Introduction
Preeclampsia is a pregnancy-specific multisystem
disorder featuring new-onset hypertension [systolic blood pressure
(SBP), ≥140 mmHg and/or diastolic blood pressure (DBP), ≥90 mmHg]
and impaired organ(s), including impaired liver function,
thrombocytopenia, pulmonary edema, cerebral/visual symptoms after
20 weeks of gestation. Proteinuria (≥300 mg/24 h or dipstick
reading >+1) is generally the most associated additional symptom
(1–4). It occurs in 5–7% of all pregnancies
worldwide.
Preeclampsia has adverse outcomes for maternal as
well as neonatal health: It causes an estimate of 50,000–100,000
deaths annually worldwide, along with severe fetal and neonatal
morbidity and mortality such as enhanced risk of fetal growth
restriction and still birth. The cost of preeclampsia is great and
includes not only the direct expense of monitoring pregnancies and
the morbidity and mortality directly caused by preeclampsia but
also the cost of associated complications such as prematurity and
growth restriction (5).
Despite the serious health, social and economic
costs of preeclampsia, no therapies are available to prevent,
stabilize or cure the disease. The only treatment is to terminate
pregnancy by delivery of the baby and placenta, which itself is
associated with a risk of premature birth (6). Therefore, an effective treatment for
preeclampsia is required.
Possible therapeutic agents for preeclampsia include
those targeting pro-angiogenic factors, vasodilators and factors
preventing inflammation and oxidative stress (6). Apelin, encoded by the APLN gene, is an
endogenous ligand of G protein-coupled receptor APJ, a putative
receptor associated with the angiotensin receptor AT1
(7). The apelin/APJ system has
multiple effects on cardiovascular physiology and pathophysiology
(8). Apelin reduced blood pressure
in a mouse model of atherosclerosis and acutely in patients with
heart failure (9–11). Furthermore, apelin promotes cardiac
contractility (12) and vessel
formation (13) and prevents aortic
inflammation by decreasing the mRNA level of interleukin 6 and
tumor necrosis factor α (14).
Prepro-apelin mRNA is ubiqutous in human tissues, with increased
levels in the placenta (15), which
suggests a potential placental production of apelin during
pregnancy. Apelin levels were reduced in serum and placental
chorionic villi of patients with preeclampsia (16–18). The
expression of APJ was also reduced in placentas of patients with
preeclampsia in association with poor fetal growth (19). However, several other studies
reported increased levels of apelin in the serum and placenta of
patients with preeclampsia (20,21).
Despite these controversial results, a potential association
between apelin and preeclampsia is indicated.
Given the pleiotropic effect of apelin on
angiogenesis, vasodilation, inhibition of inflammation and
oxidative stress, it was hypothesized that apelin ameliorated the
pathogenesis of preeclampsia. A rat model of preeclampsia induced
by reduced uterine perfusion pressure (RUPP) was used to verify the
effect of apelin on the development of preeclampsia.
Materials and methods
Animals
A total of 28 female Sprague-Dawley (SD) rats (age,
3 months; weight, 160–200 g) were obtained from the Animal Center
of Hebei Medical University (Shijiazhuang, China) and were housed
under standard conditions (temperature, 20±8°C; humidity, 60±10%;
lights on from 6:00 to 18:00) with standard rodent chow and water
provided ad libitum. All animal procedures complied with the
Animal Management Regulations of the Ministry of Health, P.R. China
(document no. 55, 2001) and the Guidelines for the Care and Use of
Laboratory Animals published by the US National Institutes of
Health (NIH; publication no. 85–23, revised 1996), and were
approved by the Animal Care Committee of Hebei Medical University
(Shijiazhuang, China).
Experimental procedure
Gestational day (GD) 0 of pregnancy was identified
by the existence of sperm in a vaginal smear after an overnight
breeding with a male rat. Pregnant rats were then randomly divided
into four groups for treatment: Control (N group; n=6), apelin
(APLN group; n=7), preeclampsia (PE group; n=7) and preeclampsia
plus apelin (PE+APLN group; n=8). The APLN and PE+ALPN rats were
administered apelin-13 (Bachem, Bubendorf, Switzerland)
subcutaneously (6×10−8 mol/kg, twice a day) from GD 11,
and the other rats were injected with an equal volume of saline. On
GD 14, preeclampsia was induced by RUPP (see procedure below). On
GD 19, blood pressure was measured in all rats and the 24-h urine
was collected using metabolism cages and urine protein was
measured, and blood and placenta were collected. Blood was
assembled for separation of serum, and fetal and placental weights
were evaluated in each rat. Placentas of rats were split, weighed,
part of them were immediately emerged into 4% paraformaldehyde for
48 h at 20°C and embedded in paraffin. serial paraffin sections
(5-µm thick) were prepared and stained with hematoxylin and eosin
(HE) for 3 min and 2 min, respectively at 20°C. On each HE section,
four high-power fields (magnification, ×200) were selected to
detect placental tissue morphology used light microscope.
CRUPP model in rats
The RUPP model in SD rats is an established
preeclampsia model (22–25). In the PE and PE+ALPN group, rats were
anesthetized by inhalation of isoflurane (4% for induction and 1–3%
for maintenance; Pharmaceutical Partners of Canada; Fresenius Kabi,
Toronto, ON, Canada), the abdominal cavity was opened by a midline
incision and the abdominal aorta was uncovered above the iliac
bifurcation. A silver clip [inner diameter (ID), 0.203 mm] was
placed around the aorta. To block compensatory flow through ovarian
arteries, two more silver clips (ID, 0.100 mm) were placed around
the left and right ovarian arteries between the ovary and uterine
horn. For rats in the N and APLN groups, arteries were similarly
manipulated and silver clips were placed on intra-abdominal
fat.
Arterial blood pressure
measurement
On GD 19, rats were anesthetized again with
isoflurane (inhalation administration), and a carotid arterial
catheter (Scientific Commodities Inc., Lake Havasu City, AZ, USA)
filled with heparin saline (50 U/ml) was then inserted for blood
pressure measurement. Arterial pressure was monitored with a
pressure transducer (Chengdu Instrument Factory, Chengdu, China)
and continuously recorded for 30 min after a 15-min stabilization
period on a recorder (Chengdu Instrument Factory). Systolic blood
pressure (SBP), diastolic blood pressure, mean arterial pressure
(MAP) and heart rate (HR) were measured.
Semi-quantitative polymerase chain
reaction (qPCR) analysis
The mRNA expression of endothelial nitric oxide
synthase (eNOS) was detected by qPCR. Placentas of rats were split,
weighed, immediately snap-frozen in liquid nitrogen and stored at
−80°C. After the tissue was crushed in liquid nitrogen by using a
mortar and pestle, total RNA was extracted by using the RNeasy
Protect Mini kit (cat. no. 74106; Qiagen, Hilden, Germany). The
isolation procedure followed the manufacturer's instructions.
Complementary DNA (cDNA) was synthesized from 1 µg mRNA by using
the Bio-Rad iScript cDNA reverse transcription kit (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). PCR amplification was then
performed using premix Taq Version (cat. no. R004A; Takara
Biotechnology Co., Ltd., Dalian, China), cDNA and primers (Sangong
Biotech, Shanghai, China) with the following sequences: eNOS sense,
5′-CCCAGGAGAGATCCACCTCA-3′ and anti-sense,
5′-CGGAAGGGTGCAATACCAGT-3′; GAPDH sense, 5′-GCAACTCCCATTCTTCCACC-3′
and anti-sense, 5′-TGGTATTCGAGAGAAGGGAGGG-3′. The condition of PCR
was as follows: 30 cycles at 95°C for 10 sec; 55°C for 30 sec; and
72°C for 1 min. Subsequently, 5 µl PCR products was subjected to
1.5% agarose gel electrophoresis (150 V for 30 min) and the results
were analyzed using a UV Gel imaging analysis system.
Western blot analysis
Placenta samples (100 mg) were frozen with liquid
nitrogen, homogenized in 1 ml RIPA buffer (high) (cat. no. R0010,
Beijing Solarbio Science Technology Co., Ltd., Beijing, China) and
then centrifuged at 12,000 × g for 10 min at 4°C, and the protein
concentration was determined using NanoDrop-1000 spectrophotometer
(Gene Co., Ltd., Beijing, China). Protein extracts were
re-suspended in a 5X sample buffer and boiled in water for 15 min
at 100°C. Total protein (100 µg) was subjected to 10% SDS-PAGE and
transferred to nitrocellulose membranes (cat. no. 1620150; Bio-Rad
Laboratories, Inc., Hercules, CA, USA), followed by incubation with
the primary antibodies anti-eNOS (cat. no. sc-654; 1:1,000
dilution) and anti-GAPDH (cat. no. sc-25778; 1:1,000; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) at 4°C overnight.
Subsequently, membranes were incubated with the IRDye 800CW goat
anti-rabbit immunoglobulin G (H+L) (Rockland Immunochemicals Inc.,
Pottstown, PA, USA) for 1 h at room temperature. Blots were
visualized by enhanced chemiluminescence (Beijing Applygen
Technologies, Beijing, China), and bands were analyzed by using
ImageJ software (vision 1.37; NIH, Bethesda, MD, USA). The protein
contents were normalized to that of GADPH.
Measurement of NO and eNOS in
serum
Contents of NO and activity of eNOS in serum were
measured by using commercial kits (cat. no. 20150205; Nanjing
Jiancheng Bioengineering Institute, Nanjing, China) according to
the manufacturer's instructions.
Measurement of total anti-oxidant
capacity (T-AOC) and malondialdehyde (MDA) levels in serum
To detect the level of oxidative stress, T-AOC and
MDA levels were measured by using commercial kits (cat. no.
20150525; Nanjing Jiancheng Bioengineering Institute) according to
the manufacturer's instructions.
Statistical analysis
Values are expressed as the mean ± standard
deviation and analyzed with SAS 9.1 software (SAS Institute Inc.,
Cary, NC, USA). One-way analysis of variance was used to compare
more than two groups, and Tukey's honest significant difference
test was used to assess between-group differences. P<0.05 was
considered to indicate a statistically significant difference.
Results
Apelin ameliorates hypertension and
proteinuria in RUPP rats
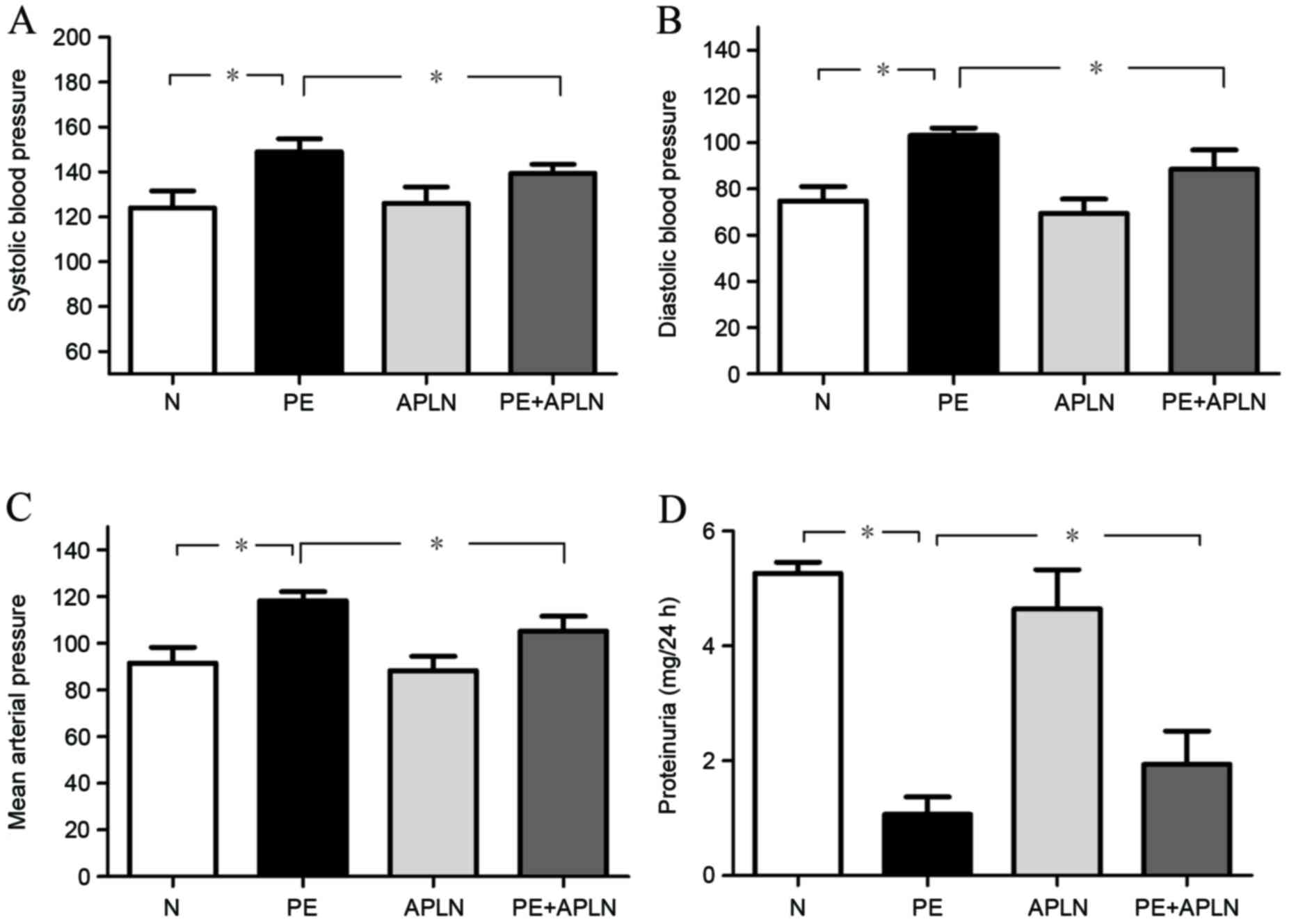
Compared with N rats, PE rats had significantly
increased SBP, DBP and MAP (P<0.05; Fig. 1A-C). Apelin administration in PE rats
(PE+APLN) significantly inhibited the elevation of SBP, DBP and MBP
(P<0.05), while no difference was observed between N and APLN
rats (P>0.05).
Compared with N rats, PE rats had significantly
decreased proteinuria (P<0.05; Fig.
1D). Apelin administration reversed the PE-associated decreases
in proteinuria (P<0.05). Proteinuria did not differ between N
and APLN rats (P>0.05).
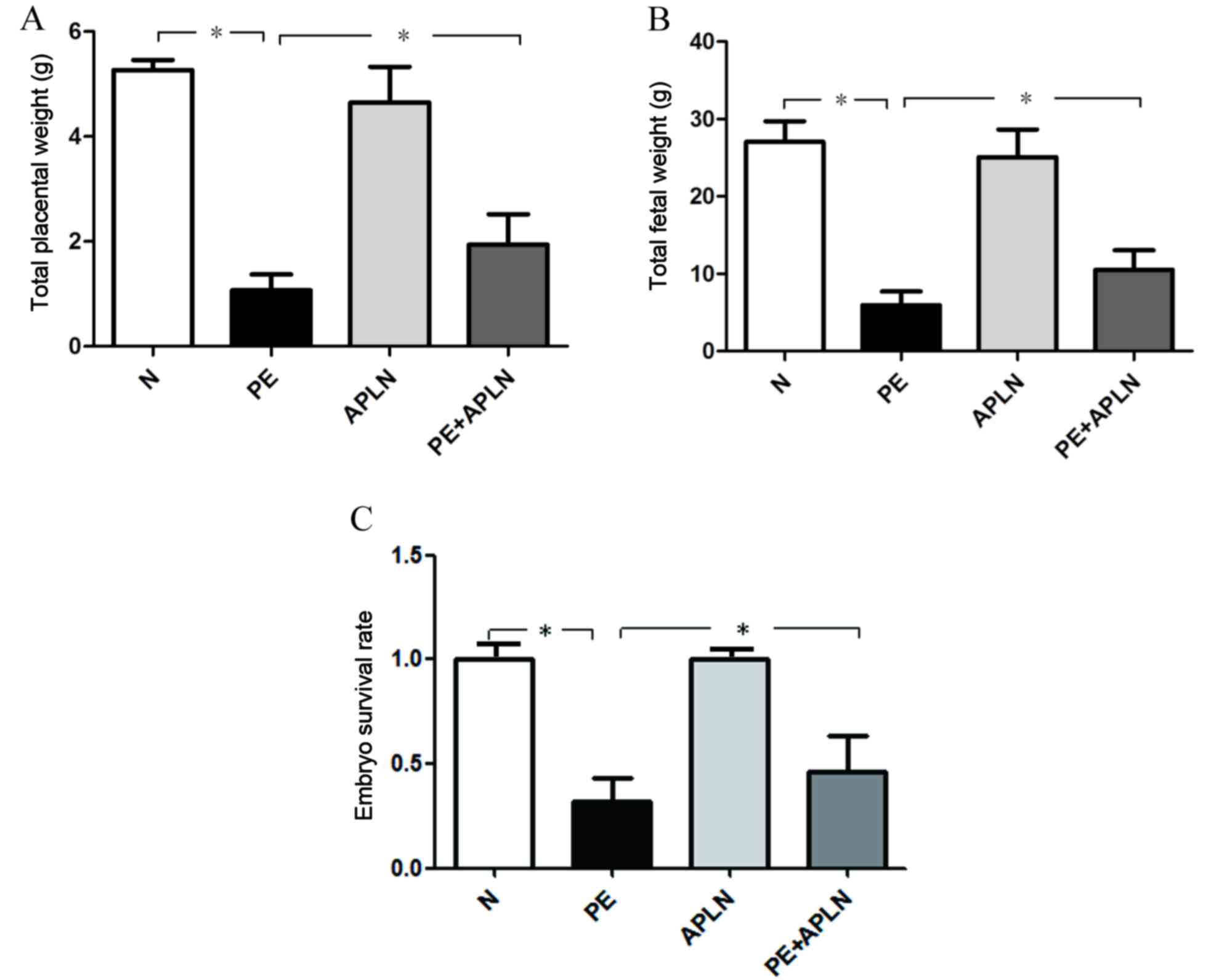
Apelin improves pregnancy outcomes in
PE rats
Compared with N rats, PE rats exhibited a
significantly decreased total fetal and total placental weight as
well as embryo survival rate (P<0.05; Fig. 2). Apelin administration reversed the
decreased total fetal weight and total placental weight, while
further increasing the embryo survival rate (P<0.05). N and APLN
rats did not exhibit any differences regarding these features
(P>0.05).
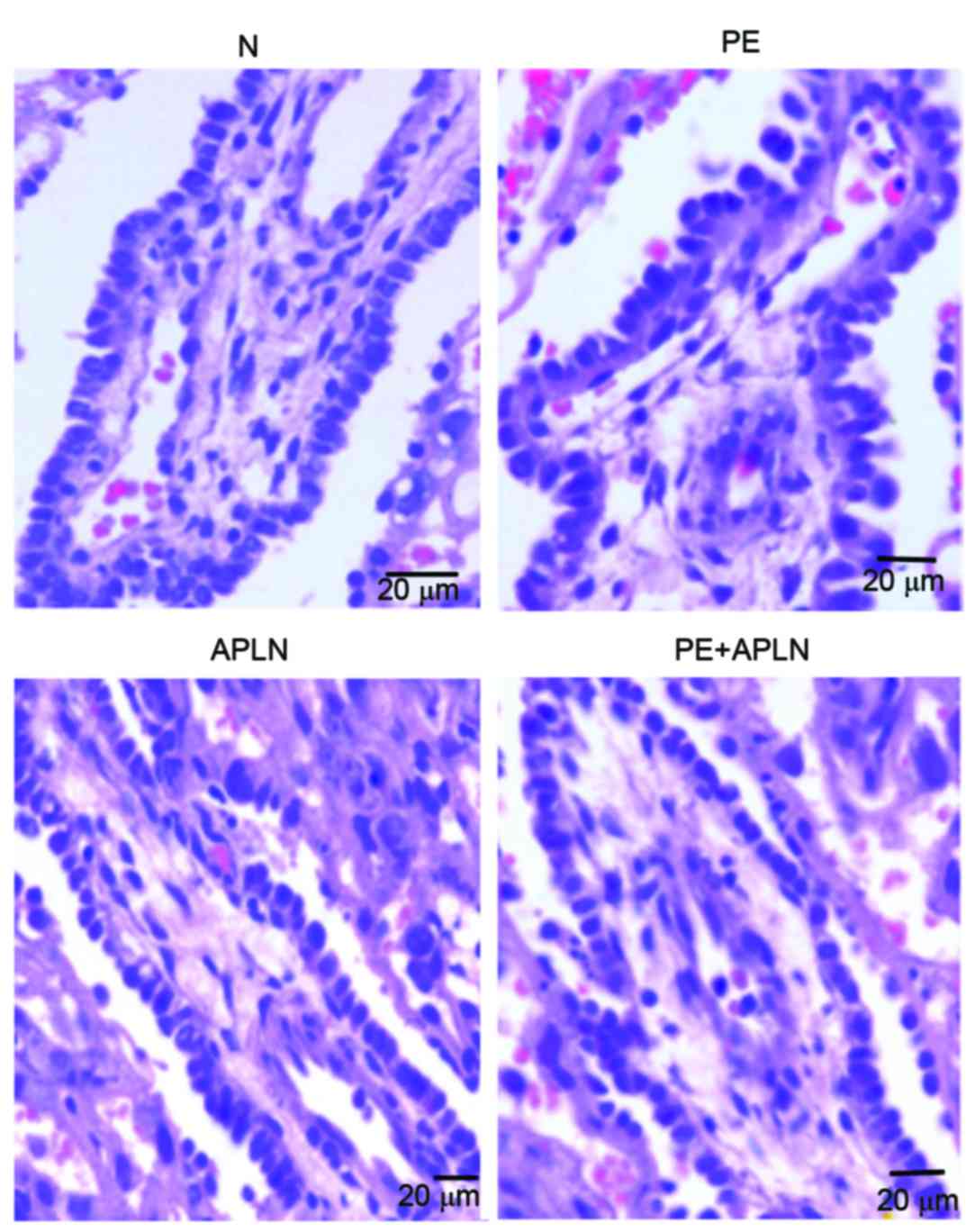
Effect of apelin on placental tissue
morphology
Villi exhibited greater differences than other parts
of the rat placenta among the groups on HE staining (Fig. 3). Compared with the placentas of N
rats, those of PE rats had villous edema, irregularly arranged
cells of various sizes and hyperchromatic nuclei, whereas PE+APLN
rats had resolved edema of the placental villi and cells were
regularly arranged with less hyperchromatic areas.
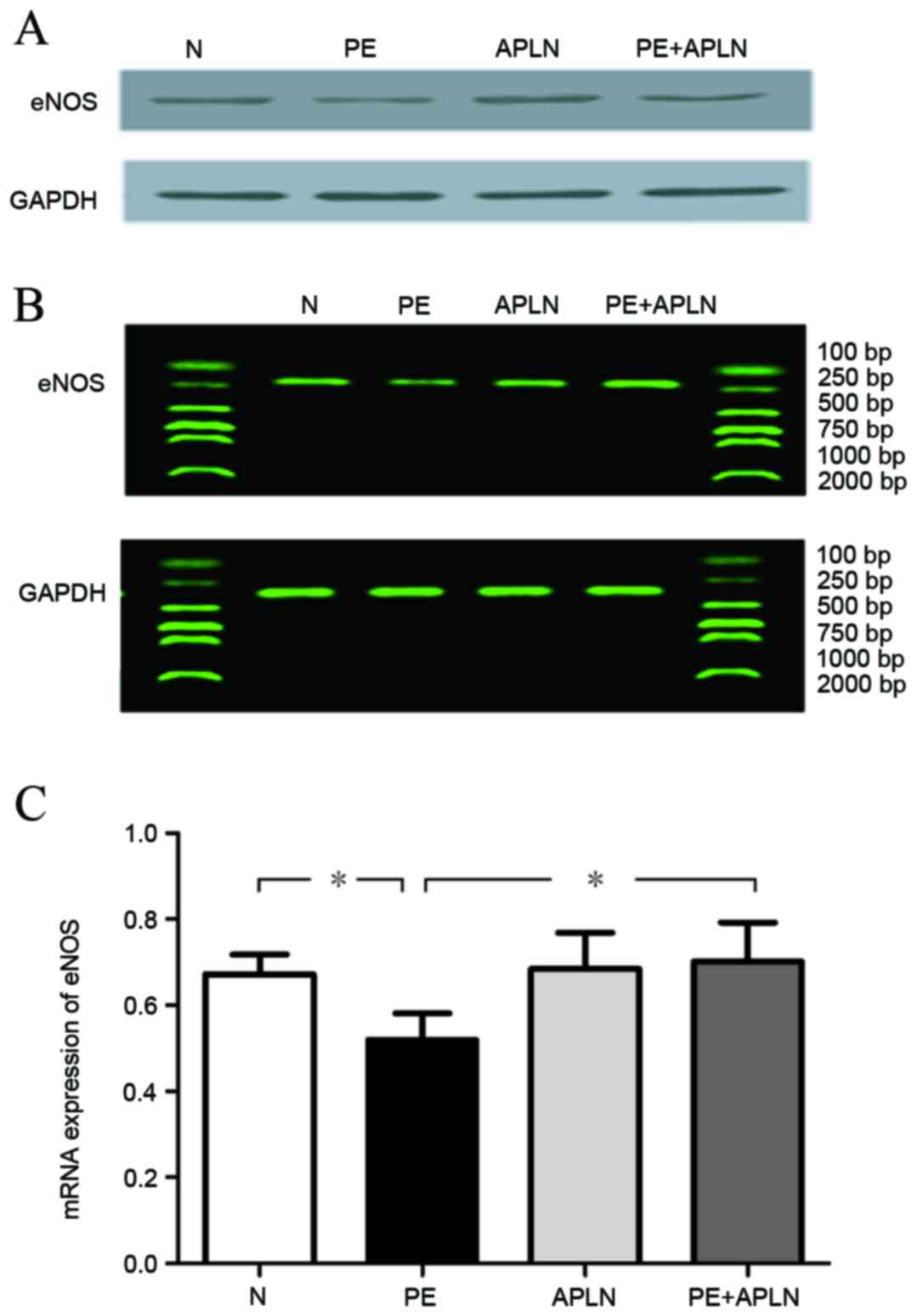
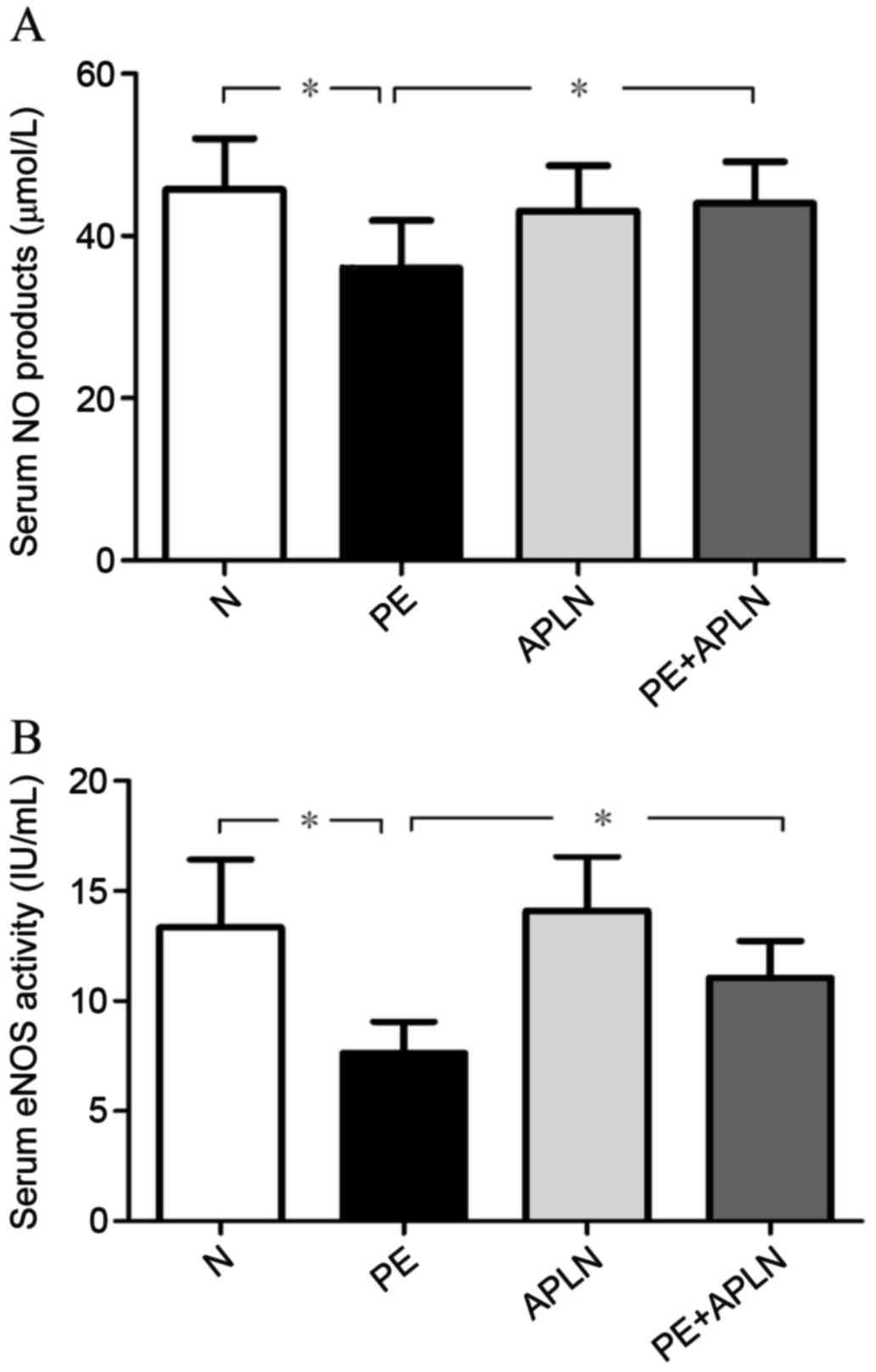
Apelin restores eNOS/NO signaling in
RUPP rats
Compared with N rats, PE rats had significantly
downregulated protein and mRNA levels of eNOS in the placenta and
decreased activity of eNOS in the serum (P<0.05). Apelin
administration to PE rats led to an upregulation of the protein and
mRNA levels of eNOS (P<0.05). N and APLN rats did not exhibit
any differences in eNOS levels (P>0.05; Fig. 4).
Accordingly, the serum content of NO in PE rats was
lower than that in N rats and the activity of serum eNOS was
decreased (P<0.05; Fig. 5).
Apelin treatment reversed the PE-induced reduction of the serum NO
content and eNOS activity (P<0.05). The serum content of NO and
the activity of eNOS did not differ between N and APLN rats
(P>0.05).
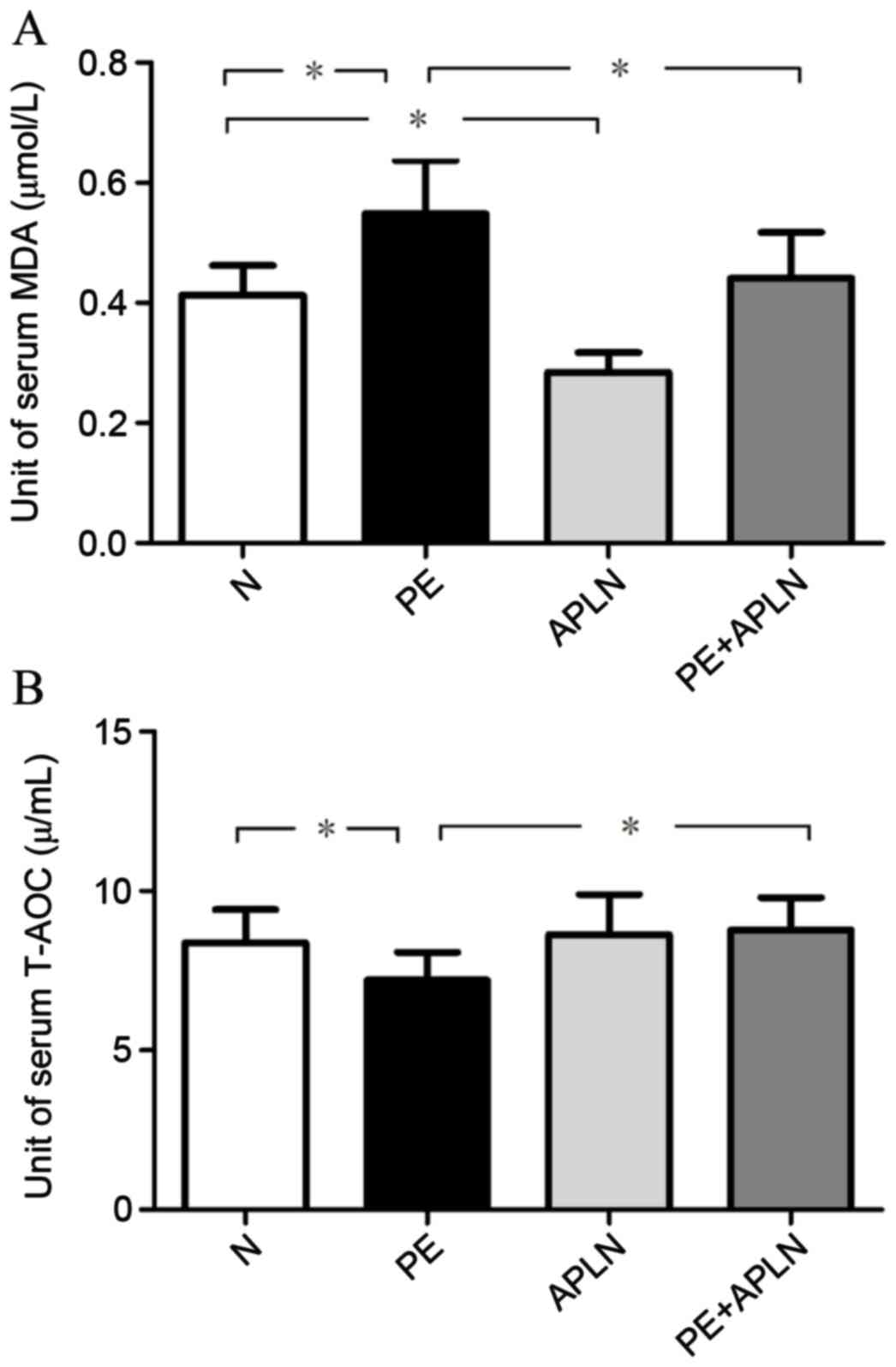
Apelin ameliorates oxidative stress in
RUPP rats
The serum T-AOC was 8.39±1.04, 7.20±0.88, 8.63±1.27
and 8.78±1.00 U/ml and serum MDA levels were 0.41±0.05, 0.55±0.09,
0.28±0.03 and 0.44±0.08 µmol/l in N, PE, APLN and PE+APLN rats,
respectively. Compared with N rats, PE rats exhibited significantly
increased serum MDA levels and decreased serum T-AOC levels
(P<0.05; Fig. 6). Apelin
administration reversed these effects (P<0.05). The N and APLN
rats did not differ in these features (P>0.05).
Discussion
In the present study, a rat preeclampsia model was
successfully established by RUPP, as demonstrated by hypertension
and poor outcomes of pregnancy. Apelin treatment significantly
ameliorated the symptoms of preeclampsia in RUPP rats. Furthermore,
it improved the impaired eNOS/NO signaling pathway and prevented
activation of oxidative stress in RUPP rats.
The RUPP animal model of placental ischemia is well
established, and in pregnant animals, this procedure invokes
numerous symptoms of preeclampsia representative of those in
humans, such as hypertension and intrauterine growth restriction
(22–25). The present study observed that blood
pressure parameters in PE rats, including SBP, DBP and MAP, were
greater than those in N rats and total fetal weight as well as the
embryo survival rate were lower. These results demonstrated the
successful establishment of the preeclampsia model in the rats of
the present study. Of note, apelin treatment reversed the
PE-associated elevation in blood pressure, increased the total
fetal weight and further increasing the embryo survival rate. These
results demonstrated the beneficial effects of apelin on
preeclampsia.
Endothelial dysfunction is considered to have a key
role in the progression of preeclampsia. Placental hypoperfusion
from a lack of spiral artery remodeling in initial placental
development releases circulating factors into the maternal
vasculature. Analysis of plasma from women with preeclampsia
indicated impaired endothelium-dependent vasodilation in healthy
resistance vessels (26,27). One critical mechanism regulating
vascular function during pregnancy is the NO vasodilation pathway.
NO, a powerful vasodilator, is produced from L-arginine by NOS and
has three isoforms: eNOS (also referred to as NOS-3), inducible NOS
(also referred to as NOS-2) and neuronal NOS (also referred to as
NOS-1). In the endothelium, NO production is primarily associated
with eNOS (28,29). NO is also a potent vasodilator in the
uterine artery vasculature and contributes to the elevated blood
flow and fetal growth during pregnancy (30). Animal models with production of NO
inhibited by genetic modification or pharmacological blockade with
L-NAME demonstrated structural and functional impairments in terms
of uterine artery adaptation and decreased uterine artery blood
flow (31). Given that the etiology
of preeclampsia is considered to be abnormal placentation and that
endothelial dysfunction is a key feature of the disease,
enhancement of NO bioavailability is an attractive strategy for
preventing and treating preeclampsia (6). One randomized controlled trial of NO
supplementation with L-arginine combined with anti-oxidant vitamins
revealed a 40% decrease in the risk of preeclampsia in women with a
personal or close family history of preeclampsia (32). Apelin has a direct activating effect
on the L-arginine/eNOS/NO pathway (33,34). The
present study found that apelin treatment significantly improved
the expression of eNOS in the placenta and the levels of NO and
eNOS in the serum, which were all decreased in PE rats. These
results suggested that restoration of the eNOS/NO pathway may be
involved in the ameliorative effects of apelin on preeclampsia.
Oxidative stress has a key role in the progression
of preeclampsia. It may trigger apoptosis of syncytium and
subsequent secretion of pro-inflammatory cytokines and
anti-angiogenic factors, which may ultimately induce endothelial
dysfunction and cause symptoms of preeclampsia (4). Accumulating evidence has demonstrated
that preeclampsia is associated with high levels of oxidative
stress markers, including lipid peroxidation products and decreased
anti-oxidant capacity (35). Given
that excessive oxidative stress is associated with the pathology of
preeclampsia, reducing oxidative stress may be one method to
prevent and treat preeclampsia (36). Accordingly, the salutary effect of
apelin on suppressing oxidative stress has been demonstrated in
numerous cell and tissue types (37,38). In
line with this, the present study also demonstrated that apelin
attenuated the increased oxidative stress in RUPP rats. These
results suggested that inhibition of oxidative stress may be
involved in the ameliorative effects of apelin on preeclampsia.
Of note, the present study had several limitations.
It investigated the therapeutic effect of apelin on preeclampsia in
rats with PE only, and further study of the effect in other animal
models of preeclampsia will be performed in the future. Due to the
transient half-life of apelin, long-acting analogs of apelin are
required. In addition, with the multiple effects of apelin, other
mechanisms by which it ameliorates progression of preeclampsia
should be thoroughly investigated.
In conclusion, the results of the present study
suggested that apelin ameliorates the pathologies of preeclampsia.
Restoring the eNOS/NO pathway and inhibiting oxidative stress may
be involved in the ameliorative effect of apelin on preeclampsia.
Apelin is a potential drug for preventing and treating
preeclampsia.
Acknowledgements
The present study was funded by the Key Research
Program of Hebei Provincial Health and Family Planning Commission
(grant no. 20150233).
References
|
1
|
Burton GJ, Jauniaux E and Watson AL:
Maternal arterial connections to the placental intervillous space
during the first trimester of human pregnancy: The boyd collection
revisited. Am J Obstet Gyneco. 181:718–724. 1999. View Article : Google Scholar
|
|
2
|
Walker JJ: Pre-eclampsia. Lancet.
356:1260–1265. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hung TH, Skepper JN and Burton GJ: In
vitro ischemia-reperfusion injury in term human placenta as a model
for oxidative stress in pathological pregnancies. Am J Pathol.
159:1031–1043. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Redman CW and Sargent IL: Latest advances
in understanding preeclampsia. Science. 308:1592–1594. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Duley L: Maternal mortality associated
with hypertensive disorders of pregnancy in africa, asia, latin
america and the caribbean. Br J Obstet Gynaecol. 99:547–553. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oyston CJ, Stanley JL and Baker PN:
Potential targets for the treatment of preeclampsia. Expert Opin
Ther Targets. 19:1517–1530. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
O'Carroll AM, Lolait SJ, Harris LE and
Pope GR: The apelin receptor APJ: Journey from an orphan to a
multifaceted regulator of homeostasis. J Endocrinol. 219:R13–R35.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee DK, Cheng R, Nguyen T, Fan T,
Kariyawasam AP, Liu Y, Osmond DH, George SR and O'Dowd BF:
Characterization of apelin, the ligand for the APJ receptor. J
Neurochem. 74:34–41. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee DK, Saldivia VR, Nguyen T, Cheng R,
George SR and O'Dowd BF: Modification of the terminal residue of
apelin-13 antagonizes its hypotensive action. Endocrinology.
146:231–236. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chun HJ, Ali ZA, Kojima Y, Kundu RK,
Sheikh AY, Agrawal R, Zheng L, Leeper NJ, Pearl NE, Patterson AJ,
et al: Apelin signaling antagonizes Ang II effects in mouse models
of atherosclerosis. J Clin Invest. 118:3343–3354. 2008.PubMed/NCBI
|
|
11
|
Japp AG, Cruden NL, Barnes G, van Gemeren
N, Mathews J, Adamson J, Johnston NR, Denvir MA, Megson IL, Flapan
AD, et al: Acute cardiovascular cffects of apelin in humans:
potential role in patients with chronic heart failure. Circulation.
121:1818–1827. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Szokodi I, Tavi P, Földes G,
Voutilainen-Myllylä S, Ilves M, Tokola H, Pikkarainen S, Piuhola J,
Rysä J, Toth M, et al: Apelin, the novel endogenous ligand of the
orphan receptor APJ, regulates cardiac contractility. Circ Res.
91:434–440. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kidoya H and Takakura N: Biology of the
apelin-APJ axis in vascular formation. J Biochem. 152:125–131.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Leeper NJ, Tedesco MM, Kojima Y, Schultz
GM, Kundu RK, Ashley EA, Tsao PS, Dalman RL and Quertermous T:
Apelin prevents aortic aneurysm formation by inhibiting macrophage
inflammation. Am J Physiol Heart Circ Physiol. 296:H1329–H1335.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Medhurst AD, Jennings CA, Robbins MJ,
Davis RP, Ellis C, Winborn KY, Lawrie KW, Hervieu G, Riley G,
Bolaky JE, et al: Pharmacological and immunohistochemical
characterization of the APJ receptor and its endogenous ligand
apelin. J Neurochem. 84:1162–1172. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bortoff KD, Qiu C, Runyon S, Williams MA
and Maitra R: Decreased maternal plasma apelin concentrations in
preeclampsia. Hypertens Pregnancy. 31:398–404. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Inuzuka H, Nishizawa H, Inagaki A, Suzuki
M, Ota S, Miyamura H, Miyazaki J, Sekiya T, Kurahashi H and Udagawa
Y: Decreased expression of apelin in placentas from severe
pre-eclampsia patients. Hypertens Pregnancy. 32:410–421. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yamaleyeva LM, Chappell MC, Brosnihan KB,
Anton L, Caudell DL, Shi S, McGee C, Pirro N, Gallagher PE, Taylor
RN, et al: Downregulation of apelin in the human placental
chorionic villi from preeclamptic pregnancies. Am J Physiol
Endocrinol Metab. 309:E852–E860. 2015.PubMed/NCBI
|
|
19
|
Furuya M, Okuda M, Usui H, Takenouchi T,
Kami D, Nozawa A, Shozu M, Umezawa A, Takahashi T and Aoki I:
Expression of angiotensin II receptor-like 1 in the placentas of
pregnancy-induced hypertension. Int J Gynecol Pathol. 31:227–235.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cobellis L, De Falco M, Mastrogiacomo A,
Giraldi D, Dattilo D, Scaffa C, Colacurci N and De Luca A:
Modulation of apelin and APJ receptor in normal and
preeclampsia-complicated placentas. Histol Histopathol. 22:1–8.
2007.PubMed/NCBI
|
|
21
|
Kucur M, Tuten A, Oncul M, Acikgoz AS,
Yuksel MA, Imamoglu M, Balci Ekmekci O, Yilmaz N and Madazli R:
Maternal serum apelin and YKL-40 levels in early and late-onset
pre-eclampsia. Hypertens Pregnancy. 33:467–475. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Alexander BT, Kassab SE, Miller MT, Abram
SR, Reckelhoff JF, Bennett WA and Granger JP: Reduced uterine
perfusion pressure during pregnancy in the rat is associated with
increases in arterial pressure and changes in renal nitric oxide.
Hypertension. 37:1191–1195. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Granger JP, LaMarca BB, Cockrell K, Sedeek
M, Balzi C, Chandler D and Bennett W: Reduced uterine perfusion
pressure (RUPP) model for studying cardiovascular-renal dysfunction
in response to placental ischemia. Methods Mol Med. 122:383–392.
2006.PubMed/NCBI
|
|
24
|
Gilbert JS, Ryan MJ, LaMarca BB, Sedeek M,
Murphy SR and Granger JP: Pathophysiology of hypertension during
preeclampsia: Linking placental ischemia with endothelial
dysfunction. Am J Physiol Heart Circ Physiol. 294:H541–H550. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Spradley FT, Tan AY, Joo WS, Daniels G,
Kussie P, Karumanchi SA and Granger JP: Placental growth factor
administration abolishes placental ischemia-induced hypertension.
Hypertension. 67:740–747. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ashworth JR, Warren AY, Johnson IR and
Baker PN: Plasma from pre-eclamptic women and functional change in
myometrial resistance arteries. Br J Obstet Gynaecol. 105:459–461.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hayman R, Warren A, Johnson I and Baker P:
Inducible change in the behavior of resistance arteries from
circulating factor in preeclampsia: An effect specific to
myometrial vessels from pregnant women. Am J Obstet. Gynecol.
184:420–426. 2001. View Article : Google Scholar
|
|
28
|
Lekontseva O, Jiang Y, Schleppe C and
Davidge ST: Altered neuronal nitric oxide synthase in the aging
vascular system: Implications for estrogens therapy. Endocrinology.
153:3940–3948. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Amaral LM, Pinheiro LC, Guimaraes DA,
Palei AC, Sertório JT, Portella RL and Tanus-Santos JE:
Antihypertensive effects of inducible nitric oxide synthase
inhibition in experimental pre-eclampsia. J Cell Mol Med.
17:1300–1307. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Eckman DM, Gupta R, Rosenfeld CR, Morgan
TM, Charles SM, Mertz H and Moore LG: Pregnancy increases
myometrial artery myogenic tone via NOS- or COX-independent
mechanisms. Am J Physiol Regul Integr Comp Physiol. 303:R368–R375.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kulandavelu S, Whiteley KJ, Bainbridge SA,
Qu D and Adamson SL: Endothelial NO synthase augments fetoplacental
blood flow, placental vascularization, and fetal growth in mice.
Hypertension. 61:259–266. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vadillo-Ortega F, Perichart-Perera O,
Espino S, Avila-Vergara MA, Ibarra I, Ahued R, Godines M, Parry S,
Macones G and Strauss JF: Effect of supplementation during
pregnancy with L-arginine and antioxidant vitamins in medical food
on pre-eclampsia in high risk population: Randomised controlled
trial. BMJ. 342:d29012011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jia YX, Lu ZF, Zhang J, Pan CS, Yang JH,
Zhao J, Yu F, Duan XH, Tang CS and Qi YF: Apelin activates
L-arginine/nitric oxide synthase/nitric oxide pathway in rat
aortas. Peptides. 28:2023–2029. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Busch R, Strohbach A, Pennewitz M, Lorenz
F, Bahls M, Busch MC and Felix SB: Regulation of the endothelial
apelin/APJ system by hemodynamic fluid flow. Cell Signal.
27:1286–1296. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Siddiqui IA, Jaleel A, Tamimi W and Al
Kadri HM: Role of oxidative stress in the pathogenesis of
preeclampsia. Arch Gynecol Obstet. 282:469–474. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu F, Tian FJ and Lin Y: Oxidative stress
in placenta: Health and diseases. Biomed Res Int. 2015:2932712015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Than A, Zhang X, Leow MK, Poh CL, Chong SK
and Chen P: Apelin attenuates oxidative stress in human adipocytes.
J Biol Chem. 289:3763–3774. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pisarenko O, Shulzhenko V, Studneva I,
Pelogeykina Y, Timoshin A, Anesia R, Valet P, Parini A and
Kunduzova O: Structural apelin analogues: Mitochondrial ROS
inhibition and cardiometabolic protection in myocardial ischaemia
reperfusion injury. Br J Pharmacol. 172:2933–2945. 2015. View Article : Google Scholar : PubMed/NCBI
|