Introduction
Inflammatory bowel disease (IBD) in humans,
including Crohn's disease and ulcerative colitis, is a complex
chronic inflammatory disorder (1).
The incidence of these diseases varies widely between different
countries, but overall has increased greatly in recent years,
making IBD a major public health problem now (2). Though its etiopathogenesis is
ambiguous, there exist a growing awareness that oxidative stress
and the resulting inflammation play an important role in the
development of IBD (3–6).
Current IBD treatments include aminosalicylates,
corticosteroids, inhibitors of tumor necrosis factor-α (TNF-α),
antibiotics and immunosuppressants (7). However, these agents have poor
tolerability and insufficient therapeutic efficacy; therefore, the
need for alternative therapeutic approaches is increasing (8,9). It is
now clear that probiotic intervention is able to prevent pouchitis
and has found to be effective in inducing and maintaining remission
in ulcerative colitis, although reports might be conflicting, as
they involve different mixtures of probiotic strains, different
protocol designs, various doses, different read-outs and different
clinical settings or patient types (10,11). The
Gram-positive spore-forming probiotic, Bacillus subtilis
(B. subtilis), has been well demonstrated to have probiotic
potential (12,13). Spore-forming bacteria may offer many
interesting advantages compared to non-sporeformers, as they are
heat-stable, resistant to low pH and other deleterious conditions
such as gastric and bile secretions in the intestinal environment.
Moreover, spores allow long term storage of preparations without
refrigeration or need for encapsulation processes (14). Notably, a number of studies have
indicated the anti-inflammatory function of some B. subtilis
strains in IBD (15,16).
Carotenoids, a subfamily of the isoprenoids
containing more than 700 members, are currently used for food
colorants and nutritional supplements (17). Carotenoids can act as antioxidants
with the potential to remove free radicals, either by a direct
reaction with radicals, resulting in the formation of harmless
products, or by disrupting radical chain reactions, avoiding
further damage of cellular compounds, such as membrane lipids. It
was noted that patients with ulcerative colitis have extremely low
concentrations of serum and mucosa carotenoids (such as lutein,
zeaxanthin, α- and β-carotene) as compared to that of healthy
individuals (18,19). Moreover, several studies have showed
that dietary carotenoids inhibited colitis and colitis-associated
colon carcinogenesis in mice (20–22).
In previous study, we had constructed a carotenoid
(4,4′-diaponeurosporene)-producing B. subtilis (B.s-Dia) by
transforming a plasmid which containing carotenoid synthase gene,
crm, and carotenoid dehydrogenase gene, crtn, into
B. subtilis strain WB800 (23). The aim of this study was to address
the protective effect of carotenoid-producing B. subtilis on
murine experimental colitis. Our results indicated that oral
administration of B.s-Dia had an improved positive effect on colon
histopathological changes and length in mice dextran sodium sulfate
(DSS)-induced colitis.
Materials and methods
Mice
C57BL/6 mice (23–24 g), 8 weeks old, were purchased
from the Animal Research Center of Yangzhou University (Jiangsu,
China). The mice were maintained under specific pathogen-free
conditions for at least 1 week before use. The animal studies were
approved by the Institutional Animal Care and Use Committee of
Nanjing Agricultural University and followed National Institutes of
Health guidelines for the performance of animal experiments.
Bacterial strains, animals
B.s-Dia was constructed as previously described. B.s
or B.s-Dia were grown in Luria-Bertani (LB) broth (10 g tryptone, 5
g yeast extract, and 5 g NaCl/l) containing 50 µg/ml kanamycin at
37°C.
Induction of experimental colitis
To inducing colitis, DSS (molecular weight 5,000; MP
Biomedicals Inc., Eschwege, Germany) was added into mouse drinking
water at the concentration of 5% (w/v) for 6 or 7 days. And mice
were randomly equally divided into four groups (n=7 in each) as
follows: Control group was just given PBS by gavage each day for 7
days and no DSS was added in drinking water. DSS group was also
given PBS by gavage once a day for 7 days but followed by DSS added
drinking water every day for successively 7 days. B.s+DSS or
B.s-Dia+DSS group was given 1×109 cfu B. subtilis
Wb800 or B.s-Dia once a day for 7 days and followed by DSS added
drinking water every day for successively 7 days. And the dose of
B.s-Dia was selected based on the results of previous studies
(24–27), and that a dose of 1×109 cfu/ml also
has been demonstrated to significantly alleviate colitis (28).
Assessment of mouse weight and colon
length
Mouse weight was measured daily from day 0 to 7 at
9:30 a.m. every day and expressed as the relative change from day
0. The colon was isolated immediately after the last weight check.
The colon was excised between the ileocaecal junction and the
proximal rectum, close to its passage under the pelvisternum. The
colon was placed on a non-absorbent surface and its length was
measured with a ruler, in such a manner that the organ was not
stretched. Mouse weight and colon length were determined as
described previously (29,30).
Histological observation and
scoring
The morphological evaluation of colon in each group
was performed using hematoxylin and eosin (H&E) staining. The
colons were fixed in 4% paraformaldehyde, embedded in paraffin and
sliced into sections of 5 µm thickness. After H&E staining,
histological analysis was performed in a blind fashion. Colitis was
assessed with tissue sections and was scored in a blinded
experimental set-up according to a standard scoring system: 0, no
thickening of colonic tissues and no inflammation (infiltration of
lymphocytes); 1, mild thickening of tissues but no inflammation; 2,
mild thickening of tissues and mild inflammation; 3, severe
thickening and severe inflammation (31).
Statistical analysis
Results were expressed as mean ± standard deviation
(SD). One-way ANOVA was employed to determine statistical
differences among multiple groups, and t-test was employed to
determine the same between two groups. P-values <0.05 were
considered statistically significant (P<0.05, P<0.01).
Histological scores were analyzed by the Mann-Whitney U test.
Results
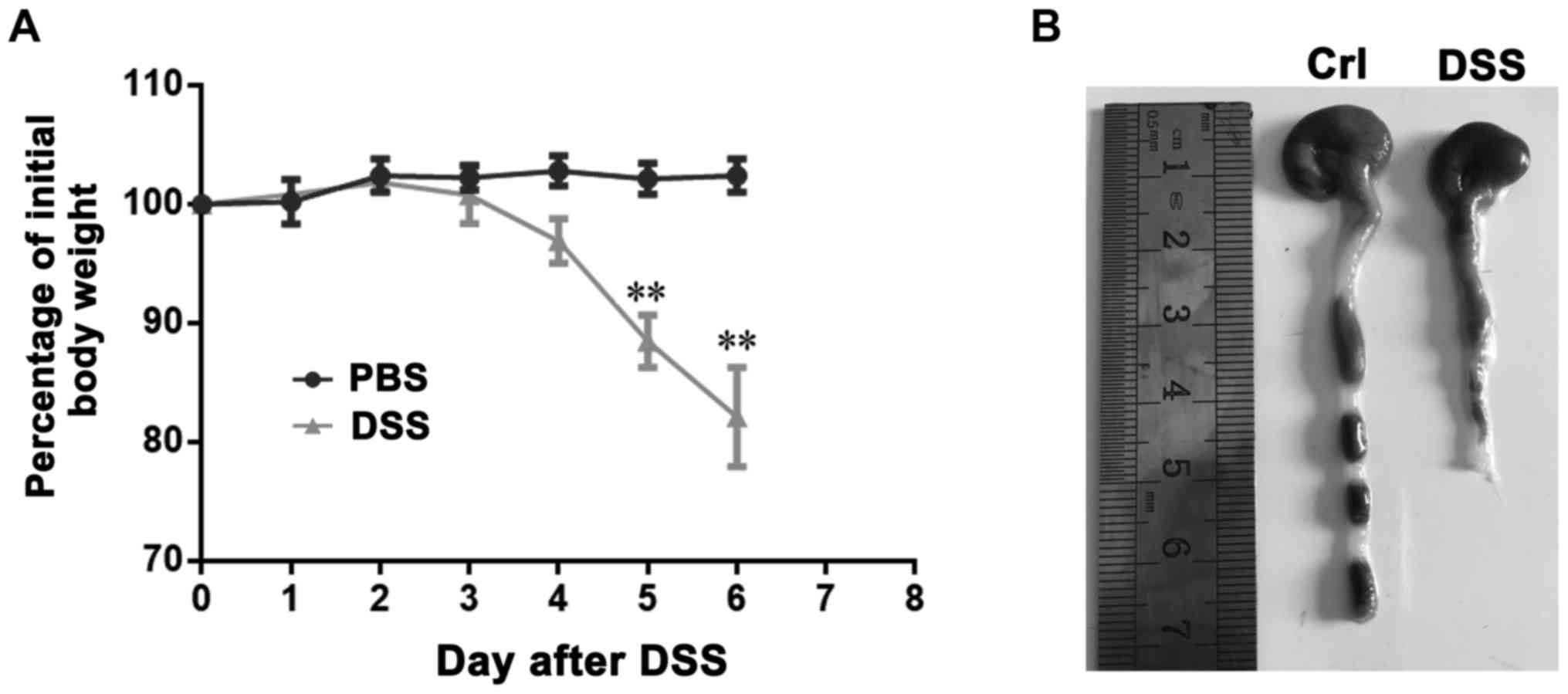
DSS-induced colitis model
In order to establish DSS-induced colitis, mice were
administered 5% DSS in the drinking fluid for 6 days and weighed
every day. DSS-treated mice drastically lost weight from the 4th
day after initiation of the medication, compared with that of
untreated animals (Fig. 1A). In
addition, DSS-treated mice also had remarkably shortened colon
(Fig. 1B). these results indicated
the successful construction of mice colitis.
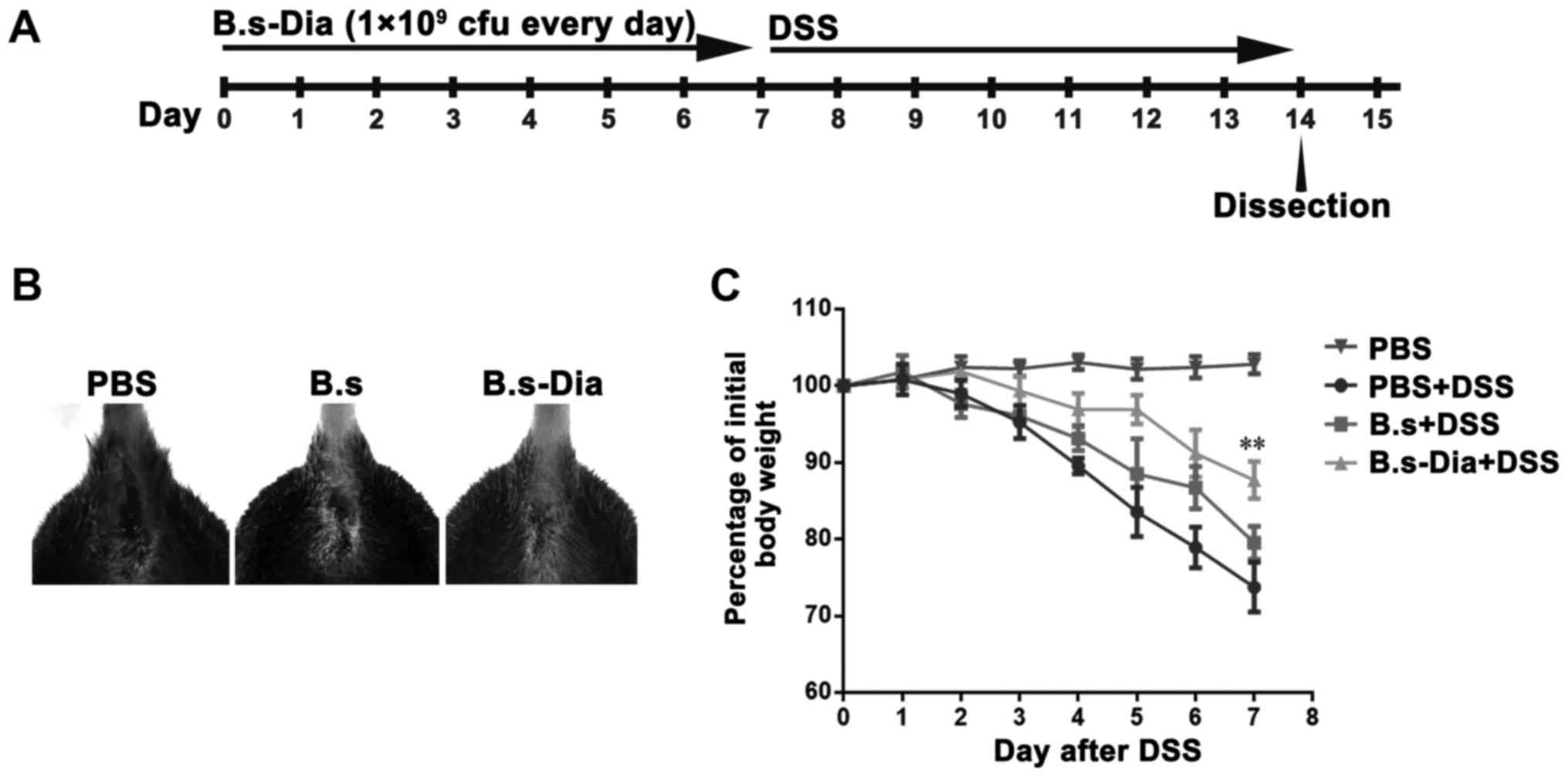
Influences of B.s-Dia oral
administration on mouse hemafecia and body weight
To evaluate the effects of B.s-Dia on colitis, mice
were orally administrated 1×109 cfu B.s-Dia every day,
followed by adding 5% DSS in the drinking water for 7 days
(Fig. 2A). Though, both B.s and
B.s-Dia oral administration observably diminished bloody stools as
showed in Fig. 2B, B.s-Dia was more
effective in this process compared with B.s. Importantly, mice in
the B.s-Dia group had significantly reduced weight loss than that
in the DSS or B.s group on day 7 (Fig.
2C).
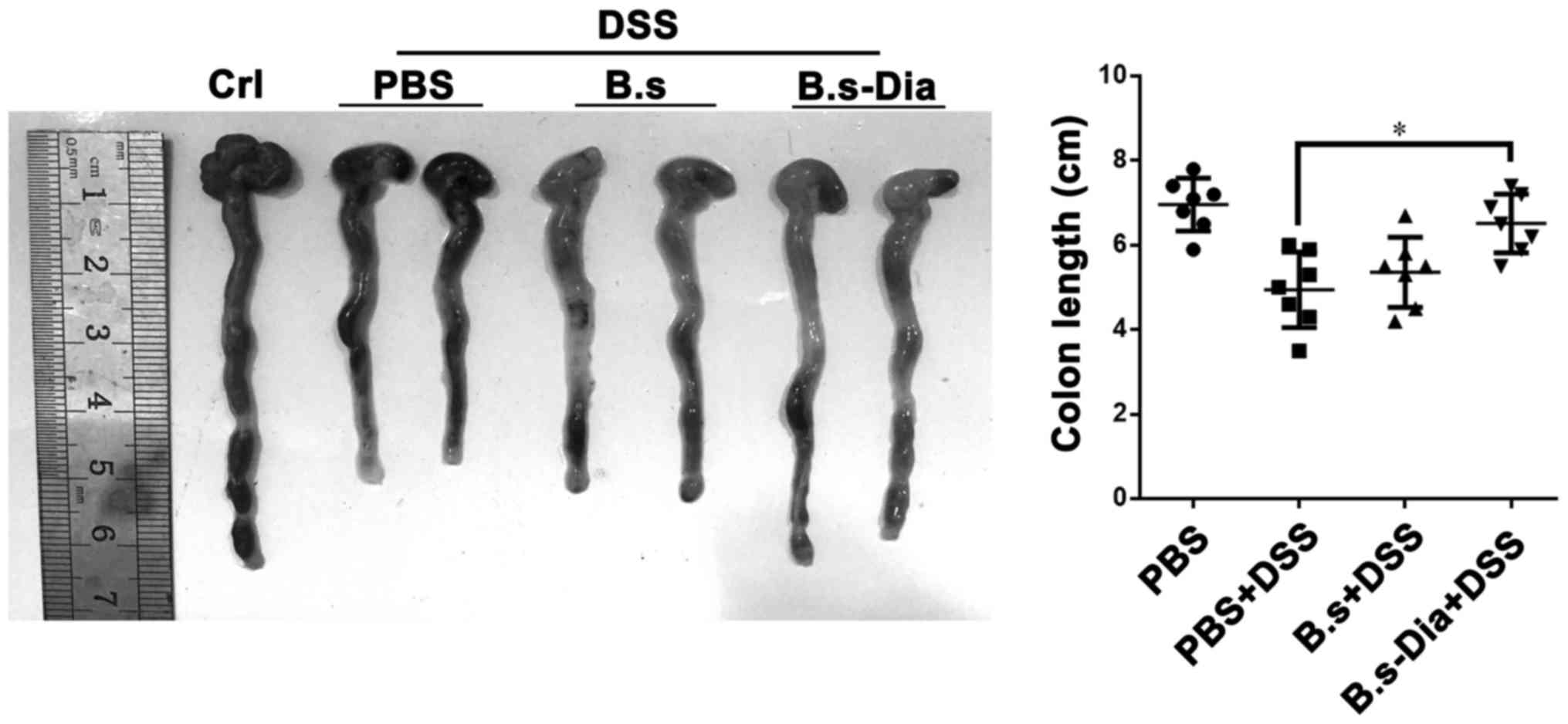
Influences of B.s-Dia oral
administration on colon length
A shorter colon length and bleeding are considered
as a hallmark of experimental colitis (32,33). Our
result revealed that oral administration of B.s-Dia significantly
increased colon length in experimental colitis, while B.s oral
administration had no significantly effect on colon length
(Fig. 3). Nevertheless, both B.s and
B.s-Dia reduced the severity of bleeding in colon wall.
Influences of B.s-Dia on histological
damage induced by DSS
To further evaluate the effects of B.s-Dia on
DSS-induced colitis, we performed histopathological examination. As
showed in Fig. 4A, the surface
epithelium, cryptal glands, mucosa, and submucosa in the normal
mice were intact. However, DSS-treated mice showed severe damage of
the surface epithelium, bleeding, disruption of the cryptal glands,
and thickened submucosal layer. Mice administered B.s showed
relatively intact surface epithelium, but the disruption of cryptal
glands and infiltration of the inflammatory cells were still
observed. However, oral administration of B.s-Dia showed more
protective effects and improved DSS-induced pathology (Fig. 4B).
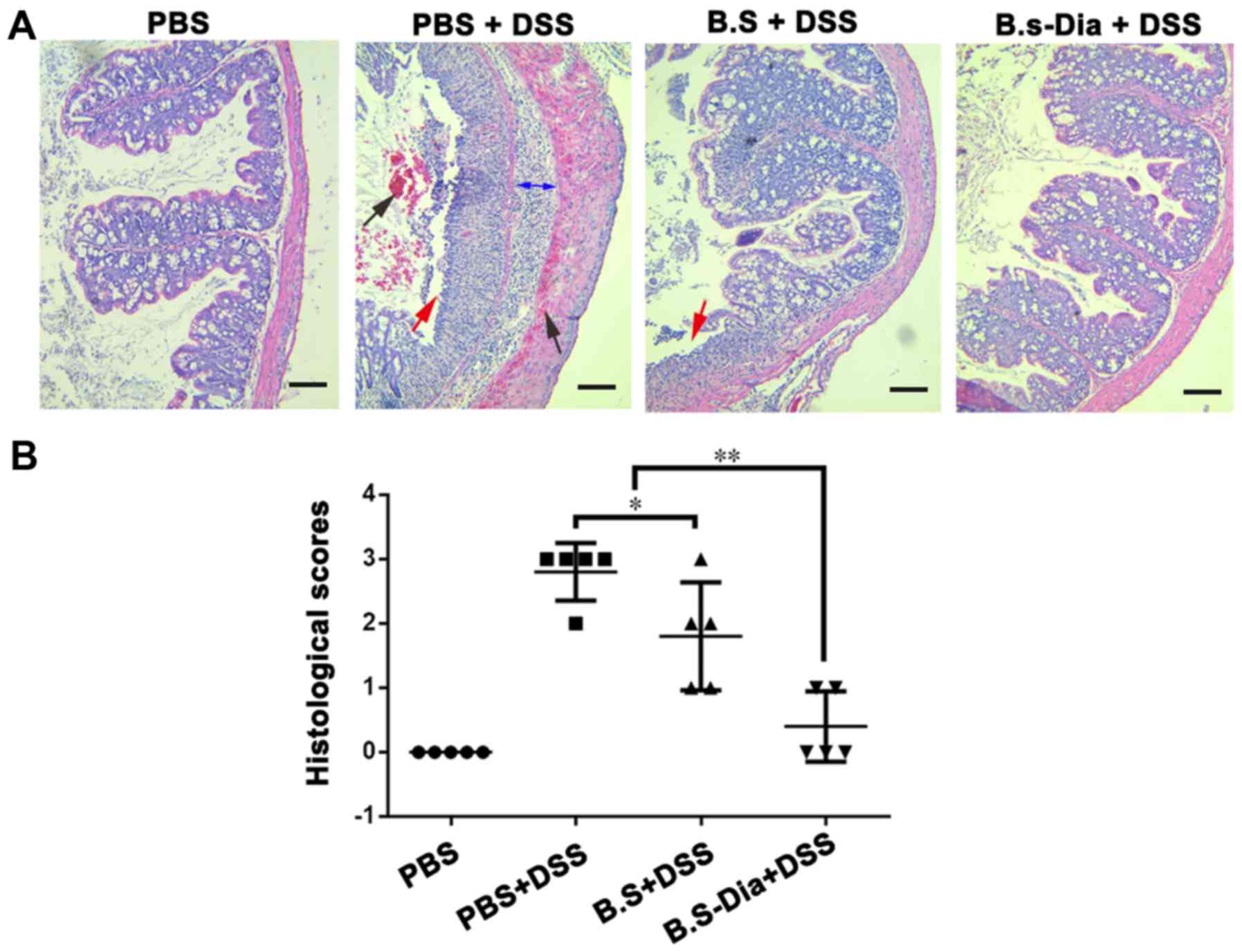 | Figure 4.Influences of B.s-Dia on histological
damage induced by DSS. (A) The colon of mice from different groups
was fixed in 4% paraformaldehyde, embedded in paraffin and sliced
into sections of 5 µm thickness, and followed by hematoxylin and
eosin staining. bleeding (black arrow), cell detachment (red
arrow), thickened submucosal layer (double sided arrow). (B)
Colitis was assessed with tissue sections and was scored in a
blinded experimental set-up as described in ‘Materials and
methods’. Histological scores were analyzed by the Mann-Whitney
U-test (*P<0.05, **P<0.01). n=5. Scale bar, 200 nm. DSS,
dextran sodium sulfate; B.s, Bacillus subtilis; B.s-Dia,
4,4′-diaponeurosporene-producing Bacillus subtilis. |
Discussion
At present, there is no known cure for human IBD
(34). Immunosuppressive therapies,
for example with TNF-α antagonists (35), are currently being used as remedies
against severe human diseases. Recent investigations have focused
on the development of new therapeutic strategies that aim to
restore the balance of the gastrointestinal microbiota to reduce or
prevent intestinal inflammation. There are growing evidence that
probiotic microorganisms might influence disease outcome of IBD in
both animal models and humans (36).
The B. subtilis species has a long history of
safe use and several studies have indicated its ameliorative effect
on murine experimental colitis (15,28), In
addition, the preventive effect of different B. subtilis
strains on colitis is not the same (14). because the protective effect of
probiotics is related with its antioxidant and anti-inflammatory
abilities (37,38). And the severity of colitis induced by
the different concentrations of DSS is not the same. Accordingly,
B. subtilis treated mice still suffer relatively severe
colitis.
Carotenoids are a kind of natural pigment with
antioxidant properties that prevent oxidative stress. It can also
effectively ameliorate symptoms of colitis. Literature shows that
β-carotene treatment ameliorated the severity of UC by modulating
various molecular targets (such as nuclear factor-κB,
cyclooxygenase-2, interleukin-17) and maintained the gut integrity
by increasing the expression of a tight junction protein,
occluding, which was decreased in the colon of mice with UC
(21). Furthermore, lycopene
(39), lutein and zeaxanthin
(40) all have effects on colitis.
In this study, we showed that 4,4′-diaponeurosporene-producing
B. subtilis had an outstanding ability to prevent
DSS-induced colitis.
4,4′-Diaponeurosporeneis is also a kind of
carotenoids. Our previous study showed that the carotenoid
4,4′-diaponeurosporene modulated hydrogen peroxide-induced
oxidative stress in mouse dendritic cells (23), indicating its antioxidant activity.
Moreover, it has been reported that the addition of B.
subtilis to chicken diets can enhance the activity of
antioxidant enzymes to scavenge free radicals (41,42). And
present study shows that B.s-Dia had a more positive effect
on DSS-induced colitis, as compared with B. subtilis.
This result implied the potential beneficial effect of
4,4′-diaponeurosporene on colitis, possibly because of its
antioxidant activity. Consequently, Bs-Dia has potential
application value for the prevention and treatment of IBD and
colitis in clinic.
In brief, the results of the present preliminary
study show that Bs-Dia can effectively improve the DSS-induced
intestinal mucosal epithelial injury, bleeding, crypts gland
rupture and submucosal thickening and other pathological
manifestations, greatly alleviate DSS-induced colitis. However, we
should note that the mechanism underlies the protection of B.
subtilis or carotenoids might be complex, and more efforts are
needed to illustrate it. Our future studies will aim to investigate
the specific factors (intrinsic regulatory mechanisms and
epithelial tight junction protein) that B.s-Dia can alleviate
colitis, and the dose-dependent effects of B.s-Dia pre-treatment on
colitis.
Acknowledgements
The present study was supported by 31372465 from the
National Science Grant of China and a project funded by the
Priority Academic Program Development of Jiangsu Higher Education
Institutions (PAPD).
References
|
1
|
Xavier RJ and Podolsky DK: Unravelling the
pathogenesis of inflammatory bowel disease. Nature. 448:427–434.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gismera CS and Aladrén BS: Inflammatory
bowel diseases: A disease(s) of modern times? Is incidence still
increasing? World J Gastroentero. 14:5491–5498. 2008. View Article : Google Scholar
|
|
3
|
Pedersen J, LaCasse EC, Seidelin JB,
Coskun M and Nielsen OH: Inhibitors of apoptosis (IAPs) regulate
intestinal immunity and inflammatory bowel disease (IBD)
inflammation. Trends Mol Med. 20:652–665. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cleynen I and Vermeire S: Paradoxical
inflammation induced by anti-TNF agents in patients with IBD. Nat
Rev Gastroenterol Hepatol. 9:496–503. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Naito Y, Takagi T and Yoshikawa T:
Molecular fingerprints of neutrophil-dependent oxidative stress in
inflammatory bowel disease. J Gastroenterol. 42:787–798. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Roessner A, Kuester D, Malfertheiner P and
Schneider-Stock R: Oxidative stress in ulcerative
colitis-associated carcinogenesis. Pathol Res Pract. 204:511–524.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rahimi R, Nikfar S and Abdollahi M:
Induction of clinical response and remission of inflammatory bowel
disease by use of herbal medicines: A meta-analysis. World J
Gastroenterol. 19:5738–5749. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rahimi R, Mozaffari S and Abdollahi M: On
the use of herbal medicines in management of inflammatory bowel
diseases: A systematic review of animal and human studies. Dig Dis
Sci. 54:471–480. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rahimi R, Shams-Ardekani MR and Abdollahi
M: A review of the efficacy of traditional iranian medicine for
inflammatory bowel disease. World J Gastroenterol. 16:4504–4514.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nell S, Suerbaum S and Josenhans C: The
impact of the microbiota on the pathogenesis of IBD: lessons from
mouse infection models. Nat Rev Microbiol. 8:564–577. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mimura T, Rizzello F, Helwig U, Poggioli
G, Schreiber S, Talbot IC, Nicholls RJ, Gionchetti P, Campieri M
and Kamm MA: Once daily high dose probiotic therapy (VSL#3) for
maintaining remission in recurrent or refractory pouchitis. Gut.
53:108–114. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hong HA, Le Duc H and Cutting SM: The use
of bacterial spore formers as probiotics. FEMS Microbiol Rev.
29:813–835. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sorokulova IB, Pinchuk IV, Denayrolles M,
Osipova IG, Huang JM, Cutting SM and Urdaci MC: The safety of two
bacillus probiotic strains for human use. Dig Dis Sci. 53:954–963.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Foligné B, Peys E, Vandenkerckhove J, Van
Hemel J, Dewulf J, Breton J and Pot B: Spores from two distinct
colony types of the strain Bacillus subtilis PB6
substantiate anti-inflammatory probiotic effects in mice. Clin
Nutr. 31:987–994. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gong Y, Li H and Li Y: Effects of
Bacillus subtilis on epithelial tight junctions of mice with
inflammatory bowel disease. J Interferon Cytokine Res. 36:75–85.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Selvam R, Maheswari P, Kavitha P,
Ravichandran M, Sas B and Ramchand CN: Effect of Bacillus
subtilis PB6, a natural probiotic on colon mucosal inflammation
and plasma cytokines levels in inflammatory bowel disease. Indian J
Biochem Biophys. 46:79–85. 2009.PubMed/NCBI
|
|
17
|
Chew BP and Park JS: Carotenoid action on
the immune response. J Nutr. 134:257S–261S. 2004.PubMed/NCBI
|
|
18
|
Nair S, Norkus EP, Hertan H and Pitchumoni
CS: Micronutrient antioxidants in gastric mucosa and serum in
patients with gastritis and gastric ulcer: Does helicobacter pylori
infection affect the mucosal levels? J Clin Gastroenterol.
30:381–385. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rumi G Jr, Szabó I, Vincze A, Matus Z,
Tóth G and Mózsik G: Decrease of serum carotenoids in crohn's
disease. J Physiology Paris. 94:159–161. 2000. View Article : Google Scholar
|
|
20
|
Yasui Y, Hosokawa M, Mikami N, Miyashita K
and Tanaka T: Dietary astaxanthin inhibits colitis and
colitis-associated colon carcinogenesis in mice via modulation of
the inflammatory cytokines. Chem Biol Interact. 193:79–87. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Trivedi PP and Jena GB: Mechanistic
insight into beta-carotene-mediated protection against ulcerative
colitis-associated local and systemic damage in mice. Eur J Nutr.
54:639–652. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bhullar KS, Parmar I and Dhillon GS:
Gastrointestinal diseases and curcumin: Developments and
challenges. Curr Res Nutr Food Sci. 2:111–113. 2014. View Article : Google Scholar
|
|
23
|
Liu H, Xu W, Chang X, Qin T, Yin Y and
Yang Q: 4,4′-diaponeurosporene, a C30 carotenoid, effectively
activates dendritic cells via CD36 and NF-κB signaling
in a ROS independent manner. Oncotarget. 7:40978–40991. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dong H, Huang Y, Yao S, Liang B, Long Y,
Xie Y, Mai J, Gong S and Zhou Z: The recombinant fusion protein of
cholera toxin B and neutrophil-activating protein expressed on
Bacillus subtilis spore surface suppresses allergic
inflammation in mice. Appl Microbiol Biotechnol. 101:5819–5829.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pazzini CA, Pereira LJ, da Silva TA,
Montalvany-Antonucci CC, Macari S, Marques LS and de Paiva SM:
Probiotic consumption decreases the number of osteoclasts during
orthodontic movement in mice. Arch Oral Biol. 79:30–34. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Starosila D, Rybalko S, Varbanetz L,
Ivanskaya N and Sorokulova I: Anti-influenza activity of a
Bacillus subtilis probiotic strain. Antimicrob Agents
Chemother. 61:e00539–e00617. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wu Z, Tang Z, Shang M, Zhao L, Zhou L,
Kong X, Lin Z, Sun H, Chen T, Xu J, et al: Comparative analysis of
immune effects in mice model: Clonorchis sinensis cysteine
protease generated from recombinant Escherichia coli and
Bacillus subtilis spores. Parasitol Res. 116:1811–1822.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang HL, Li WS, Xu DN, Zheng WW, Liu Y,
Chen J, Qiu ZB, Dorfman RG, Zhang J and Liu J: Mucosa-reparing and
microbiota-balancing therapeutic effect of Bacillus subtilis
alleviates dextrate sulfate sodium-induced ulcerative colitis in
mice. Exp Ther Med. 12:2554–2562. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Song WJ, Li Q, Ryu MO, Ahn JO, Bhang Ha D,
Jung Chan Y and Youn HY: TSG-6 Secreted by human adipose
tissue-derived mesenchymal stem cells ameliorates DSS-induced
colitis by inducing M2 macrophage polarization in mice. Sci Rep.
7:51872017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang B and Wu C: Dietary soy isoflavones
alleviate dextran sulfate sodium-induced inflammation and oxidative
stress in mice. Exp Ther Med. 14:276–282. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Mazmanian SK, Round JL and Kasper DL: A
microbial symbiosis factor prevents intestinal inflammatory
disease. Nature. 453:620–625. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chassaing B, Aitken JD, Malleshappa M and
Vijay-Kumar M: Dextran sulfate sodium (DSS)-induced colitis in
mice. Curr Protoc Immunol. 15:252014.
|
|
33
|
Ko SJ, Bu Y, Bae J, Bang YM, Kim J, Lee H,
Beom-Joon L, Hyun YH and Park JW: Protective effect of Laminaria
japonica with probiotics on murine colitis. Mediators Inflamm.
2014:4178142014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Prisciandaro L, Geier M, Butler R, Cummins
A and Howarth G: Probiotics and their derivatives as treatments for
inflammatory bowel disease. Inflamm Bowel Dis. 15:1906–1914. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Feagan BG, Sandborn WJ, Baker JP,
Cominelli F, Sutherland LR, Elson CD, Salberg B, Archambault A,
Bernstein CA, Lichtenstein GR, et al: A randomized, double-blind,
placebo-controlled, multi-center trial of the engineered human anti
body to TNF (CDP571) for steroid sparing and maintenance of
remission in patients with steroid-dependent Crohn's disease.
Gastroenterology. 118:A6552000. View Article : Google Scholar
|
|
36
|
Damaskos D and Kolios G: Probiotics and
prebiotics in inflammatory bowel disease: Microflora ‘on the
scope’. Br J Clin Pharmacol. 65:453–467. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ghouri YA, Richards DM, Rahimi EF, Krill
JT, Jelinek KA and DuPont AW: Systematic review of randomized
controlled trials of probiotics, prebiotics and synbiotics in
inflammatory bowel disease. Clin Exp Gastroenterol. 7:473–487.
2014.PubMed/NCBI
|
|
38
|
Paynich ML, Jones-Burrage SE and Knight
KL: Exopolysaccharide from Bacillus subtilis induces
anti-inflammatory M2 macrophages that prevent T cell-mediated
disease. J Immunol. 198:2689–2698. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Baykalir Gul B, Aksit D, Dogru MS, Yay
Hanım A, Aksit H, Seyrek K and Attesahin A: Lycopene ameliorates
experimental colitis in rats via reducing apoptosis and oxidative
stress. Int J Vitam Nutr Res. 1–9. 2017. View Article : Google Scholar
|
|
40
|
Glabska D, Guzek D, Zakrzewska P, Wlodarek
D and Lech G: Lycopene, lutein and zeaxanthin may reduce faecal
blood, mucus and pus but not abdominal pain in individuals with
ulcerative colitis. Nutrients. 8:E6132016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bai K, Huang Q, Zhang J, He J, Zhang L and
Wang T: Supplemental effects of probiotic Bacillus subtilis
fmbJ on growth performance, antioxidant capacity and meat quality
of broiler chickens. Poult Sci. 96:74–82. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bai WK, Zhang FJ, He TJ, Su PW, Ying XZ,
Zhang LL and Wang T: Dietary probiotic Bacillus subtilis
strain fmbj increases antioxidant capacity and oxidative stability
of chicken breast meat during storage. PLoS One. 11:e01673392016.
View Article : Google Scholar : PubMed/NCBI
|