Introduction
Endometriosis is defined as the growth of functional
endometrial tissue outside the uterine cavity, and is characterized
by the presence of chronic pelvic pain and infertility in females
of a reproductive age (1). This
disease affects women in the 15–49 years age group and presents a
serious clinical problem with reproductive consequences. Current
treatments for endometriosis, including surgery and hormonal
therapy, are often insufficient, and result in a high rate of
relapse and various side effects, such as hepatic injury and
osteoporosis (2). Furthermore, the
pathogenesis of endometriosis has not yet been fully elucidated;
however, the most widely-accepted hypothesis involves retrograde
menstruation, which was initially proposed by Sampson in 1927
(3).
Recent studies have demonstrated the importance of
neovascularization within the peritoneal cavity for endometriosis
pathogenesis. Endometriotic lesions require an adequate blood
supply to survive in their ectopic sites (4). Therefore, angiogenesis has become a
promising target candidate for endometriosis therapy. Vascular
endothelial growth factor (VEGF) is a heparin-binding glycoprotein
with potent angiogenic, endothelial cell-specific mitogenic and
vascular permeability activities (5). The concentration of VEGF is increased
in human endometrium, and it may be important in physiological and
pathological angiogenesis (6). VEGF
expression varies among different types of lesions, with the early
and highly vascularized red lesions exhibiting a greater VEGF
expression in comparison with the later-stage and more inactive
black lesions (7). In eutopic as
well as ectopic endometria, no significant cyclic variations were
observed throughout the cycle (7).
However, VEGF content was found to be higher in the eutopic
glandular epithelium of women with endometriosis during the late
secretory phase, possibly suggesting a greater chance of
implantation (7). Following the
attachment phase, the high VEGF levels may provoke an increase in
the subperitoneal vascular network, facilitating implantation and
viability in the retroperitoneal space (8).
Neovascularization is considered to be a key factor
in the progression of endometriosis; this pathological condition
has been defined as an angiogenic disease (9). The angiopoietin (Ang) family of growth
factors is known to mainly promote vessel maturation and remodeling
(10). Ang-2 expression in the
ectopic and eutopic endometria serves an important role in the
pathogenesis and development of endometriosis (11). Ang-2 upregulates the production of
proteases, including matrix metalloproteinases (MMPs) (12) in the presence of VEGF. Increased
proteolytic activity due to MMPs may help to explain the invasive
factors that result in endometriosis (13). Furthermore, Ang-2 promotes vessel
sprouting in conjunction with VEGF by blocking the stabilization
signal of Ang-1 at the level of Ang-1's endothelial cell-specific
tyrosine kinase receptor (Tie2) (14). This suggests that there is an
association between VEGF and Ang-2.
In traditional Chinese medicine (TCM), endometriosis
is considered to be a syndrome caused by deficiency of the kidney
with blood stasis (15). Treatment
of endometriosis using TCM is common in China, and considerable
research has been conducted on the role of such formulas in
promoting fertility, alleviating pain and preventing relapse
(16,17). Hua Yu Xiao Zheng (HYXZ) decoction
(Table I), a Chinese medicinal
formula for endometriosis treatment, is prescribed based on our
clinical experience. HYXZ contains Salvia miltiorrhizae
radix (10.7%), Morindae officinalis radix (7.2%), Panax
notoginseng radix (6.4%), Semen coicis (17.9%),
Fritillariae thunberg bulbus (7.2%), Spica prunellae
(10.7%), Polygoni aviculare herba (10.7%), Panta rhei
radix (3.6%), Alternaria dianthi herba (10.7%), Corydalis
sp. rhizome (7.1%), Hirudo sp. (2.1%), Typhae sp.
pollen (4.3%) and Draconis sanguis (1.4%). In clinical practice, it
has been observed that HYXZ can alleviate endometriosis-associated
symptoms, including severe dysmenorrhea, dyspareunia, menstrual
irregularities and infertility. However, the mechanisms underlying
the effect of HYXZ decoction on endometriosis remain unknown.
 | Table I.Composition of Hua Yu Xiao Zheng
decoction. |
Table I.
Composition of Hua Yu Xiao Zheng
decoction.
| Herb | Component | Grams |
|---|
| Danshen | Salviae
miltiorrhizae Bunge, root | 15 |
| Bajitian | Morinda
officinalis How, root | 10 |
| Sanqi | Panax
notoginseng (Burk.) F.H. Chen, root | 9 |
| Yiyiren | Coix
lacryma-jobi L. var. ma-yuen (Roman.) Stapf, seed | 25 |
| Zhebeimu | Fritillaria
thunbergii Miq., tuber | 10 |
| Xiakucao | Prunella
vulgaris L., cluster | 15 |
| Bianxu | Polygonum
aviculare L., overground part | 15 |
| Dahuang | Rheum
palmatum L., root | 5 |
| Qumai | Dianthus
superbus L., overground part | 15 |
| Yanhusuo | Corydalis
yanhusuo W.T. Wang, tuber | 10 |
| Shuizhi | Whitmania
pigra Whitman, whole body | 3 |
| Puhuang | Typha
angustifolia L., pollen | 6 |
| Xuejie | Daemonorops
draco Bl., resin | 2 |
| Total amount |
| 140 |
In the present study, the effects of HYXZ on the
expression levels of VEGF and Ang-2 in endometriosis tissues were
investigated in a rat model, in an attempt to clarify the
mechanisms underlying the action of HYXZ in the treatment of
endometriosis.
Materials and methods
Animals
Specific-pathogen-free grade female, nonpregnant
Sprague-Dawley rats (n=108; body weight, 190–230 g) were obtained
from Vital River Laboratory Animal Technology Co., Ltd (Beijing,
China; SCXK 2012–0001). Animals were raised at a constant
temperature of 25°C, 50% humidity and 12-h light/dark cycles.
Standard rat feed and water were provided ad libitum. All
rats were acclimatized for 7 days to ascertain health before the
experiments were performed. All procedures described in the present
study were reviewed and approved by the Ethical Committee of
Capital Medical University (Beijing, China; approval no.
AEEI-2015-100).
Preparation of formula
All the medicinal plants used to prepare the HYXZ
decoction were obtained from the Pharmacy Department of Dong Fang
Hospital of Beijing University of Chinese Medicine (Beijing,
China). The quality of the raw herbs was controlled according to
the Pharmacopoeia of the People's Republic of China (18). The aqueous extract of HYXZ was
prepared according to a previously described procedure (19). Briefly, the mixture of herbs listed
in Table I was macerated in
distilled water for 0.5 h. Following this, the herbs were decocted
for 0.5 h, and the residue was then decocted again for 0.5 h. The
final extract was subsequently filtered, combined and concentrated
to 2.1 g/ml (crude dosage) by heating.
Rat model of endometriosis
The animal model was established by
autotransplantation of endometrial tissues, as previously reported
by Zhang et al (20).
Briefly, 96 rats were anesthetized with intraperitoneal
administration of 10% chloral hydrate (m/v) at a dose of 0.35
ml/100 g body weight. Prior to surgery, the abdominal skin was
disinfected with 75% ethanol and the abdominal cavity was opened.
The uterine vessel was ligated with 5-0 polypropylene sutures. The
right uterine horn was removed and placed in a Petri dish
containing phosphate-buffered saline (PBS) at 37°C. Approximately 1
cm of the uterine horn was cut along the longitudinal axis, and
then divided in half. Two squares of 5×5 mm open uterus were
prepared. Each endometrium segment was fixed with 6-0 sutures to
the peritoneal side of the bilateral abdominal wall with the
endometrium facing the abdominal wall. Subsequently, the abdominal
muscle and skin was closed using 4-0 polypropylene sutures. The
control group underwent sham surgery, consisting solely of the
unilateral hemihysterectomy without autotransplantation of
endometrial tissues. Following surgery, all rats were treated with
cephalosporin (0.1 g; Tianjin Pharmaceutical Holdings Gencom
Pharmacy Co., Ltd., Tianjin, China) by intraperitoneal injection
for 3 days. After a recovery period of 21 days, the endometriosis
model rats underwent a second exploratory laparotomy to examine
whether the models of experimental endometriosis had been
successfully established, which was determined by observation of
red/brown color and cystic formation on the implant surfaces
(21). In the present experiment, 72
out of 96 model rats survived, with a success percentage of 75%,
and these rats were used in subsequent experiments.
Treatment
The 72 rats with successful endometriosis model were
randomly divided into four groups as follows (n=18 in each group):
Endometriosis (EM group), endometriosis + low-dose HYXZ (EM + L
group), endometriosis + mid-dose HYXZ (EM + M group), endometriosis
+ high-dose HYXZ (EM + H group). Rats in the EM + L, EM + M and EM
+ H groups were administered with HYXZ at a dose of 7, 14 and 21
g/kg/day, respectively. The mid-dose group, which was similar to
the dose administered to female patients, was calculated using the
formula of dose translation among different species based on body
surface area (22). Rats in the
control and EM groups were administered with double-distilled water
(10 ml/kg). The drugs and double-distilled water were administered
daily by oral gavage for 28 days.
Specimen collection
After treatment for 28 days, the rats were
sacrificed. Ectopic endometrial tissues were measured and dissected
from the implant sites. Half of the lesion was immediately fixed in
4% paraformaldehyde and then embedded in paraffin for
hematoxylin-eosin (HE) staining and immunohistochemical assays. The
remaining half of the lesion was stored at −80°C for analysis of
the protein and mRNA levels of VEGF and Ang-2.
H&E staining
Both eutopic endometrium and ectopic endometria of
rats were surgically detached. The endometriotic implants were
first fixed in 10% formaldehyde solution, embedded in paraffin and
cut into 4-µm sections. These sections were stained withH&Eand
examined under a light microscope. The morphology was captured with
a digital camera (Nikon 4500; Nikon Corp., Tokyo, Japan).
Immunohistochemical analysis
The paraffin-embedded sections of ectopic
endometrial tissue were subjected to antigen retrieval with citric
acid buffer, and were then incubated overnight at 4°C with the
following primary antibodies: VEGF antibody (Ab46154; dilution,
1:200; Abcam, Cambridge, UK); and Ang-2 antibody (Ab180820;
dilution, 1:200; Abcam). Subsequent to washing in PBS (pH 7.4)
three times, the sections were incubated with secondary antibody
(Ab205718; dilution, 1:50; Abcam) for 60 min at 37°C. Following
incubation, the slides were rewashed with PBS and incubated with
0.01% 3,3-diaminobenzidine tetrahydrochloride hydrate for ~1 min.
Sections were then washed thoroughly in PBS three times for 5 min
each, and counterstained in hematoxylin for 20 sec. Finally, the
tissues were dehydrated, rendered transparent and mounted prior to
being analyzed under a microscope. Negative control tissues were
treated with the same procedure, using PBS instead of primary
antibody. Images of the tissues were captured by a digital camera
(Nikon 4500). The positive area and optical density (OD) of
positive cells were determined by measuring three randomly selected
microscopic fields for each slide. The immunohistochemical index
was defined as the average integral OD (AIOD), as follows: AIOD =
positive area × OD/total area (23).
Western blot analysis
The tissues of the ectopic endometria were sectioned
and lysed in radioimmunoprecipitation assay lysis buffer (C1053;
Applygen Technologies, Inc., Beijing, China). The concentration of
protein used for western blot analysis was first quantified by a
bicinchoninic acid assay (P1511; Applygen Technologies, Inc.).
Next, protein samples (20 µg) were subjected to 10% sodium dodecyl
sulfate-polyacrylamide gel electrophoresis and then transferred to
a polyvinylidene difluoride (PVDF) membrane. The membrane was
blocked for 1 h at room temperature in 5% skimmed milk. Subsequent
to blocking, the membranes were incubated at 4°C overnight with the
following primary antibodies: VEGF (Ab46154; dilution, 1:2,000),
Ang-2 (Ab180820; dilution, 1:2,000) and GAPDH (Ab181602; dilution,
1:20,000). All the primary antibodies were obtained from Abcam.
Following rinsing in Tris-Buffered Saline and Tween-20 three times,
the PVDF membranes were incubated with the secondary antibodies
(C1633; dilution, 1:2,000; Applygen Technologies, Inc.) at room
temperature for 1 h. Images were obtained using
electrochemiluminescence (NCI4106; Pierce; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), prior to being analyzed using
Image J software v1.46r (National Institutes of Health, Bethesda,
MD, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Total RNA was isolated from ectopic endometrial
tissues using TRIzol reagent (15596–026; Thermo Fisher Scientific,
Inc.), and then reverse transcribed into cDNA with a Reverse
Transcription kit (D6130; Takara Bio, Inc., Otsu, Japan). The
resulting cDNA was diluted 10-fold in sterile water, and aliquots
were subjected to qPCR. The PCR primer pairs used in the analysis
were designed and synthetized by Taihe Biotechnology Co., Ltd.
(Beijing, China; Table II). PCR
cycles were composed of one cycle of 95°C for 5 min to denature all
proteins, 40 cycles of 95°C for 20 sec, and then 65°C for 30 sec.
The reaction was terminated at 72°C for 5 min and quenched at 4°C.
qPCR reactions were performed with an AMI Prism 7700 sequence
detection system (Applied Biosystems; Thermo Fisher Scientific,
Inc.). The relative expression of each target gene compared with
GAPDH was analyzed using the 2−ΔΔCq method (24).
 | Table II.Quantitative polymerase chain
reaction primer sequences. |
Table II.
Quantitative polymerase chain
reaction primer sequences.
| Gene | Primer sequences
(5′→3′) | Product size
(bp) |
|---|
| VEGF | F:
GGCTCACTTCCAGAAACACG | 165 |
|
| R:
GTGCTCTTGCAGAATCTAGTGG |
|
| Ang-2 | F:
CGGACTCTGTCACAAGCAAGAA | 237 |
|
| R:
AGCACAAGACGGAACAACGAA |
|
| GAPDH | F:
TGCTGAGTATGTCGTGGAG | 288 |
|
| R:
GTCTTCTGAGTGGCAGTGAT |
|
Statistical analysis
All statistical analyses were performed using the
SPSS software, version 17.0 (SPSS, Inc., Chicago, IL, USA). Values
are expressed as the mean ± standard deviation. The analysis of
variance test was used to compare the differences among the animal
groups. Differences with a P<0.05 were considered to be
statistically significant.
Results
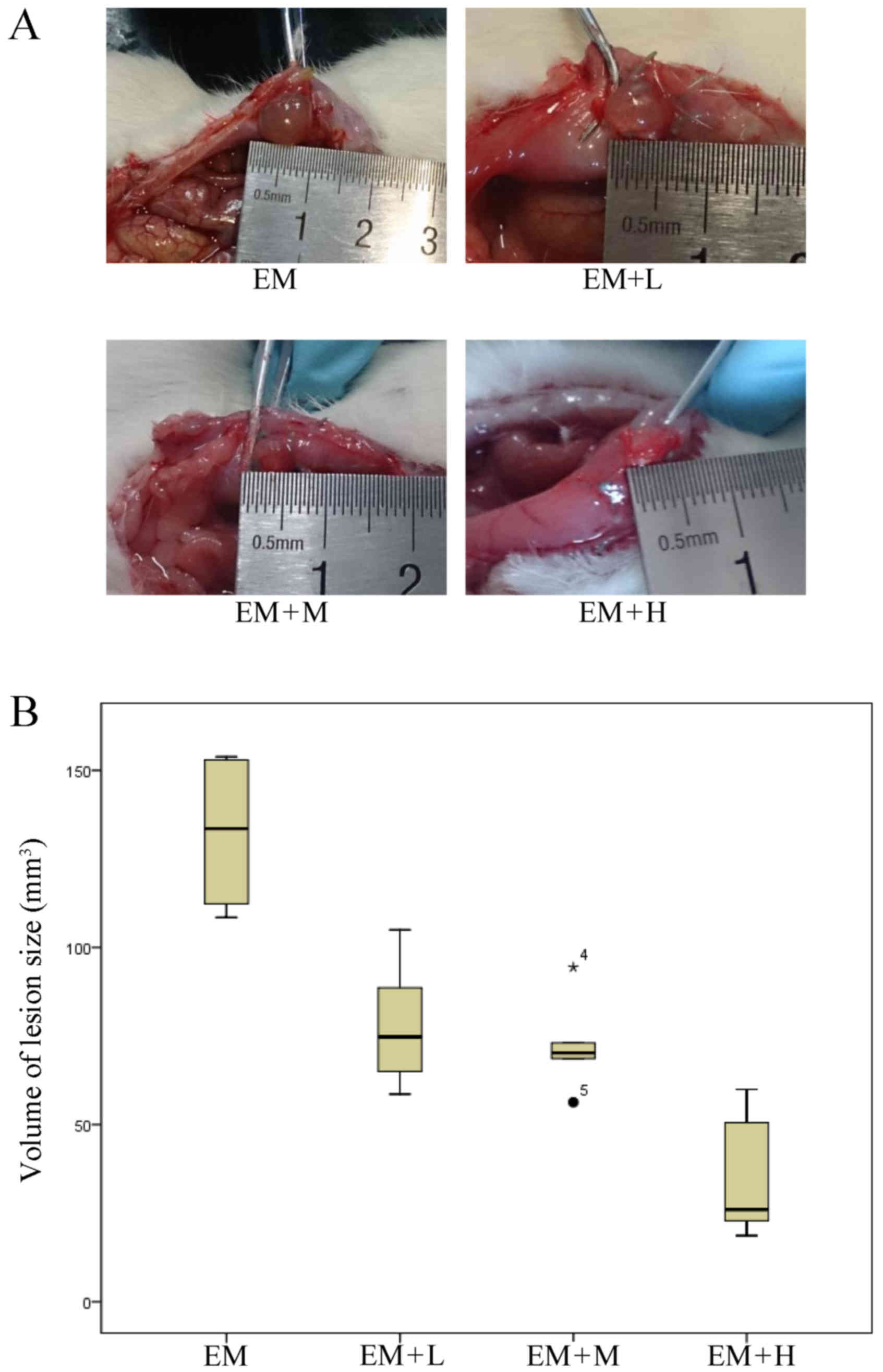
Treatment with HYXZ reduces the volume
of the lesion
After the 28-day treatment with HYXZ, the longest
lengths and perpendicular widths of the lesions were measured by a
vernier calliper. The volume of the endometriotic lesion was
calculated according to the following formula: 0.52 ×
width2 × length (25). A
comparison of the HYXZ-treated groups and the EM model group
demonstrated a statistically significant reduction in the lesion
size following the treatment (P<0.05). As the dose increased,
the volume of the lesion size was further reduced (Fig. 1; Table
III).
 | Table III.Volume of lesions following HYXZ
decoction treatment. |
Table III.
Volume of lesions following HYXZ
decoction treatment.
| Group | Median
(mm3) | Percentiles
(P25-P75) |
|---|
| EM | 133.48 | 111.35–153.11 |
| EM + L | 74.75a | 63.40–92.68 |
| EM + M | 70.20a | 65.58–78.38 |
| EM + H | 26.03a | 21.79–52.88 |
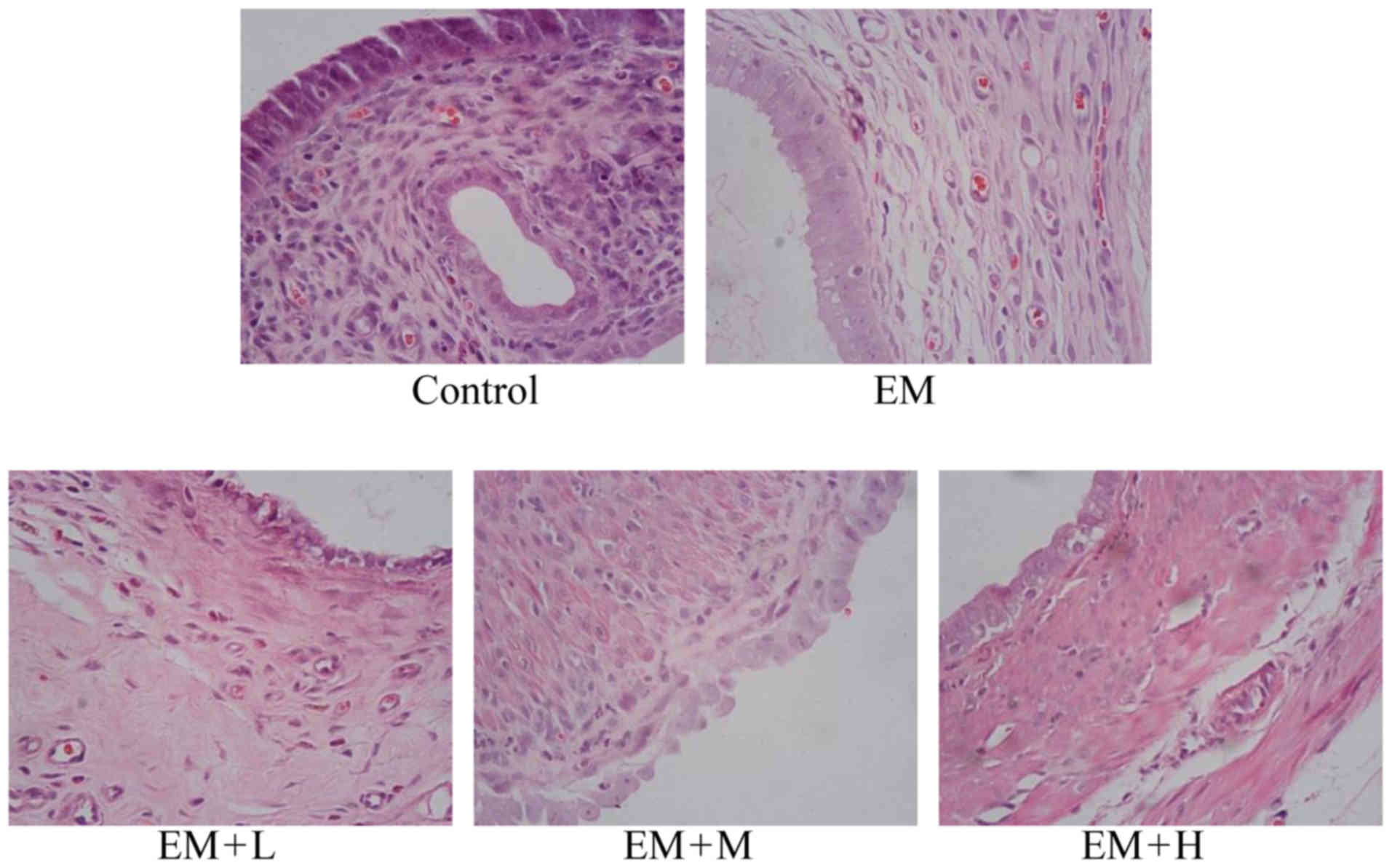
Light microscopy analysis
The H&Estaining results demonstrated that the
shape of the eutopic endometrium epithelial cells was columnar, and
the glandular epithelial and stromal cells were normal in the
control group. In the EM model group, H&E staining revealed
that the ectopic endometrium was covered with connective tissues
and that the glands and intercellular substances grew well and
intensively. The intima was thick and the glandular and superficial
epithelia formed a high column. Evident hyperplasia and
angiopoiesis were also observed. In the EM + L, EM + M and EM + H
treatment groups, the columnar shape was nearly natural, while the
number of glands and glandular epithelial cells was almost normal
(Fig. 2).
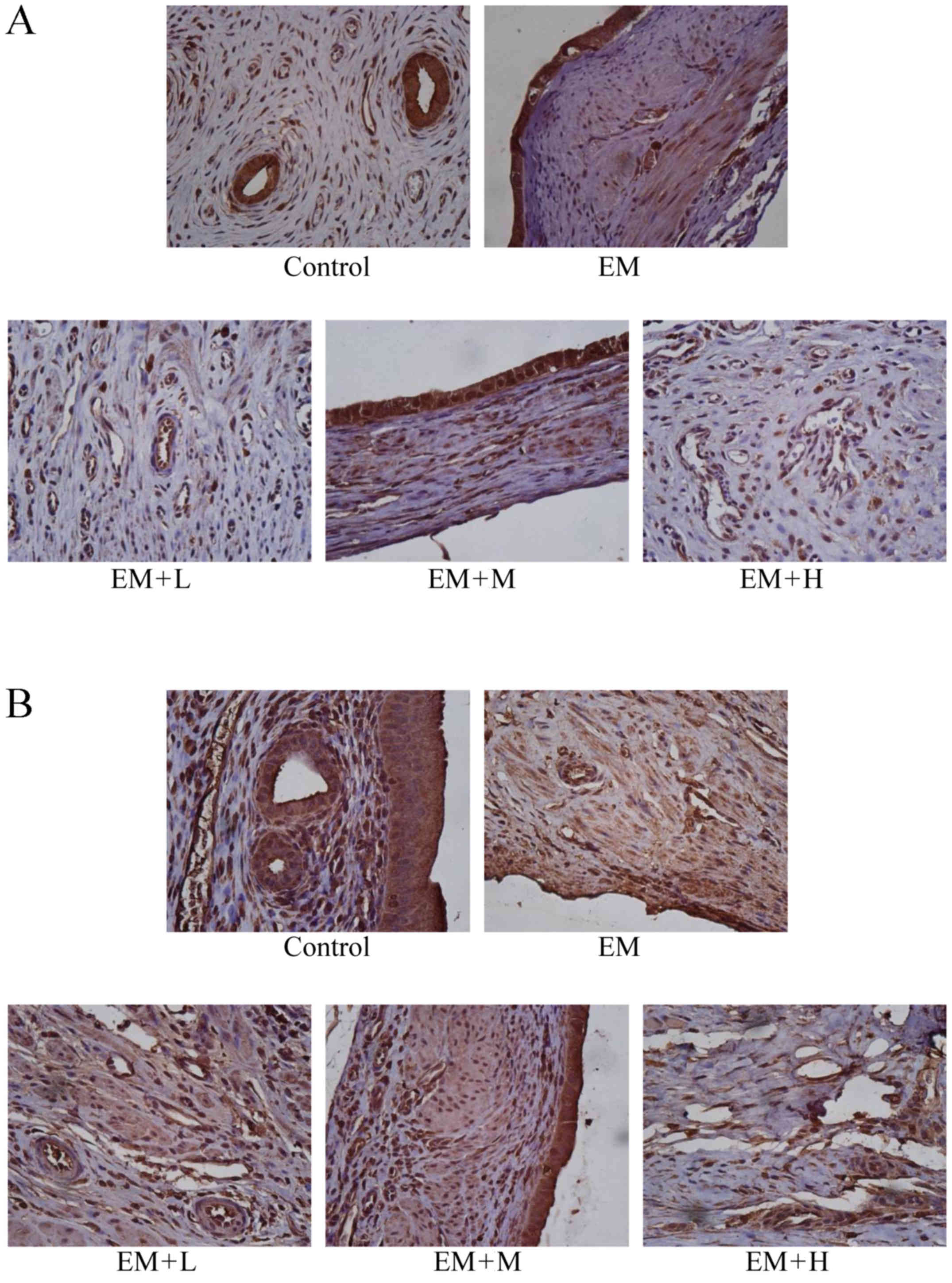
Expression levels of VEGF and Ang-2 as
evaluated by immunohistochemistry
As illustrated in Fig.
3 and Table IV, the
immunohistochemical scores of VEGF and Ang-2 were higher in the EM
model group compared with the control sham-operated group
(P<0.05). Following HYXZ administration (EM + L, EM + M and EM +
H groups), the Ang-2 immunohistochemical score was significantly
decreased compared with that of the EM group (P<0.05).
Similarly, the VEGF immunohistochemical score was significantly
decreased in the EM + H group compared with the EM group
(P<0.05), but not markedly altered in the EM + L and EM + H
groups.
 | Table IV.Scores of immunohistochemical
staining for VEGF and Ang-2 in the endometriotic lesions. |
Table IV.
Scores of immunohistochemical
staining for VEGF and Ang-2 in the endometriotic lesions.
|
| AIOD |
|---|
|
|
|
|---|
| Group | VEGF | Ang-2 |
|---|
| Control | 4.30±1.51 | 2.37±0.29 |
| EM |
12.33±7.82a |
8.86±1.94a |
| EM + L | 9.05±3.22 |
6.46±1.90b |
| EM + M | 10.66±3.65 |
5.55±2.23b |
| EM + H |
6.44±1.33b |
3.44±1.10b |
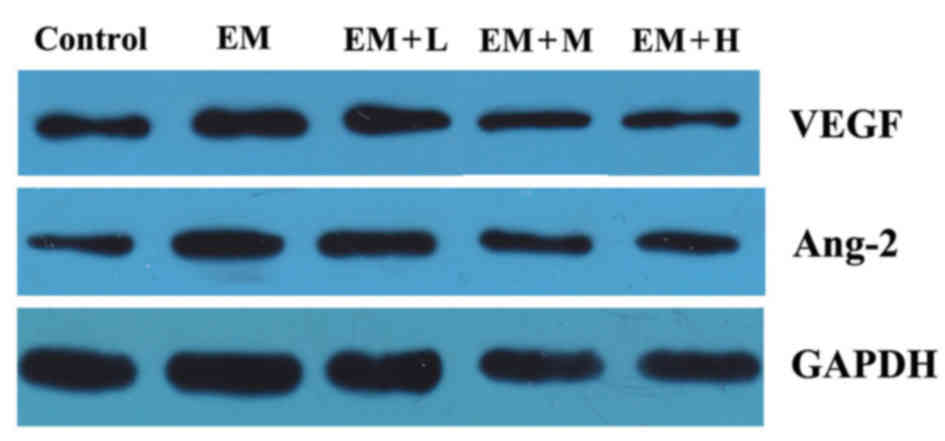
Protein levels of VEGF and Ang-2
As shown in Fig. 4
and Table V, the protein levels of
VEGF and Ang-2 in the EM group manifested a statistically
significant increase in comparison with those in the control group
(P<0.05). Compared with the EM group, the protein levels of VEGF
markedly decreased in all the HYXZ-treated groups (EM + L, EM + M
and EM + H) in a dose-dependent manner (P<0.05). Furthermore, it
was also observed that the protein levels of Ang-2 in the EM + M
and EM + H groups were significantly decreased compared with those
in the EM group (P<0.05).
 | Table V.Effect of Hua Yu Xiao Zheng decoction
on protein levels of VEGF and Ang-2. |
Table V.
Effect of Hua Yu Xiao Zheng decoction
on protein levels of VEGF and Ang-2.
| Group | Dose
(g/kg/day) | VEGF/GAPDH | Ang-2/GAPDH |
|---|
| Control | – | 0.30±0.03 | 0.73±0.09 |
| EM | – |
0.49±0.06a |
0.92±0.05a |
| EM + L | 7 |
0.41±0.04b | 0.86±0.07 |
| EM + M | 14 |
0.38±0.03b |
0.74±0.07b |
| EM + H | 21 |
0.36±0.02b |
0.70±0.06b |
Expression of VEGF and Ang-2 mRNA
Results of RT-qPCR demonstrated a pronounced
difference in the expression levels of VEGF and Ang-2 mRNA in the
EM model and HYXZ-treated groups when compared with the control
group. As shown in Table VI, the
expression levels of VEGF and Ang-2 mRNA in the EM group were
significantly upregulated in comparison with the control group
(P<0.05). Furthermore, the expression of VEGF and Ang-2 mRNA in
the EM + M and EM + H treatment groups was markedly downregulated
in comparison with the EM model group (P<0.05).
 | Table VI.Effect of Hua Yu Xiao Zheng decoction
on mRNA levels of VEGF and Ang-2. |
Table VI.
Effect of Hua Yu Xiao Zheng decoction
on mRNA levels of VEGF and Ang-2.
| Group | Dose
(g/kg/day) | VEGF/GAPDH | Ang-2/GAPDH |
|---|
| Control | – | 0.76±0.24 | 0.78±0.35 |
| EM | – |
8.92±3.73a |
7.01±2.29a |
| EM + L | 7 | 7.02±2.66 | 6.59±2.47 |
| EM + M | 14 |
4.47±1.51b |
3.89±1.35b |
| EM + H | 21 |
3.14±0.98b |
3.32±1.19b |
Discussion
Endometriosis is a common gynecological condition
with diverse clinical manifestations, as well as highly variable
and unpredictable clinical course with decreased quality of life.
Although endometriosis is a benign disorder, the process through
which endometrial cells attach to and invade surface shares
features of malignancy (26).
Current treatments, including surgery and hormonal therapy, are
often insufficient, as well as present a high rate of relapse and
various side effects, such as weight gain, abnormal lipid
metabolism, hepatic injury and osteoporosis. Therefore, it is
necessary to explore novel medical strategies and treatment
approaches to improve the treatment of endometriosis lesions.
TCM herbs have become popular for relieving
endometriosis-associated symptoms, including dysmenorrhea,
dyspareunia, menorrhagia and chronic pain, which typically require
surgical or medical intervention (27,28).
Chinese herbal formulas for endometriosis therapy are designed to
alleviate blood stasis and nourish the kidney (29). The present study aimed to examine the
therapeutic potential of HYXZ, determine its ability to reduce
endometrial explants growth and determine the possible mechanism of
action in EM model rats, in order to provide a theoretical
foundation for endometriosis treatment. To the best of our
knowledge, the current study demonstrated for the first time that a
physiological dose of HYXZ (which is six times greater than the
amount administered to humans) administered for 28 days inhibited
the growth of endometriotic tissues in a dose-dependent manner.
Furthermore, the results demonstrated that HYXZ did not suppress
the growth of the eutopic endometrium inside the uterine cavity of
the rat model. These findings indicate that HYXZ specifically
targets the endometriotic tissues with minimal side effects on
normal endometrium.
The development of new blood vessels is a complex
dynamic process, which is characterized by a coordinated sequence
of humoral and cellular interactions. It is widely accepted that
endometriotic lesions are typically characterized by a dense
vascularization that occurs through the angiogenesis process
(30,31). The establishment and development of
an effective blood supply and angiogenesis are essential for the
growth of endometriotic implants (32). Endometrial expression of VEGF, which
is a potent vascular permeability-inducing, mitogen and morphogen
agent, is thus an important mediator of angiogenesis (33). Furthermore, Ang proteins are potent
angiogenic factors that are expressed in the normal human
endometrium. Ang-2 activates in response to hypoxia and induces
vessel destabilization upon binding the Tie2 receptor (34). VEGF and Ang-2 have been reported to
independently induce angiogenesis (35). Furthermore, they have been
demonstrated to act synergistically to induce endothelial
destabilization, increase vascular branching, and increase
angiogenesis (36). In addition, the
two growth factors independently induce formation of endothelial
branches in ex vivo aortic ring assays, suggesting an
angiogenic effect on perivascular cells as well (37).
Endometriotic lesions are highly vascularized, and
angiogenesis is considered as a critical step for the establishment
of endometriosis (38,39). VEGF stimulates endothelial cell
proliferation and migration, while elevated levels of VEGF have
been reported in the peritoneal fluid of women with endometriosis
(7), with the same increase observed
for Ang-2. In the present study, the protein and mRNA expression
levels of VEGF and Ang-2 were significantly higher in the ectopic
endometrium as compared with those in the normal endometrium. Thus,
these results suggest that VEGF and Ang-2 serve an important role
in the pathogenesis of endometriosis. The findings of the present
study offer a novel perspective on the underlying mechanisms of
endometriosis and indicate that anti-angiogenic therapy may be a
potentially promising treatment method for patients with
endometriosis. Furthermore, the current study observed that HYXZ
was able to significantly reduce the protein and mRNA expression of
VEGF and Ang-2 in the ectopic endometrium. The results also
indicated that inhibition of angiogenesis may lead to regression of
the lesion size following HYXZ treatment. Compared with other TCM
formulas previously reported, HYXZ reduced the volume of
endometriosis lesions to a similar extent as Guizhi Fuling capsules
(40), and also demonstrated a
significant inhibition of the angiogenesis process.
Although the present study offered a unique
evaluation of the effects of HYXZ decoction on the endometriosis
model, the small number of animals in the groups is a limitation of
the study. Larger prospective controlled clinical trials are
required in order to further elucidate the findings.
In conclusion, the current study revealed that HYXZ
decoction significantly reduced the size of the endometrial
explants in endometriosis model rats by decreasing the protein and
mRNA levels of VEGF and Ang-2 in the ectopic endometrium. HYXZ may
be a promising TCM formula to treat endometriosis, however, further
clinical trials are required to confirm its efficacy.
References
|
1
|
Burney RO and Giudice LC: Pathogenesis and
pathophysiology of endometriosis. Fertil Steril. 98:511–519. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jacobson TZ, Duffy JM, Barlow DH, Koninckx
PR and Garry R: WITHDRAWN: Laparoscopic surgery for pelvic pain
associated with endometriosis. Cochrane Database Syst Rev:
CD001300. 2014. View Article : Google Scholar
|
|
3
|
Sampson JA: Metastatic or embolic
endometriosis, due to the menstrual dissemination of endometrial
tissue into the venous circulation. Am J Pathol. 3:93–110.43.
1927.PubMed/NCBI
|
|
4
|
Groothuis PG, Nap AW, Winterhager E and
Grümmer R: Vascular development in endometriosis. Angiogenesis.
8:147–156. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mueller MD, Vigne JL, Minchenko A, Lebovic
DI, Leitman DC and Taylor RN: Regulation of vascular endothelial
growth factor (VEGF) gene transcription by estrogen receptors alpha
and beta. Proc Natl Acad Sci USA. 97:pp. 10972–10977. 2000,
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rocha AL, Reis FM and Taylor RN:
Angiogenesis and endometriosis. Obstet Gynecol Int.
2013:8596192013.PubMed/NCBI
|
|
7
|
Donnez J, Smoes P, Gillerot S,
Casanas-Roux F and Nisolle M: Vascular endothelial growth factor
(VEGF) in endometriosis. Hum Reprod. 13:1686–1690. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Charnock-Jones DS, Sharkey AM,
Rajput-Williams J, Burch D, Schofield JP, Fountain SA, Boocock CA
and Smith SK: Identification and localization of alternately
spliced mRNAs for vascular endothelial growth factor in human
uterus and estrogen regulation in endometrial carcinoma cell lines.
Biol Reprod. 48:1120–1128. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Healy DL, Rogers PA, Hii L and Wingfield
M: Angiongenesis: A new theory for endometriosis. Hum Reprod
Update. 4:736–740. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yancopoulos GD, Davis S, Gale NW, Rudge
JS, Wiegand SJ and Holash J: Vascular-specific growth factors and
blood vessel formation. Nature. 407:242–248. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jingting C, Yangde Z, Yi Z, Mengxiong L,
Rong Y, Yu Z, Guoqing P and Lixiu P: Expression of heparanase and
angiopoietin-2 in patients with endometriosis. Eur J Obstet Gynecol
Reprod Biol. 136:199–209. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Loukovaara S, Robciuc A, Holopainen JM,
Lehti K, Pessi T, Liinamaa J, Kukkonen KT, Jauhiainen M, Koli K,
Keski-Oja J and Immonen I: Ang-2 upregulation correlates with
increased levels of MMP-9, VEGF, EPO and TGFβ1 in diabetic eyes
undergoing vitrectomy. Acta Ophthalmol. 91:531–539. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Weigel MT, Krämer J, Schem C, Wenners A,
Alkatout I, Jonat W, Maass N and Mundhenke C: Differential
expression of MMP-2, MMP-9 and PCNA in endometriosis and
endometrial carcinoma. Eur J Obstet Gynecol Reprod Biol. 74–78.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hur SE, Lee JY, Moon HS and Chung HW:
Angiopoietin-1, angiopoietin-2 and Tie-2 expression in eutopic
endometrium in advanced endometriosis. Mol Hum Reprod. 12:421–426.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wieser F, Cohen M, Gaeddert A, Yu J,
Burks-Wicks C, Berga SL and Taylor RN: Evolution of medical
treatment for endometriosis: Back to the roots? Hum Reprod Update.
13:487–499. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang M: Treatment of endometriosis by
comprehensive TCM therapy: A clinical observation of 35 cases. New
J Tradit Chin Med. 2004.PubMed/NCBI
|
|
17
|
Tsai PJ, Lin YH, Chen JL, Yang SH, Chen YC
and Chen HY: Identifying Chinese herbal medicine network for
endometriosis: Implications from a population-based database in
Taiwan. Evid Based Complement Alternat Med. 2017:75010152017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gao H, Wang Z, Li Y and Qian Z: Overview
of the quality standard research of traditional Chinese medicine.
Frontiers of Medicine. 5:195–202. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gong X, Lou J, Lu Q, Huang H and Jin Z: Bu
Shen Huo Xue decoction restores endometrial leukemia-inhibitory
factor but not Angiopoietin-2 expression, and improves uterine
receptivity in the controlled ovarian stimulation rat model. Exp
Ther Med. 9:751–757. 2015.PubMed/NCBI
|
|
20
|
Zhang H, Li J, Sun W, Hu Y, Zhang G, Shen
M and Shi X: Hyaluronic acid-modified magnetic iron oxide
nanoparticles for MR imaging of surgically induced endometriosis
model in rats. PLoS One. 9:e947182014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jiao L, Qi X, Lu G, Zhang Q, Zhang C and
Gao J: Effect of traditional Chinese medicine (Xiaochaihu Tang) on
the expression of MMP-2 and MMP-9 in rats with endometriosis. Exp
Ther Med. 6:1385–1389. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Reagan-Shaw S, Nihal M and Ahmad N: Dose
translation from animal to human studies revisited. FASEB J.
22:659–661. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yu J, Zhao L, Zhang D, Zhai D, Shen W, Bai
L, Liu Y, Cai Z, Li J and Yu C: The effects and possible mechanisms
of puerarin to treat endometriosis model rats. Evid Based
Complement Alternat Med. 2015:2691382015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Jiang HQ, Li YL and Zou J: Effect of
recombinant human endostatin on endometriosis in mice. Chin Med J
(Engl). 120:1241–1246. 2007.PubMed/NCBI
|
|
26
|
Lucidi RS, Witz CA, Chrisco M, Binkley PA,
Shain SA and Schenken RS: A novel in vitro model of the early
endometriotic lesion demonstrates that attachment of endometrial
cells to mesothelial cells is dependent on the source of
endometrial cells. Fertil Steril. 84:16–21. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Anderson FW and Johnson CT: Complementary
and alternative medicine in obstetrics. Int J Gynaecol Obstet.
91:116–124. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tindle HA, Davis RB, Phillips RS and
Eisenberg DM: Trends in use of complementary and alternative
medicine by US adults: 1997–2002. Altern Ther Health Med. 11:42–49.
2005.PubMed/NCBI
|
|
29
|
Flower A, Liu JP, Lewith G, Little P and
Li Q: Chinese herbal medicine for endometriosis. Cochrane Database
Syst Rev: CD006568. 2012. View Article : Google Scholar
|
|
30
|
Giudice LC: Clinical practice.
Endometriosis. N Engl J Med. 362:2389–2398. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
McLaren J: Vascular endothelial growth
factor and endometriotic angiogenesis. Hum Reprod Update. 6:45–55.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Folkman J and Shing Y: Angiogenesis. J
Biol Chem. 267:10931–10934. 1992.PubMed/NCBI
|
|
33
|
Shifren JL, Tseng JF, Zaloudek CJ, Ryan
IP, Meng YG, Ferrara N, Jaffe RB and Taylor RN: Ovarian steroid
regulation of vascular endothelial growth factor in the human
endometrium: Implications for angiogenesis during the menstrual
cycle and in the pathogenesis of endometriosis. J Clin Endocrinol
Metab. 81:3112–3118. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Niedźwiecki S, Stepień T, Kopeć K, Kuzdak
K, Komorowski J, Krupiński R and Stepień H: Angiopoietin 1 (Ang-1),
angiopoietin 2 (Ang-2) and Tie-2 (a receptor tyrosine kinase)
concentrations in peripheral blood of patients with thyroid
cancers. Cytokine. 36:291–295. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hur SE, Lee JY, Moon HS and Chung HW:
Angiopoietin-1, angiopoietin-2 and Tie-2 expression in eutopic
endometrium in advanced endometriosis. Mol Hum Reprod. 12:421–426.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
He LL, Zhang WJ, Su H and Xu DG: Synergism
between Ang-2 and VEGF and its application of anti-angiogenesis in
tumor therapy - review. Zhongguo Shi Yan Xue Ye Xue Za Zhi.
15:445–448. 2007.(In Chinese). PubMed/NCBI
|
|
37
|
Bohn KA, Adkins CE, Nounou MI and Lockman
PR: Inhibition of VEGF and angiopoietin-2 to reduce brain
metastases of breast cancer burden. Front Pharmacol. 8:1932017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yeniel AÖ, Erbas O, Ergenoglu AM, Aktug H,
Taskiran D, Yildirim N and Ulukus M: Effect of oxytocin treatment
on explant size, plasma and peritoneal levels of MCP-1, VEGF, TNF-α
and histopathological parameters in a rat endometriosis model. Eur
J Obstet Gynecol Reprod Biol. 175:134–139. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Liu S, Xin X, Hua T, Shi R, Chi S, Jin Z
and Wang H: Efficacy of Anti-VEGF/VEGFR agents on animal models of
endometriosis: A systematic review and meta-analysis. PLoS One.
11:e01666582016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hu C, Wang Z, Pang Z, Lu W, Cai X, Yang J,
Wang D and Cao P: Guizhi fuling capsule, an ancient Chinese
formula, attenuates endometriosis in rats via induction of
apoptosis. Climacteric. 17:410–416. 2014. View Article : Google Scholar : PubMed/NCBI
|