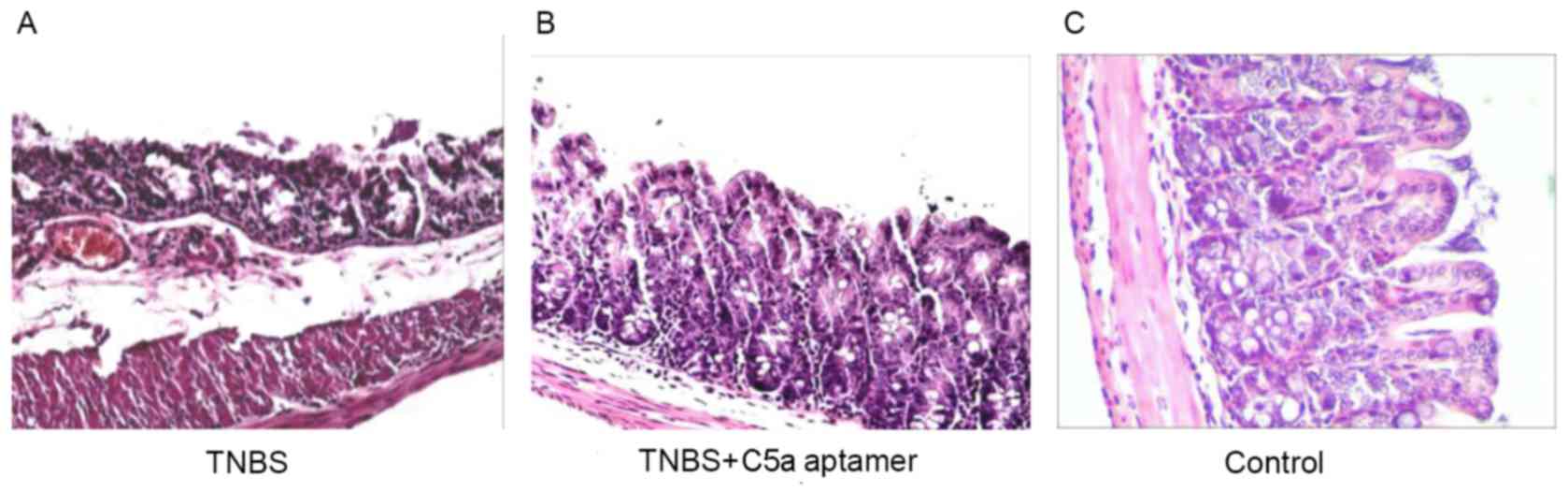
Colon tissue samples from mice in all groups were
histopathologically analyzed by hematoxylin and eosin staining. In
the TNBS group, the epithelial layer was damaged and the villi were
absent; there was also evidence of edema in the submucosa and
lamina propria, with congested and dilated blood vessels (Fig. 3A). By contrast, no evidence of
congestion and vasodilation in the lamina propria was observed in
the C5a aptamer-treated group (Fig.
3B). The histological appearance of the colonic walls from the
mice in the control group was normal (Fig. 3C).
Treatment with C5a aptamers attenuates
the TNBS-induced increase in MPO expression
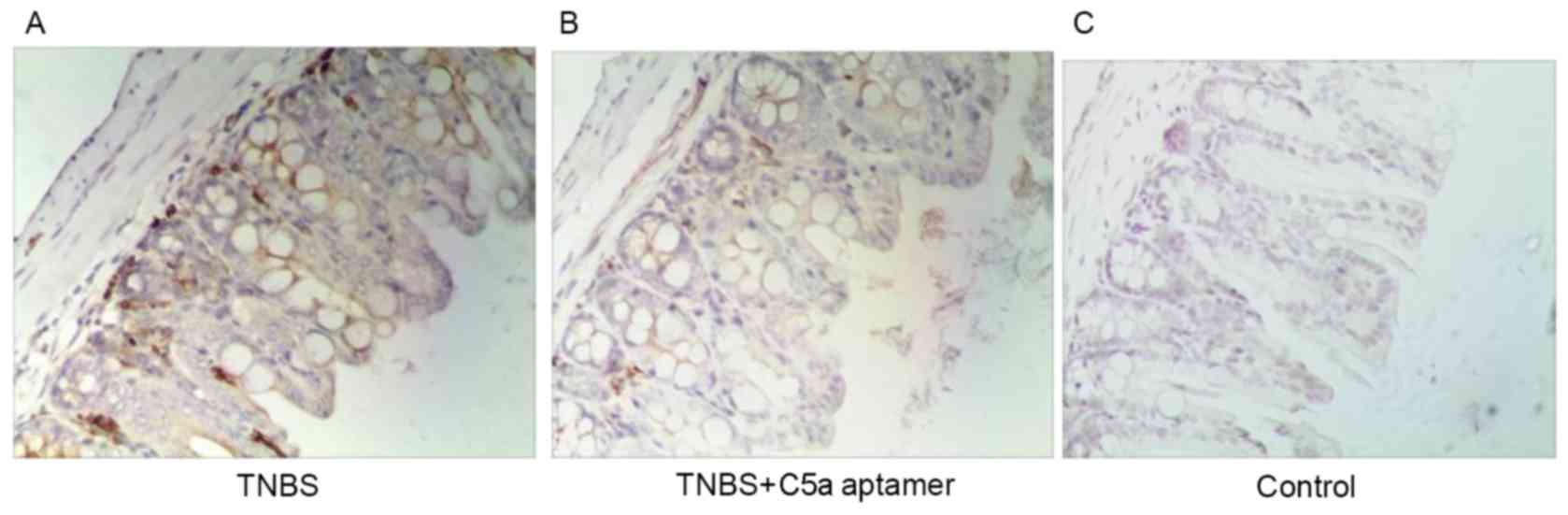
MPO expression was observed in lymphocytes within
the colonic mucosal lamina propria in mice from the TNBS group
(Fig. 4A). However, reduced MPO
expression was observed in the colons of mice from the TNBS+C5a
aptamer group (Fig. 4B), whereas no
MPO expression was observed in colon tissue samples from mice in
the control group (Fig. 4C). These
results indicate that C5a aptamers exhibit therapeutic effects in
IBD.
C5a treatment altered the level of
cytokines in the serum
The level of the majority of cytokines in mice with
TNBS-induced colitis was observed to increase when compared with
mice in the control group (Table I).
The level of macrophage inflammatory protein (MIP)-1γ was decreased
in the TNBS group compared with the control and TNBS+C5a aptamer
groups, whereas the serum concentration of B lymphocyte
chemoattractant (BLC) and interferon-γ (IFN-γ) in the TNBS group
was >25-fold higher when compared with the TNBS+C5a aptamer
group (Table I). Following treatment
with C5a aptamer, the concentration of ~50% of the inflammatory
cytokines decreased to a level similar to that observed in the
control group. By contrast, interleukin (IL)-7, IL-10 and IL-21,
platelet-derived growth factor-inducible protein KC (KC), MIP-1α,
MIP-1γ and T-cell activation protein-3 (TCA-3) concentrations in
mice from the TNBS+C5a aptamer group were higher compared with the
TNBS and control groups (Table
I).
 | Table I.Level of inflammatory cytokines. |
Table I.
Level of inflammatory cytokines.
| Cytokine (pg/ml) | Control | TNBS | TNBS+C5a
aptamers |
|---|
| BLC | 0.0 | 86,452.2 | 1,936.7 |
| Eotaxin | 20.3 | 2,070.4 | 327.1 |
| Eotaxin-2 | 0.0 | 2,597.2 | 507.2 |
| IFNγ | 611.7 | 4,771.8 | 170.8 |
| IL-6 | 0.0 | 189.6 | 17.3 |
| IL-7 | 0.0 | 9.4 | 824.0 |
| IL-10 | 0.0 | 22.1 | 168.1 |
| IL-12p70 | 128.3 | 14,934.7 | 1,269.5 |
| IL-21 | 0.0 | 393.7 | 6,659.0 |
| KC | 12.5 | 358.0 | 696.5 |
| MCP-1 | 0.0 | 107.0 | 30.7 |
| MCP-5 | 20.6 | 575.0 | 131.8 |
| MCSF | 0.0 | 12.1 | 0.0 |
| MIG | 1,204.0 | 8,552.2 | 2,931.8 |
| MIP-1α | 0.0 | 54.8 | 1,831.9 |
| MIP-1γ | 969.3 | 37.4 | 1,672.2 |
| TARC | 0.0 | 24.2 | 10.3 |
| TCA-3 | 0.3 | 3.3 | 11.0 |
Discussion
IBD is used to describe two chronic diseases that
lead to inflammation of the intestines, including Crohn's disease
and ulcerative colitis (4). Although
the diseases share common features, there are important
differences. The results of previous studies suggest that specific
C3 and mannose-binding lectin genotypes, as well as associated
multi-gene regions that encode complement molecules, are associated
with IBD (4,16). Despite these observations, complement
molecules have not emerged as a cause of monogenic IBD (16,17).
Instead, hyperactivation of the complement system or the lack of
regulators, may underlie chronic inflammatory diseases such as IBD
(18,19).
The complement system is considered to serve a
central role in the innate immune system, which functions as a host
defense mechanism against invading pathogens and in the clearance
of potentially damaging cell debris (20–23).
However, it has been confirmed that complement activation is also
involved in the pathogenesis of several inflammatory and
immunological diseases (24). A
previous study has indicated that the establishment of a
TNBS-induced experimental colitis in the intestinal mucosa was
associated with the increased production of the pro-inflammatory
cytokine, IL-6, and decreased production of the anti-inflammatory
cytokine, IL-10 (25). The present
study demonstrated that the C5a aptamers reduced the level of
specific cytokines in the serum of mice with TNBS-induced colitis,
including BLC, eotaxin, eotaxin-2, IL-6, IFNγ, IL-12p70, monocyte
chemoattractant protein 1 and 5, macrophage colony-stimulating
factor, monokine induced by IFN-γ, and thymus and
activation-regulated chemokine, when compared with the TNBS group
and control group. By contrast, treatment with a C5a aptamer
increased the production of IL-7, IL-10, KC, IL-21, MIP-1α, MIP-1γ
and TCA-3 in mice with TNBS-induced colitis. Colitis is
characterized by an imbalance in pro-inflammatory and
anti-inflammatory mediators, which may result in an exaggerated
pro-inflammatory response, anti-inflammatory response syndrome,
immunosuppression, apoptosis, organ dysfunction or clinical
manifestations such as weight loss (26). The effect of C5a aptamer treatment in
a mouse model of colitis was examined in the present study.
Following C5a aptamer treatment, the body weight and levels of
inflammatory cytokines in mice with colitis decreased and were
closer to the values observed in mice from the control group.
Currently, immunomodulators are commonly used to
treat IBD. The side effects of these agents include nephrotoxicity,
nausea, tremor, headaches and gingival hyperplasia (27). Oligonucleotides have emerged as a
promising class of biopharmaceuticals. Aptamers constitute a novel
class of oligonucleotides that have gained therapeutic importance
(28). In the present study, the
importance of C5a in IBD was addressed by treating mice with
TNBS-induced colitis using C5a aptamers. Aptamers are selected from
random-sequence oligonucleotide pools to bind a wide range of
molecules, ranging from small inorganic molecules to
biomacromolecules with affinities and specificities that are
comparable to antibodies (29). Some
aptamers have entered the clinical trials process (30). The greatest success in the
therapeutic application of an aptamer, which was approved by the US
Food and Drug Administration, was an anti-vascular endothelial
growth factor aptamer for the treatment of age-associated macular
degeneration (31). A number of
aptamers are currently being evaluated in clinical trials (32). In addition, numerous aptamers have
exhibited efficacy in tissue culture experiments and animal models,
including those targeting thrombin, factor IXa, IFN-γ and epidermal
growth factor receptor (33–36). In the current study, the mouse C5a
aptamers were selected to bind to the C5a protein, and were
revealed to markedly reduce inflammation in mice with TNBS-induced
colitis. Therefore, C5a aptamers may present a potential
therapeutic candidate to treat patients with IBD.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 31402167), the ‘3551
Optics Valley Schema for Talents’ by the Wuhan East Lake Hi-tech
Development Zone [grant no. J(Q)H20140716-006], and the Achievement
Transformation Program by the Wuhan Junke Boyuan Biosciences Corp
(grant no. JH20140627-002).
References
|
1
|
Xavier RJ and Podolsky DK: Unravelling the
pathogenesis of inflammatory bowel disease. Nature. 448:427–434.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sartor RB: Pathogenesis and immune
mechanisms of chronic inflammatory bowel diseases. Am J
Gastroenterol. 92 12 Suppl:5S–11S. 1997.PubMed/NCBI
|
|
3
|
Saeed SA and Kugathasan S: Epidemiology of
pediatric inflammatory bowel disease. Pediatr Inflam Bowel Dis.
71–86. 2017.
|
|
4
|
Singh UP, Singh NP, Busbee B, Guan H,
Singh B, Price RL, Taub DD, Mishra MK, Nagarkatti M and Nagarkatti
PS: Alternative medicines as emerging therapies for inflammatory
bowel diseases. Int Rev Immunol. 31:66–84. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thiel S, Vorup-Jensen T, Stover CM,
Schwaeble W, Laursen SB, Poulsen K, Willis AC, Eggleton P, Hansen
S, Holmskov U, et al: A second serine protease associated with
mannan-binding lectin that activates complement. Nature.
386:506–510. 1997. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mastellos DC, Ricklin D, Hajishengallis E,
Hajishengallis G and Lambris JD: Complement therapeutics in
inflammatory diseases: Promising drug candidates for C3-targeted
intervention. Mol Oral Microbiol. 31:3–17. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo RF and Ward PA: Role of C5a in
inflammatory responses. Annu Rev Immunol. 23:821–852. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Czermak BJ, Sarma V, Bless NM, Schmal H,
Friedl HP and Ward PA: In vitro and in vivo dependency of chemokine
generation on C5a and TNF-alpha. J Immunol. 162:2321–2325.
1999.PubMed/NCBI
|
|
9
|
Mollnes TE, Brekke OL, Fung M, Fure H,
Christiansen D, Bergseth G, Videm V, Lappegård KT, Köhl J and
Lambris JD: Essential role of the C5a receptor in E coli-induced
oxidative burst and phagocytosis revealed by a novel
lepirudin-based human whole blood model of inflammation. Blood.
100:1869–1877. 2002.PubMed/NCBI
|
|
10
|
Dang L, He L, Wang Y, Xiong J, Bai B and
Li Y: Role of the complement anaphylatoxin C5a-receptor pathway in
atopic dermatitis in mice. Mol Med Rep. 11:4183–4189. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bamberg CE, Mackay CR, Hyun L, Zahra D,
Jackson J, Lim YS, Whitfeld PL, Craig S, Corsini E, Lu B, Gerard C
and Gerard NP: The C5a receptor (C5aR) C5L2 is a modulator of
C5aR-mediated signal transduction. J Biol Chem. 285:7633–7644.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang Y, Rollins SA, Madri JA and Matis LA:
Anti-C5 monoclonal antibody therapy prevents collagen-induced
arthritis and ameliorates established disease. Proc Natl Acad Sci
USA. 92:pp. 8955–8959. 1995, View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Makrides SC: Therapeutic inhibition of the
complement system. Pharmacol Rev. 50:59–87. 1998.PubMed/NCBI
|
|
14
|
Kaur G and Roy I: Therapeutic applications
of aptamers. Expert Opin Investig Drugs. 17:43–60. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Parashar A: Aptamers in therapeutics. J
Clin Diagn Res. 10:BE01–BE06. 2016.PubMed/NCBI
|
|
16
|
Jain U, Otley AR, Van Limbergen J and
Stadnyk AW: The complement system in inflammatory bowel disease.
Inflamm Bowel Dis. 20:1628–1637. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rioux JD, Silverberg MS, Daly MJ,
Steinhart AH, McLeod RS, Griffiths AM, Green T, Brettin TS, Stone
V, Bull SB, et al: Genomewide search in Canadian families with
inflammatory bowel disease reveals two novel susceptibility loci.
Am J Hum Genet. 66:1863–1870. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Speidl WS: Atherosclerosis and complement:
Anaphylatoxin C5a as a new risk marker and therapeutic target. Clin
Lipidol. 6:123–126. 2011. View
Article : Google Scholar
|
|
19
|
Holers VM: Complement and its receptors:
New insights into human disease. Annu Rev Immunol. 32:433–859.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gasque P: Complement: A unique innate
immune sensor for danger signals. Mol Immunol. 41:1089–1098. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dempsey PW, Allison ME, Akkaraju S,
Goodnow CC and Fearon DT: C3d of complement as a molecular
adjuvant: Bridging innate and acquired immunity. Science.
271:348–350. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ogden CA and Elkon KB: Role of complement
and other innate immune mechanisms in the removal of apoptotic
cells. Curr Dir Autoimmun. 9:120–142. 2006.PubMed/NCBI
|
|
23
|
Woodruff TM, Ager RR, Tenner AJ, Noakes PG
and Taylor SM: The role of the complement system and the activation
fragment C5a in the central nervous system. Neuromolecular Med.
12:179–192. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Guo RF, Riedemann NC and Ward PA: Role of
C5a-C5aR interaction in sepsis. Shock. 21:1–7. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee T, Lee E, Irwin R, Lucas PC, McCabe LR
and Parameswaran N: β-Arrestin-1 deficiency protects mice from
experimental colitis. Am J Pathol. 182:1114–1123. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Margonis GA, Christoloukas N, Antoniou E,
Arkadopoulos N, Theodoropoulos G, Agrogiannis G, Pikoulis E,
Patsouris ES, Zografos GC and Papalois AE: Effectiveness of
sildenafil and U-74389G in a rat model of colitis. J Surg Res.
193:667. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Afif W and Loftus EV Jr: Safety profile of
IBD therapeutics: Infectious risks. Gastroenterol Clin North Am.
38:691–709. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Keefe AD, Pai S and Ellington A: Aptamers
as therapeutics. Nat Rev Drug Discov. 9:537–550. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hermann T and Patel DJ: Adaptive
recognition by nucleic acid aptamers. Science. 287:820–825. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nimjee SM, Rusconi CP and Sullenger BA:
Aptamers: An emerging class of therapeutics. Annu Rev Med.
56:555–583. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cunningham ET Jr, Adamis AP, Altaweel M,
Aiello LP, Bressler NM, D'Amico DJ, Goldbaum M, Guyer DR, Katz B,
Patel M, et al: A phase II randomized double-masked trial of
pegaptanib, an anti-vascular endothelial growth factor aptamer, for
diabetic macular edema. Ophthalmology. 112:1747–1757. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bunka DH, Platonova O and Stockley PG:
Development of aptamer therapeutics. Curr Opin Pharmacol.
10:557–562. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dobrovolsky AB, Titaeva EV, Khaspekova SG,
Spiridonova VA, Kopylov AM and Mazurov AV: Inhibition of thrombin
activity with DNA-aptamers. Bull Exp Biol Med. 148:33–36. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rusconi CP, Scardino E, Layzer J, Pitoc
GA, Ortel TL, Monroe D and Sullenger BA: RNA aptamers as reversible
antagonists of coagulation factor IXa. Nature. 419:90–94. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kubik MF, Bell C, Fitzwater T, Watson SR
and Tasset DM: Isolation and characterization of 2′-fluoro-,
2′-amino-, and 2′-fluoro-/amino-modified RNA ligands to human
IFN-gamma that inhibit receptor binding. J Immunol. 159:259–267.
1997.PubMed/NCBI
|
|
36
|
Li N, Nguyen HH, Byrom M and Ellington AD:
Inhibition of cell proliferation by an anti-EGFR aptamer. PLoS One.
6:e202992011. View Article : Google Scholar : PubMed/NCBI
|