Introduction
Adenomyosis is a common chronic gynecological
disorder characterized by penetration of ectopic endometrium into
the surrounding myometrium. As a special form of endometriosis, the
typical clinical features of adenomyosis include menorrhagia,
dysmenorrhea, metrorrhagia and infertility (1–3). The
medications most commonly used to treatments for adenomyosis are
hormonal, including the levonorgestrel intrauterine system and
gonatropin-releasing hormone agonists. In consideration of the
inflammatory pathology of adenomyosis, other non-hormonal
treatments, including non-steroidal anti-inflammatory drugs, are
also effective for dysmenorrhea in adenomyosis. However, the exact
pathogenesis of adenomyosis has remained elusive. Emerging evidence
suggested that this disease may be linked to the expression of
inflammatory mediators and the induction of an immune response
(4–6). A previous study by our group has
confirmed that lipopolysaccharide (LPS) stimulates inflammatory
cell proliferation and invasive growth of stromal cells in
adenomyosis by activation of the Toll-like receptor (TLR4)/myeloid
differentiation primary response gene 88/nuclear factor (NF)-κB
signaling pathway, indicating that LPS/TLR4 signaling is implicated
in the pathogenesis of adenomyosis (7). This provided a novel therapeutic
strategy for adenomyosis via targeting TLR4 signaling.
Berberine (BBR), extracted from rhizoma
coptis (Huanglian in Chinese) and other Chinese medicinal
herbs, has been extensively used for thousands of years in the
treatment of infectious diarrhea, heat-clearing and detoxification
in Traditional Chinese Medicine (8,9). Studies
have demonstrated that BBR possesses different pharmacological
activities involving multiple biological mechanisms, and may be
utilized for the treatment of various diseases, including diabetes,
metabolic syndrome and various types of cancer (9–14). It
was reported that BBR has a beneficial effect on patients with
diabetes and obesity, partly by stimulating adenosine monophosphate
kinase activity (15). Other studies
have demonstrated that BBR sensitized ovarian cancer cells to
cisplatin through the microRNA-93/phosphatase and tensin
homologue/Akt signaling axis (13,16,17).
However, whether BBR exerts a suppressive effect on
LPS-induced adenomyosis via inhibiting TLR4-mediated stromal cell
invasion has remained elusive. The aim of the present study was to
investigate the effect of BBR on LPS-induced ectopic endometrial
stromal cells (EESCs) and the development of adenomyosis.
Materials and methods
Reagents
BBR and LPS were purchased from Sigma-Aldrich (Merck
KGaA, Darmstadt, Germany). ELISA kits for interleukin (IL)-6 (cat.
no. HM10205), IL-8 (cat. no. HM10222) and transforming growth
factor (TGF)-β (cat. no. HM10058) were purchased from Bio-Swamp
(Shanghai, China). Antibodies against epithelial growth factor
(EGF) (cat. no. Ab9695) and matrix metalloproteinase (MMP)2 (cat.
no. Ab92536) were purchased from Abcam (Cambridge, MA, USA).
Antibodies against vascular endothelial growth factor (VEGF) (cat.
no. AF5131) were purchased from Affinity Biosciences (Cincinnati,
OH, USA), while antibody against GAPDH (cat. no. 5174) was
purchased from Cell Signaling Technologies (Danvers, MA, USA). The
secondary antibodies donkey anti-goat horseradish peroxidase (HRP)
(cat. no. A0181), goat anti-rabbit HRP (cat. no. A0208) and goat
anti-mouse HRP (cat. no. A0216) were purchased from Beyotime
Institute of Technology (Haimen, China).
Subjects and specimens
Between August 2016 to October 2016, a total of
three patients with adenomyosis between 22 and 40 years of age
undergoing laparoscopy for pelvic pain or/and dysmenorrhea at
Yangpu Hospital affiliated to Tongji University School of Medicine
(Shanghai, China) were recruited. All of them had regular menstrual
cycles (28–32 days) and had not been on any hormonal medication 3
months prior to the initiation of the study. The phase of the
menstrual cycle was the early proliferative phase. The diagnosis
was confirmed by pathology, which excluded the presence of any
other gynecological diseases. Adenomyosis was diagnostically
confirmed by the macroscopic appearance of the biopsy samples
according to the published criteria (18) and the final diagnosis was mainly
based on histological examination. All fresh tissue specimens were
collected in PBS with added 100 U/ml penicillin, 100 µg/ml
streptomycin, stored at 4°C, and processed within 1–6 h. The study
was approved by the Research Ethics Committee of Tongji University
(Shanghai, China) and written informed consent was obtained from
all patients.
Cell collection and culture
EESCs (EESC1, EESC2 and EESC3, respectively) were
isolated and characterized as described previously (19–21). In
brief, the tissues were washed twice with sterile PBS under aseptic
conditions, minced into small pieces, digested by incubation with
Dulbecco's modified Eagle's medium (DMEM)/F12 (Hyclone; GE
Healthcare Life Sciences, South Logan, UT, USA) supplemented with 1
mg/ml collagenase (Serva Electrophoresis GmbH, Heidelberg, Germany)
and 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) for 2 h at 37°C and subsequently filtrated
with a 70-mm nylon cell strainer. The resultant supernatant was
centrifuged at 111 × g for 5 min at room temperature, and the
pellets were re-suspended and cultured with DMEM/F12 containing 10%
FBS, 100 IU/ml penicillin and 100 mg/ml streptomycin at 37°C in a
humidified air atmosphere containing 5% CO2.
Identification of the isolated cells was performed with
anti-vimentin and mouse anti-CK19 antibodies by
immunohistochemistry (22).
Cell proliferation assay
Cells (5.0×103/well) were seeded in
96-well plates and stimulated with different concentrations (50,
100 and 200 µM) of BBR or/and LPS (100 ng/ml) for 24, 48 or 72 h.
Cell proliferation was detected by using the Cell Count Kit-8
(CCK-8; Signalway Antibody, LLC, College Park, MD, USA) according
to the manufacturer's protocol. The optical density (OD) was
detected at a wavelength of 450 nm with a microplate reader
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The relative cell
viability was calculated using following formula: (OD of sample/OD
of blank control) ×100%. Each assay was performed in
triplicate.
Cell cycle analysis
The cell cycle was evaluated by propidium iodide
(PI; 7 Sea Biotech, Shanghai, China) staining and analysis using a
flow cytometer (BD Biosciences). Cells were seeded on 6-well plates
at 3×104 cells/well, treated with BBR (200 µM) or/and
LPS (100 ng/ml) for 48 h, washed in PBS and re-suspended in
staining solution containing 20 µg/ml PI and 100 µg/ml RNase A
(Beijing Solarbio Science & Technology Co., Ltd., Beijing,
China). Experiments were performed in triplicate. G1, S and G2/M
fractions were quantified with FlowJo 7.6.1 software (FlowJo, LLC,
Ashland, OR, USA). The experiments were performed in
triplicate.
Annexin V-fluorescein isothiocyanate
(FITC)/PI apoptosis assay
Apoptotic cells were analyzed using an Annexin
V-FITC/PI double staining procedure. Cells were treated with BBR
(200 µM) and/or LPS (100 ng/ml) for 48 h. Subsequently, cells were
digested into single cell suspensions using EDTA-free trypsin
(Beijing Solarbio Science & Technology Co., Ltd.) and then
stained according to the instructions provided with the Annexin
V-FITC/PI Apoptosis Detection kit (Beyotime Institute of
Biotechnology). The stained cells were analyzed within 10–15 min by
flow cytometry (BD Biosciences). At least 2×104 cells
were acquired for each sample. The experiments were performed in
triplicate.
Reverse-transcription quantitative
polymerase chain reaction (RT-qPCR)
PCR was performed using an ABI 7300 instrument
(Applied Biosystems; Thermo Fisher Scientific, Inc.) and SYBR-Green
Master Mix (cat. no. K0223; Thermo Fisher Scientific, Inc.). The
primers had the following sequences: IL-6 forward,
5′-AGCCACTCACCTCTTCAGAAC-3′ and reverse,
5′-GCCTCTTTGCTGCTTTCACAC-3′; IL-8 forward, 5′-CAAGAGCCAGGAAGAAAC-3′
and reverse, 5′-TGGTCCACTCTCAATCAC-3′; TGF-β1 forward,
5′-GACTACTACGCCAAGGAGGTC-3′ and reverse, 5′-GAGAGCAACACGGGTTCAG-3′;
VEGF forward, 5′-ATTTCTGGGATTCCTGTAG-3′ and reverse,
5′-CAGTGAAGACACCAATAAC-3′; EGF forward,
5′-GAAACTGTTGGGAGAGGAATCG-3′ and reverse,
5′-AGAGCAAGGCAAAGGCTTAG-3′; MMP2 forward,
5′-TTGACGGTAAGGACGGACTC-3′ and reverse, 5′-GGCGTTCCCATACTTCACAC-3′;
GAPDH forward, 5′-CACCCACTCCTCCACCTTTG-3′ and reverse,
5′-CCACCACCCTGTTGCTGTAG-3′. GAPDH was used as an internal control.
EESCs (5.0×105/well) were seeded in 6-well plates and
treated with BBR (200 µM) for 48 h. Total RNA was extracted using
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. Complementary DNA was
synthesized by using RT Reagent kit (cat. no. K1622; Fermentas,
Thermo Fisher Scientific, Inc.) at 37°C for 1 h and 85°C for 5 min.
The PCR cycling conditions were as follows: 95°C for 10 min,
followed by 40 cycles of 15 sec at 95°C and 60°C for 1 min.
Verification of specific product amplification was performed by
dissociation curve analysis. The gene expression was calculated
using the 2−ΔΔCq method (23). All results are expressed as the mean
of three replicates.
Western blot analysis
Cell lysates were prepared with
radioimmunoprecipitation assay buffer. The protein concentration
was measured by a bicinchoninic acid assay (Thermo Fisher
Scientific, Inc.). The supernatant with an equal amount of protein
(15 µg protein/lane) was separated by 10% SDS-PAGE. Proteins then
were blotted onto nitrocellulose membranes (EMD Millipore,
Billerica, MA, USA) and incubated with primary antibodies for 2 h
at room temperature, followed by the corresponding secondary
antibodies for 1 h at room temperature. The bound antibodies were
visualized by enhanced chemiluminescence and quantified by
densitometry using ChemiDocTM XRS+ image analyzer (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). Densitometric analyses of
the bands were adjusted with GAPDH. Each assay was performed in
triplicate.
ELISA
The secretion of IL-6, IL-8 and TGF-β in culture
medium was respectively determined using the human IL-6, IL-8 and
TGF-β ELISA kits (Bio-Swamp), according to the protocol provided by
the manufacturer. The EESCs were treated with LPS or/and BBR. The
culture medium was collected and centrifuged for 1 min at 111 × g
at 4°C, and the secreted IL-6, IL-8 and TGF-β in the culture medium
were measured spectrophotometrically in an ELISA reader at 450 nm.
The absolute concentration of IL-6, IL-8 and TGF-β in the culture
medium was calculated from a standard curve.
Statistical analysis
Data analysis was performed using SPSS 20.0 software
(IBM Corp., Armonk, NY, USA). Values are expressed as the mean ±
standard error of the mean. Comparisons between multiple groups
were made by analysis of variance followed by Tukey's honest post
hoc test, or by the Wilcoxon test. P<0.05 was considered to
indicate a statistically significant difference.
Results
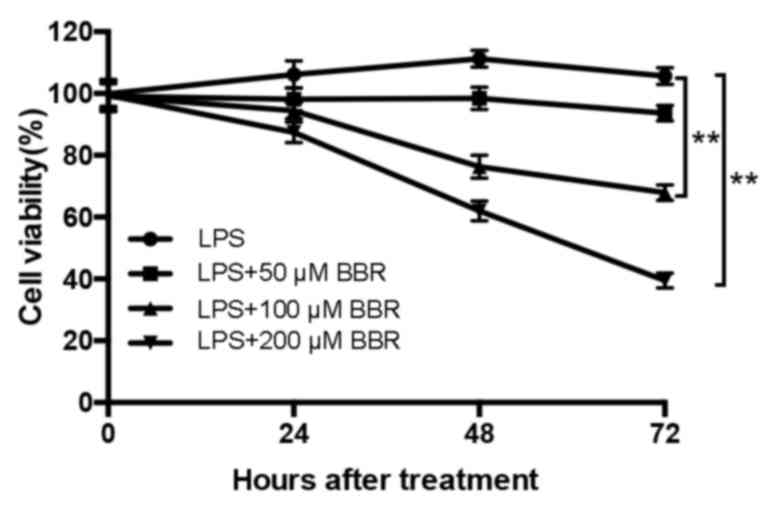
BBR inhibits the proliferation of
LPS-induced EESCs
To evaluate the effects of BBR on the proliferation
of LPS-induced EESCs, the amount of viable cells was detected by a
CCK-8 assay. In accordance with the results of a previous study by
our group, EESCs presented with a maximum increase in the levels of
cell growth at 48 h and in response to 10–100 ng/ml LPS (7), and thus, 100 ng/ml was selected as the
optimal concentration of LPS in the subsequent experiments. As
presented in Fig. 1, EESCs were
treated with 100 ng/ml LPS with or without different concentrations
of BBR for 24, 48 and 72 h. The results revealed that LPS
significantly increased the proliferation and viability of EESCs
compared with that in the blank group (P<0.05). Combined
treatment with BBR markedly decreased the LPS-induced cell
proliferation in a time- and dose-dependent manner. The amount of
viable EESCs after treatment with 100 or 200 µM BBR combined with
LPS was significantly lower than that after treatment with 50 µM
BBR combined with LPS. Compared with 24-h BBR+LPS treatment, 48-
and 72-h BBR+LPS treatment had a significantly higher inhibitory
effect on the growth of EESCs. Therefore, the concentration of 200
µM was selected as the optimal BBR concentration combined with LPS
in the subsequent experiments.
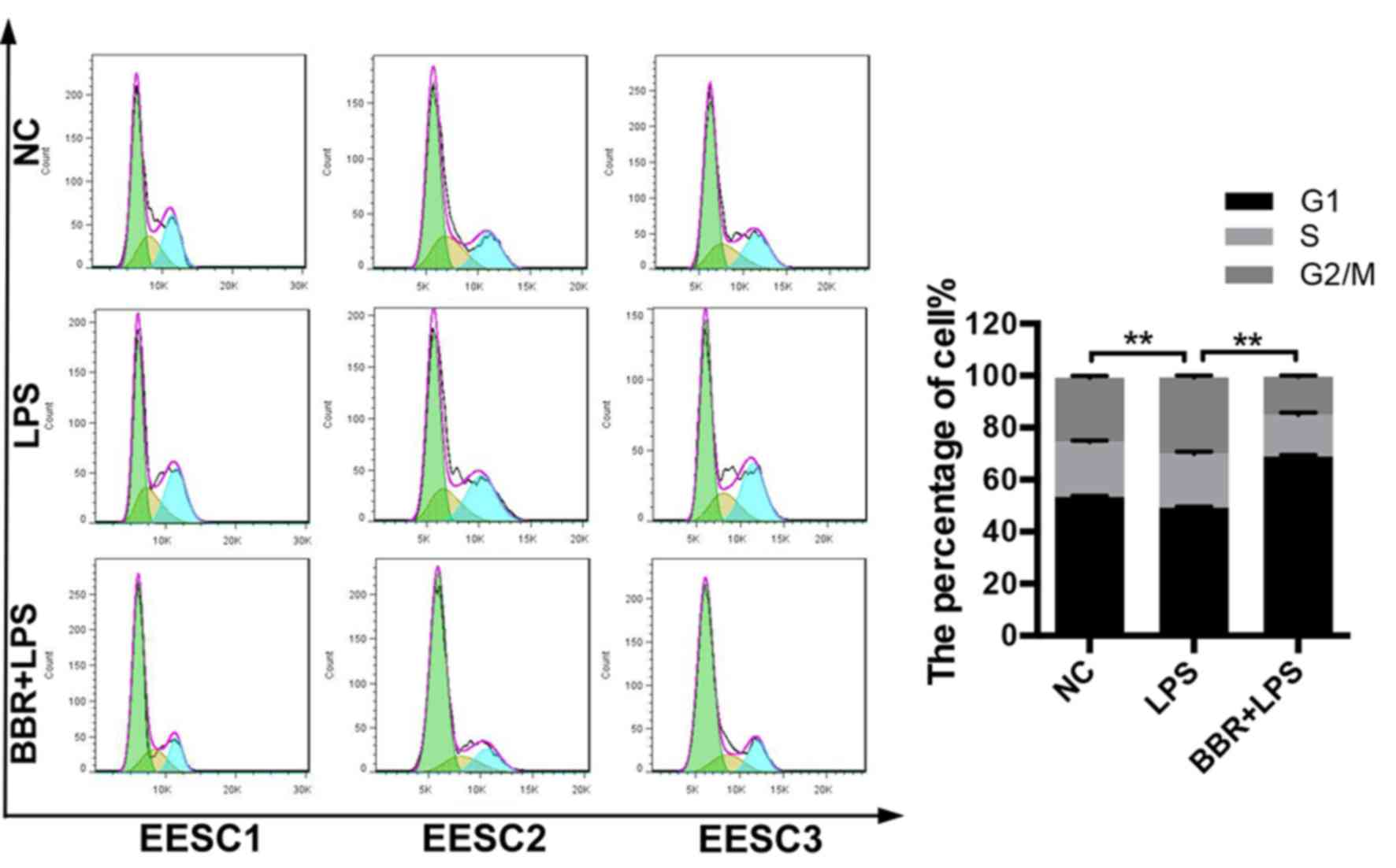
BBR induces cell cycle arrest of
LPS-induced EESCs
To determine whether BBR affected the cell cycle of
LPS-induced EESCs, the cell cycle distribution was evaluated by
flow cytometry using PI staining. As presented in Fig. 2, LPS treatment significantly
decreased the number of EESCs in G0/G1 phase and increased the
number of EESCs in G2/M phase as compared with that in the control
group. The combination of BBR and LPS could markedly induced cell
cycle arrest of EESCs in G0/G1 phase, which may have been the cause
of BBR-induced inhibition of cell proliferation.
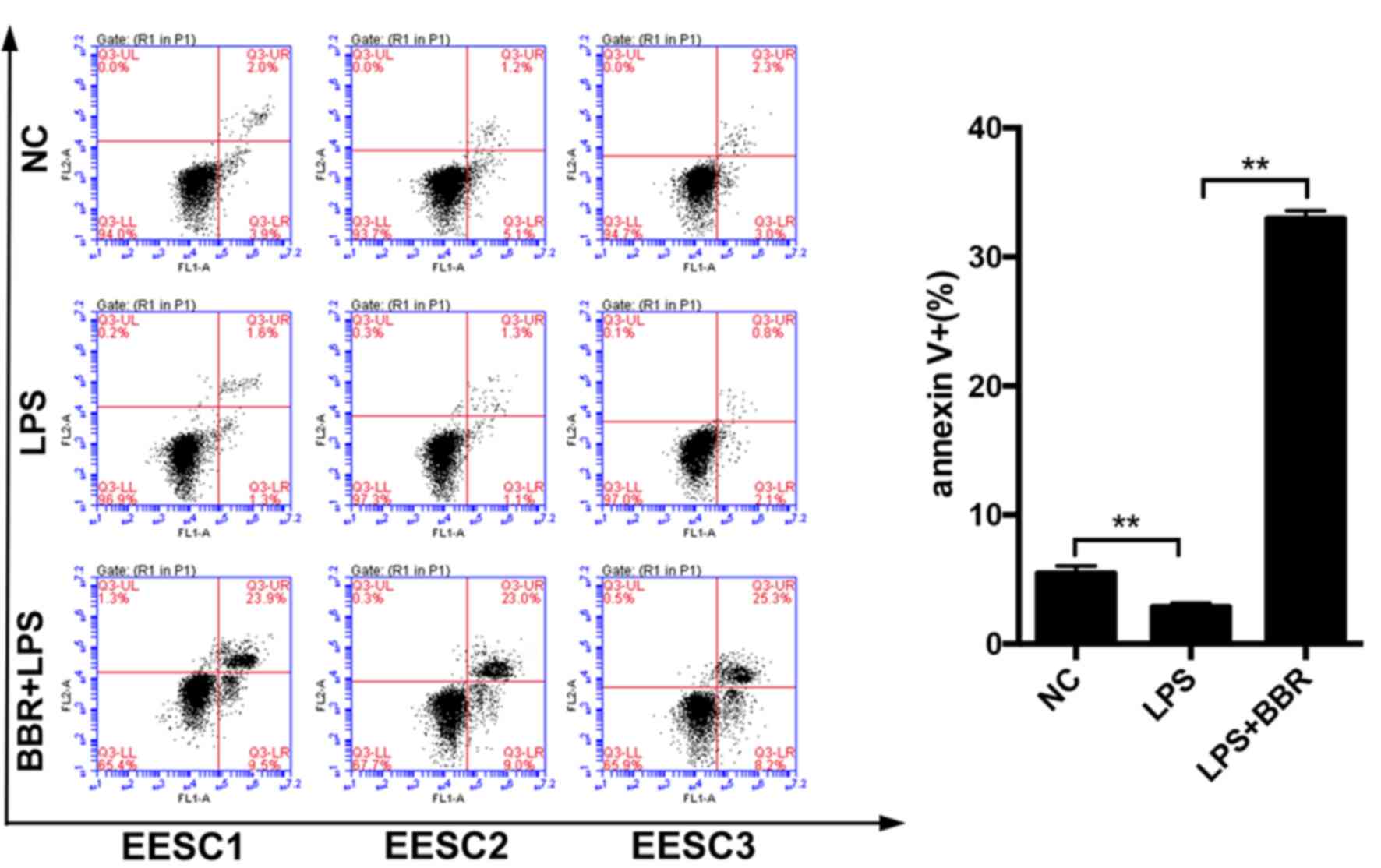
BBR increases apoptosis of LPS-induced
EESCs
To explore the apoptotic effect of BBR on
LPS-induced EESCs, apoptosis was evaluated by using an Annexin
V-FITC/PI staining assay. As presented in Fig. 3, compared with the control cells,
treatment of EESCs with LPS for 48 h led to a decreased early
(lower right quadrant) and late (upper right quadrant) apoptotic
rate. The combination of BBR and LPS significantly increased the
number of apoptotic EESCs as compared with that in the LPS-induced
group. These results suggested that BBR has a marked
apoptosis-promoting effect on LPS-induced EESCs.
BBR inhibits the expression of IL-6,
IL-8, TGF-β, EGF, VEGF and MMP2 in LPS-induced EESCs
To evaluate whether BBR affected the development of
LPS-induced EESCs, the expression of relevant markers, which are
crucial for the infiltration and proliferation of EESCs, were
detected by RT-qPCR, ELISA and western blot analysis. The results
demonstrated that LPS significantly increased the mRNA (Fig. 4A) and protein (Fig. 4B-D) expression of IL-6, IL-8, TGF-β,
EGF, VEGF and MMP2 as compared with that in the control group.
After treatment of EESCs with BBR and LPS, the mRNA (Fig. 4A) and protein (Fig. 4B-D) levels of IL-6, IL-8, TGF-β, EGF,
VEGF and MMP2 were significantly decreased as compared with those
in the LPS-treated group. These results indicated that BBR may
inhibit the LPS-induced invasion and proliferation of EESCs, which
is associated with adenomyosis.
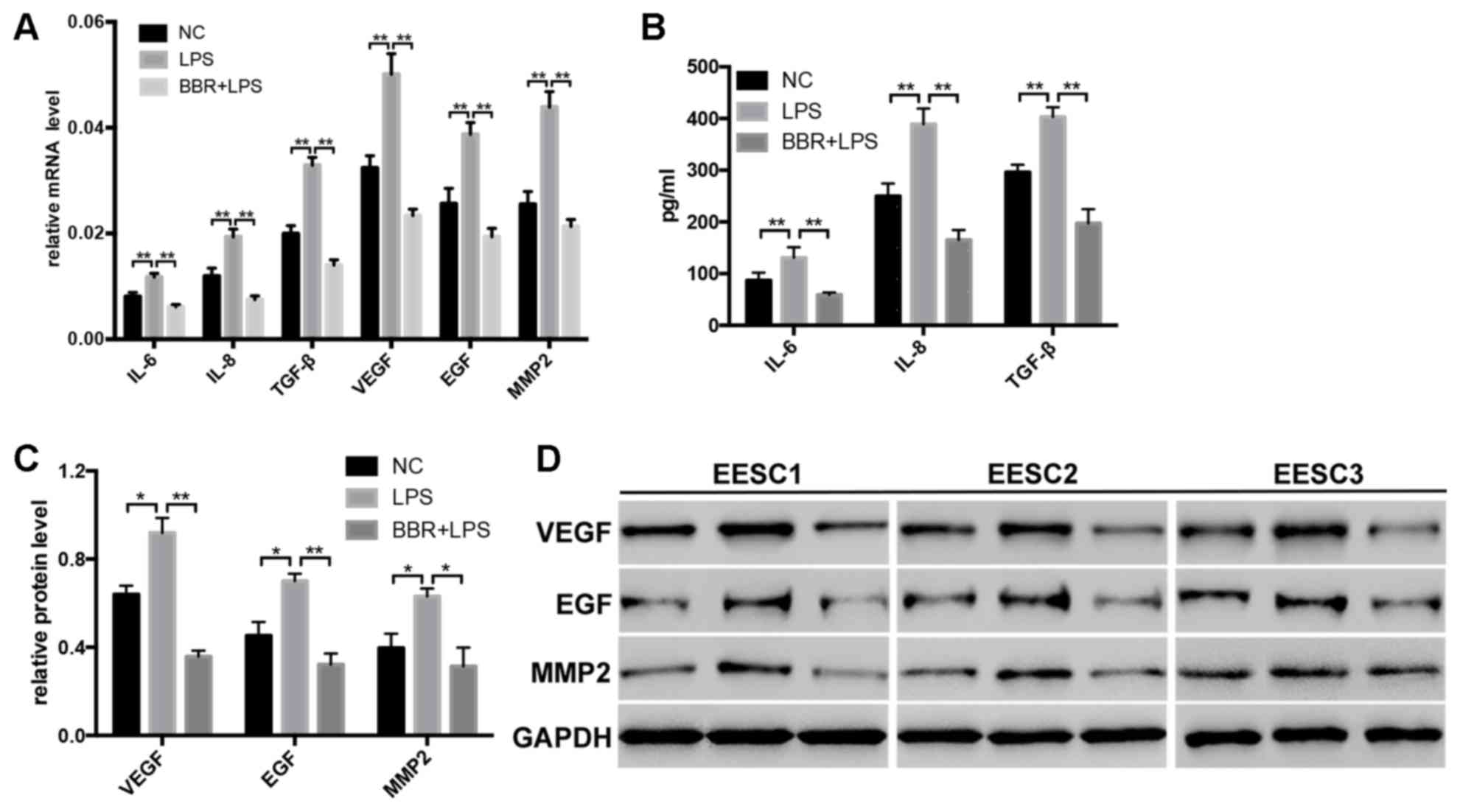 | Figure 4.BBR inhibits the expression of IL-6,
IL-8, TGF-β, EGF, VEGF and MMP2 in LPS-induced EESCs. EESCs from 3
different patients were treated with LPS or LPS+BBR for 48 h. The
expression levels of IL-6, IL-8 and TGF-β in EESCs were detected by
(A) RT-qPCR and (B) ELISA. The expression levels of EGF, VEGF and
MMP2 in EESCs was detected by (A) RT-qPCR and (C and D) western
blot analysis. Data were obtained from at least three independent
experiments. *P<0.05, **P<0.01. BBR, berberine; LPS,
lipopolysaccharide; EESCs, ectopic endometrial stromal cells; NC,
negative control; IL, interleukin; TGF, transforming growth factor;
EGF, epithelial growth factor; VEGF, vascular endothelial growth
factor; MMP, matrix metalloproteinase; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction. |
Discussion
LPS-mediated TLR4 signaling as a first line of
defense against microbial infection has been extensively studied in
the past decades. The improper inflammatory response induced by
LPS/TLR4 signaling has the potential to cause inflammatory
disorders (24,25). Various studies have indicated that
local and chronic inflammatory reactions in the uterus and
peritoneal environment are likely to be associated with the
pathogenesis of endometriosis/adenomyosis (7,26,27).
Azuma et al (26) reported
that LPS promoted the development of endometriosis through the
NF-κB pathway in a murine model. A previous study by our group
demonstrated that LPS/TLR4-mediated stromal cells acquire an
invasive phenotype and are implicated in the pathogenesis of
adenomyosis, which shed light on the mechanism underlying
adenomyosis and provided a novel potential strategy for the
clinical treatment of the disease (7).
BBR as an isoquinoline derivative alkaloid has been
demonstrated to have diverse pharmacological activities involving
multiple biological mechanisms, which may be utilized for the
treatment of a wide variety of diseases (9–14).
Previous studies have demonstrated that BBR acts as an LPS
antagonist and blocks LPS/TLR4 signaling in an LPS-induced animal
model (28–30). Li et al (30) reported that BBR protects against
LPS-induced intestinal injury in mice via reducing enterocyte
apoptosis, inhibiting the TLR4 pathway and decreasing neutrophil
infiltration. Huang et al (29) reported that BBR exerted a potent
anti-inflammatory effect by suppressing the LPS-evoked secretion of
the proinflammatory cytokines IL-1β, IL-6 and TNFα and attenuated
the activation of LPS-TLR4-cytokines/NF-κB signaling pathways in
the mouse macrophage-like cell line RAW264.7. However, the effect
of BBR on LPS-induced adenomyosis has remained elusive. In the
present study, a CCK-8 assay and flow cytometric analysis
demonstrated that BBR significantly and time- and dose-dependently
inhibited the proliferation and significantly inhibited the cell
cycle progression of LPS-induced EESCs.
Adenomyosis, as a specific type of endometriosis,
features certain alterations in apoptosis, invasion and
angiogenesis compared with healthy controls. Indeed, numerous
cytokines have been demonstrated to have a major role in the
pathogenesis of adenomyosis (7,21,31,32).
The present study focused on IL-6, IL-8, TGF-β, EGF, VEGF and MMP2
to investigate whether BBR inhibited the invasion, inflammatory
response and maintenance of LPS-induced stromal cells from
adenomyosis tissues. The results revealed that BBR significantly
inhibited the mRNA and protein expression of IL-6, IL-8, TGF-β,
EGF, VEGF and MMP2 in LPS-induced EESCs. Various studies confirmed
that various cytokines, including IL-6, IL-8, IL-10, TNF-α and
TGF-β, synthesized and secreted by peritoneal macrophages may
contribute to the adhesion, invasion and proliferation of
endometrial cells and the progression of endometriosis (33–36).
Yang et al (32) reported
that abnormal secretion of IL-6 and IL-8 from endometrial stromal
cells may have a role in the formation of ectopic islands in
adenomyosis. Furthermore, the role of EGF, VEGF and TGF-β
implicated in inflammation, angiogenesis and the pathophysiology of
endometriosis has been well established (36–38).
Cell invasion is a vital hallmark of tumor formation and
progression. Indeed, upregulation of several MMPs has also been
linked to the pathogenesis of adenomyosis (39,40).
Weigel et al (41)
demonstrated obvious differences in the expression pattern of MMP2
and MMP9 in different stages of endometriosis and suggested that
these markers may be used to evaluate the aggressiveness and
invasiveness of endometriotic lesions.
In conclusion, the present study indicated that BBR
has the ability to induce apoptosis and inhibit the proliferation
and invasive phenotypes of LPS-induced stromal cells of adenomyosis
tissues. The present findings shed light on the potential mechanism
of BBR targeting the adenomyosis cells stimulated by LPS and
provided a novel therapeutic strategy for the clinical treatment of
the disease. However, further investigation of the molecular
mechanism in vivo and randomized controlled trials are
required for the clinical application of BBR.
Acknowledgements
This study was supported by grants from the Shanghai
Municipal Commission of Health and Family Planning (grant no.
ZA2015A33).
References
|
1
|
Harvey J and Warwick I: Endometriosis.
BMJ. 340:c26612010. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Korzekwa A, Łupicka M, Socha B, Mannelli
Ch and Skarzynski DJ: Is adenomyosis a problem in reproduction and
fertility? Pol J Vet Sci. 17:187–194. 2014.PubMed/NCBI
|
|
3
|
Farquhar C and Brosens I: Medical and
surgical management of adenomyosis. Best Pract Res Clin Obstet
Gynaecol. 20:603–616. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lousse JC, Van Langendonckt A, Defrere S,
Ramos RG, Colette S and Donnez J: Peritoneal endometriosis is an
inflammatory disease. Front Biosci (Elite Ed). 4:23–40. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sheldon IM and Bromfield JJ: Innate
immunity in the human endometrium and ovary. Am J Reprod Immunol.
66 Suppl 1:S63–S71. 2011. View Article : Google Scholar
|
|
6
|
Bertschi D, McKinnon BD, Evers J,
Bersinger NA and Mueller MD: Enhanced inflammatory activity of
endometriotic lesions from the rectovaginal septum. Mediators
Inflamm. 2013:4509502013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Guo J, Chen L, Luo N, Li C, Chen R, Qu X,
Liu M, Kang L and Cheng Z: LPS/TLR4-mediated stromal cells acquire
an invasive phenotype and are implicated in the pathogenesis of
adenomyosis. Sci Rep. 6:214162016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Pan L, Wang W, Shi F, Zhou J, Zhang M, Zhu
H and Zeng M: Exploratory pharmacokinetics of Geniposide in rat
model of cerebral ischemia orally administered with or without
Baicalin and/or Berberine. Evid Based Complement Alternat Med.
2013:3495312013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun Y, Xun K, Wang Y and Chen X: A
systematic review of the anticancer properties of berberine, a
natural product from Chinese herbs. Anticancer Drugs. 20:757–769.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kong WJ, Zhang H, Song DQ, Xue R, Zhao W,
Wei J, Wang YM, Shan N, Zhou ZX, Yang P, et al: Berberine reduces
insulin resistance through protein kinase C-dependent up-regulation
of insulin receptor expression. Metabolism. 58:109–119. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Singh IP and Mahajan S: Berberine and its
derivatives: A patent review (2009–2012). Expert Opin Ther Pat.
23:215–231. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yin J, Zhang H and Ye J: Traditional
chinese medicine in treatment of metabolic syndrome. Endoc Metab
Immune Disord Drug Targets. 8:99–111. 2008. View Article : Google Scholar
|
|
13
|
Jin P, Zhang C and Li N: Berberine
exhibits antitumor effects in human ovarian cancer cells.
Anticancer Agents Med Chem. 15:511–516. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li J, Cao B, Liu X, Fu X, Xiong Z, Chen L,
Sartor O, Dong Y and Zhang H: Berberine suppresses androgen
receptor signaling in prostate cancer. Mol Cancer Ther.
10:1346–1356. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ,
Shen Y, Ye JM, Lee CH, Oh WK, Kim CT, et al: Berberine, a natural
plant product, activates AMP-activated protein kinase with
beneficial metabolic effects in diabetic and insulin-resistant
states. Diabetes. 55:2256–2264. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen Q, Qin R, Fang Y and Li H: Berberine
sensitizes human ovarian cancer cells to cisplatin through
miR-93/PTEN/Akt signaling pathway. Cell Physiol Biochem.
36:956–965. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Marverti G, Ligabue A, Lombardi P, Ferrari
S, Monti MG, Frassineti C and Costi MP: Modulation of the
expression of folate cycle enzymes and polyamine metabolism by
berberine in cisplatin-sensitive and -resistant human ovarian
cancer cells. Int J Oncol. 43:1269–1280. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Exacoustos C, Manganaro L and Zupi E:
Imaging for the evaluation of endometriosis and adenomyosis. Best
Pract Res Clin Obst Gynaecol. 28:655–681. 2014. View Article : Google Scholar
|
|
19
|
Rajaei S, Mirahmadian M, Jeddi-Tehrani M,
Tavakoli M, Zonoobi M, Dabbagh A and Zarnani AH: Effect of
1,25(OH)2 vitamin D3 on cytokine production by endometrial cells of
women with repeated implantation failure. Gynecol Endocrinol.
28:906–911. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wan L, Zou Y, Wan LH, Wang LQ, Huang MZ,
Wu J, Zhu YB and Huang OP: Tanshinone IIA inhibits the
proliferation, migration and invasion of ectopic endometrial
stromal cells of adenomyosis via 14-3-3zeta downregulation. Arch
Gynecol Obstet. 292:1301–1309. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang JH, Wu MY, Chen CD, Chen MJ, Yang YS
and Ho HN: Altered apoptosis and proliferation in endometrial
stromal cells of women with adenomyosis. Hum Reprod. 22:945–952.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bruse C, Guan Y, Carlberg M, Carlström K
and Bergqvist A: Basal release of urokinase plasminogen activator,
plasminogen activator inhibitor-1, and soluble plasminogen
activator receptor from separated and cultured endometriotic and
endometrial stromal and epithelial cells. Fertil Steril. 83 Suppl
1:S1155–S1160. 2005. View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Miller SI, Ernst RK and Bader MW: LPS,
TLR4 and infectious disease diversity. Nat Rev Microbiol. 3:36–46.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lu YC, Yeh WC and Ohashi PS: LPS/TLR4
signal transduction pathway. Cytokine. 42:145–151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Azuma Y, Taniguchi F, Nakamura K, Nagira
K, Khine YM, Kiyama T1, Uegaki T, Izawa M and Harada T:
Lipopolysaccharide promotes the development of murine
endometriosis-like lesions via the nuclear factor-kappa B pathway.
Am J Reprode Immunol. 77:2017.
|
|
27
|
Allhorn S, Böing C, Koch AA, Kimmig R and
Gashaw I: TLR3 and TLR4 expression in healthy and diseased human
endometrium. Reprod Biol Endocrinol. 6:402008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chu M, Ding R, Chu ZY, Zhang MB, Liu XY,
Xie SH, Zhai YJ and Wang YD: Role of berberine in anti-bacterial as
a high-affinity LPS antagonist binding to TLR4/MD-2 receptor. BMC
Complement Alternat Med. 14:892014. View Article : Google Scholar
|
|
29
|
Huang LH, Pan XP, Gong KR and Shao G:
Anti-inflammatory effects of three kinds of traditional Mongolian
medicine monomer and its combination on LPS-stimulated RAW264.7
macrophages. Eur Rev Med Pharmacol Sci. 20:950–958. 2016.PubMed/NCBI
|
|
30
|
Li HM, Wang YY, Wang HD, Cao WJ, Yu XH, Lu
DX, Qi RB, Hu CF and Yan YX: Berberine protects against
lipopolysaccharide-induced intestinal injury in mice via alpha 2
adrenoceptor-independent mechanisms. Acta Pharmacol Sin.
32:1364–1372. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Benagiano G, Brosens I and Habiba M:
Structural and molecular features of the endomyometrium in
endometriosis and adenomyosis. Hum Reprod Update. 20:386–402. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang JH, Chen MJ, Wu MY, Chen YC, Yang YS
and Ho HN: Decreased suppression of interleukin-6 after treatment
with medroxyprogesterone acetate and danazol in endometrial stromal
cells of women with adenomyosis. Fertil Steril. 86:1459–1465. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
May KE, Conduit-Hulbert SA, Villar J,
Kirtley S, Kennedy SH and Becker CM: Peripheral biomarkers of
endometriosis: A systematic review. Hum Reprod Update. 16:651–674.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Younis A, Hawkins K, Mahini H, Butler W
and Garelnabi M: Serum tumor necrosis factor-α, interleukin-6,
monocyte chemotactic protein-1 and paraoxonase-1 profiles in women
with endometriosis, PCOS, or unexplained infertility. J Assist
Reprod Genet. 31:1445–1451. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Khan KN, Masuzaki H, Fujishita A, Kitajima
M, Hiraki K, Sekine I, Matsuyama T and Ishimaru T: Interleukin-6-
and tumour necrosis factor alpha-mediated expression of hepatocyte
growth factor by stromal cells and its involvement in the growth of
endometriosis. Hum Reprod. 20:2715–2723. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sotnikova N, Antsiferova I and Malyshkina
A: Cytokine network of eutopic and ectopic endometrium in women
with adenomyosis. Am J Reprod Immunol. 47:251–255. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Omwandho CO, Konrad L, Halis G, Oehmke F
and Tinneberg HR: Role of TGF-betas in normal human endometrium and
endometriosis. Hum Reprod. 25:101–109. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kang S, Zhao J, Liu Q, Zhou R, Wang N and
Li Y: Vascular endothelial growth factor gene polymorphisms are
associated with the risk of developing adenomyosis. Environ Mol
Mutagen. 50:361–366. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yang JH, Wu MY, Chen MJ, Chen SU, Yang YS
and Ho HN: Increased matrix metalloproteinase-2 and tissue
inhibitor of metalloproteinase-1 secretion but unaffected
invasiveness of endometrial stromal cells in adenomyosis. Fertil
Steril. 91 5 Suppl:2193–2198. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yi KW, Kim SH, Ihm HJ, Oh YS, Chae HD, Kim
CH and Kang BM: Increased expression of p21-activated kinase 4 in
adenomyosis and its regulation of matrix metalloproteinase-2 and −9
in endometrial cells. Fertil Steril. 103:1089–1097.e2. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Weigel MT, Krämer J, Schem C, Wenners A,
Alkatout I, Jonat W, Maass N and Mundhenke C: Differential
expression of MMP-2, MMP-9 and PCNA in endometriosis and
endometrial carcinoma. Eur J Obstet Gynecol Reprod Biol. 160:74–78.
2012. View Article : Google Scholar : PubMed/NCBI
|