Introduction
Lung cancer is a major cause of cancer-related
mortality worldwide (1), despite
advances in surgery, radiotherapy and chemotherapy. The majority of
patients present at an advanced stage of the disease, and advanced
lung cancer remains an important area of research (2). Advanced lung cancer is frequently
treated with radiation; however, the use of high radiation doses
and large lung volumes results in a high likelihood of radiation
pneumonitis, which is defined as inflammation in the lung following
radiation (3,4). Radiation pneumonitis has become a
clinical problem due to its high incidence, reported to be
29.6–50.3% in Japan from 1983 to 1992, and due to the difficulty in
treating it (5,6). Previous studies have demonstrated that
antioxidant molecules are able to protect the lung from
radiation-induced damage (7,8). There has been a growing interest in the
use of natural antioxidants derived from plants as additives in
cosmetics and food (9), and
traditional Chinese medicines, including quercetin (Que;
3,3,4,5,7-pentahydroxyflavone), curcumin and resveratrol are able
to mitigate the inflammation induced by irradiation (10,11).
Que is a member of the flavonol subclass of
flavonoids, and is usually present in a glycosylated form (12). The major sources of Que are fruits,
including apples, berries and cherries, vegetables, including
onions and broccoli, and beverages, including red wine and tea
(13). Que has been also identified
in several medicinal plants, such as Ginkgo biloba,
Aesculus hippocastanum and Hypericum perforatum
(14). Que has been applied in
various fields, due to its anti-inflammatory, antiviral,
anti-allergy, antioxidant, anti-asthmatic and antitumor activities
(15). Que has been reported to have
protective effects against liver injury caused by chronic toluene
exposure in rats (16). Despite this
wide spectrum of pharmacological properties, the use of Que in the
pharmaceutical field is limited due to its poor aqueous solubility,
instability in physiological media and rapid photodegradation
(17–19). The clinical application of Que is
limited by its poor bioavailability and low stability in aqueous
medium (20). The achievement of
effective concentrations of Que in the lung tissue is difficult;
therefore, higher doses and administration frequency are required,
which leads to higher treatment costs as well as increased side
effects, such as arthralgia and inflammatory response (21). Thus, it is necessary to develop new
dosage forms and administration routes for Que with increased
solubility and improved bioavailability. Inhalation therapy holds
great potential for the effective treatment of various diseases,
such as lung cancer. An inhalation therapy approach to respiratory
disease, such as asthma, chronic obstructive pulmonary disease and
lung cancer, is a promising means of improving therapeutic
efficiency and minimizing unwanted systemic toxicity (22). A number of drugs, including
dichromate flavonoid derivative and inhalative beclomethasone, have
been investigated in vitro, in animal models and in human
trials as targeted aerosol chemotherapies for lung cancer (23–25).
Previous studies have also reported that lipid microparticles
loaded with Que have enhanced stability in topical formulations
(26–29). However, studies on the use of Que
inhalation to treat radiation pneumonitis are lacking.
The aim of the present was to evaluate the
preventive effect of inhaled Que on radiation pneumonitis using a
rat model of induced radiation pneumonitis, and to identify a novel
route of administration for Que.
Materials and methods
Materials
Pentobarbital sodium was purchased from Sinopharm
Chemical Reagent Co., Ltd. (Shanghai, China); immunohistochemistry
kits for the detection of interleukin (IL)-6 (cat no. EIA-1008) and
transforming growth factor (TGF)-β1 (cat no. EIA-1122)
were supplied by Beijing Zhongshan Golden Bridge Biotechnology Co.,
Ltd. (Beijing, China); Que was supplied by Wuhan Tianyu Technology
Co., Ltd. (Wuhan, China). Sodium dihydrogen phosphate and sodium
hydrogen phosphate were purchased from Shanghai Chemical Reagent
Co., Ltd. (Shanghai, China; batch nos. 20070604 and 20070509,
respectively). All other chemicals and solvents were reagent
grade.
Animals
A total of 48 male Wistar rats (5–6 weeks old;
150–180 g) were obtained from Shandong Lukang Animal Center (animal
license no., Slxklu no. 2011002; Jining, China). The animals were
maintained in a room at 23±2°C, with a relative humidity of 50±5%,
and a 12-h light/dark cycle. The rats were given a standard
laboratory diet and water ad libitum. The study was approved
by the Ethics Committee of Weifang Medical University (Weifang,
China). All animal procedures were conducted in accordance with the
rules and regulations approved by the Animal Care and Use Committee
for Biological Studies of Shandong Province (Weifang, China).
Rat model of radiation
pneumonitis
The 48 Wistar rats were randomly divided into four
groups (each n=12) for treatment via inhalation as follows: i) No
treatment (normal group); ii) 20 ml 0.9% normal saline (model
group); iii) 20 ml inhaled Que at a dose of 10 mg/100 g (Que
group), as previously described (30); and iv) 20 ml dexamethasone at a dose
of 5×10−4 mg/100 g (positive control group). Rats in the
normal, model, Que and positive control groups were exposed to
X-ray irradiation of the pulmonary apex (15 Gy) using a 6-MV linear
accelerator (ELEKTA AB, Stockholm, Sweden) to establish a radiation
pneumonitis model. The 15-Gy dose was delivered to the pulmonary
apex in a single fraction. For administration via inhalation,
animals were placed in a 15×15×20 cm Plexiglass box, and treatment
was administered using an air compressor pump atomizer (PARI
TurboBoy nebulizer; PARI GmbH, Starnberg, Germany) from a week
before irradiation. Rats were administered the treatment by
atomization for 30 min once a day, for 4 months.
Erythrocyte and leukocyte counts in
blood
At 1, 2, 3 and 4 months, a quarter of the rats from
each group were anesthetized with 0.3% pentobarbital (3 mg/100 g)
by intraperitoneal injection, the chest was opened to expose the
heart, and a 2 ml sample of blood was taken from the heart. The
blood was placed in an EDTA-K2 vacuum tube for measurement of the
number of erythrocytes and leukocytes using a Sysmex KX-21
automated hematology analyzer (Sysmex Corporation, Osaka,
Japan).
Bronchoalveolar lavage fluid (BALF)
analysis
At 1, 2, 3 and 4 months, a quarter of the rats from
each group were anesthetized and the trachea was cannulated. BALF
was collected by flushing the lung with 2 ml PBS via the trachea
immediately following sacrifice. Approximately 5.0 ml BALF was
recovered following three lavages. The BALF was centrifuged (4°C,
3,230 × g, 10 min) and the supernatant was stored at −80°C. The
pellet was resuspended in 5 ml PBS. The number of leukocytes was
measured using a Sysmex KX-21 automated hematology analyzer.
Histological analysis with hematoxylin
and eosin staining
Following the collection of BALF, lung tissues were
fixed in 10% paraformaldehyde for 24 h at room temperature, then
paraffin-embedded, sectioned at 5–8 µm thickness, and subjected to
routine hematoxylin (5 min) and eosin staining (2–3 min) (31) at room temperature for observation
using a 37XC microscope (Shanghai Optical Instrument Factory Co.,
Ltd., Shanghai, China).
Immunohistochemistry
Immunohistochemical detection of IL-6 and
TGF-β1 was performed using immunohistochemistry kits.
The lung sections (prepared using the same steps mentioned above)
were incubated with 0.3% hydrogen peroxide for 20 min at room
temperature to block endogenous peroxidase activity, followed by
rinsing with PBS, pH 7.4, and the absorption of excess fluid using
filter paper. The sections were then treated with rabbit
anti-murine IL-6 (EIA: 1008) or TGF-β1 (EIA: 1122)
antibody (1:10 dilution; Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd., Beijing, China) overnight at 4°C.
Subsequently, the sections were treated with polymer enhancer
(Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.) for 20
min at 37°C, followed by rinsing with PBS and the absorption of
excess fluid using filter paper. The sections were then treated
with murine anti-rabbit antibody (1:100 dilution) for 30 min at
37°C, washed and visualized by 3,3-diaminobenzidine
tetrahydrochloride DAB. Finally, the sections were counter-stained
with hematoxylin for 5 min at room temperature. For each section,
five random microscopic fields were selected and observed. The
total cell count and positive cell count were calculated in order
to determine the percentage of positive cells. The positive cells
were counted using the system: − −(strong negative sample),
−(negative sample), +(positive sample) and ++ (strong positive
sample).
Statistical analysis
Data are reported as the mean ± standard deviation.
Statistical analysis was performed using SPSS 13.0 software (SPSS,
Inc., Chicago, IL, USA). Data were analyzed using one-way analysis
of variance followed by the Least Significant Difference post hoc
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Behavior and general observations
Rats in the normal group exhibited significant
activity, fine and smooth fur, and normal body weight. In all
groups exposed to radiation, arched backs, piloerection, quietness
and a reduction in body weight were observed in the first month.
For rats in the Que group, the symptoms of arched back and
piloerection were alleviated and body weight was gradually
increased in the second month. Rats in the model group exhibited
significantly delayed reactions, dispiritedness, and a reduction in
body weight compared with the normal group. In the Que inhalation
group, weight loss was prevented, and the rats had significantly
higher body weights in comparison with the model group from the
second month after irradiation until the end of the study. The
positive control group was generally comparable with the Que
group.
Erythrocyte and leukocyte counts in
blood
Erythrocytes and leukocytes were counted following
irradiation of the whole thorax (Tables
I and II, respectively). There
were a significantly higher number of erythrocytes in the positive
control group compared with the model group at 3 months
(P<0.05). However, the number of leukocytes was decreased
following irradiation in the model, Que and positive control groups
during the first three months. There was a significant difference
in the number of leukocytes between the model and normal groups at
1, 2 and 3 months (P<0.05), and no significant difference
between the normal and Que groups at any time-point (P>0.05).
Furthermore, at 4 months after irradiation, the numbers of
erythrocytes and leukocytes in irradiated rats treated with Que was
increased compared with those in the model group. These data
indicate that Que inhalation ameliorates the inflammatory response
in the blood of rats following whole-thorax irradiation.
 | Table I.Effect of Que on radiation-induced
erythrocytes in blood (mean ± standard deviation,
×1012/l). |
Table I.
Effect of Que on radiation-induced
erythrocytes in blood (mean ± standard deviation,
×1012/l).
| Time (months) | Normal | Model | Que | Positive
control |
|---|
| 1 | 6.75±0.37 | 7.38±1.15 | 7.40±0.41 | 6.89±0.34 |
| 2 | 7.23±0.16 | 7.34±0.40 | 7.04±0.10 | 7.12±0.21 |
| 3 | 7.10±0.14 | 6.88±0.29 | 7.13±0.18 |
7.43±0.11a |
| 4 | 7.46±0.49 | 7.91±0.89 | 8.13±1.09 | 7.63±0.51 |
 | Table II.Effect of Que on radiation-induced
leukocytes in blood (mean ± standard deviation,
×109/l). |
Table II.
Effect of Que on radiation-induced
leukocytes in blood (mean ± standard deviation,
×109/l).
| Time (months) | Normal | Model | Que | Positive
control |
|---|
| 1 |
7.87±1.20a | 4.84±0.74 | 5.72±1.84 |
4.62±0.84b |
| 2 |
10.55±0.30a | 5.98±1.38 |
6.80±0.95b |
6.82±0.92b |
| 3 |
6.54±0.96a | 4.66±0.47 | 5.08±0.72 | 4.97±1.04 |
| 4 | 4.28±1.40 | 4.93±0.58 | 5.20±1.49 | 4.68±0.24 |
Leukocytes in BALF
To evaluate the effect of Que on inflammatory cells
in BALF, leukocytes were measured following irradiation of the
whole thorax (Table III). The
number of leukocytes in the BALF peaked in the model group at 2
months after irradiation, and then decreased at 3 and 4 months. At
2 months, the number of leukocytes in the model group was
significantly increased compared with that in the normal group
(P<0.05). However, no significant difference was observed
between the Que and normal groups (P>0.05), or between the Que
and positive control groups (P>0.05) at this time-point. There
were significant differences in the number of leukocytes between
the model group and normal, Que, positive control group at 3 months
(P<0.05). At 4 months after irradiation, the number of
leukocytes of the model and Que groups was markedly decreased
compared with that at the first month.
 | Table III.Effect of Que on radiation-induced
leukocytes in bronchoalveolar lavage fluid (mean ± standard
deviation, ×109/l). |
Table III.
Effect of Que on radiation-induced
leukocytes in bronchoalveolar lavage fluid (mean ± standard
deviation, ×109/l).
| Time (months) | Normal | Model | Que | Positive
control |
|---|
| 1 |
2.82±0.95a | 5.80±0.89 |
2.52±0.79a |
2.62±0.48a |
| 2 |
2.47±1.06a | 10.55±1.47 |
2.94±1.27a |
2.42±0.12a |
| 3 |
2.44±0.05a | 6.98±1.04 |
1.31±0.25a |
2.37±1.54a |
| 4 |
2.58±0.22a | 4.81±0.47 |
1.10±1.13a,b |
2.78±0.54a |
Lung histopathological changes
To examine the changes in lung tissue following
thoracic irradiation combined with Que treatment, the extent of
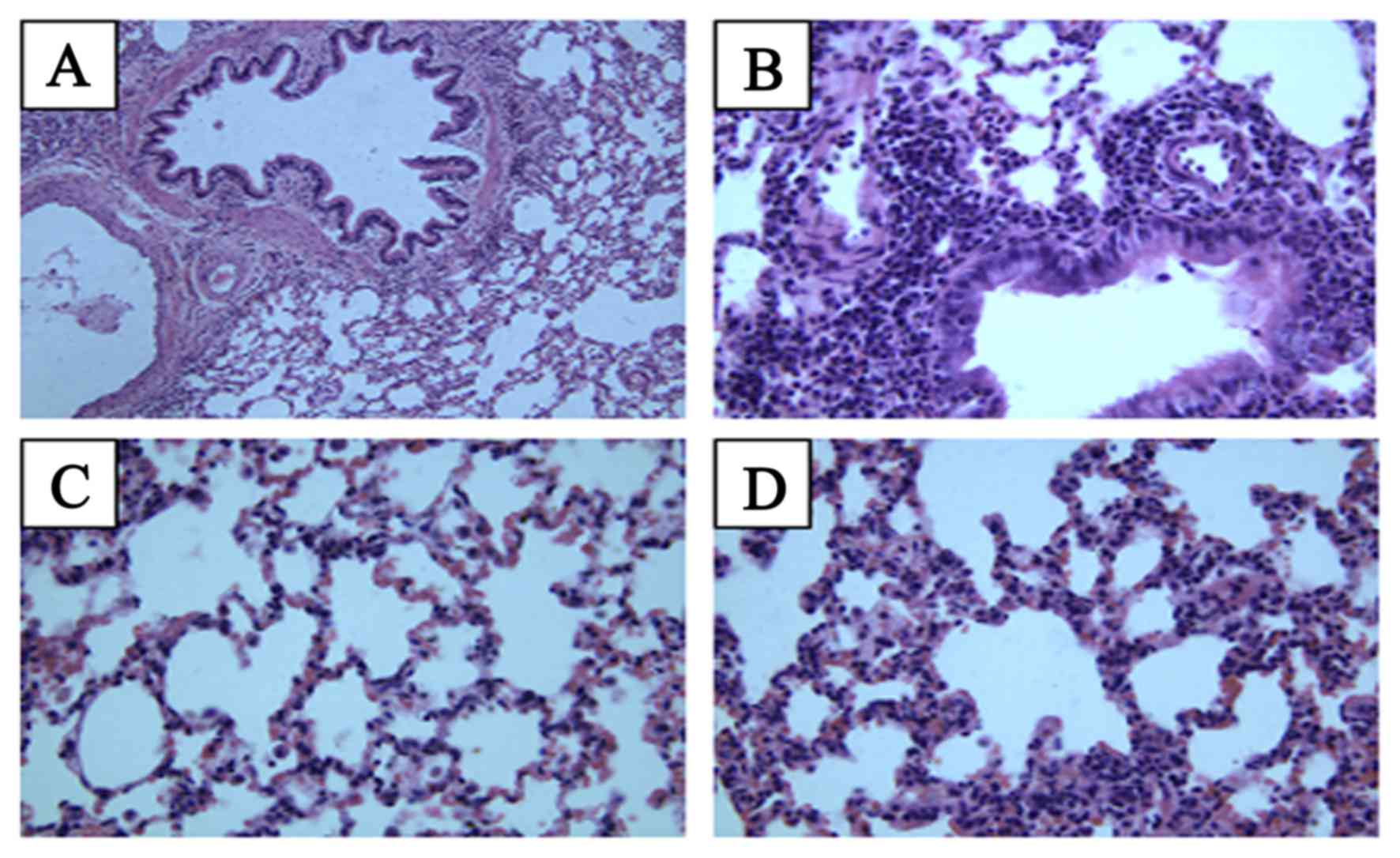
lung inflammation was assessed histologically at 2 months (Fig. 1) and 4 months (Fig. 2) following irradiation. In the normal
group, the lung tissue exhibited normal lung alveoli, normal
alveolar septum and well-distributed alveolar walls at 2 months;
infiltration of inflammatory cells was not evident, and slight
hemorrhaging was observed. In the model group, infiltration of
inflammatory cells was evident at 2 months after thoracic
irradiation, and extensive hemorrhaging were observed. In the model
group, lung injury was evident with destruction of the alveoli,
thickened alveolar septum and decreased alveolar spaces. In the Que
group, slight hemorrhages and normal lung alveoli were observed,
and infiltration of inflammatory cells was not evident. There was
no clear difference between the Que group and the positive control
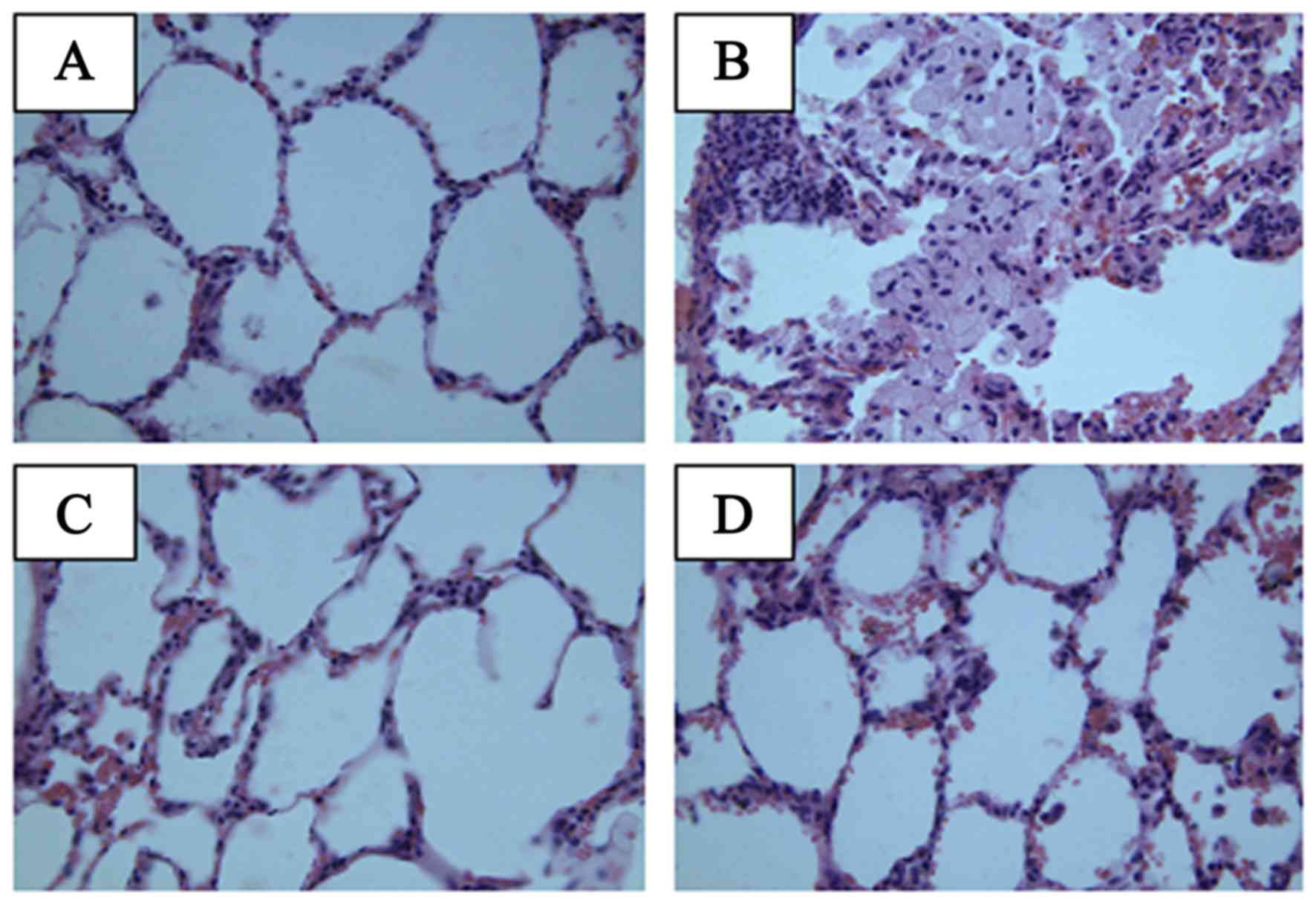
group. At 4 months, the lung tissue exhibited normal lung alveoli,
thinning of the alveolar septum and increased alveolar spaces in
the normal group. In the lung tissue of the model group, slight
hemorrhages, thin alveolar septum and increased alveolar spacing
were observed. However, Que clearly reduced the local inflammation
caused by irradiation, and infiltration of inflammatory cells was
not evident.
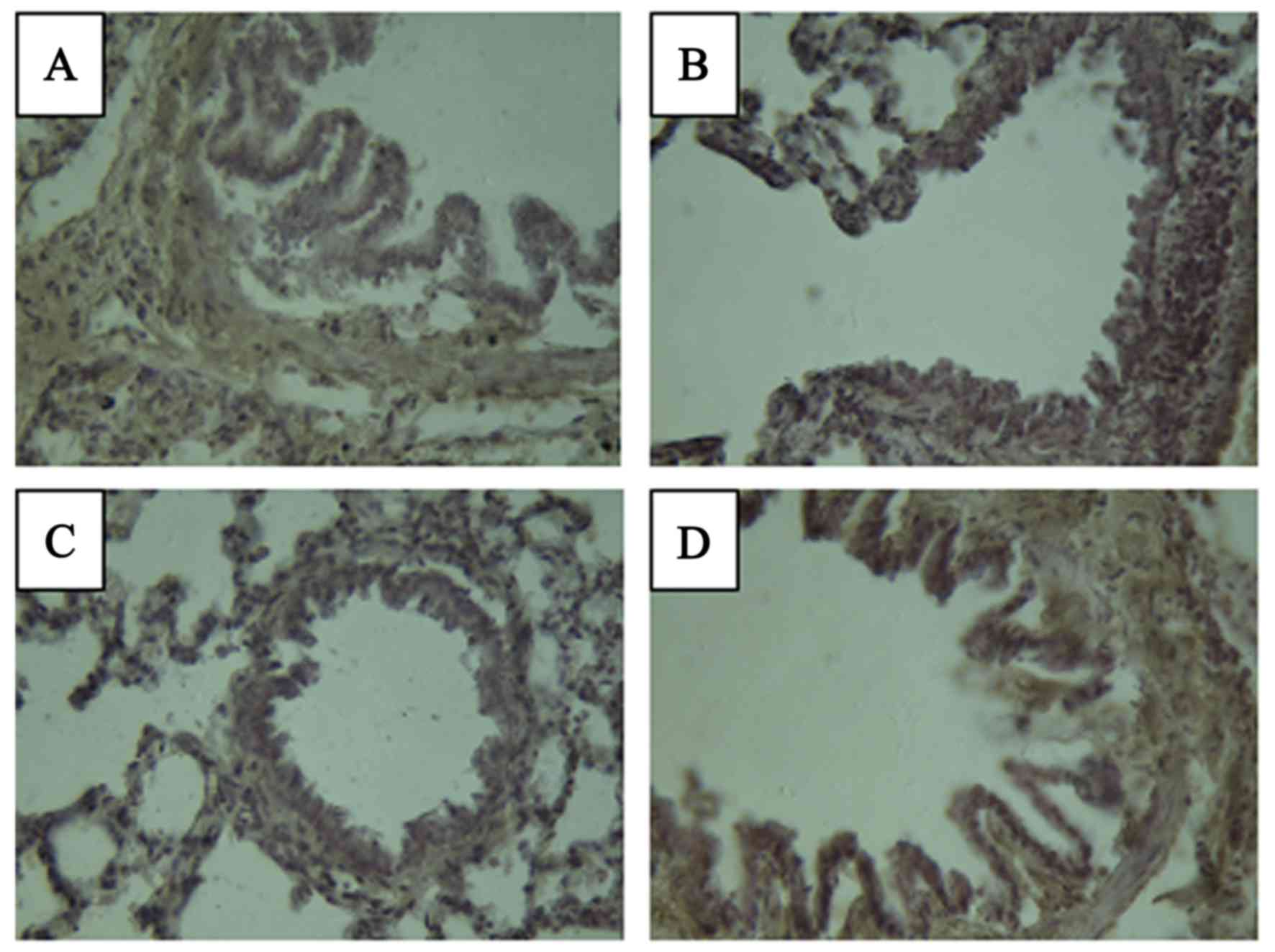
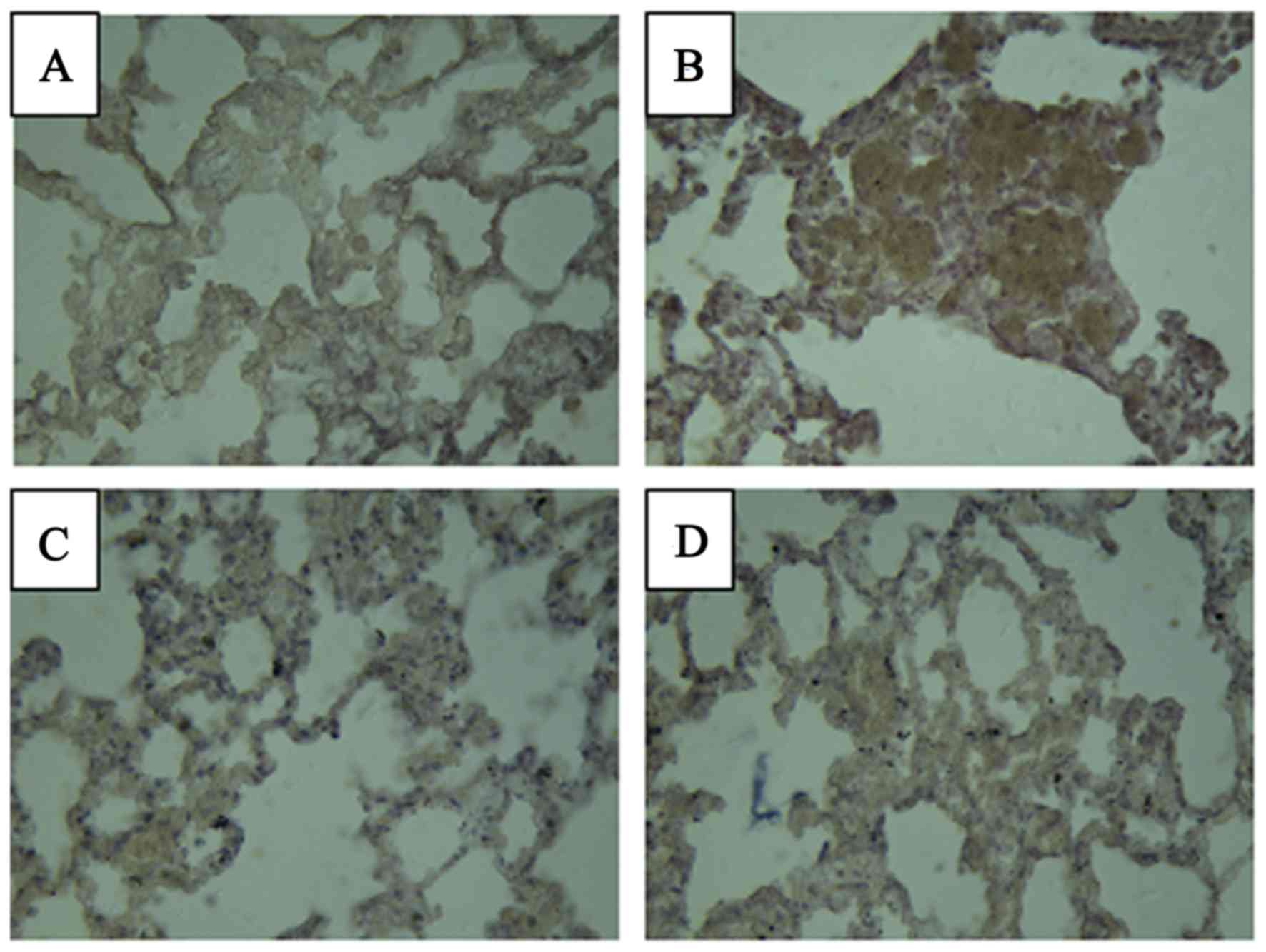
Levels of TGF-β1 and
IL-6
The levels of TGF-β1 and IL-6 in the lung
tissue were measured at 4 months after radiation (Figs. 3 and 4
and Table IV). In the normal group,
brown staining indicating the presence of inflammatory cytokines
was not visible. In the model group, the expression of
TGF-β1 was observed primarily in bronchial epithelial
cells, peripheral lymphocyte and macrophages as brown staining of
the lung tissue. However, the group treated with Que had only a
small amount of brown staining of TGF-β1 in the
bronchial epithelial cells. Expression of IL-6 was not observed in
the normal group, as brown staining was not evident in the
cytoplasm. In the model group, the expression of IL-6 was primarily
observed in the alveolar space. The group treated with Que had only
a small amount of brown staining of IL-6. No notable differences
were observed in the levels of TGF-β1 and IL-6 in the
Que and positive control groups compared with the normal group.
 | Table IV.Effect of Que on radiation-induced
levels of TGF-β1 and IL-6 in bronchoalveolar lavage
fluid. |
Table IV.
Effect of Que on radiation-induced
levels of TGF-β1 and IL-6 in bronchoalveolar lavage
fluid.
| Cytokine | Normal | Model | Que | Positive
control |
|---|
|
TGF-β1 | − − | ++ | + | + |
| IL-6 | − − | ++ | + | + |
Discussion
Radiation pneumonitis is a fairly common subacute
side effect of radiotherapy in patients with lung cancer and the
incidence is high. The incidence likely arises from different
patient populations, the subjective scoring of radiation
pneumonitis and treatment-related factors (32). In the present study, a rat model was
used to investigate the effect of inhaled Que on the adverse
effects of radiotherapy for lung cancer. The results demonstrated
that the administration of Que by inhalation decreased inflammation
in the lung. Previous studies have shown that rats survived
pneumonitis following exposure to irradiation doses of 12, 13 and
20 Gy (33,34). In the present study, rats were
exposed to radiation of the apex pulmonis (15 Gy) using 6-MV
linearly accelerated X-rays to establish a radiation pneumonitis
model. In the model group, numerous symptoms were evident following
irradiation, including piloerection, arching of the back, quietness
and weight loss, which demonstrated that the model was successful.
Numerous studies have administered agents prior to radiation
exposure to evaluate their ability to act as radiation protective
agents, such as TNF-α and TGF-β inhibitors (35,36).
However, it is necessary to investigate and identify agents that
are effective for the reduction of radiation-induced lung injury
when administered following irradiation (37).
The effects of Que on radiation-induced lung injury
are unclear (30). The present study
demonstrated that Que inhalation decreased the number of
inflammatory cells in rats irradiated with X-rays. Que reduced the
characteristics of inflammation, including the infiltration of
inflammatory cells. The number of inflammatory cells, including
lymphocytes (38), neutrophils
(39) and alveolar macrophages
(40) increases following
irradiation. However, the effect of Que on airway inflammation
remains largely unknown (30). Que
has the ability to suppress radiation-induced inflammation and has
beneficial effects on the lungs; however, it also has poor
solubility, low bioavailability and low absorption (41,42). In
the present study, Que was administered by nebulization to maintain
Que at high local concentrations (29). Drug delivery to the respiratory tract
via inhalation is influenced by the properties of the inhaled
particles as well as the breathing patterns of the experimental
animals (43). The ability of a
system to efficiently deliver a drug to the animal respiratory
tract depends particularly on the inhaled particles' properties
(44). Compressed air nebulizers
produce aerosols with small-sized particles from drug-containing
solutions and these aerosols have suitable characteristics for
animal testing (27). In the present
study, rats were treated using compressor pump atomization.
In all groups exposed to radiation, the rats
exhibited arched backs, piloerection and a reduction in body
weight. In the model group, infiltration of inflammatory cells was
evident following thoracic irradiation, and extensive hemorrhaging
was observed. Lung injury was manifested by destruction of the
alveoli, thickened alveolar septa and decreased alveolar spaces.
Histopathological examination revealed that Que reduced the
radiation-induced histopathological changes compared with those in
saline-treated model rats.
The present study demonstrated that irradiation of
the thorax affected the chronic inflammatory response in the lung
tissue. Previous studies have shown that pro-inflammatory
cytokines, including TGF-β1, IL-6 and tumor necrosis
factor-α, are induced by irradiation of the thorax (34,42). In
the present study, the levels of TGF-β1 and IL-6 were
elevated by lung tissue challenge with radiation, and were reduced
in the Que group compared with the model group. Histopathological
investigation revealed increased submucosal and airway wall
thickening following irradiation. In the model group, the levels of
TGF-β1 and IL-6 were increased compared with those in
the normal group. The infiltration of inflammatory cells was
markedly attenuated by Que administration. Brown staining of
lymphocytes and macrophages was observed in the lung tissue of
model group. However, the group treated with Que inhalation only
had a small amount of brown staining. Radiation injury is
characterized by capillary permeability, edema and excessive
fibroblast proliferation within the alveolar septa (34,42). The
levels of TGF-β1 and IL-6 were markedly inhibited
following Que administration compared with those in the model
group. These results suggest that Que is an excellent candidate for
use as an adjuvant therapy for radiation pneumonitis.
In conclusion, the present study demonstrated the
preventive effect of Que inhalation on radiation pneumonitis in a
rat model. The administration of Que using a nebulizer inhibited
the inflammatory reaction of radiation pneumonitis by increasing
the number of leukocytes and erythrocytes in the blood and
decreasing the number of leukocytes in the BALF, and decreasing the
levels of TGF-β1 and IL-6. The results of histological
observation also indicated that Que inhalation ameliorated
radiation-induced hemorrhage, inflammation and fibrosis, and
reduced inflammatory cell infiltration in the respiratory tract.
These findings suggest that Que inhalation may be effective in
protecting the lung tissue of radiation pneumonitis and is
potentially clinically useful for use in patients with lung cancer
who require radiotherapy.
Acknowledgements
The authors are grateful for generous financial
support from the National Natural Science Foundation of China
(grant nos. 81573717 and 81774125), the National Key Technology
R&D Program of the Ministry of Science and Technology (grant
no. 2013GA740103), the Visiting Scholar Project of Weifang Medical
University and project of Collaborative Innovation Center for
Target Drug Delivery System of Weifang Medical University (no.
2017).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kuhar M, Sen S and Singh N: Role of
mitochondria in quercetin-enhanced chemotherapeutic response in
human non-small cell lung carcinoma H-520 cells. Anticancer Res.
26:1297–1303. 2006.PubMed/NCBI
|
|
3
|
Graham MV, Purdy JA, Emami B, Harms W,
Bosch W, Lockett MA and Perez CA: Clinical dose-volume histogram
analysis for pneumonitis after 3D treatment for non-small cell lung
cancer (NSCLC). Int J Radiat Oncol Biol Phys. 45:323–329. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kwa SL, Lebesque JV, Theuws JC, Marks LB,
Munley MT, Bentel G, Oetzel D, Spahn U, Graham MV, Drzymala RE, et
al: Radiation pneumonitis as a function of mean lung dose: An
analysis of pooled data of 540 patients. Int J Radiat Oncol Biol
Phys. 42:1–9. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nair MP, Mahajan S, Reynolds JL, Aalinkeel
R, Nair H, Schwartz SA and Kandaswami C: The flavonoid quercetin
inhibits proinflammatory cytokine (tumor necrosis factor alpha)
gene expression in normal peripheral blood mononuclear cells via
modulation of the NF-kappa beta system. Clin Vaccine Immunol.
13:319–328. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Watanabe H, Suga A, Tsuchihashi Y, Hori A,
Kawakami K, Masaki H, Akiyama M, Ohishi K, Takahashi A, Nagatake T,
et al: Clinical study of radiation pneumonitis over 10 years. Nihon
Kyobu Shikkan Gakkai Zasshi. 33:384–388. 1995.(In Japanese).
PubMed/NCBI
|
|
7
|
Lee JC, Kinniry PA, Arguiri E, Serota M,
Kanterakis S, Chatterjee S, Solomides CC, Javvadi P, Koumenis C,
Cengel KA and Christofidou-Solomidou M: Dietary curcumin increases
antioxidant defenses in lung, ameliorates radiation-induced
pulmonary fibrosis, and improves survival in mice. Radiat Res.
173:590–601. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu L, Huang Z, Qin P, Yao Y, Meng X, Zou
J, Zhu K and Ren G: Chemical characterization of a procyanidin-rich
extract from sorghum bran and its effect on oxidative stress and
tumor inhibition in vivo. J Agric Food Chem. 59:8609–8615. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sharma SD, Meeran SM and Katiyar SK:
Proanthocyanidins inhibit in vitro and in vivo growth of human
non-small cell lung cancer cells by inhibiting the prostaglandin
E(2) and prostaglandin E(2) receptors. Mol Cancer Ther. 9:569–580.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Comalada M, Camuesco D, Sierra S,
Ballester I, Xaus J, Gálvez J and Zarzuelo A: In vivo quercitrin
anti-inflammatory effect involves release of quercetin, which
inhibits inflammation through down-regulation of the NF-kappaB
pathway. Eur J Immunol. 35:584–592. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bournival J, Plouffe M, Renaud J,
Provencher C and Martinoli MG: Quercetin and sesamin protect
dopaminergic cells from MPP+-induced neuroinflammation
in a microglial (N9)-neuronal (PC12) coculture system. Oxid Med
Cell Longev. 2012:921–941. 2012. View Article : Google Scholar
|
|
12
|
Grande F, Parisi OI, Mordocco RA, Rocca C,
Puoci F, Scrivano L, Quintieri AM, Cantafio P, Ferla S, Brancale A,
et al: Quercetin derivatives as novel antihypertensive agents:
Synthesis and physiological characterization. Eur J Pharrm Sci.
82:161–170. 2016. View Article : Google Scholar
|
|
13
|
Boots AW, Haenen GR and Bast A: Health
effects of quercetin: From antioxidant to nutraceutical. Eur J
Pharmacol. 585:325–337. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grande F, Parisi OI, Mordocco RA, Rocca C,
Puoci F, Scrivano L, Quintieri AM, Cantafio P, Ferla S, Brancale A,
et al: Quercetin derivatives as novel antihypertensive agents:
Synthesis and physiological characterization. Eur J Pharm Sci.
82:161–170. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brito AF, Ribeiro M, Abrantes AM, Pires
AS, Teixo RJ, Tralhão JG and Botelho MF: Quercetin in cancer
treatment, alone or in combination with conventional therapeutics?
Curr Med Chem. 22:3025–3039. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kanter M: Protective effect of quercetin
on liver damage induced by chronic toluene exposure in rats.
Toxicol Ind Health. 28:483–491. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fasolo D, Schwingel L, Holzschuh M,
Bassani V and Teixeira H: Validation of an isocratic LC method for
determination of quercetin and methylquercetin in topical
nanoemulsions. J Pharm Biomed Anal. 44:1174–1177. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kumari A, Yadav SK, Pakade YB, Singh B and
Yadav SC: Development of biodegradable nanoparticles for delivery
of quercetin. Colloids Surf B Biointerfaces. 80:184–192. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Aytac Z, Kusku SI, Durgun E and Uyar T:
Quercetin/β-cyclodextrin inclusion complex embedded nanofibres:
Slow release and high solubility. Food Chem. 197:864–871. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chimento A, Sala M, Gomez-Monterrey IM,
Musella S, Bertamino A, Caruso A, Sinicropi MS, Sirianni R, Puoci
F, Parisi OI, et al: Biological activity of 3-chloro-azetidin-2-one
derivatives having interesting antiproliferative activity on human
breast cancer cell lines. Bioorg Med Chem Lett. 23:6401–6405. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao J, Liu J, Wei T, Ma X, Cheng Q, Huo
S, Zhang C, Zhang Y, Duan X and Liang XJ: Quercetin-loaded
nanomicelles to circumvent human castration-resistant prostate
cancer in vitro and in vivo. Nanoscale. 8:5126–5138. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Crompton G: A brief history of inhaled
asthma therapy over the last fifty years. Prim Care Respir J.
15:326–331. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Otterson GA, Villalona-Calero MA, Sharma
S, Kris MG, Imondi A, Gerber M, White DA, Ratain MJ, Schiller JH,
Sandler A, et al: Phase I study of inhaled Doxorubicin for patients
with metastatic tumors to the lungs. Clin Cancer Res. 13:1246–1252.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Otterson GA, Villalona-Calero MA, Hicks W,
Pan X, Ellerton JA, Gettinger SN and Murren JR: Phase I/II study of
inhaled doxorubicin combined with platinum-based therapy for
advanced non-small cell lung cancer. Clin Cancer Res. 16:2466–2473.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Moon H, Choi HH, Lee JY, Moon HJ, Sim SS
and Kim CJ: Quercetin inhalation inhibits the asthmatic responses
by exposure to aerosolized-ovalbumin in conscious guinea-pigs. Arch
Pharm Res. 31:771–778. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Scalia S, Haghi M, Losi V, Trotta V, Young
PM and Traini D: Quercetin solid lipid microparticles: A flavonoid
for inhalation lung delivery. Eur J Pharm Sci. 49:278–285. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Scalia S, Trotta V, Traini D, Young PM,
Sticozzi C, Cervellati F and Valacchi G: Incorporation of quercetin
in respirable lipid microparticles: Effect on stability and
cellular uptake on A549 pulmonary alveolar epithelial cells.
Colloids Surf B Biointerfaces. 112:322–329. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Scalia S and Mezzena M: Incorporation of
quercetin in lipid microparticles: Effect on photo- and
chemical-stability. J Pharm Biomed Anal. 49:90–94. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Takashima K, Matsushima M, Hashimoto K,
Nose H, Sato M, Hashimoto N, Hasegawa Y and Kawabe T: Protective
effects of intratracheally administered quercetin on
lipopolysaccharide-induced acute lung injury. Respir Res.
15:1502014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chang YC, Tsai MH, Sheu WH, Hsieh SC and
Chiang AN: The therapeutic potential and mechanisms of action of
quercetin in relation to lipopolysaccharide-induced sepsis in vitro
and in vivo. PLoS One. 8:e807442013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Fischer AH, Jacobson KA, Rose J and Zeller
R: Hematoxylin and eosin staining of tissue and cell sections. CSH
Protoc. 2008:pdb.prot49862008.PubMed/NCBI
|
|
32
|
Henkenberens C, Janssen S, Lavae-Mokhtari
M, Leni K, Meyer A, Christiansen H, Bremer M and Dickgreber N:
Inhalative steroids as an individual treatment in symptomatic lung
cancer patients with radiation pneumonitis grade II after
radiotherapy - a single-centre experience. Radiat Oncol. 11:122016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chiang CS, Liu WC, Jung SM, Chen FH, Wu
CR, McBride WH, Lee CC and Hong JH: Compartmental responses after
thoracic irradiation of mice: Strain differences. Int J Radiat
Oncol Biol Phys. 62:862–871. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rübe CE, Wilfert F, Palm J, König J,
Burdak-Rothkamm S, Liu L, Schuck A, Willich N and Rübe C:
Irradiation induces a biphasic expression of pro-inflammatory
cytokines in the lung. Strahlenther Onkol. 180:442–448. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tsoutsou PG: The interplay between
radiation and the immune system in the field of post-radical
pneumonitis and fibrosis and why it is important to understand it.
Expert Opin Pharmacother. 15:1781–1783. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Henkenberens C, Janssen S, Lavae-Mokhtari
M, Leni K, Meyer A, Christiansen H, Bremer M and Dickgreber N:
Inhalative steroids as an individual treatment in symptomatic lung
cancer patients with radiation pneumonitis grade II after
radiotherapy-a single-centre experience. Radiat Oncol. 11:122016.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pietrofesa R, Turowski J, Tyagi S, Dukes
F, Arguiri E, Busch TM, Gallagher-Colombo SM, Solomides CC, Cengel
KA and Christofidou-Solomidou M: Radiation mitigating properties of
the lignan component in flaxseed. BMC Cancer. 13:1792013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Adawi A, Zhang Y, Baggs R, Rubin P,
Williams J, Finkelstein J and Phipps RP: Blockade of CD40-CD40
ligand interactions protects against radiation-induced pulmonary
inflammation and fibrosis. Clin Immunol Immunopathol. 89:222–230.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Thrall RS, Phan SH, McCormick JR and Ward
PA: The development of bleomycin-induced pulmonary fibrosis in
neutrophil-depleted and complement-depleted rats. Am J Pathol.
105:76–81. 1981.PubMed/NCBI
|
|
40
|
Rubin P, Finkelstein J and Shapiro D:
Molecular biology mechanisms in the radiation induction of
pulmonary injury syndromes: Interrelationship between the alveolar
macrophage and the septal fibroblast. Int J Radiat Oncol Biol Phys.
24:93–101. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cai X, Fang Z, Dou J, Yu A and Zhai G:
Bioavailability of quercetin: Problems and promises. Curr Med Chem.
20:2572–2582. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu H, Xue JX, Li X, Ao R and Lu Y:
Quercetin liposomes protect against radiation-induced pulmonary
injury in a murine model. Onocl Lett. 6:453–459. 2013.
|
|
43
|
Suarez S and Hickey AJ: Drug properties
affecting aerosol behavior. Respir Care. 45:652–666.
2000.PubMed/NCBI
|
|
44
|
Hrvacić B, Bosnjak B, Tudja M, Mesić M and
Merćep M: Applicability of an ultrasonic nebulization system for
the airways delivery of beclomethasone dipropionate in a murine
model of asthma. Pharm Res. 23:1765–1775. 2006. View Article : Google Scholar : PubMed/NCBI
|