Introduction
Osteoporosis is a progressive skeletal disorder
characterized by reduced bone mineral density (BMD) and
microarchitectural deterioration, which typically leads to
increased bone fragility and susceptibility to fracture (1). In patients with osteoporosis, the
differentiation, proliferation and osteogenic capabilities of bone
marrow-derived mesenchymal stem cells (BMSCs) are inhibited during
bone healing, which results in reduced bone formation and
compromised osseointegration of bone implants (2,3). In
addition, the regulation of bone tissue regeneration and remodeling
in the bone marrow, particularly regarding the quantity and quality
of crucial biological factors, such as VEGF-A and TGF-beta1, is
markedly altered in ageing animals (4). As a result, bone healing and
regeneration around dental implants are substantially decreased in
patients with osteoporosis, resulting in higher rates of implant
failure (5–7). A previous study demonstrated that
osseointegration in osteoporosis is associated with multiple
complications, including bone graft failure, prolonged bone healing
time and a high bone implant failure rate in clinical dentistry
(8). Results of a retrospective
clinical study have also suggested that certain general disorders,
particularly osteoporosis, may contribute to implant failure
following maxillary sinus bone grafting (9). However, histological analyses of
osseointegrated implants removed from patients with osteoporosis
have identified a close contact between healthy bone and the
implant surface, with this bone-implant contact (BIC) confirming
that osseointegration had been achieved (10,11).
These inconsistencies in previous results indicate that further
experimental studies are warranted to determine the influence of
osteoporosis on bone regeneration around implants.
Two established animal models currently exist for
the study of osteoporosis, the OVX model and the
immobilization-induced bone loss model. Neither of these animal
models identically represent the stages of osteoporosis in humans
(12); however, they do provide an
experimental comparison. Small and large animals, including rats,
rabbits and sheep, may be used for these models depending on which
aspects of osteoporosis are being investigated. Among these models,
the OVX rat model, with a short generation time and low cost, is
the most popular animal model that has been validated to represent
bone loss in women (13),
particularly during the early stages of osteoporosis (14,15). The
OVX rat model exhibits significant loss of volume and strength in
the vertebral cancellous bone as early as 3 months after
ovariectomy; a relatively shorter generation period compared with
larger animal models of osteoporosis (16,17). In
addition, the diameter of the rat tibia is typically ~5 mm, which
is larger than the implant diameter of 2.6 mm, thus leaving >1
mm of surrounding bone to maintain the stability of the implant.
For these reasons, the OVX rat model of osteoporosis was adopted in
the present study.
Two methods are typically administered to treat
patients with osteoporosis who exhibit impaired osteogenesis. The
first aims to improve patients' osteogenic capability through
systemic medicinal treatment of osteoporosis, and the second aims
to improve the implant biocompatibility by modifying its surface.
At present, treatment for osteoporosis primarily involves the
systemic administration of medication. For example, bisphosphonates
are used to improve osseous healing potential, possibly through
effects on BMSC mitogenesis, and proproliferative and antiapoptotic
effects on osteoblasts, though their underlying mechanisms of
action remain unknown (18). In
modern implant dentistry, studies have focused on the surface
modification of implant materials, with the aim of improving the
biocompatibility and in vivo performance of implants. In
particular, studies have focused on various methods that alter the
surface characteristics of implants, including modification of the
implant surface roughness (19).
However, it is difficult to improve implant osseointegration
through physiochemical modifications alone (20), as bone healing and growth is a
complicated process, involving migration, proliferation and
differentiation of osteogenic cells.
Tissue engineering-based approaches have been
documented to improve local osteogenesis around an implant. BMSCs
are among the most commonly used cells in such approaches (21), due to their high differentiation
potential (including osteogenic differentiation), proliferative
ability and suitability for autologous transplantation due to their
ability to avoid an immunologic reaction (22). In animal models of osteoporosis,
dental implant modifications using cell-based tissue engineering
techniques have demonstrated potential for the repair of bone
defects (23). Previous studies by
our group have evaluated an implant technique involving BMSC
sheets, whereby the modified constructs were characterized by a
higher cell density, greater content of extracellular matrix (ECM)
and growth factors, the ability for facile harvesting without the
need for chemical treatment and stability around the BIC (24). Our previous studies have also
demonstrated that BMSC sheets may be used to create a BMSC-implant
construct with osteogenic potential in vivo and in
vitro (25). However, the
ability of a BMSC-based tissue engineering approach to improve the
osseointegration of dental implant materials in patients with
osteoporosis remains unknown. Therefore, in the present study, a
rat model of osteoporosis was used to evaluate the osseointegration
of a BMSC sheet-titanium implant complex. The data obtained suggest
that this novel BMSC sheet-based tissue engineering strategy may
enhance bone regeneration around titanium implants.
Materials and methods
Preparation of implant samples
The surfaces of 60 polished titanium implants were
rinsed in ethanol twice and distilled water twice. Then, an MJ2000
ultrasonic machine (Wuxi Meijie Ultrasonic Cleaning Equipment Co.,
Ltd., Wuxi, China) was used for deep cleaning of the implants.
Animal model preparation
A total of 40 female Sprague-Dawley rats (Medical
Laboratory Animal Center, The Fourth Military Medical University,
Xi'an, China; weight, 110±8.73 g; age, 10 weeks old) were used in
the current study, according to institutional guidelines for the
care of experimental animals of the Fourth Military Medical
University (Xi'an, China). Animal experiments were performed
according to an animal study protocol approved by the Ethics
Committee of the Fourth Military Medical University (approval no.
2015065). The rats were housed individually in the cages with the
room temperature ~18–24°C and relative humidity between 40–60%.
Fluorescent lighting was provided on a 12-h light/dark cycle. Free
access to tap water and standard rodent feed (CE-2; CLEA Japan,
Inc., Tokyo, Japan) was given to all rats. Rats were randomly
divided into the following two groups: An ovariectomized (OVX)
group in which a bilateral ovariectomy was performed (n=20); and a
sham operation group (n=10). After intramuscular injection of 1%
pentobarbital (20 mg/kg), rats were under deep of anesthesia. Skin
preparation and sterilization was performed and ophthalmic scissors
were used to cut ~2 mm at both sides of the rat dorsalis, exposing
the psoas muscle layer. The psoas muscle was longitudinally cut to
exposure the abdominal cavity (1.5 mm), exposing the bilateral
ovaries attached with mesentery and ligation was performed. The
same procedure was performed in the sham group without ligation
after exposure. After the suture, iodophor was used to disinfect
the incision area. The two groups of rats were housed under the
conditions mentioned above. In the following surgical procedures,
the BMSC-based implant was inserted into the right tibia (the test
group); and the titanium control implant was inserted into the left
tibia (the negative control group) of the same OVX rat. Two rats in
the OVX group were excluded as they succumbed to anesthesia-related
fatality. Therefore, a total 18 rats (36 samples) were analyzed in
the present study.
BMSC isolation and culture
From the total Sprague-Dawley rats originally
obtained from the Animal Experiment Center at the Fourth Military
Medical University, 10 female rats (10 weeks old; weighing 80–120
g) were used from bone marrow harvesting. Approval for bone marrow
harvesting was obtained from the Institutional Animal Care and Use
Committee of the Fourth Military Medical University. Rat BMSCs were
isolated and harvested as previously described (26). Briefly, rats were sacrificed by an
overdose of pentobarbital (1%, 100 mg/kg) that was
intraperitoneally injected. Then, bilateral tibiae and femora were
dissected and tissue scissors were used to cut the ends of the long
bones. Bone marrow was extruded into Dulbecco's modified Eagle
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
using a syringe. After centrifugation (560 × g for 4 min at room
temperature), 5×106 bone marrow cells were seeded into
T25 flasks. Cells were incubated at 37°C with 5% CO2 for
24 h. Following incubation, DMEM supplemented with 1 ng/ml b-FGF
was added to replenish and remove non-adherent cells. The medium
was then replaced every 3 days. Cells were passaged after reaching
90% confluency. Cells from the fourth passage were used for
experiments in the present study.
Construction of the osteoporosis rat
model
No rats experienced complications during the 3-month
post-operative recovery period and their weight continued to
increase until the time of sacrifice. A total of 4 samples could
not be included in the analysis as 2 rats in the OVX group died
upon anesthesia induction. The remaining 36 samples were used for
subsequent experiments.
Using micro-computed tomography (CT), reconstruction
of the three-dimensional (3D) bone structure and related
quantitative analysis may be performed based on selected images,
and the bone density in live rats following ovariectomy may be
measured. In the current study, the osteoporosis model produced by
overiectomy in Sprague-Dawley rats was verified at 3 months
post-operation by evaluation of BMD using micro-CT analysis.
Phenotypic analysis of rat BMSCs
Attached BMSCs were trypsinized, centrifuged (560 ×
g for 4 min at 37°C) and stained with phycoerythrin-conjugated rat
antibodies directed against CD-90, CD-45, CD-29 and CD-34
respectively (1:500; Cat. nos. 553014, 561588, 562154 and 551387;
BD Biosciences, San Jose, CA, USA) for 30 min at room temperature.
BMSCs were then rinsed twice with PBS. After centrifugation at 560
× at room temperature, cells were re-suspended in 500 µl PBS to a
concentration of ~5×105 cells/100 µl. Cells incubated
without antibodies served as controls. Flow cytometric analysis of
cell surface protein expression was performed using an Epics XL
flow cytometer with CXP Analysis Software 15 Network (Beckman
Coulter, Inc., Brea, CA, USA), as described previously (27).
Multilineage differentiation potential
of isolated BMSCs in vitro
To induce osteogenic differentiation, rat BMSCs were
seeded into 6-well plates at a density of 1×105
cells/well and cultured in α-Minimum Essential Medium (α-MEM;
Sigma-Aldrich; Merck KGaA Darmstadt, Germany) in an atmosphere
containing 10% CO2 at 37°C for 24 h until cells had
adhered. Medium was then replenished with the following
osteoinductive medium: α-MEM supplemented with 10% fetal bovine
serum (FBS), 0.1 mM dexamethasone (Sigma-Aldrich; Merck KGaA), 50
mM ascorbate-2-phosphate (Sinopharm Chemical Reagent Co., Ltd.,
Shanghai, China), 10 mM β-glycerophosphate (Alfa Aesar; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), and 1%
penicillin-streptomycin-glutamine liquid (Gibco; Thermo Fisher
Scientific, Inc.). α-MEM medium was used as the control group. The
osteoinductive medium was replaced every 3 days. After 10 days of
culture (10% CO2 at 37°C), BMSCs in parallel wells were
examined for mineralized nodule formation using light microscopy.
Cells were fixed after 28 days of osteoinduction in 70% ethanol at
room temperature for 15 min and then subjected to Alizarin Red S
staining (pH 4; Sigma-Aldrich; Merck KGaA). Images were captured
using an inverted phase-contrast microscope.
To induce adipogenic differentiation, rat BMSCs were
seeded into 6-well plates at a density of 1×105
cells/well for the induction of lipid formation in vitro.
Cells were cultured in α-MEM (Sigma-Aldrich; Merck KGaA) culture
(10% CO2 at 37°C) until they reached 60% confluency. The
medium was then replaced with the following adipogenic induction
medium: α-MEM supplemented with 10% FBS, 200 mM indomethacin, 10 mM
insulin, 1 mM dexamethasone, 0.5 mM isobutyl methylxanthine and 1%
antibiotic/antimycotic solution (10,000 U penicillin and 10 mg
streptomycin; all Sigma-Aldrich; Merck KGaA). The adipogenic
induction medium was refreshed every 2–3 days. After 21 days, the
cells were fixed in 70% ethanol for 15 min at room temperature and
stained with Oil Red O (Sigma-Aldrich; Merck KGaA) for 20 min.
Images were captured using an inverted phase-contrast
microscope.
Induction of BMSC sheet formation
Allogenic BMSCs were seeded into 6-well plates at a
density of 2×105 cells/well and cultured in
osteoinductive medium for 4 weeks in an atmosphere containing 10%
CO2 at 37°C until cell layers had formed. Intact layers
of BMSCs were then detached from the substratum using a cell
scraper and forceps, and combined with other detached layers to
form multilayer BMSC sheets. The BMSC sheets were wrapped tightly
around titanium implants (6 mm in length, 2.6 mm in diameter, 0.2
mm thread depth and 0.5 mm thread pitch; Northwest Institute for
Nonferrous Metal Research, Xi'an, China) that were specifically
designed for the present study and the BMSC-implant constructs were
placed in an incubator containing 10% CO2 at 37°C for 1
h to enhance the stability of the construct prior to implantation
into the tibiae of rats in the OVX and sham operation groups.
Implantation of the BMSC-implant
constructs
In preparation for surgery, rats were anesthetized
with an intraperitoneal injection of a mixed narcotic, consisting
of xylazine (5 mg/kg; Bayer AG, Leverkusen, Germany) and ketamine
(75 mg/kg; Pfizer, Inc., New York, NY, USA). The hind limbs were
shaved and disinfected with lodophor (Mundipharma GmbH, Limburg,
Germany). A longitudinal medial incision was made on the skin. With
the knee in flexion, a 2 mm diameter canal was drilled through the
intercondylar notch of the femur using an implant drill
(NobelReplace Tapered Surgery Kit; Nobel Biocare, Zürich,
Switerland) and monitor (Nobel Biocare, Zürich, Switzerland). Each
rat received an unmodified control titanium implant and a BMSC
sheet-coated implant in the left and right tibiae, respectively.
The skin and soft tissue were closed with a single subcuticular
stitch (1-0 vicryl suture; Johnson & Johnson Corp., Shanghai,
China). Before the rats awoke, postoperative motion of the knee
joint was verified.
BMD measurement
High-resolution micro-CT was performed using an
Inveon Siemens micro-CT scanner (Siemens AG, Munich, Germany) in
the small animal scanning mode (80 kV; 500 mA; 800 msec integration
time) to measure BMD (mg/cm2). Rats were anesthetized by
intra-abdominal injection of pentobarbital sodium solution (1%, 20
mg/kg) and kept in a limited motion holder to ensure image clarity.
BMD measurements were also performed in live animals prior to
implant surgery to identify the implant insertion area. Scans and
analyses were conducted twice; 12 weeks after OVX animal model
formation and 8 weeks after implantation (the implant healing
period).
Evaluation of BIC interfaces: Micro-CT
reconstruction and measurement
The two rat groups were subjected to local micro-CT
after 8 weeks of bone healing to evaluate hard tissue regeneration
around the implants. A circle around the implant (5-mm in size) was
chosen as the region of interest, and a 3D image was reconstructed
and evaluated using Inveon Research Workplace software (version
2.2.0; Siemens AG). Specifically, the bone formation indices of
trabecular thickness (Tb.Th), trabecular spacing (Tb.Sp),
trabecular number (Tb.N), bone volume/total volume (BV/TV) and bone
surface/bone volume (BS/BV) were measured.
Evaluation of bone-implant interfaces:
Histological and histomorphometric analysis
After an implant healing period of 8 weeks, all rats
were sacrificed with an intra-abdominal injection of 1%
pentobarbital sodium solution 100 mg/kg. The tibiae of rats were
then harvested and fixed with 10% formalin solution (pH ~7) for 3
weeks at 4°C before being embedded in resin as a cylinder (diameter
20 mm, height 40 mm). Two sections (~120-µm thick) were made from
every one sample. All sections were cut through the long axis of
the implant using a LEICA SP1600 high-speed precision microtome
(Leica Microsystems GmbH, Wetzlar, Germany), then polished to 100
µm thickness for observation under a light microscope. One section
was stained using Masson-Ponceau Tri-Chrome method and the other
section from the same sample was stained by Van Gieson's method
(28). A histomorphometric
evaluation with a Leica Qwin Pro-Image Analysis system (Leica QWin
Pro 16 system; Leica Microsystems GmbH) was used to quantify the
image data. Specifically, the rate of BIC was measured for
subsequent quantitative analysis of new bone formation.
Statistical analysis
All data are expressed as the mean ± standard
deviation. All statistical analyses were performed using SPSS
(version 20.0; IBM SPSS, Armonk, NY, USA). Analysis of the variance
were used, specifically, one-way ANOVA was performed on the
micro-CT data and two-way ANOVA for BIC ratios in order to compare
the differences between the two implant groups through the 8-week
healing period. P<0.05 was considered to indicate a
statistically significant difference.
Results
Phenotype and differentiation of the
isolated rat BMSCs
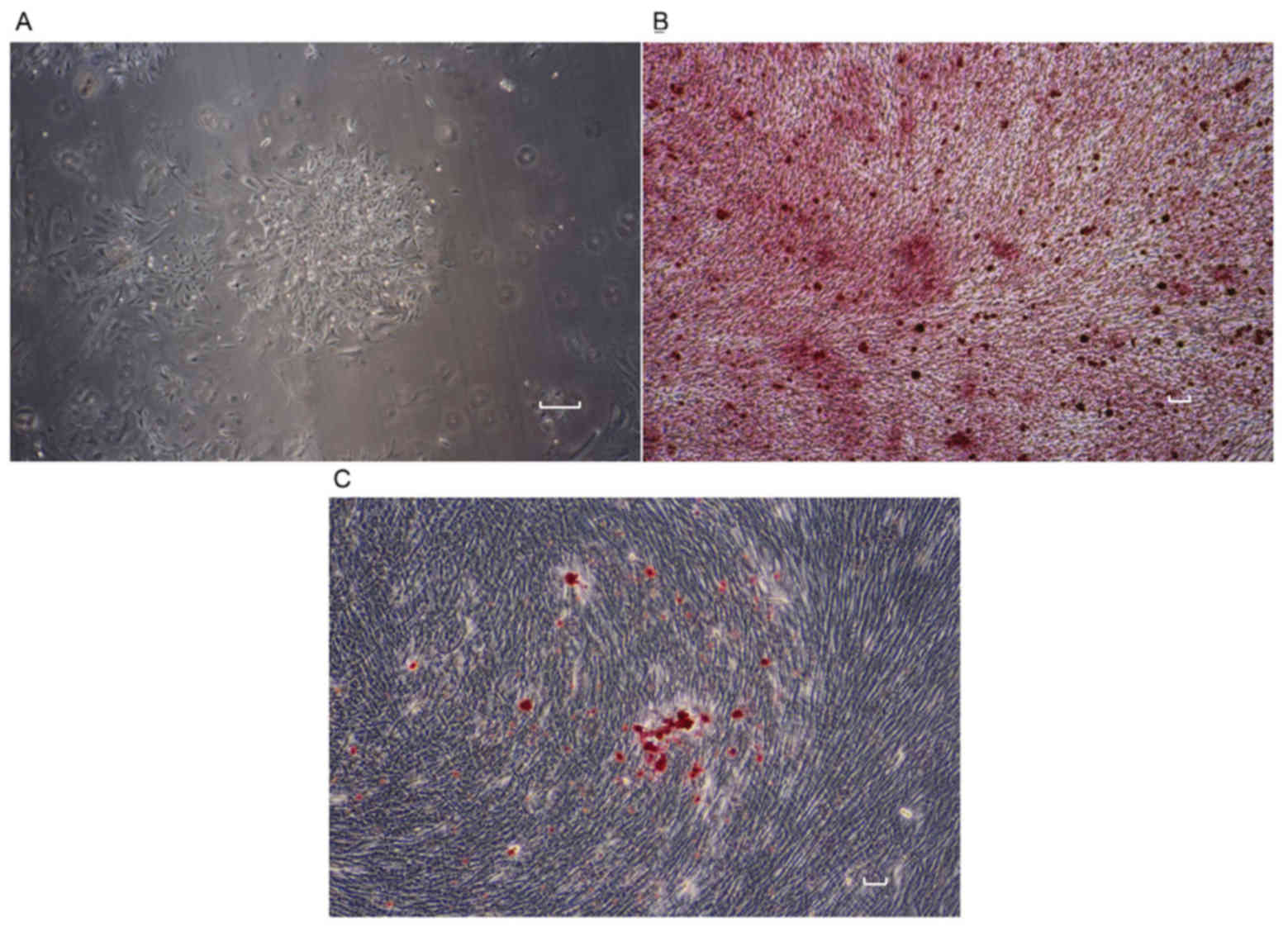
Isolated rat BMSCs exhibited a polygonal shape and
adhered to the bottom of culture flasks after 24 h of culture. The
BMSCs proliferated in α-MEM to reach a confluency of 80% in 7–10
days (Fig. 1A). Calcium nodes were
stained by Alizarin Red (Fig. 1B).
By contrast, Oil Red O staining of BMSCs revealed cellular lipids
characteristic of adipocytes (Fig.
1C).
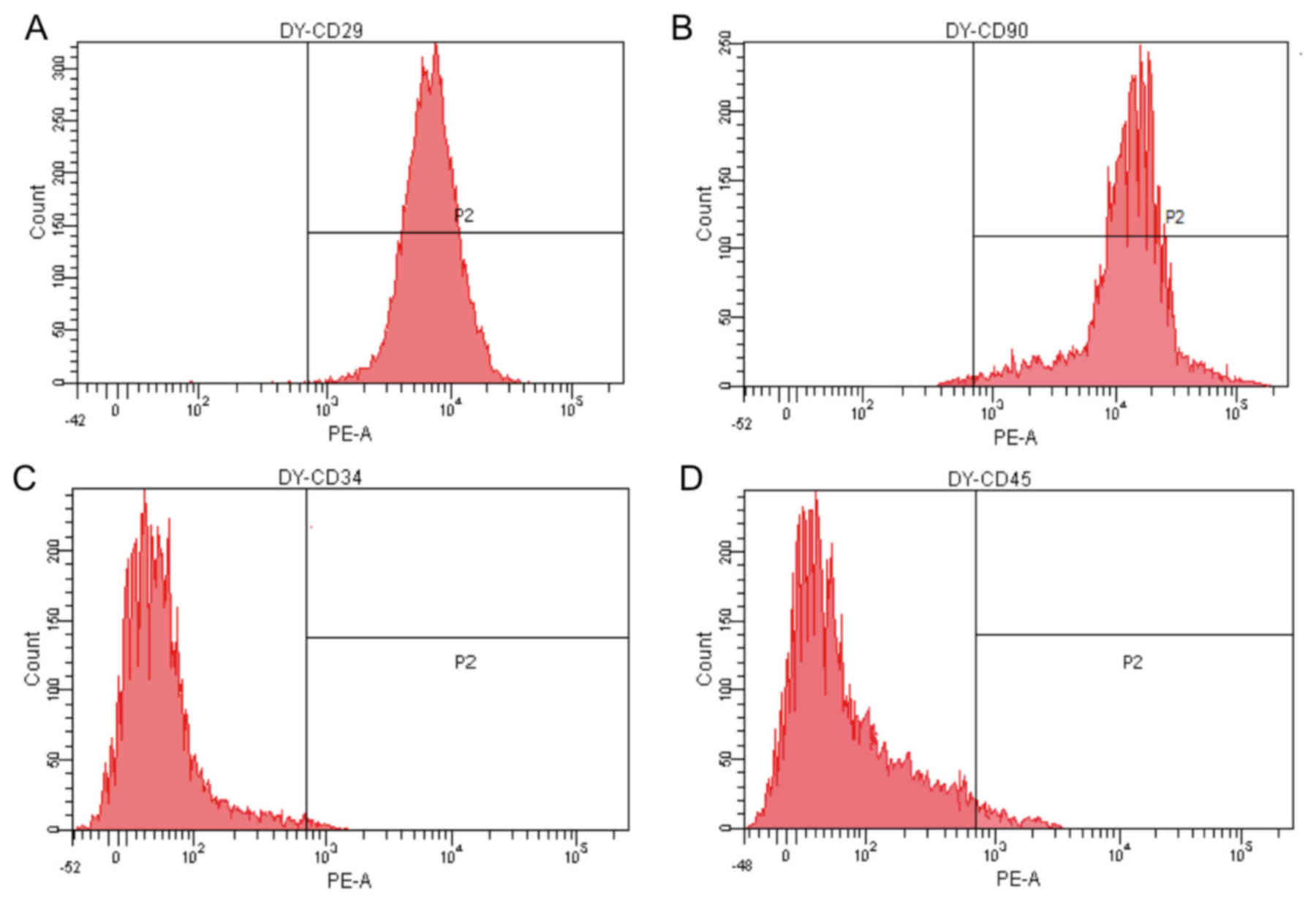
Prior to differentiation, expanded BMSCs were
uniformly positive for the mesenchymal stem cell (MSC) markers CD29
and CD90, and negative for the hematopoietic lineage marker CD34
and leukocyte common antigen CD45 (Fig.
2).
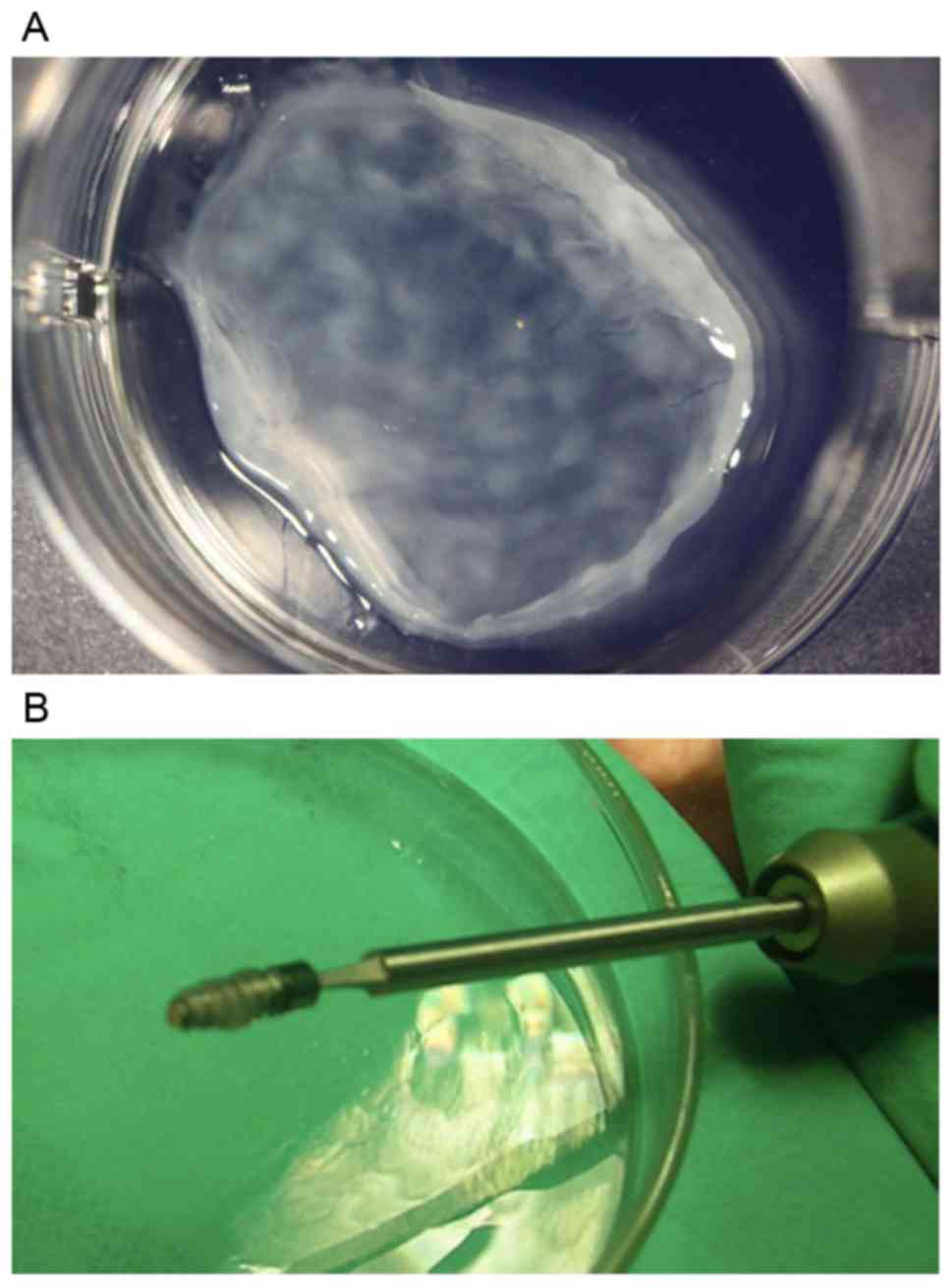
Multilayer BMSC sheet formation and
combining with titanium implants
After the culture in osteoinductive medium for 4
weeks, BMSCs reached over 90% confluency on the bottom of the
culture dish and proliferated over the basal cell layer to form a
high-density 3D sheet-like structure. Three layers of the cell
sheets were combined and wrapped around titanium implants (Fig. 3).
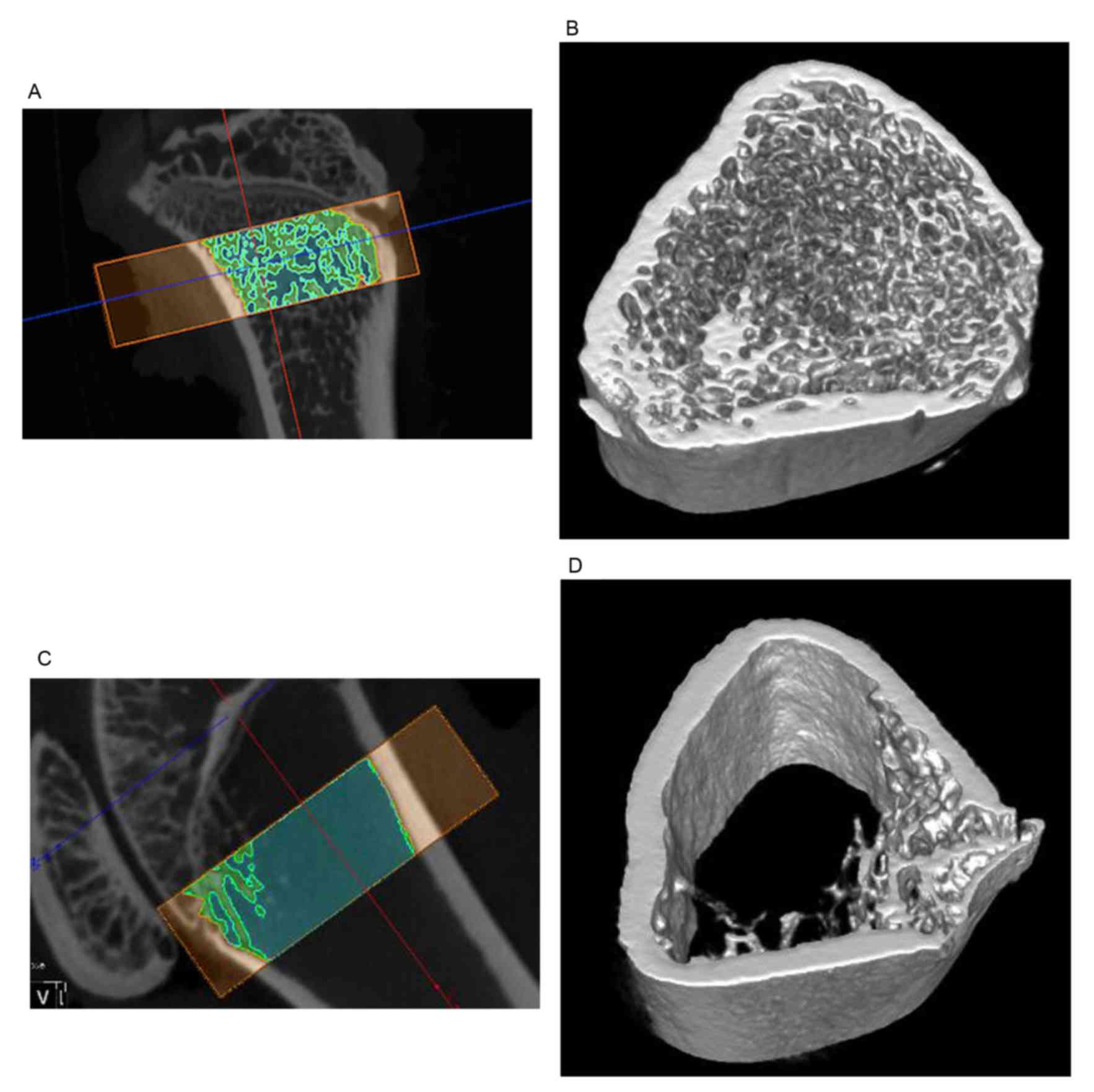
Establishment of OVX model
As depicted in Table
I, at 3 months after ovariectomy, the bone density index in the
OVX group for BV/TV and Tb.Th were significantly decreased compared
with the sham operation group, and significantly increased for
Tb.sp. The micro CT analysis also show the loss of trabecular
framework (Fig. 4).
 | Table I.Bone density indices in the control
and OVX groups. |
Table I.
Bone density indices in the control
and OVX groups.
| Bone density
index | Control | OVX |
|---|
| BV/TV, % |
55.27±2.43 |
9.78±0.06a |
| BS/BV, % |
20.29±1.86 |
23.25±0.57a |
| Tb.Th, mm |
0.99±0.25 |
0.09±0.01a |
| Tb.N, mm |
5.85±0.36 |
1.14±0.04a |
| Tb.sp, mm |
0.07±0.01 |
0.78±0.05a |
| Tb.P.F,1/mm |
5.81±0.08 |
6.75±0.04a |
Micro-CT evaluation of bone formation
in vivo
Rats in the two groups were given an implant healing
period of 8 weeks to allow the host bone tissue regeneration around
the implants. The images obtained by micro-CT indicated that
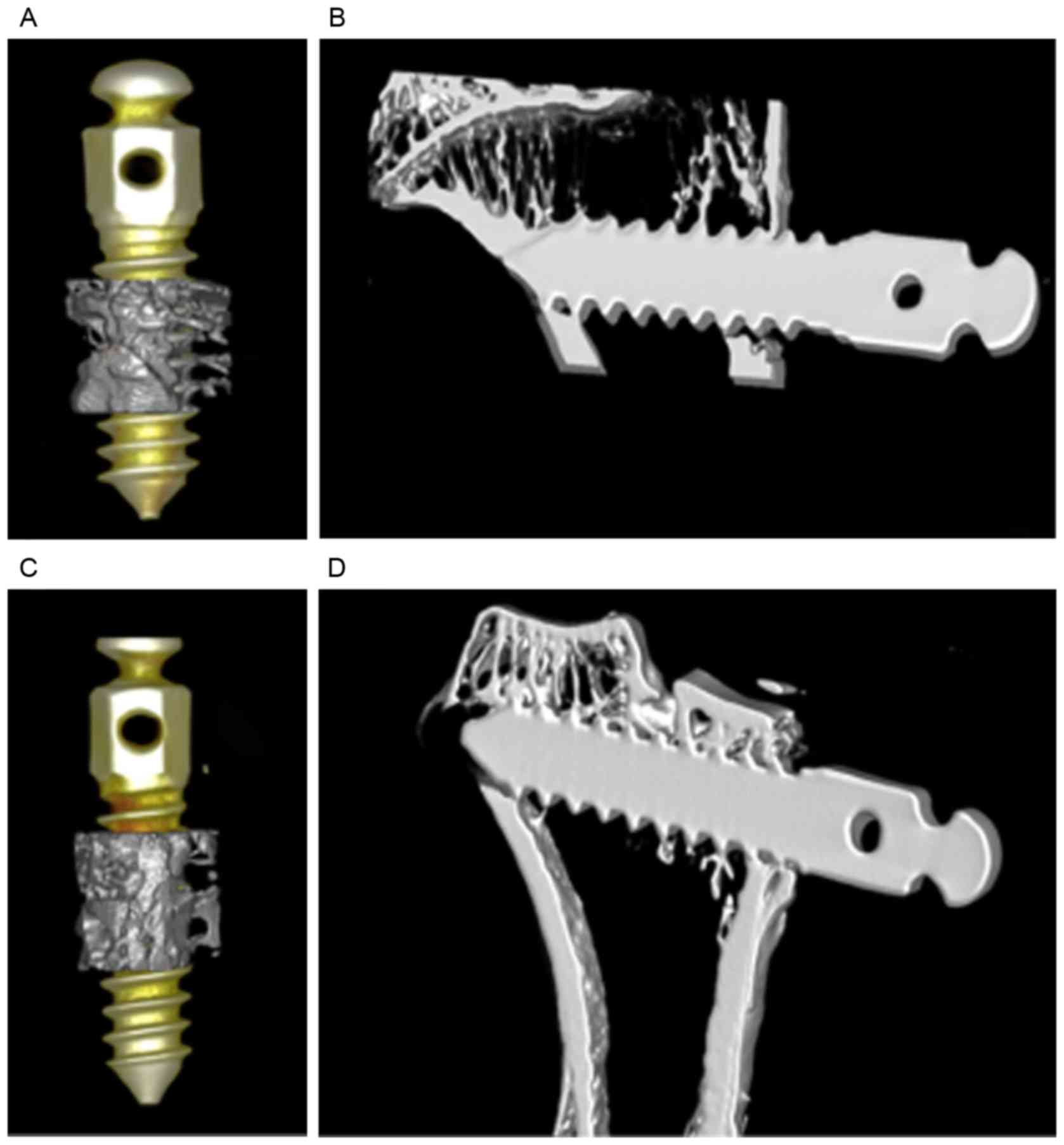
osseointegration of implants had occurred in both groups (Fig. 5). In the test group, newly formed
bone surrounding the BMSC-implant construct (Fig. 5A and B) was greater in quality and
quantity compared with that in the control group (Fig. 5C and D). In addition, more organized
supporting bone and greater trabecular construction was observed
around the BMSC-implant construct in the test group. The bone
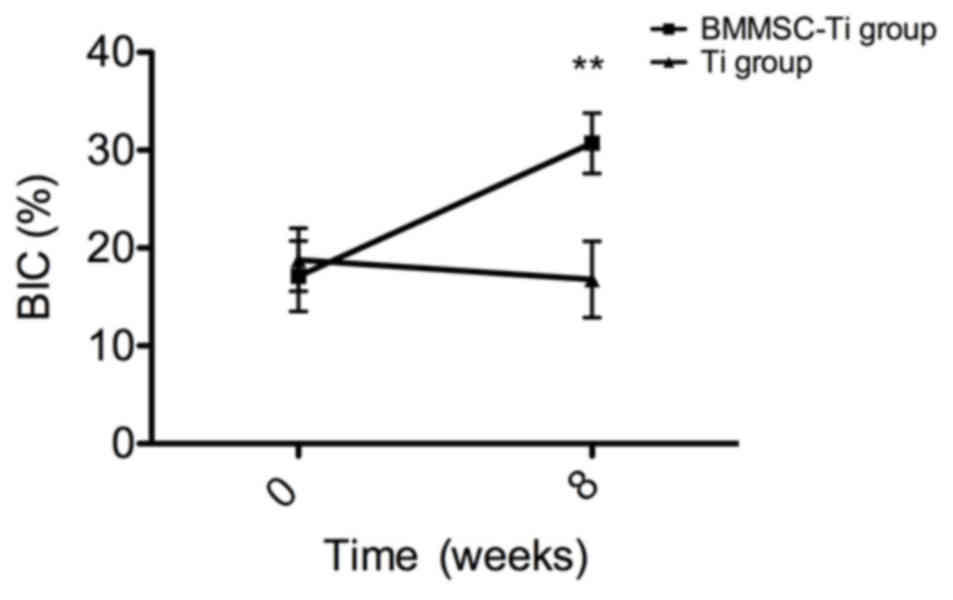
density index around the implants, BIC (Fig. 6), BV/TV, Tb.Th, Tb.Sp, Tb.N and BS/BV
were measured by micro CT analysis (Table II). The BIC, BV/TV and Tb.Th of the
BMSC-implant test group were significantly higher, while the Tb.Sp
was significantly lower, compared with those in the control group
(all P<0.05). However, there was no significant difference in
Tb.N and BS/BV values between the two groups in this study, which
was probably caused by insufficient sample volume.
 | Table II.Bone density indices at the
bone-implant interface after 8 weeks of healing. |
Table II.
Bone density indices at the
bone-implant interface after 8 weeks of healing.
| Bone density
index | Ti implant | BMSC-Ti
construct |
|---|
| BV/TV, % |
12.82±0.07 |
30.71±0.23a |
| BS/BV, % |
10.25±0.07 |
12.82±0.11 |
| Tb.Th, mm |
0.09±0.01 |
0.16±0.01a |
| Tb.N, mm |
1.14±0.05 |
1.97±0.05 |
| Tb.sp, mm |
0.79±0.06 |
0.35±0.03a |
| Tb.P.F,1/mm |
6.75±0.06 |
3.46±0.03a |
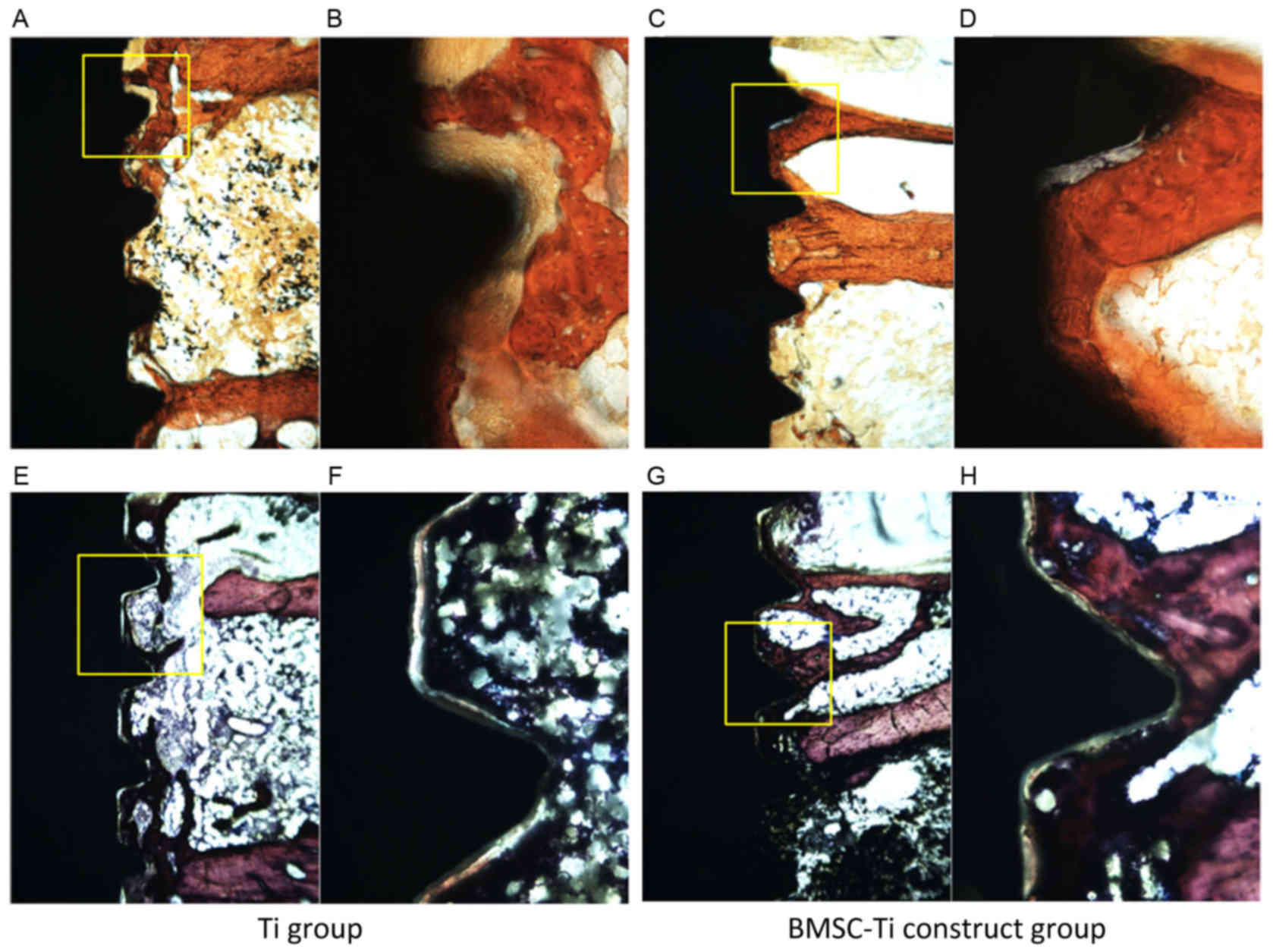
Histological evaluation of bone
formation in vivo
Following Masson-Ponceau Tri-Chrome and Van Gieson's
staining, all the sections were evaluated by light microscopy
(Fig. 7). At 8 weeks
post-implantation, it was observed that bone regeneration occurred
more rapidly in the test group, and newly formed bone around the
BMSC-implant constructs exhibited a greater BMD compared with the
control group. In addition, bone tissue was closely adhered to the
BMSC-implant constructs and there was no apparent gap around the
BMSC-implant constructs. Subsequent histomorphometric analysis of
18 BMSC-implant construct specimens indicated that the BIC ratio in
the BMSC-implant group (30.7±3.1%) was significantly higher
compared with that in the control group (16.8±3.9%) after 8 weeks
of healing in vivo (P<0.001; Fig. 6).
Discussion
Regarding dental implants, adequate osseointegration
with the host bone tissue is of great importance and required for
successful restoration. Scholars in implant dentistry have so far
designed and evaluated a variety methods aimed at enhancing
osseointegration, primarily by improving the speed and quality of
bone regeneration (29). However, in
medically compromised patients suffering from a systematic disease
such as osteoporosis, diabetes and periodontal inflammation,
osseointegration of dental implants is unpredictable and remains a
clinical challenge (2,30,31).
Osteoporosis, one of the most prevalent bone diseases, is
principally caused by an imbalance between bone formation and
resorption, resulting in the weaker structure of the host
trabecular bone (32). In patients
with osteoporosis, the success rate of dental implant treatment is
primarily comprised of two factors: i) The bone density of the
mandible and/or maxilla is reduced along with other parts of the
skeleton; and ii) impaired bone metabolism in osteoporosis may
reduce the healing capacity of bone around dental implants and
prolong the healing process (33).
To solve these problems, mutiple methods and ideas have been
considered and proposed. Oral administrations of ibandronate or
bisphosphonate are typically suggested for patients with
osteoporosis, which are considered to be beneficial in increasing
BMD and bone healing capacity. However, the final drug distribution
in the alveolar bone and the effect on the bone healing ability
around the implant remains unknown (34).
In our previous study, multilayer BMSC sheets, which
were applied within the edentulous region, have showed positive
effects on bone regeneration in the bone defect area (35). Moreover, the combination of the
multilayer BMSC sheets and dental implant were proved to enhance
the implant-bone osseointegration in both healthy and diabetic
animals (36,37). The improvement in the bone
regeneration resulted from the BMSCs sheets probably lied in
numerous cell factors released by BMSCs, including platelet-derived
growth factors, bone morphogenetic protein-2, transforming growth
factor-β and insulin-like growth factor-I (38). These growth factors may enhance
osteoblast differentiation and bone matrix mineralization (39). In addition, the superimposition of
dense cell-ECM layers to form a 3D structure is currently being
considered as a method of building tissues and scaffold materials
in vitro for tissue regeneration (40). These 3D cell-based structures allow
for a large number of cells and close cell-cell conjunctions. A
study by Kii et al (41)
demonstrated that a number of cadherins responsible for mediating
cell-cell interactions, e.g. the M-cadherin and cadherin-11are
associated with ECM stability. Therefore, a BMSC-implant structure
that supports cell-to-cell interactions may also support osteogenic
differentiation and proliferation (25). Furthermore, one more advantage of
using the BMSC sheets was that the mechanical strength of the BMSCs
sheets were relatively favorable, which could tolerate the friction
during the surgical manipulation and insertion.
In the present study, the BMSC from healthy rats
were used because it is a specific type of adult stem cell that
differentiate into other cell types when a tissue has been lost
and/or damaged due to trauma or disease, and their multilineage
differentiation potential may be maintained during their lifespan.
In addition, the BMSCs could also ensure the osteogenesis of
transplanted cells. With the use of allogenic BMSCs in the
sheet-implant constructs, no signs of rejection were observed
during the experiment.
According to the micro-CT analysis and histologucal
evaluation, at 8 weeks post-implantation, faster bone regeneration
around BMSC-implant constructs was observed. Analysis of
high-resolution 3D images obtained from micro-CT also demonstrated
that all bone formation indices, including BV/TV, Tb.Th and BIC,
were improved in newly formed areas around BMSC-implant constructs.
Although the inherent biocompatibility of pure titanium implants
also enables them to induce bone regeneration (42), the usage of multilayer BMSC sheets
around titanium implants showed improved implant osseointegration,
which may consequently improve the success rate of implant-based
treatments in patients with low BMD.
Regarding the use of the present animal model, it
may have been more appropriate and favorable to use larger OVX
animal models in the present study, in which the alveolar bone
would be a preferred choice for the installation of dental implant.
Bone tissue in larger OVX animal models may exhibit closer
similarities to humans regarding osteoporosis. However, the useage
of live micro-CT scanning, which observed and analyzed the bone
healing process atraumatically, limits the size of the sample being
scanned. Therefore, the OVX rat model was adopted in the present
study.
Further studies are required to investigate the
combination of BMSCs with different cell types, such as periodontal
ligament stem cells, dental pulp stem cells or gingiva-derived
mesenchymal stem cells, which may produce higher levels of
osteogenic factors or activate specific gene expression signaling
pathways to release desirable osteogenic proteins. The BMSC-implant
constructs should also be evaluated in different systematic
diseases that interfere with bone healing in future studies.
In conclusion, the BMSC sheet-implant constructs
improved the implant osseointegration in the rat model of
osteoporosis. Micro-CT and histological analyses identified higher
levels of BIC and new bone regeneration around BMSC-implant
constructs. These results indicate that titanium implants combined
with multilayer BMSC sheets may be considered in implant
restoration for patients with osteoporosis on the basis of clinical
trials.
Acknowledgements
The present study was partly supported by the
Shaanxi provincial Science & Technology Research Project,
P.R.China (grant no. 2016SF-011).
References
|
1
|
Mendoza-Edroso C, Sánchez Garrido-Lestache
N and Lopez-Picado A: Postmenopausal osteoporosis: Primary
prevention or excessive use of medications. Semergen. 39:123–129.
2013.(In Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Esposito M, Hirsch JM, Lekholm U and
Thomsen P: Biological factors contributing to failures of
osseointegrated oral implants. (II). Etiopathogenesis. Eur J Oral
Sci. 106:721–764. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Esposito M, Hirsch JM, Lekholm U and
Thomsen P: Biological factors contributing to failures of
osseointegrated oral implants. (I). Success criteria and
epidemiology. Eur J Oral Sci. 106:527–551. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Olivares-Navarrete R, Raines AL, Hyzy SL,
Park JH, Hutton DL, Cochran DL, Boyan BD and Schwartz Z: Osteoblast
maturation and new bone formation in response to titanium implant
surface features are reduced with age. J Bone Miner Res.
27:1773–1783. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Shibli JA, Aguiar KC, Melo L, d'Avila S,
Zenóbio EG, Faveri M, Iezzi G and Piattelli A: Histological
comparison between implants retrieved from patients with and
without osteoporosis. Int J Oral Maxillofac Surg. 37:321–327. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Marco F, Milena F, Gianluca G and Vittoria
O: Peri-implant osteogenesis in health and osteoporosis. Micron.
36:630–644. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Devlin H: Identification of the risk for
osteoporosis in dental patients. Dent Clin North Am. 56:847–861.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Erdoğan O, Shafer DM, Taxel P and Freilich
MA: A review of the association between osteoporosis and alveolar
ridge augmentation. Oral Surg Oral Med Oral Pathol Oral Radiol
Endod. 104:738.e1–13. 2007. View Article : Google Scholar
|
|
9
|
Blomqvist JE, Alberius P, Isaksson S,
Linde A and Hansson BG: Factors in implant integration failure
after bone grafting: An osteometric and endocrinologic matched
analysis. Int J Oral Maxillofac Surg. 25:63–68. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
de Melo L, Piattelli A, Lezzi G, d'Avila
S, Zenóbio EG and Shibli JA: Human histologic evaluation of a
six-year-old threaded implant retrieved from a subject with
osteoporosis. J Contemp Dent Pract. 9:99–105. 2008.PubMed/NCBI
|
|
11
|
Shibli JA, Grande PA, d'Avila S, Iezzi G
and Piattelli A: Evaluation of human bone around a dental implant
retrieved from a subject with osteoporosis. Gen Dent. 56:64–67.
2008.PubMed/NCBI
|
|
12
|
Thompson DD, Simmons HA, Pirie CM and Ke
HZ: FDA Guidelines and animal models for osteoporosis. Bone. 4
Suppl:17:125S–133S. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ikebe K, Wada M, Kagawa R and Maeda Y: Is
old age a risk factor for dental implants? Jpn Dent Sci Rev.
45:59–64. 2009. View Article : Google Scholar
|
|
14
|
Namkung-Matthai H, Appleyard R, Jansen J,
Hao Lin J, Maastricht S, Swain M, Mason RS, Murrell GA, Diwan AD
and Diamond T: Osteoporosis influences the early period of fracture
healing in a rat osteoporotic model. Bone. 28:80–86. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kalu DN: The ovariectomized rat model of
postmenopausal bone loss. Bone Miner. 15:175–191. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mosekilde L, Danielsen CC and Knudsen UB:
The effect of aging and ovariectomy on the vertebral bone mass and
biomechanical properties of mature rats. Bone. 14:1–6. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoshitake K, Yokota K, Kasugai Y, Kagawa
M, Sukamoto T and Nakamura T: Effects of 16 weeks of treatment with
tibolone on bone mass and bone mechanical and histomorphometric
indices in mature ovariectomized rats with established osteopenia
on a low-calcium diet. Bone. 25:311–319. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Vieira HP, Leite IA, Araújo Sampaio TM,
Dos Anjos de Paula J, do Nascimento Andrade A, de Abreu LC, Valenti
VE, Goulart FC and Adami F: Bisphosphonates adherence for treatment
of osteoporosis. Int Arch Med. 6:242013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Le Guéhennec L, Soueidan A, Layrolle P and
Amouriq Y: Surface treatments of titanium dental implants for rapid
osseointegration. Dent Mater. 23:844–854. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu Y, Huse RO, de Groot K, Buser D and
Hunziker EB: Delivery mode and efficacy of BMP-2 in association
with implants. J Dent Res. 86:84–89. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu Y, Wu J, Zhu Y and Han J: Therapeutic
application of mesenchymal stem cells in bone and joint diseases.
Clin Exp Med. 14:13–24. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nelson TJ, Behfar A, Yamada S,
Martinez-Fernandez A and Terzic A: Stem cell platforms for
regenerative medicine. Clin Transl Sci. 2:222–227. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pino AM, Rosen CJ and Rodríguez JP: In
osteoporosis, differentiation of mesenchymal stem cells (MSCs)
improves bone marrow adipogenesis. Biol Res. 45:279–287. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yamato M and Okano T: Cell sheet
engineering. Mater Today. 7:42–47. 2004. View Article : Google Scholar
|
|
25
|
Zhou W, Han C, Song Y, Yan X, Li D, Chai
Z, Feng Z, Dong Y, Li L, Xie X, et al: The performance of bone
marrow mesenchymal stem cell-implant complexes prepared by cell
sheet engineering techniques. Biomaterials. 31:3212–3221. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mandal BB and Kundu SC: Osteogenic and
adipogenic differentiation of rat bone marrow cells on non-mulberry
and mulberry silk gland fibroin 3D scaffolds. Biomaterials.
30:5019–5030. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Agata H, Yamazaki M, Uehara M, Hori A,
Sumita Y, Tojo A and Kagami H: Characteristic differences among
osteogenic cell populations of rat bone marrow stromal cells
isolated from untreated, hemolyzed or Ficoll-treated marrow.
Cytotherapy. 14:791–801. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Apgar JM, Juarranz A, Espada J, Villanueva
A, Cañete M and Stockert JC: Fluorescence microscopy of rat embryo
sections stained with haematoxylin-eosin and Masson's trichrome
method. J Microsc. 191:20–27. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sykaras N, Iacopino AM, Marker VA,
Triplett RG and Woody RD: Implant materials, designs, and surface
topographies: Their effect on osseointegration. A literature
review. Int J Oral Maxillofac Implants. 15:675–690. 2000.PubMed/NCBI
|
|
30
|
Mombelli A and Cionca N: Systemic diseases
affecting osseointegration therapy. Clin Oral Implants Res. 17
Suppl 2:S97–S103. 2006. View Article : Google Scholar
|
|
31
|
Porter JA and von Fraunhofer JA: Success
or failure of dental implants? A literature review with treatment
considerations. Gen Dent. 53(423–432): quiz 433. 4462005.
|
|
32
|
Muñoz-Torres M, Alonso G and Raya MP:
Calcitonin therapy in osteoporosis. Treat Endocrinol. 3:117–132.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Dao TT, Anderson JD and Zarb GA: Is
osteoporosis a risk factor for osseointegration of dental implants?
Int J Oral Maxillofac Implants. 8:137–144. 1993.PubMed/NCBI
|
|
34
|
Recknor C, Czerwinski E, Bone HG, Bonnick
SL, Binkley N, Palacios S, Moffett A, Siddhanti S, Ferreira I,
Ghelani P, et al: Denosumab compared with ibandronate in
postmenopausal women previously treated with bisphosphonate
therapy: A randomized open-label trial. Obstet Gynecol.
121:1291–1299. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu Y, Ming L, Luo H, Liu W, Zhang Y, Liu
H and Jin Y: Integration of a calcined bovine bone and BMSC-sheet
3D scaffold and the promotion of bone regeneration in large
defects. Biomaterials. 34:9998–10006. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu B, Zhang J, Brewer E, Tu Q, Yu L, Tang
J, Krebsbach P, Wieland M and Chen J: Osterix enhances
BMSC-associated osseointegration of implants. J Dent Res.
88:1003–1007. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kotsovilis S, Karoussis IK and Fourmousis
I: A comprehensive and critical review of dental implant placement
in diabetic animals and patients. Clin Oral Implants Res.
17:587–599. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lalani Z, Wong M, Brey EM, Mikos AG and
Duke PJ: Spatial and temporal localization of transforming growth
factor-beta1, bone morphogenetic protein-2, and platelet-derived
growth factor-A in healing tooth extraction sockets in a rabbit
model. J Oral Maxillofac Surg. 61:1061–1072. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yamada Y, Ueda M, Naiki T and Nagasaka T:
Tissue-engineered injectable bone regeneration for osseointegrated
dental implants. Clin Oral Implants Res. 15:589–597. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gkantidis N, Schauseil M, Pazera P, Zorkun
B, Katsaros C and Ludwig B: Evaluation of 3-dimensional
superimposition techniques on various skeletal structures of the
head using surface models. PloS One. 10:e01188102015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kii I, Amizuka N, Shimomura J, Saga Y and
Kudo A: Cell-cell interaction mediated by cadherin-11 directly
regulates the differentiation of mesenchymal cells into the cells
of the osteo-lineage and the chondro-lineage. J Bone Miner Res.
19:1840–1849. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Oron A, Agar G, Oron U and Stein A:
Correlation between rate of bony ingrowth to stainless steel, pure
titanium, and titanium alloy implants in vivo and formation of
hydroxyapatite on their surfaces in vitro. J Biomed Mater Res A.
91:1006–1009. 2009. View Article : Google Scholar : PubMed/NCBI
|