Introduction
Immunoglobulin light chain amyloidosis (AL)
amyloidosis is a monoclonal plasma cell disorder in which the
precursor protein is an immunoglobulin light chain or light chain
fragment. It may occur as a primary disease or in association with
multiple myeloma or other plasma cell dyscrasias. In primary
amyloidosis, there is 2:1 preponderance for λ over κ light chain
synthesis (1). AL amyloidosis is the
commonest type of amyloidosis, accounting for about 85% of all
newly diagnosed cases. Cardiac amyloidosis (CA) describes a disease
in which cardiac extracellular space is expanded by a type of
amorphous, fibril protein known as amyloid (2). While 31 subtypes of protein are known
to deposit as amyloid, only 11 have been identified involving the
heart (3), and AL amyloidosis is the
most common subtypes (4). The heart
is affected in >50% of cases (5),
and symptomatic cardiac involvement portends a worse prognosis
(6,7). Conversely, involvement limited to the
heart constitutes <5% of patients with AL amyloidosis. Cardiac
contractile function and electrical conduction can be impaired with
amyloid infiltration. At a cellular level, amyloid infiltration
results in abnormal cellular metabolism, calcium transport and
receptor regulation. Cardiac involvement with resultant heart
failure or arrhythmia accounts for >50% of the mortality in
patients with AL amyloidosis (1,7).
Histological examination remains the definitive diagnostic modality
in CA (8). While not definitive,
certain non-invasive imaging and laboratory findings may guide
further diagnostic testing and management and assess the severity
of the disease for prognostic purposes (9). Appropriate treatment of amyloid
disorders requires the correct identification of the subtype of
amyloid protein and severity of cardiac involvement, as patients
with advanced cardiomyopathy are unsuitable candidates for
effective hematological treatment including autologous stem cell
transplantation (10).
Thromboembolism also contributes significantly to
morbidity and mortality. Intracardiac thrombosis was found in 51%
(11) and 35% (12) patients with AL-type CA in the Mayo
amyloid autopsy study and in a group of patients undergoing follow
up echocardiographic imaging respectively. Cardiogenic ischemic
stroke is a rare, underappreciated complication of CA. A research
for cause of ischemic stroke may contribute to discovery of CA, and
therefore benefits for later treatment and prognosis. Herein, we
report a patient who suffered from embolic cerebral infarct causing
by intra-cardiac thrombus, as a result of CA, and review
literatures focusing on documented cases of ischemic stroke
secondary to CA, in order to highlight the rare cause of ischemic
stroke, emphasize the importance of transesophageal
echocardiography (TEE) and cardiac magnetic resonance (CMR) imaging
in discovering intra-cardiac thrombi.
Case report
A 50-year-old man was admitted to our department
presenting with exertional dyspnea for >1 year. Physical
examination was as follows: BP 100/60 mmHg, HR 90 bpm, normal
cardiac auscultation with scattered purpura periorbitally and on
the neck and abdomen.
Laboratory tests including routine blood culture,
hepatic and renal functions, and coagulation test were within
normal values. At 6 months before admission, 3 months before
admission and at the point of admission, test results were as
follows: Uric acid, 266, 364 and 428 µmol/l; cardiac troponin I
(cTnI), 0.02, 0.03 and 0.05 µg/l; N-terminal pro-brain natriuretic
peptide (NT-proBNP), 2,080, 4,351 and 7,821 pg/ml. Serum
immunofixation electrophoresis was normal. Urine immunofixation
electrophoresis was negative for free light chain κ (FLCκ) and
monoclonal protein, but positive for free light chain λ (FLCλ).
Serum FLCκ was 6.93 mg/dl, FLCλ140.75 mg/dl, κ/λ 0.049↓; serum
total LCκ427 mg/dl↓, total LCλ165 mg/dl↓, κ/λ 2.59.
Electromyography showed neurogenic injury of bilateral tibialis
anterior muscle. 99mTc-MDP bone scan, bone marrow and
abdominal fat aspiration biopsies showed no abnormality. Cerebral
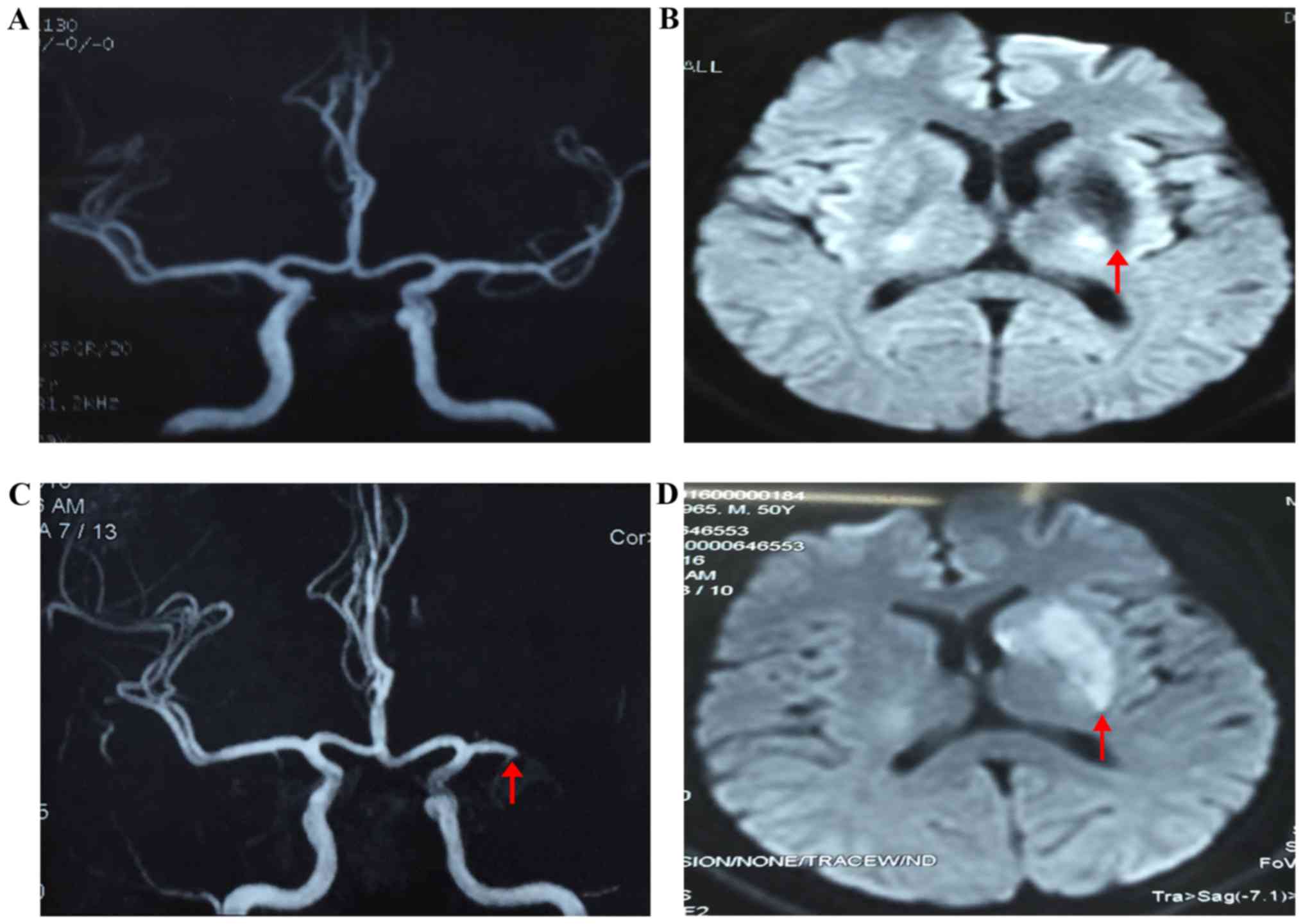
magnetic resonance imaging (MRI) showed an old infarction in the
left basal ganglia, however, the appearance of magnetic resonance
angiography (MRA) was normal (Fig.
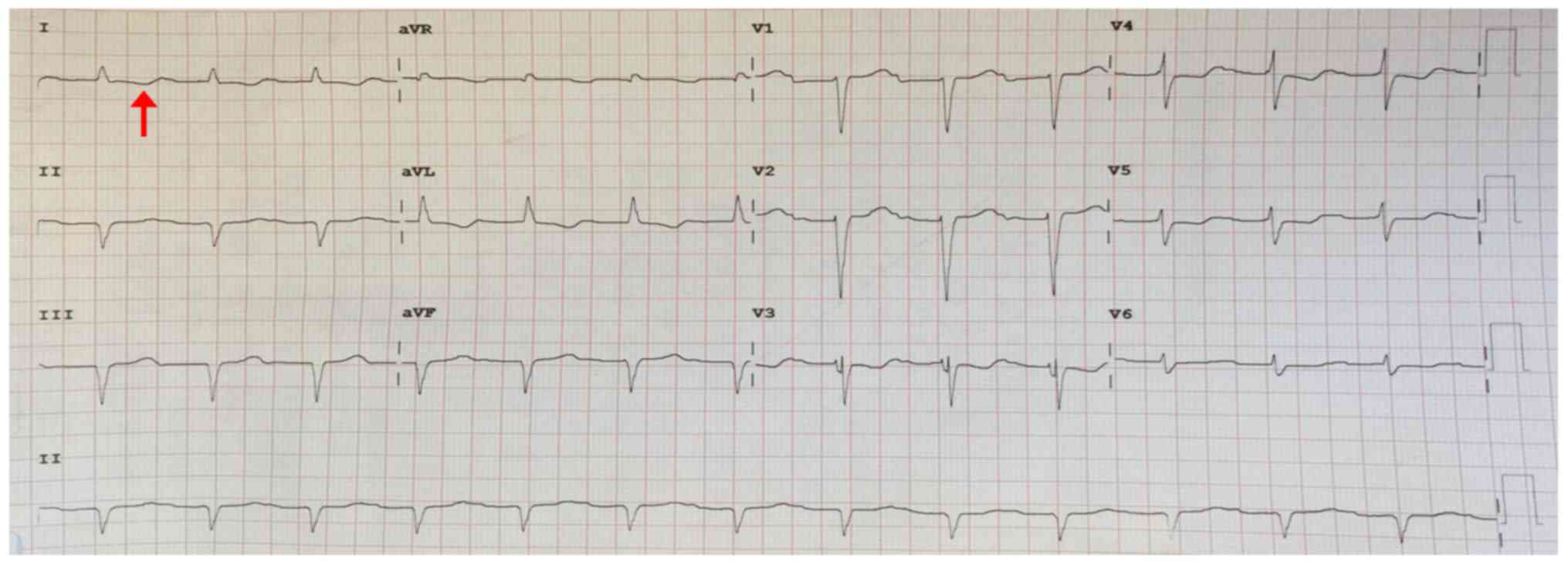
1A). Electrocardiogram and 24-h Holter monitoring found first
degree atrioventricular block (Fig.
2). 6-min walk distance test indicated a reducing exercise
capacity (distance walked 330 meters; prediction = [(7.57 × height
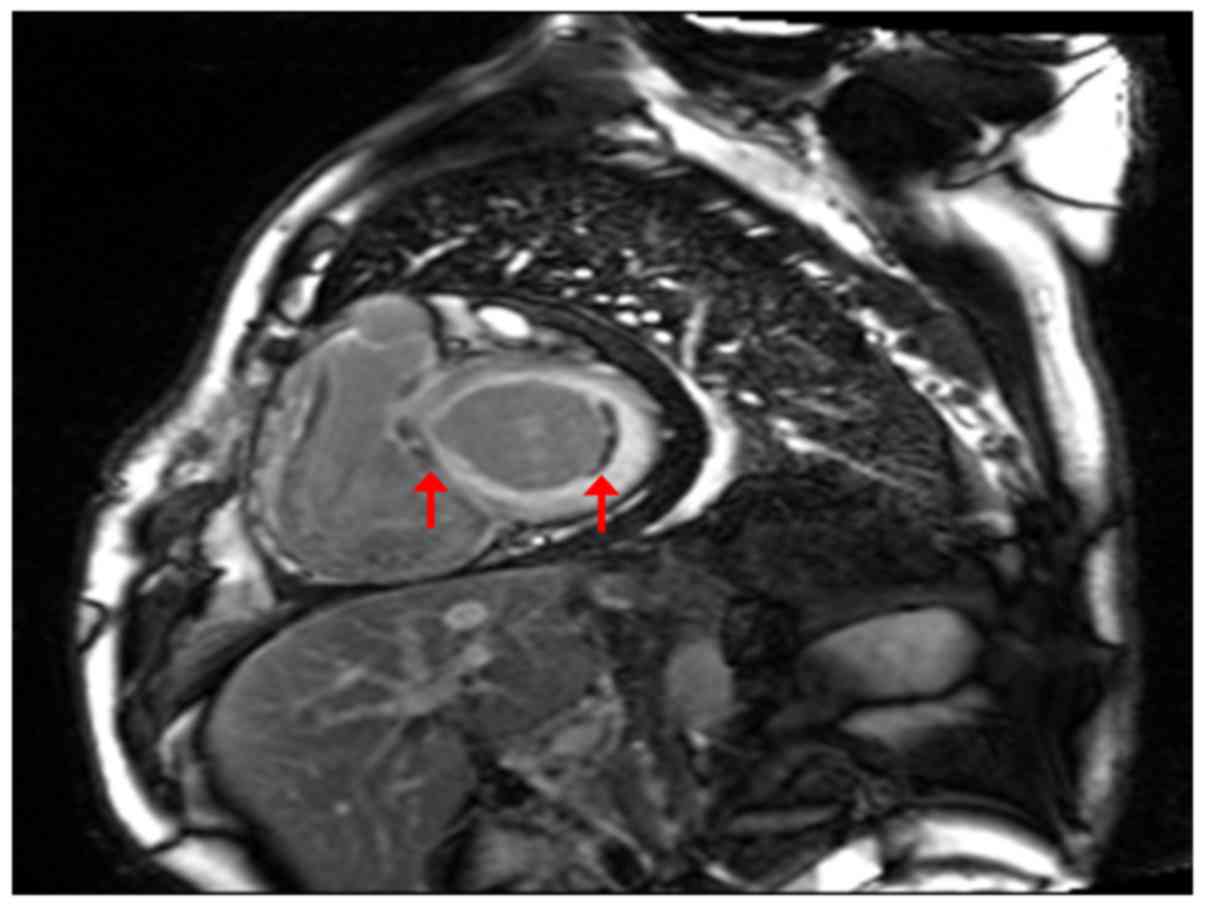
in cm) - (5.02 × age) - (1.76 × weight in kg) - 309)]. CMR showed
bilateral atrial enlargement, mitral and tricuspid insufficiency,
bilateral ventricular diastolic and systolic functional impairment;
thickened bilateral atrial and ventricular walls with diffusing
delayed enhancement, especially under the endocardium. There was a
localized mural thrombus in basal segment of left ventricular
lateral wall (Fig. 3). Transthoracic
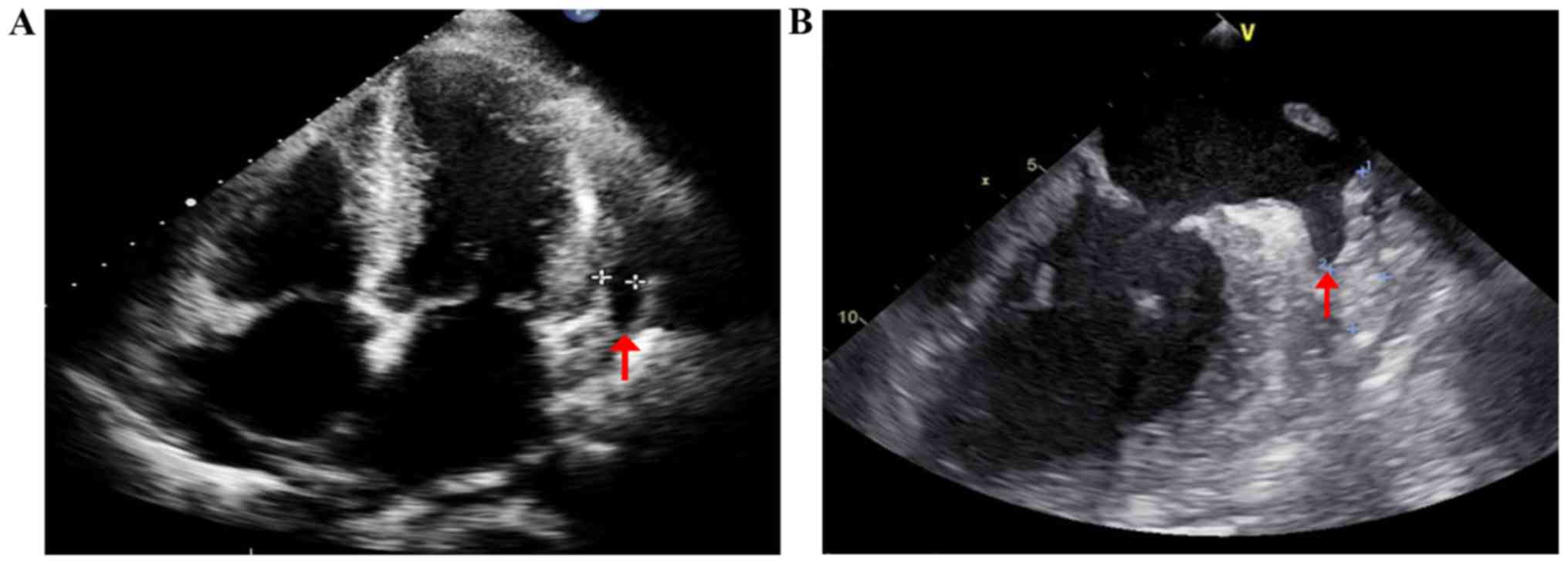
echocardiography (TTE) showed a left ventricular ejection fraction
of 46%, symmetrical thickening of both ventricular walls with
characteristic ‘granular sparkling’ appearance; both atrial
enlargement and restrictive diastolic dysfunction of the left
ventricle (Fig. 4A). TEE showed slow
flow and thrombus in the left auricle (Fig. 4B). Amyloid deposits and Congo red
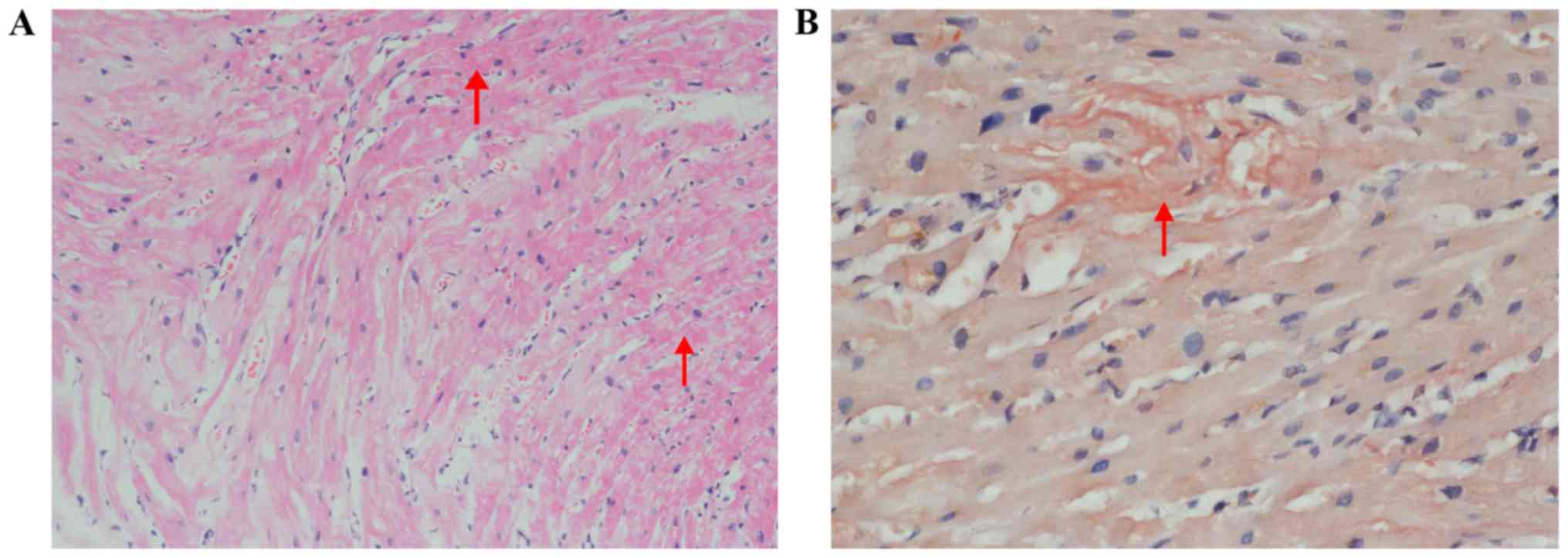
staining was positive in myocardial biopsy (Fig. 5).
Based on the above findings, diagnoses of CA
(AL-type), restrictive cardiomyopathy and chronic heart failure
were made.
Benazepril, spironolactone and metoprolol were
prescribed for chronic heart failure; for anticoagulation, with
consideration of old cerebral embolism, the risk of recurrent
stroke, and the existing thrombus in atrium and left ventricle, low
molecular weight heparin was prescribed; bortezomib was chosen as
the first line chemotherapy for amyloidosis. Autologous stem cell
transplant was under consideration, however, after 6 months, the
patient deteriorated and died of decompensate heart failure.
Half a year before admission, the patient visited a
local hospital for right extremity aphasia and dyskinesia. Cerebral
MRI detected new infarction in the left basal ganglia. MRA showed
nearly total occlusion of the distal left middle cerebral artery
(MCA) (Fig. 1B). After medical
treatment with aspirin and statins, he completely recovered without
any neurological deficits. The patient had no history of
hypertension or diabetes. He had never had smoking or drinking
habits. Ultrasonic Doppler imaging of carotid and subclavian
arteries were normal.
Literature review
A literature search on Pubmed and Medline database
was performed from January 1980 to December 2016 using key words
‘cerebral infarct/ischemic stroke/cerebral embolism/cerebral
thrombosis’, and ‘cardiac amyloidosis’, and ‘heart plus
amyloidosis’, resulting a total of 35 articles. Case reports were
included, and reports without adequate details or full text were
excluded. A total of 7 articles with reference information on 9
patients (including our patient in the present study) qualified for
the literature review and served as the study population for the
purpose of analysis (Table I)
(13–19).
 | Table I.Summary of literature reports on CA
with ischemic stroke. |
Table I.
Summary of literature reports on CA
with ischemic stroke.
| Author, year | No. of reported
patients | Refs. |
|---|
| Cho et al,
2005 | 2 | (13) |
| Rice et al,
1981 | 1 | (14) |
| Bøtker et
al, 1986 | 1 | (15) |
| Owa et al,
2001 | 1 | (16) |
| Yamano et
al, 2008 | 1 | (17) |
| Gillmore et
al, 1999 | 1 | (18) |
| Saux et al,
2006 | 1 | (19) |
| Present case,
2016 | 1 |
|
In the 9 patients, 4 were male, with a median age of
52.9 years (range, 29–83 years), and ~80% of them were diagnosed at
>40 years of age. AL-type amyloidosis was relatively common,
accounting for 57% of discovered cerebral embolic events, while
other types, such as familial amyloidosis and senile systemic
amyloidosis were also involved, especially in older patients
(17,18).
Symptoms of patients with CA and cerebral embolism
were nonspecific, including manifestations of heart failure and
ischemic stroke. Recurrent strokes (14–16)
happened in one-third of the patients and 2 patients accompanied
with infarction of other organs, such as kidney (15) and spleen (15,16). In
addition, 4 patients were diagnosed with CA with stroke as the
first presentation (15,17–19).
AL-type CA with ischemic stroke had worse outcomes, with short-term
survival of few months after the stroke events (15,19). By
contrast, acquired amyloidosis accounting for 28.6% of reviewed
cases, had better outcomes with longer ages over 65 years, however,
it exhibited a high risk of amyloid cardiomyopathy development
(17,18).
All 9 patients were negative for intra-cardiac
thrombus by TTE. However, 4 of whose (including our case) medical
history and electrocardiogram showing no evidence of atrial
fibrillation, revealed intra-cardiac thrombus (13,15,16). 2
patients were proved by TEE (13,16), 1
by autopsy (16), and the other was
not elaborated. As to the present case, he was found to have
intra-cardiac thrombus by both TEE and CMR.
In addition to the routine treatment of heart
failure, 4 patients (13,16,19),
including the present case, were prescribed with immunosuppressive
therapy, and one patient underwent liver transplantation (16). 5 patients (13,14,18) and
the present case received anticoagulation therapy, 4 of whom
survived (13,14,18), 1
of whom died of heart failure (our case), 1 of whom had recurrent
stroke but was still alive (14) at
the time of press. Oppositely, 3 of the 4 patients (15–17,19)
without anticoagulation therapy died of heart and kidney failure
(15), disseminated intravascular
coagulation (16) and multiple
myeloma (19) respectively. Clinical
data of the patients are presented in Table II.
 | Table II.Clinical data of patients with
cardiac amyloidosis and ischemic stroke. |
Table II.
Clinical data of patients with
cardiac amyloidosis and ischemic stroke.
| A, Age, sex and
clinical observations |
|---|
|
|---|
| Author, year | Patient no. | Age (years) |
| Sex (F/M) | Atrial
fibrillation | Intracardiac
thrombus | Ischemic
stroke | Organ
infarction | Refs. |
|---|
| Cho et al,
2005 | 1 | 29 |
| F | No | No | Right basal ganglia
and corona radiata | No | (13) |
| Cho et al,
2005 | 2 | 73 |
| F | No | Left atrial
appendage thrombi | Right PCA and left
MCA territories | No | (13) |
| Rice et al,
1981 | 3 | 34 |
| M | No | No | Right frontal and
left MCA territory (recurrent) | NA | (14) |
| Bøtker et
al, 1986 | 4 | 46 |
| F | No | Mural thrombi in
the left atrium | Left and right
temporoparietal area (recurrent) | Kidney, spleen | (15) |
| Owa et al,
2001 | 5 | 47 |
| F | No | Mitral valve and
left atrium | Both cerebral
hemispheres (recurrent) | Spleen | (16) |
| Yamano et
al, 2008 | 6 | 67 |
| M | No | No | Right temporal
lobe | NA | (17) |
| Gillmore et
al, 1999 | 7 | 83 |
| M | Yes | NA | Right cerebral
infarct | NA | (18) |
| Saux et al,
2006 | 8 | 55 |
| NA | No | No | Left MCA
territory | NA | (19) |
| Present case,
2016 | 9 | 50 |
| M | No | Left atrium, and
both ventricles | Left basal ganglia
and ventricular lateral wall | No | Present |
|
| B, Risk factors,
treatment, diagnosis and outcomes |
|
| Author,
year | Patient
no. | Risk factors for
cerebrovascular diseases | Anticoagulation
treatment | Outcome | Type | TTE | TEE | CMR | Refs. |
|
| Cho et al,
2005 | 1 | No | Heparin and
warfarin | Alive | NA | Yes | No | No | (13) |
| Cho et al,
2005 | 2 | Hypertension | LMWH and
warfarin | Alive | AL amyloidosis with
multiple myeloma | Yes | Yes | No | (13) |
| Rice et al,
1981 | 3 | No | Heparin and
Coumadin | Alive | NA | Yes | No | No | (14) |
| Bøtker et
al, 1986 | 4 | No | No | Deceased | AL | Yes | No | No | (15) |
| Owa et al,
2001 | 5 | No | No | Deceased | Familial amyloid
polyneuropathy | Yes | No | No | (16) |
| Yamano et
al, 2008 | 6 | Hypertension | No | Alive | Senile systemic
amyloidosis | Yes | No | No | (18) |
| Gillmore et
al, 1999 | 7 | NA | Warfarin | Alive | Mutant
transthyretin amyloidosis | Yes | No | No | (129 |
| Saux et al,
2006 | 8 | NA | No | Deceased | AL amyloidosis with
multiple myeloma | Yes | Yes | No | (13) |
| Present | 9 | No | LMWH and
dabigatran | Deceased | AL | Yes | Yes | Yes | Present |
Discussion
Amyloidosis is a group of diseases that result from
the extracellular deposition of amyloid, a fibrillar material
derived from various precursor proteins that self-assemble with
highly ordered abnormal cross β-sheet conformation (7). Amyloidosis is a multiorgan disease,
that results in nonspecific symptoms, including dyspnea, weight
loss, edema, proteinuria, bleeding tendency, orthostatic
hypotension, and other features of autonomic or peripheral
neuropathy. Cardiac involvement is the major determinant of
survival. Deposition of amyloid fibrils in the extracellular space
causes separation and distortion of the existing tissues and
eventually causes irreversible cardiac dysfunction. This may occur
in the myocardium, pericardium, small vessels and conduction system
(20). When the myocardium is
primarily affected, the ventricles may become stiff, leading to
restrictive cardiomyopathy. The stiff ventricles may impair
ventricular filling during diastole, leading to a clinical
presentation of diastolic heart failure, and later systolic
dysfunction (20); Angina is common
in CA and is thought to be frequently secondary to amyloid
deposition in small coronary arteries and infrequently coronary
artery disease; Deposition of amyloid can also lead to conduction
system dysfunction, with arrhythmias and sudden death (21,22). In
our study, clinical features of the patient was accordant with AL
amyloidosis with significant heart involvement.
Prognosis of CA is generally poor. For patients with
AL amyloidosis, the median survival is 6 months after appearance of
congestive heart failure (13,23),
however, patients with senile systemic amyloidosis and heart
failure live with longer survival of 5 years (24). Cardiac troponins and NT-proBNP are
important prognostic indicators in CA, and also allow for
monitoring progression of disease or efficacy of therapy (25). Serum uric acid is a novel independent
prognostic factor in AL amyloidosis and the median overall survival
was lower in patients with uric acid levels ≥8 mg/dl (26). A combination of uric acid, troponin T
and N-terminal-pro-B-type natriuretic peptide provides a strong
predictive model for early mortality (27). The serum immunoglobulin free light
chain assay enables quantification of aberrant circulating
amyloidogenic fibril protein precursors and serial monitoring of
amyloidogenic light chain production during chemotherapy (28). In the present study, cTnI, NT-proBNP
and uric acid of our patient (results of other patients were not
obtained) were all elevated and progressively increased along with
disease exacerbation. Delayed diagnosis leads to prognosis of CA
with cerebral embolism even worsen, resulting from the low
incidence of cerebral embolism in CA, less cognition of physician
and insufficient examination (such as TEE, CMR). The true incidence
of ischemic strokes in patients with CA is unknown. One prospective
study including 40 patients with systemic amyloidosis and ischemic
stroke described 37 of AL, 2 of SSA and 1 of FA; 13 patients
presented ischemic stroke as the first manifestation (11 were AL),
with average survival of 6.9 months after diagnosis of amyloidosis;
37% of the 13 patients experienced recurrent ischemic stroke; while
70% of them had cardioembolic infarctions (29).
Intra-cardiac thrombus in patients of CA are common.
Roberts and Waller demonstrated that 14 patients (26%) were
presented with at least one intra-cardiac thrombus in 54 necropsy
patients with CA (30). Feng et
al discovered 38 patients (33%) with thrombus through a total
of 116 autopsy or transplanted cases of CA (55 of AL and 61 of
other type), including 23 of 1 thrombus and 15 of 2–5 thrombi; for
a total of 63 thrombi, 36 were in the right atrium, 19 in the left
atrium, 4 on the coronary sinus valve, 3 in the right ventricle,
and 1 in the left ventricle. The author thought AL type, atrial
fibrillation, left ventricular diastolic dysfunction, higher heart
rate, right ventricular wall thickness were independently
associated with thromboembolism (11). In this study, despite the strong
consideration of cerebral embolic stroke, all the reviewed 9 cases
were negative of intra-cardiac thrombus by TTE. However, 4 patients
was revealed of intra-cardiac thrombus by other methods: 2 patients
were proved by TEE (13,16), 1 by autopsy (16), while the last one was not described
clearly. In addition, our case was also discovered with
intra-cardiac thrombus by CMR. Summing up the above, TTE is not as
sensitive as enough to display intra-cardiac thrombus, especially
special thrombi in the left auricle. So, we suggest that it is
necessary to carry out TEE or CMR in CA patients with risk factors
of atrial embolism, in order to avoid missing intra-cardiac
thrombus or severe cerebral embolic events (31).
Mechanisms of intra-cardiac thrombus in CA includes
the following points: First, blood stasis and associated cardiac
intracavitary turbulence may contribute to mural thrombosis
(15). Second, focal endocardial and
subendocardial areas are consistently involved by severe deposition
of amyloid, with fibrous thickening and impaired wall motion
attributed to organized endocardial thrombi (14,16).
Third, arrhythmia is another established culprit of embolic stroke.
Atrial fibrillation may due to left atrial dilatation and failure
with increasing wall stress, which contributes to thrombosis in
left atrium (13,14). Forth, amyloid may infiltrate into the
intima of coronary arteries, which may lead to myocardial ischemia,
endocardial damage, mural thrombosis and subsequent cerebral
embolism (13). Lastly, the plasma
hypercoagulability state resulted from nephrotic syndrome,
thrombocytosis related to hyposplenism, thrombin-antithrombin
pathway impairment or, blood viscosity or procoagulant activity
related to circulating monoclonal component may also be a
contributor (32).
In fact, due to deficiency of coagulation factors,
abnormal fibrin polymerization, hyperfibrinolysis, platelet
dysfunction, and vascular damage caused by local amyloid
deposition, hemorrhage tendency in patients with amyloidosis is
more common than thrombophilia (33). Physical examination also revealed
typical cutaneous bleeding, including periorbital, cervical and
abdominal purpura in our case. Meanwhile, mural thrombi in left
atrium and both ventricles were found, which further even resulted
in embolic cerebral infarction.
If intra-cardiac thrombus is detected,
anticoagulation therapy may be indicated to prevent systemic
circulation embolism; however, anticoagulation therapy may
exacerbate the hemorrhagic tendency that is known in amyloidosis
due to fragile blood vessel walls secondary to amyloid deposition
and the coexisting coagulopathy. Melo et al once reported a
case with recurrent cardiac embolic infarcts, developing fatal
intracerebral hemorrhage 3 days after intravenous anticoagulation
therapy. Anticoagulation therapy and cerebral amyloid angiopathy
were demonstrated to trigger cerebral hemorrhage (34). However, in the reviewed 9 patients, 5
patients received anticoagulation therapy, 4 of whom were alive
without any visceral hemorrhage. On the contrary, 3 of the 4
patients not prescribed with anticoagulation therapy were deceased,
2 of whom underwent recurrent strokes and other systemic organ
infarcts (15,16). These results indicate the probable
effect of anticoagulation therapy on decreasing the incidence of
arterial embolic complications, and benefits to improve prognosis
and survival time. Given the unhealed feature and poor prognosis in
amyloidosis, more profound researches about occasion to start and
terminate, frequency and dose of anticoagulation therapy in CA
patients with ischemic stroke are required.
In conclusion, this report presents a case of CA
with cardioembolic cerebral stroke although no clues of atrial
fibrillation, while subcutaneous bleeding tendency occurs
simultaneously. Cardiac embolus is a rare cause of ischemic stroke
in adults but could be progressive rapidly, recurrent and fatal.
Summarizing the key aspects of this rare disease based on a review
of available literatures, we emphasize the importance of making a
thorough search for cardiac problems in all stroke patients with
low risk of cerebrovascular diseases, and the crucial role of TEE
and CMR in detecting the intra-cardiac thrombus of CA patients.
References
|
1
|
Shah KB, Inoue Y and Mehra MR: Amyloidosis
and the heart: A comprehensive review. Arch Intern Med.
166:1805–1813. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Falk RH, Alexander KM, Liao R and Dorbala
S: AL (light-chain) cardiac amyloidosis: A review of diagnosis and
therapy. J Am Coll Cardiol. 68:1323–1341. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Maleszewski JJ: Cardiac amyloidosis:
Pathology, nomenclature, and typing. Cardiovasc Pathol. 24:343–350.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rosenbaum AN and Edwards BS: Current
indications, strategies, and outcomes with cardiac transplantation
for cardiac amyloidosis and sarcoidosis. Curr Opin Organ
Transplant. 20:584–592. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Merlini G: CyBorD: Stellar response rates
in AL amyloidosis. Blood. 119:4343–4345. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Desport E, Bridoux F, Sirac C, Delbes S,
Bender S, Fernandez B, Quellard N, Lacombe C, Goujon JM, Lavergne
D, et al: Al amyloidosis. Orphanet J Rare Dis. 7:542012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wechalekar AD, Gillmore JD and Hawkins PN:
Systemic amyloidosis. Lancet. 387:2641–2654. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wechalekar AD, Gillmore JD and Hawkins PN:
Cardiac amyloidosis: An approach to diagnosis and management.
Expert Rev Cardiovasc Ther. 8:1007–1013. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Selvanayagam JB, Hawkins PN, Paul B,
Myerson SG and Neubauer S: Evaluation and management of the cardiac
amyloidosis. J Am Coll Cardiol. 50:2101–2110. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Karafiatova L and Pika T: Amyloid
cardiomyopathy. Biomed Pap Med Fac Univ Palacky Olomouc Czech
Repub. 161:117–127. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Feng D, Edwards WD, Oh JK, Chandrasekaran
K, Grogan M, Martinez MW, Syed IS, Hughes DA, Lust JA, Jaffe AS, et
al: Intracardiac thrombosis and embolism in patients with cardiac
amyloidosis. Circulation. 116:2420–2426. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Feng D, Syed IS, Martinez M, Oh JK, Jaffe
AS, Grogan M, Edwards WD, Gertz MA and Klarich KW: Intracardiac
thrombosis and anticoagulation therapy in cardiac amyloidosis.
Circulation. 119:2490–2497. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cho KH, Cho YM and Kim JS: Embolic
infarction associated with cardiac amyloidosis. J Clin Neurol.
1:92–96. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rice GP, Ebers GC, Newland F and Wysocki
GP: Recurrent cerebral embolism in cardiac amyloidosis. Neurology.
31:904–907. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bøtker HE and Rasmussen OB: Recurrent
cerebral embolism in cardiac amyloidosis. Int J Cardiol. 13:81–83.
1986. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Owa M, Takei Y, Hashikura Y, Kawasaki S,
Koyama M and Ikeda S: Recurrent cerebral embolism in a familial
amyloid polyneuropathy patient who received partial liver
transplantation from a living donor. Intern Med. 40:259–264. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yamano M, Azuma A, Yazaki M, Ikeda S,
Sawada T and Matsubara H: Early cardiac involvement in senile
systemic amyloidosis: A case report. Amyloid. 15:54–59. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gillmore JD, Booth DR, Pepys MB and
Hawkins PN: Hereditary cardiac amyloidosis associated with the
transthyretin Ile122 mutation in a white man. Heart. 82:e21999.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Saux A, Heroum C, Sportouch C, De Graeve
F, Lequellec A and Pagès M: Amyloid cardiomyopathy: A rare cause of
cerebral embolism. Rev Neurol (Paris). 162:1128–1130. 2006.(In
French). View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yusuf SW, Solhpour A, Banchs J,
Lopez-Mattei JC, Durand JB, Iliescu C, Hassan SA and Qazilbash MH:
Cardiac amyloidosis. Expert Rev Cardiovasc Ther. 12:265–277. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ritts AJ, Cornell RF, Swiger K, Singh J,
Goodman S and Lenihan DJ: Current concepts of cardiac amyloidosis:
Diagnosis, clinical management, and the need for collaboration.
Heart Fail Clin. 13:409–416. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mankad AK, Sesay I and Shah KB:
Light-chain cardiac amyloidosis. Curr Probl Cancer. 41:144–156.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rahman JE, Helou EF, Gelzer-Bell R,
Thompson RE, Kuo C, Rodriguez ER, Hare JM, Baughman KL and Kasper
EK: Noninvasive diagnosis of biopsy-proven cardiac amyloidosis. J
Am Coll Cardiol. 43:410–415. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cacoub P, Axler O, De Zuttere D, Hausfater
P, Amoura Z, Walter S, Wechsler B, Godeau P and Piette JC:
Amyloidosis and cardiac involvement. Ann Med Interne (Paris).
151:611–617. 2000.PubMed/NCBI
|
|
25
|
Palladini G, Barassi A, Klersy C,
Pacciolla R, Milani P, Sarais G, Perlini S, Albertini R, Russo P,
Foli A, et al: The combination of high-sensitivity cardiac troponin
T (hs-cTnT) at presentation and changes in N-terminal natriuretic
peptide type B (NT-proBNP) after chemotherapy best predicts
survival in AL amyloidosis. Blood. 116:3426–3430. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kumar S, Dispenzieri A, Lacy MQ, Hayman
SR, Leung N, Zeldenrust SR, Buadi FK, Kyle RA, Rajkumar SV and
Gertz MA: Serum uric acid: Novel prognostic factor in primary
systemic amyloidosis. Mayo Clin Proc. 83:297–303. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kumar SK, Gertz MA, Lacy MQ, Dingli D,
Hayman SR, Buadi FK, Short-Detweiler K, Zeldenrust SR, Leung N,
Greipp PR, et al: Recent improvements in survival in primary
systemic amyloidosis and the importance of an early mortality risk
score. Mayo Clin Proc. 86:12–18. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Dispenzieri A, Lacy MQ, Katzmann JA,
Rajkumar SV, Abraham RS, Hayman SR, Kumar SK, Clark R, Kyle RA,
Litzow MR, et al: Absolute values of immunoglobulin free light
chains are prognostic in patients with primary systemic amyloidosis
undergoing peripheral blood stem cell transplantation. Blood.
107:3378–3383. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zubkov AY, Rabinstein AA, Dispenzieri A
and Wijdicks EF: Primary systemic amyloidosis with ischemic stroke
as a presenting complication. Neurology. 69:1136–1141. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Roberts WC and Waller BF: Cardiac
amyloidosis causing cardiac dysfunction: Analysis of 54 necropsy
patients. Am J Cardiol. 52:137–146. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Santarone M, Corrado G, Tagliagambe LM,
Manzillo GF, Tadeo G, Spata M and Longhi M: Atrial thrombosis in
cardiac amyloidosis: Diagnostic contribution of transesophageal
echocardiography. J Am Soc Echocardiogr. 12:533–536. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hausfater P, Costedoat-Chalumeau N, Amoura
Z, Cacoub P, Papo T, Grateau G, Leblond V, Godeau P and Piette JC:
AL cardiac amyloidosis and arterial thromboembolic events. Scand J
Rheumatol. 34:315–319. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Sucker C, Hetzel GR, Grabensee B,
Stockschlaeder M and Scharf RE: Amyloidosis and bleeding:
Pathophysiology, diagnosis, and therapy. Am J Kidney Dis.
47:947–955. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Melo TP, Bogousslavsky J, Regli F and
Janzer R: Fatal hemorrhage during anticoagulation of cardioembolic
infarction: Role of cerebral amyloid angiopathy. Eur Neurol.
33:9–12. 1993. View Article : Google Scholar : PubMed/NCBI
|