Introduction
Acquired hemophilia A (AHA) is a coagulopathy that
results in soft tissue and mucocutaneous hemorrhage, and possibly
even life-threatening intracranial hemorrhage (1). The present study reported a case of AHA
with acquired factor VIII deficiency associated with hemorrhagic
pericardial effusions. It is necessary to uncover the primary cause
of this disease in order to effectively manage it; however, the
causes are variable and there is no evidence to suggest why factor
VIII deficiency is associated with hemorrhagic pericardial
effusions. The major treatment for acquired hemophilia A with
acquired factor VIII deficiency is management to avoid bleeding,
eradicating the inhibitor, treating underlying diseases and
decreasing the risk of injuries that may cause iatrogenic bleeding
(1). Bypassing agents are currently
the most commonly used first line treatment; recombinant activated
factor VII and factor VIII inhibitor bypassing activity (FEIBA)
have been demonstrated to be effective treatments for acquired
hemophilia (1). In the present
study, the patient was treated with infusion of cryoagglutinin to
prevent the production of continuous bloody pericardial effusions.
This treatment was successful. The present study presents a case
with hemorrhagic pleural effusions related to acquired coagulation
factor VIII deficiency.
Case report
A 39-year-old Chinese man who had experienced
progressive dyspnea for one week was admitted to the outpatient
clinic of Xiamen Chang Gung Hospital (Xiamen, China) in August
2015. The patient had a history of hematoma of scrotum requiring
negative pressure suction 2 years prior to admission to the
hospital. Over the course of the week prior to admittance to the
hospital, the patient became short of breath, experienced
paroxysmal nocturnal dyspnea and was unable lie down on the bed.
Furthermore, the patient was unable to climb the stairs. The
patient complained of chest pain, which he described as a sense of
constriction in his chest that gradually worsened. The patient had
no notable medical history or familial genetic diseases.
On physical examination, the patient's vital signs
included a heart rate of 101 beats per min, blood pressure of
108/51 mmHg and temperature of 36.4°C. When receiving oxygen via a
nasal cannula (2 l/min), the patient was mildly tachypneic (19
breaths/min) with an oxygen saturation of 98%. Cardiovascular
examination demonstrated distant heart sounds, normal first and
second heart sounds and no murmurs, rubs or knocks. There was
jugular venous distention to 16 cm above the manubriosternal angle.
Lung examination did not demonstrate anything abnormal. The
patient's abdomen was soft and nondistended. There was no
peripheral edema, adenopathy or hepatosplenomegaly.
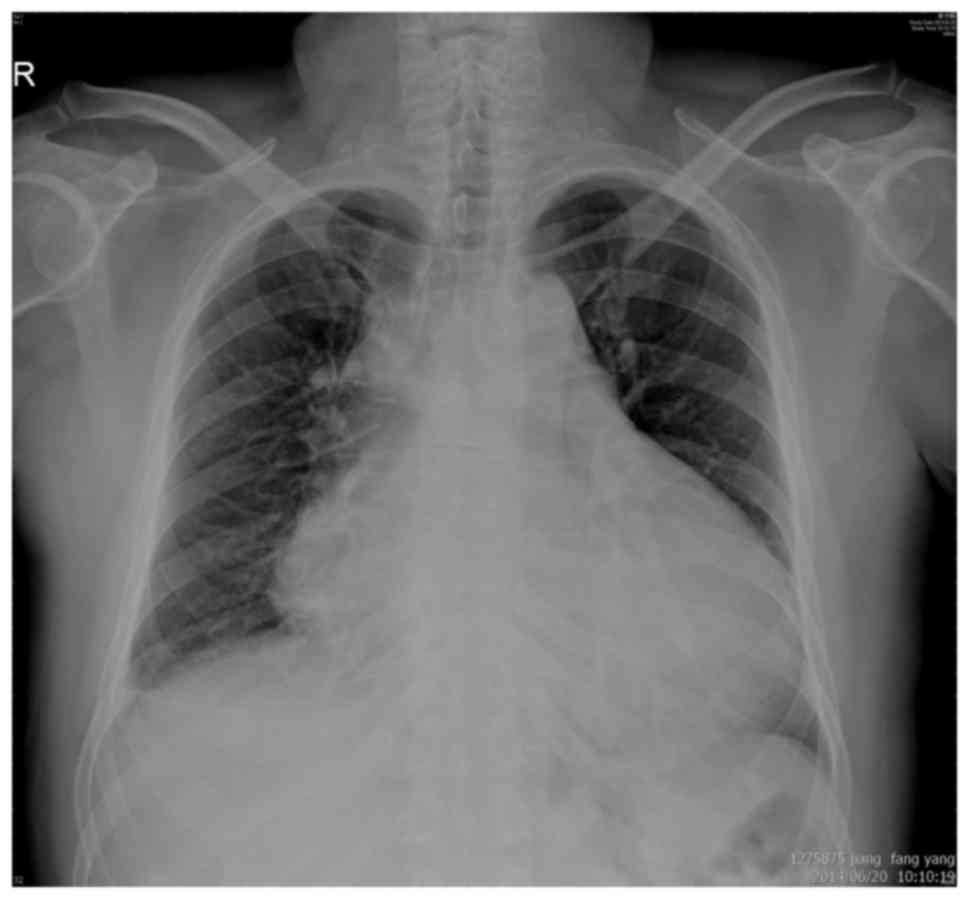
A chest X-ray revealed cardiomegaly and a medium
left pleural effusion (Fig. 1).
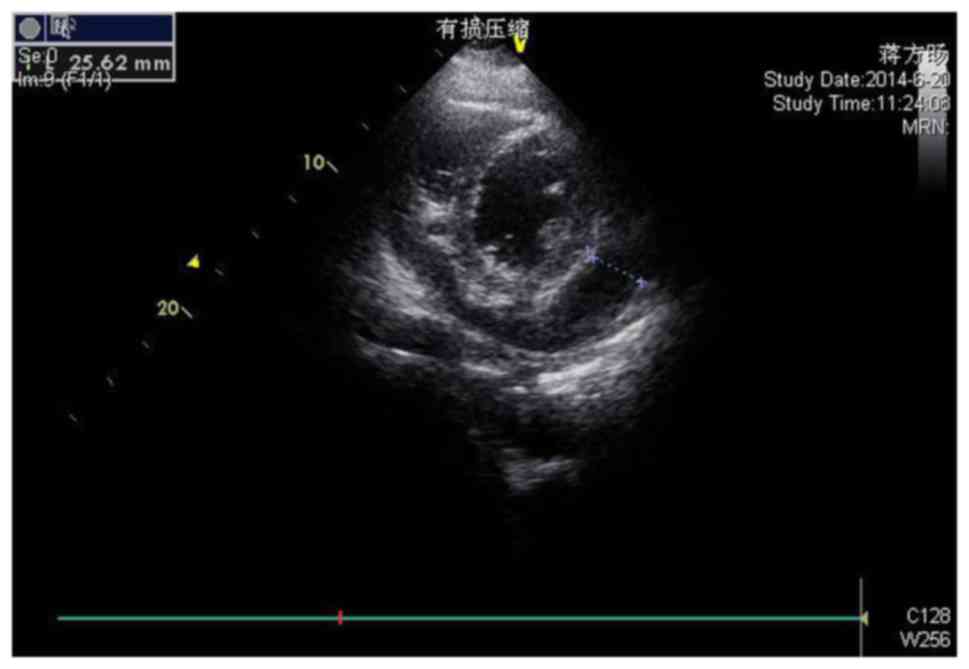
Echocardiography demonstrated sinus tachycardia. A subsequent
transthoracic echocardiogram (Fig.
2) revealed a large circumferential pericardial effusion, with
the area of the darkest liquid measuring 25 mm in the right
ventricular anterior wall near to the apex, with evidence of right
atrial and ventricular collapse and pericardial thickening.
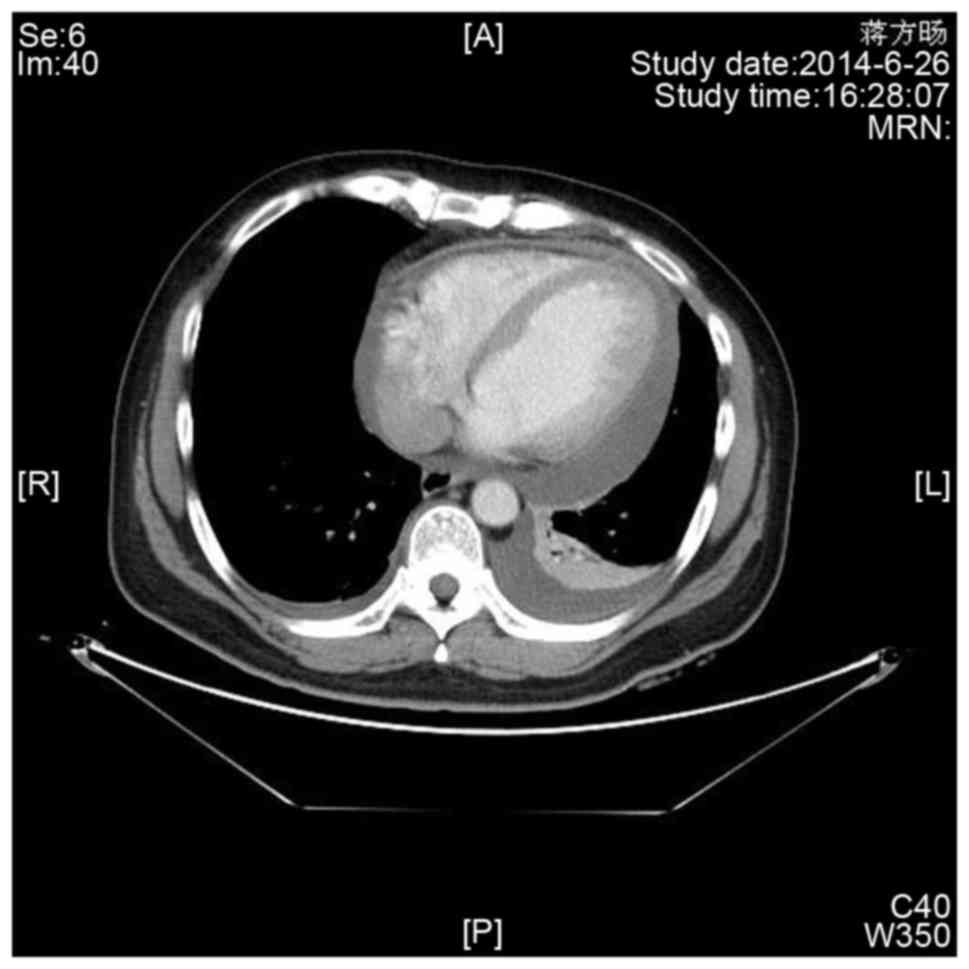
Enhanced chest computed tomography (Fig.
3) demonstrated evident pericardial effusion, and a small
amount of pleural effusion on the left side. No evidence of
malignant or metastatic lesions was found elsewhere in the
body.
A routine blood test revealed a white blood cell
count of 10.2×109 cells/l with 66.6% segment, 109 g/l
hemoglobin and a platelet count of 240×109 platelets/l.
A laboratory assessment was performed to determine coagulation
function. Results were as follows: Prothrombin time, 17.2 sec
(reference, 10–13 sec); plasma fibrinogen, 13.0 mg/l (reference,
<5); active partial thromboplastin time, 42.8 sec (reference,
23.3–39.3 sec); plasma D-dimer, 3.36 mg/l (reference, 0–1 mg/l);
coagulation factor IX, normal; and factor VIII activity, 14%.
Coagulation factor antibodies were negative in coagulation factor
inhibitor screening and titer tests in a Bethesda assay performed
as previously described (2). Factor
VIII 1 and 2 inversion analysis reports were negative. The patient
was diagnosed with coagulation factor VIII deficiency.
Although the patient's blood pressure was normal,
the jugular venous distention and distant heart sounds exhibited by
the patient were consistent with progression to tamponade, thus
pericardiocentesis was performed. Bloody effusion, amounting to
~6.3 l, was drained from the membrane surrounding the heart over a
period of 20 days. Following drainage, the patient's health
gradually improved and the patient exhibited resolution of dyspnea
and normalization of physical examination after 3 months.
The pericardial fluid contained 1,950,000 red blood
cells and 3,960 leukocytes/µl. The leukocyte composition was 64%
neutrophils, 29% lymphocytes, 5% monocytes and 2% eosinophils.
Fluid analysis demonstrated that fluid glucose was 6.03 mmol/l,
fluid lactate dehydrogenase was 353.4 IU/l and total protein was 57
g/dl. Acid fast staining of the fluid was performed to identify
acid-fast bacilli for 20 min at room temperature and the result was
negative, as were the bacterial cultures. Fluid cytology revealed
only reactive mesothelial cells.
Due to concern about possible hemorrhagic fluid,
coagulopathy was performed using a transfusion approach. On gross
examination, the thickened pericardium measured between 0.1 and 0.3
cm; however, acid-fast stains and culture remained negative.
On the sixth day after admission, the daily complete
blood count was notable for the presence of immunoblasts. Flow
cytometry of peripheral blood for lymphocyte subsets was performed.
A repeat echocardiogram did not demonstrate re-accumulation of
pericardial fluid; the patient remained asymptomatic and was
discharged for outpatient evaluation. Informed consent was obtained
from the patient.
Discussion
Certificating the etiological spectrum of
pericardial bloody effusions is essential to allow for the
diagnosis and treatment of the disease. Common causes of
pericardial bloody effusions are malignancy, uremia and
tuberculosis. Patients suffering with this symptom are often
transferred to a cardiologist, respiratory physician or oncologist
(3). Pericardial bloody effusion
associated with factor VIII deficiency is a rare complication and
may often be overlooked. Patients with factor VIII deficiency may
develop serious and fatal complications, such as intracerebral
hemorrhage, muscle bleeding, hematuria, epistaxis and
gastrointestinal bleeding (4). The
present study reported a life-threatening case of pericardial
bloody effusion following cardiac tamponade and arrhythmia in a
39-year-old male whose factor VIII activity measured 14%.
Coagulation factor VIII, also known as Hageman
factor, participates in the intrinsic coagulation system and
initiates the intrinsic coagulation pathway by activating
coagulation factor XI (5). Acquired
coagulation factor VIII deficiency is rare and has various causes,
including autoimmune disease, malignancy, pregnancy, skin
disorders, drugs and infection. Secondary to anti-factor VIII
antibodies, which are usually diagnosed following the clinical
presentation of cutaneous or soft tissue bleeding in adults,
activated partial thromboplastin time (aPTT) showed prolongation as
it did not correct with normal plasma addition incubation periods
later (6). Finally, factor VIII
deficiency was confirmed by the presence of very low levels of
factor VIII in a Bethesda assay (2).
Genetic testing has recently been made available to determine an
individual's risk of developing or passing on hemophilia (7).
In the present case, the patient experienced
hematoma of the scrotum 2 years prior to admission to Xiamen Chang
Gung Hospital. The patient did not bleed during surgery until
pericardiocentesis was performed, which revealed pericardial
non-clotting red fluid with a red blood cell count of ≥100,000
cells/mm3. Furthermore, investigations were conducted to
determine the presence of tumor markers or pathogens and a
tuberculosis smear and culture was performed. All of these
investigations yielded negative results. Finally, it was discovered
that aPTT prolongation of 42.8 sec (reference, 23.3–39.3 sec) was
unable to be corrected by plasma mixing. Coagulation factor
antibodies were negative in coagulation factor inhibitor screening
and titer tests. The patient was diagnosed with coagulation factor
VIII deficiency.
For the patient, the most effective treatment
focused on eradication of the inhibitor in order to remiss the
production of continuous bloody pericardial effusions. Along with
pericardiocentesis, the coagulopathy was treated by infusion of
cryoagglutinin and steroids to eradicate the coagulation inhibitor.
After 3 months, the pericardium did not produce cardiac bloody
effusions according to the echocardiogram and the patient had been
successfully treated.
In conclusion, coagulation factor XIII deficiency is
may cause hemorrhagic cardiac effusions. Hemorrhagic cardiac
effusions are rare and may result in high mortality. The present
case report highlights the successful management of an AHA patient
who exhibited life-threatening hemorrhagic pericardial effusions.
Although the specific coagulation inhibitor was not identified in
the patient, immunosuppressive therapy (steroids) was used
according to treatment guidelines to suppress the inhibitor. In
future, the inhibitor levels should be measured again to determine
if the treatment was effective in the long-term in preventing
recurrence of disease. Early recognition and intervention of
coagulopathy and fatal cardiac effusions is essential for
increasing the survival rate for this disorder.
Acknowledgements
We would like to thank the staff of the Intensive
Care Unit, Xiamen Chang Gung Hospital, for their help in the
management of this patient. We would like to thank the Hematology
and Cardiology units at Xiamen Chang Gung Hospital for arranging
investigations and assisting with patient management.
References
|
1
|
Higasa S: Diagnosis and treatment of
congenital and acquired hemophilia. Rinsho Ketsueki. 58:857–865.
2017.PubMed/NCBI
|
|
2
|
Collins PW and Percy CL: Advances in the
understanding of acquired haemophilia A: Implications for clinical
practice. Br J Haematol. 148:183–194. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Atar S, Chiu J, Forrester JS and Siegel
RJ: Bloody pericardial effusions in patients with cardiac
tamponade: Is the cause cancerous, tuberculosis, or iatrogenic in
the 1990s? Chest. 116:1564–1569. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ma AD and Carrizosa D: Acquired factor
VIII inhibitors: Pathophysiology and treatment. Hematology Am Soc
Hematol Educ Program. 432–437. 2006.PubMed/NCBI
|
|
5
|
Fay PJ: Factor VIII structure and
function. Int J Hematol. 83:103–108. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huth-Kühne A, Baudo F, Collins P,
Ingerslev J, Kessler CM, Lévesque H, Castellano ME, Shima M and
St-Louis J: International recommendations on the diagnosis and
treatment of patients with acquired hemophilia A. Haematologica.
94:566–575. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Matino D, Makris M, Dwan K, D'Amico R and
Iorio A: Recombinant factor VIIa concentrate versus plasma-derived
concentrates for treating acute bleeding episodes in people with
haemophilia and inhibitors. Cochrane Database Syst Rev: CD004449.
2015. View Article : Google Scholar
|