Introduction
Intracerebral hemorrhage is the most common human
cerebrovascular disease and the subtype of hemorrhagic stroke that
is most difficult to treat (1).
Patients with acute intracerebral hemorrhage were frequently
monitored in the intensive care unit (2,3).
Spontaneously sudden intracerebral hemorrhage is associated with
higher rates of mortality and morbidity in comparison with other
intracephalic diseases (4). Cerebral
hemorrhage is frequently induced by exertion and emotion, and the
majority of patients demonstrate sudden onset during activity
(5). In addition, cerebral
hemorrhage usually causes severe dysfunction of the cerebral
nervous system and loss of working and self-care abilities,
consequently further increasing the burden of the patient's family
(6,7). Previous studies have observed that the
neuroprotective functions contributed to the downregulation of
inflammatory (CD68-positive) cells in a mouse model of
intracerebral hemorrhage (2,8).
Simvastatin is a statin drug used to manage the
blood cholesterol levels and prevent cardiovascular disease due to
its inhibitory effect on 3-hydroxy-3-methylglutaryl coenzyme A
reductase (9,10). To date, simvastatin has been reported
to increase the survival of septic or infectious patients by
improvement of the sepsis-induced mortality and acute kidney injury
via its renal vascular effects (11). Karki et al (12) have demonstrated that simvastatin and
atorvastatin significantly improved neurological recovery,
decreased tissue loss and increased neurogenesis when administered
for 1 week following intracerebral hemorrhage. In addition, Lapchak
and Han (13) have suggested that
the 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor
simvastatin was able to reduce the thrombolytic-induced
intracerebral hemorrhage in embolized rabbits. Furthermore, another
study indicated that vascular recovery was promoted by simvastatin
following experimental intracerebral hemorrhage, as determined by
magnetic resonance imaging and histological examinations (14). Therefore, simvastatin may be an
efficient agent for intracerebral hemorrhage therapy.
In the present study, the anti-apoptotic properties
of simvastatin in the progression of intracerebral hemorrhage were
investigated in a mouse model. It was hypothesized that simvastatin
may be able to protect neurons by regulating neuronal apoptosis.
The study reports that simvastatin led to the attenuation of brain
edema and reduced cellular apoptosis by suppressing the nuclear
factor (NF)-κB-mediated myeloid differentiation primary response 88
(MyD88)/TIR domain-containing adaptor protein inducing interferon-β
(TRIF) signaling pathway subsequent to the induction of
intracerebral hemorrhage in mice.
Materials and methods
Ethical statement
All animal protocols were performed in accordance
with the recommendations of the Guide for the Care and Use of
Laboratory Animals of the National Institutes of Health (Bethesda,
MD, USA). Experiments were approved by the Committee on the Ethics
of Animal Experiments Defense Research of Ningbo No. 2 Hospital
(Ningbo, China).
Intracerebral hemorrhage animal
model
Intracerebral hemorrhage was established using the
stereotaxic intrastriatal injection of collagenase type IV as
described previously with certain modifications (15). Briefly, a total of 60 CD-1 mice (6–8
weeks old; 300–350 g body weight) were purchased from the Institute
of Biophysics at the Chinese Academy of Sciences (Beijing, China),
and housed in a temperature-controlled facility at 23±1°C with
relative humidity of 50±5% and a 12 h light/dark cycle. The mice
were anesthetized with 10% chloral hydrate (0.3 ml/100 g,
intraperitoneally; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany).
The rectal temperature was maintained at 37°C throughout the
surgical procedure using a heating lamp. Collagenase type IV (0.5
IU; Sigma-Aldrich; Merck KGaA) in 2 µl saline was injected into the
left striatum to induce intracerebral hemorrhage over a period of 5
min. Mice were divided into two groups (the model + vehicle group
and the model + simvastatin group; n=30 in each group) and
intracerebral hemorrhage animals received intragastric
administration of the vehicle (vehicle-treated model) or
simvastatin (50 µg dissolved in 500 µl saline) once a day
immediately following intracerebral hemorrhage.
Western blot analysis
Following treatment with simvastatin for 10 days,
the experimental animals were sacrificed and neuronal cells were
isolated as previously described (16). Cells were collected and lysed in a
radioimmunoprecipitation assay buffer (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) followed homogenization at 4°C for 10 min.
Protein concentration was measured using a BCA kit (cat no. 23225;
Thermo Fisher Scientific, Inc.). A total of 20 µg protein extract
underwent 12.5% SDS-PAGE and was then transferred to a
nitrocellulose membrane. The membrane was incubated in blocking
buffer (5% milk) for 30 min at 37°C prior to incubation with
primary antibodies at 4°C overnight. The primary rabbit anti-mouse
antibodies used in the immunoblotting assays were as follows: NF-κB
(1:1,200; ab32360), aquaporin-4 (AQP4; 1:1,200; ab9512), matrix
metallopeptidase 9 (MMP-9; 1:1,000; ab54230), caspase-3 (1:1,200;
ab2171), B-cell lymphoma-2 (Bcl-2; 1:1,000; ab692), MyD88 (1:1,200;
ab2068), TRIF (1:1,200; ab13810) and β-actin (1:500; ab8226; all
from Abcam, Cambridge, MA, USA). Horseradish peroxidase-conjugated
anti-rabbit IgG secondary antibody (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA) was then added at a dilution of 1:5,000, and
proteins were detected by enhanced chemiluminescence using a
Western Blotting Luminol reagent (Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA). The density of the bands was analyzed by Quantity
One software version 4.62 (Bio-Rad Laboratories).
Terminal deoxynucleotidyl
transferase-mediated dUTP nick end labeling (TUNEL) assay for
apoptosis detection
Apoptotic neuronal cells in the hippocampus of the
intracerebral hemorrhage animal model were analyzed using TUNEL
assay (DeadEnd™ Colorimetric TUNEL System; Promega Corporation,
Madison, WI, USA) according to the manufacturer's protocol. The
slides were then analyzed with fluorescence microscopy (Bx51;
Olympus Corporation, Tokyo, Japan).
Analysis of cerebral water content
(CWC)
The CWC was measured on day 10 after treatment with
simvastatin following intracerebral hemorrhage as described
previously (17). Briefly, the
brains of the mice were isolated and divided into the two
hemispheres. The hemispheres were weighed using an electronic
analytical balance to obtain the wet weights. Subsequently, the
brain tissues were dried in an electric oven at 100°C for 24 h to
analyze the CWC in the intracerebral hemorrhage mouse model. The
CWC was calculated according to the following formula: CWC (%)=(Wet
weight-dry weight)/wet weight ×100.
Quantitative analysis of blood-brain
barrier (BBB) permeability
BBB leakage was assessed as previously described
with a slight modification (18).
Briefly, all experimental mice received 100 µl of a 5% solution of
Evans blue (cat. no. E2129; Sigma-Aldrich; Merck KGaA) in
simvastatin or saline administered intravenously with the final
dose of simvastatin or vehicle at 10 days following intracerebral
hemorrhage. At 2 h following Evans blue injection, cardiac
perfusion was performed under deep anesthesia with 200 ml saline to
clear the cerebral circulation of the Evans blue. Following
sacrifice, the brain was isolated, embedded in paraffin and cut
into 20-µm-thick cryosections. The two hemispheres were homogenized
in 750 µl N, N-dimethylformamide (cat. no. D4551; Sigma-Aldrich;
Merck KGaA). The samples obtained were incubated in a 50°C water
bath for 48 h and subsequently centrifuged at 12,000 × g for 30 min
at 25°C. The resultant supernatant was spectrophotometrically
quantified for the extravasated Evans blue dye at 620 nm.
NF-κB activity
Neuronal cells in experimental mice were isolated as
described above. A total of 1×106 cells were cultured in
Dulbecco's modified Eagle's medium (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum
(Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) and
incubated at 37°C for 12 h. Neuronal cells were transfected with
plentivirus (p)-NF-κB-Lucreporter plasmid using the VigoFect
Transfection reagent (Vigorous Biotechnology, Beijing, China). A
total of 24 h following transfection, cells were lysed and the
NF-κB luciferase activity was determined using the Dual-Luciferase
Reporter Assay system (Promega Corporation, Madison, WI, USA)
according to the manufacturer's protocol.
NF-κB overexpression
Neuronal cells (1×105) were cultured in
6-well culture plates until 85% confluence, when the media was
removed and the plates were washed three times with PBS. Neuronal
cells were transfected with pNF-κB using Lipofectamine®
2000 (Sigma-Aldrich; Merck KGaA) according to manufacturer's
protocol. Cells with NF-κB overexpression were treated with
simvastatin (200 µM) for further analysis.
Behavioral assessments
Animal behavioral assessment was performed on
postoperative day 10. The assessment parameters, including the left
limb movement and coordination of movement, were evaluated using
the modified neurological severity score (mNSS) as previously
described (19). Each animal in the
present study was analyzed using mNSS scores. The total possible
score was 15, mNSS scores of ≥10 indicated normal behavior, scores
≥5 indicated behavioral difficulties and mNSS scores <5
indicated dysfunctional behavior.
Statistical analysis
Data are presented as the mean ± standard deviation
of triplicate experiments. All data were analyzed by SPSS version
13.0 software (SPSS, Inc., Chicago, IL, USA). Comparisons between
the groups were assessed by Student's t-test or one-way analysis of
variance. A P-value of <0.05 was considered to indicate a
statistically significant result.
Results
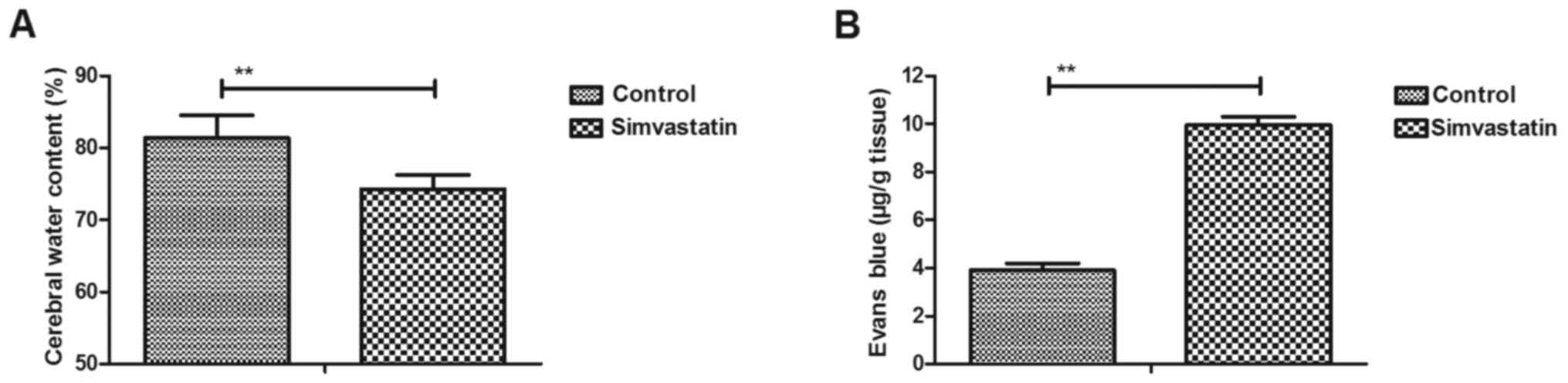
Simvastatin improves the CWC and BBB
disruption in intracerebral hemorrhage animals
An intracerebral hemorrhage mouse model was used to
analyze the efficacy of simvastatin treatment. It was observed that
simvastatin treatment significantly decreased the CWC in
intracerebral hemorrhage animals compared with the vehicle-treated
model group (Fig. 1A). The results
also revealed that simvastatin treatment improved BBB disruption in
the intracerebral hemorrhage animals compared with the
vehicle-treated model group, as observed by the marked increase in
the Evans blue content of brain tissues (Fig. 1B). These results suggested that
simvastatin treatment significantly improved the CWC and BBB
disruption in intracerebral hemorrhage animals.
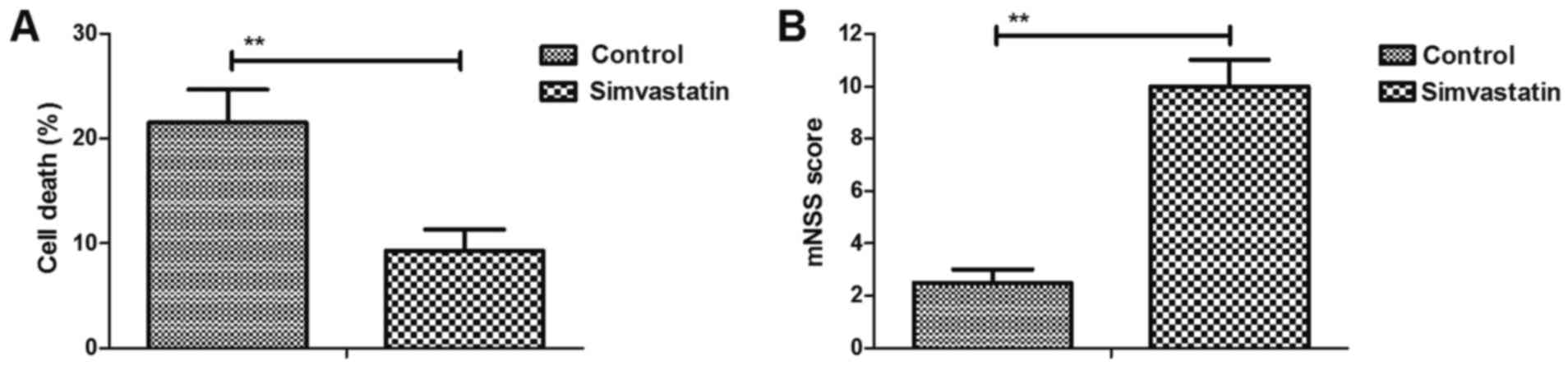
Simvastatin decreases intracerebral
hemorrhage-induced neuronal cell death
The efficacy of simvastatin on neuronal cell death
was investigated in intracerebral hemorrhage mice. As shown in
Fig. 2A, simvastatin treatment
significantly reduced cell death in the injured hemispheres.
Furthermore, it was demonstrated that simvastatin markedly improved
the motor function in intracerebral hemorrhage animals compared
with the vehicle-treated model group, as observed by the marked
increase in mNSS values (Fig. 2B).
These results suggested that simvastatin decreased the
intracerebral hemorrhage-induced neuronal cell death.
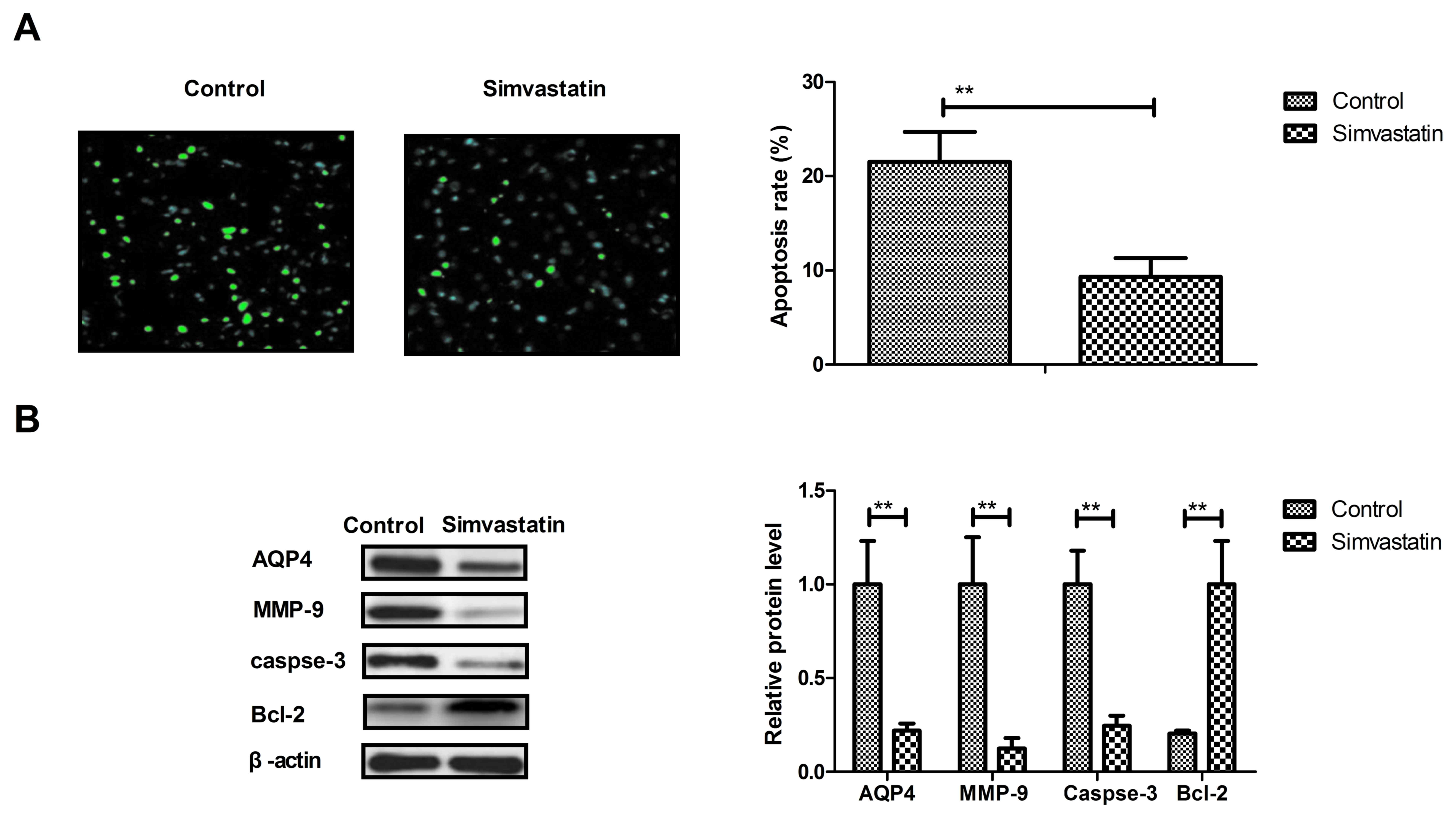
Simvastatin decreases the apoptosis of
neurons in intracerebral hemorrhage animals
The anti-apoptotic effects of simvastatin were
analyzed in intracerebral hemorrhage animals, and it was observed
that simvastatin treatment significantly decreased the apoptosis of
neuronal cells in intracerebral hemorrhage animals (Fig. 3A). In addition, the expression levels
of AQP4, MMP-9 and caspase-3 were downregulated and Bcl-2 were all
upregulated by simvastatin treatment compared with the levels in
the vehicle-treated model group (Fig.
3B). These results suggest that simvastatin treatment can
decrease the apoptosis of neurons in animals suffering
intracerebral hemorrhage.
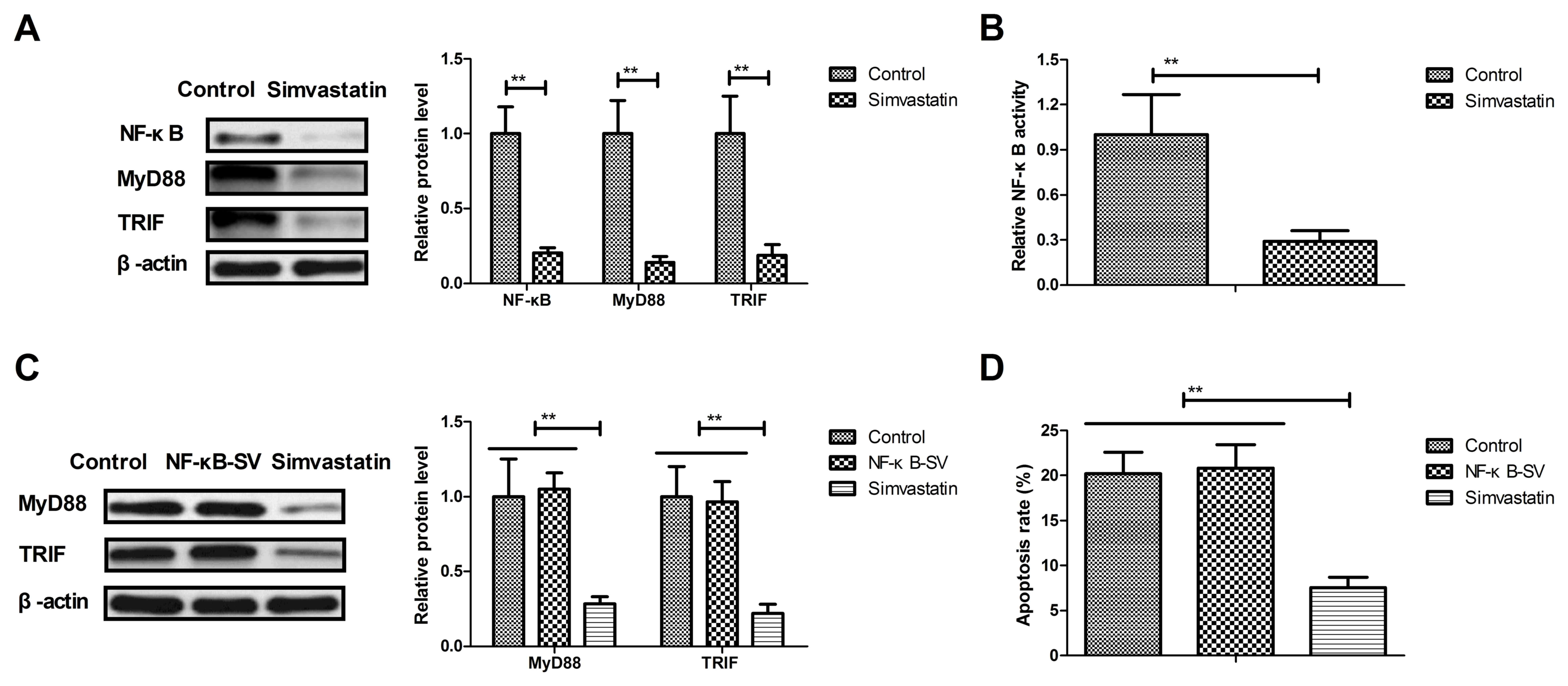
Simvastatin reduced cellular apoptosis
by suppressing NF-κB-mediated MyD88/TRIF
The potential mechanism underlying the effect of
simvastatin in neurons in the intracerebral hemorrhage animals was
also examined in the present study. Mechanism analysis indicated
that simvastatin treatment significantly downregulated the NF-κB,
MyD88 and TRIF expression levels in the neuronal cells in
experimental mice as compared with the vehicle-treated model mice
(Fig. 4A). Furthermore, the NF-κB
activity was also decreased by simvastatin in the neuronal cells of
the experimental mice (Fig. 4B).
The results also demonstrated that NF-κB
overexpression abolished simvastatin-downregulated MyD88 and TRIF
expression in the neurons of the NF-κB + simvastatin group
(Fig. 4C). Simvastatin-inhibited
apoptosis was also prevented by the NF-κB overexpression in
neuronal cells (Fig. 4D). These
results suggested that simvastatin treatment reduced cellular
apoptosis by suppressing the NF-κB-mediated MyD88/TRIF signaling
pathway in the neurons of the intracerebral hemorrhage animals.
Discussion
Intracerebral hemorrhage is frequently induced by
exertion and emotion, and the majority of patients demonstrate
sudden onset during activity. Previous evidence revealed that
simvastatin exhibits beneficial effects for patients with
intracerebral hemorrhage (20). In
addition, simvastatin has been demonstrated to attenuate cerebral
vasospasm and improve the treatment outcomes by upregulation of
various signaling pathways in a rat model of subarachnoid
hemorrhage (21). In the present
study, the potential mechanism mediated by simvastatin was analyzed
in a CD-1 mouse model of intracerebral hemorrhage. It was
demonstrated that simvastatin treatment significantly improved the
CWC and BBB disruption in this mouse model. The findings also
suggested that intracerebral hemorrhage-induced neuronal cell death
was decreased by the simvastatin treatment. Notably, simvastatin
treatment significantly reduced the Evans blue leakage into the
injured hemispheres and improved the motor function. Mechanism
analysis indicated that simvastatin treatment was able to reduce
cellular apoptosis by suppressing the NF-κB-mediated MyD88/TRIF
signaling pathway subsequent to intracerebral hemorrhage in
mice.
Subarachnoid hemorrhage is one of the most severe
cerebral hemorrhage types and usually leads to mortality due to
bleeding into the subarachnoid space (22,23).
However, simvastatin treatment was observed to attenuate the
cerebral vasospasm following subarachnoid hemorrhage in rats via
increased phosphorylation of protein kinase B and endothelial
nitric oxide synthase (24). Lin
et al (25) have also
demonstrated the therapeutic effects of simvastatin on delayed
cerebral vasospasm following subarachnoid hemorrhage in rabbits. In
the present study, simvastatin treatment markedly improved CWC and
BBB disruption, which further improved the motor function in
intracerebral hemorrhage animals.
The preventive and therapeutic effects of
simvastatin on secondary inflammatory damage have previously been
investigated in rats with cerebral hemorrhage (26). Another study observed that NF-κB
activation was closely associated with cell death and served an
important function in secondary brain damage following
intracerebral hemorrhage in patients (27). Furthermore, Shen et al
(28) indicated that NF-κB regulates
the intracerebral hemorrhage-induced neuronal damage via apoptosis.
A further study also indicated that simvastatin reduces the NF-κB
activity in peripheral mononuclear and in plaque cells of rabbit
atheroma (29). In the current
investigation, it was demonstrated that simvastatin treatment
downregulated the NF-κB expression, and upregulated the MyD88 and
TRIF expression levels in neuronal cells in the experimental mice.
In addition, NF-κB overexpression abolished the simvastatin-induced
downregulation of MyD88 and TRIF levels in neurons.
In conclusion, the present study reported the
therapeutic effects of simvastatin on experimental mice with
intracerebral hemorrhage. The anti-apoptotic efficacy of
simvastatin on neurons in the progression of intracerebral
hemorrhage was also discussed. The study findings indicated that
simvastatin treatment attenuated brain edema and reduced cellular
apoptosis by suppressing the NF-κB-mediated MyD88/TRIF signaling
pathway following intracerebral hemorrhage in mice. These data
suggest that simvastatin is an efficient drug for intracerebral
hemorrhage therapy and identified that NF-κB may be a potential
target for the treatment of intracerebral hemorrhage.
References
|
1
|
Sussman ES and Connolly ES Jr: Hemorrhagic
transformation: A review of the rate of hemorrhage in the major
clinical trials of acute ischemic stroke. Front Neurol. 4:692013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ma B and Zhang J: Nimodipine treatment to
assess a modified mouse model of intracerebral hemorrhage. Brain
Res. 1078:182–188. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Manaenko A, Fathali N, Chen H, Suzuki H,
Williams S, Zhang JH and Tang J: Heat shock protein 70 upregulation
by geldanamycin reduces brain injury in a mouse model of
intracerebral hemorrhage. Neurochem Int. 57:844–850. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee HJ, Kim KS, Kim EJ, Choi HB, Lee KH,
Park IH, Ko Y, Jeong SW and Kim SU: Brain transplantation of
immortalized human neural stem cells promotes functional recovery
in mouse intracerebral hemorrhage stroke model. Stem Cells.
25:1204–1212. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kumar N Ganesh, Zuckerman SL, Khan IS,
Dewan MC, Morone PJ and Mocco J: Treatment of intracerebral
hemorrhage: A selective review and future directions. J Neurosurg
Sci. 61:523–535. 2017.PubMed/NCBI
|
|
6
|
Lee WJ, Yeon JY, Jo KI, Kim JS and Hong
SC: Reversible cerebral vasoconstriction syndrome and posterior
reversible encephalopathy syndrome presenting with deep
intracerebral hemorrhage in young women. J Cerebrovasc Endovasc
Neurosurg. 17:239–245. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kanamaru K, Suzuki H and Taki W: Cerebral
infarction after aneurysmal subarachnoid hemorrhage. Acta Neurochir
Suppl. 121:167–172. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu L, Barfejani AH, Qin T, Dong Q, Ayata C
and Waeber C: Fingolimod exerts neuroprotective effects in a mouse
model of intracerebral hemorrhage. Brain Res. 1555:89–96. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ramadan WH and Kabbara WK:
Sitagliptin/simvastatin: A first combination tablet to treat type 2
diabetes and hypercholesterolemia-a review of its characteristics.
Vasc Health Risk Manag. 11:125–132. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Taveira-DaSilva AM, Jones AM,
Julien-Williams PA, Stylianou M and Moss J: Retrospective review of
combined sirolimus and simvastatin therapy in
lymphangioleiomyomatosis. Chest. 147:180–187. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yasuda H, Yuen PS, Hu X, Zhou H and Star
RA: Simvastatin improves sepsis-induced mortality and acute kidney
injury via renal vascular effects. Kidney Int. 69:1535–1542. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Karki K, Knight RA, Han Y, Yang D, Zhang
J, Ledbetter KA, Chopp M and Seyfried DM: Simvastatin and
atorvastatin improve neurological outcome after experimental
intracerebral hemorrhage. Stroke. 40:3384–3389. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lapchak PA and Han MK: The
3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor
simvastatin reduces thrombolytic-induced intracerebral hemorrhage
in embolized rabbits. Brain Res. 1303:144–150. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang D, Knight RA, Han Y, Karki K, Zhang
J, Ding C, Chopp M and Seyfried DM: Vascular recovery promoted by
atorvastatin and simvastatin after experimental intracerebral
hemorrhage: Magnetic resonance imaging and histological study. J
Neurosurg. 114:1135–1142. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shimamura N, Kakuta K, Wang L, Naraoka M,
Uchida H, Wakao S, Dezawa M and Ohkuma H: Neuro-regeneration
therapy using human Muse cells is highly effective in a mouse
intracerebral hemorrhage model. Exp Brain Res. 235:565–572. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Park JS, Kim S, Han DK, Lee JY and Ghil
SH: Isolation of neural precursor cells from skeletal muscle
tissues and their differentiation into neuron-like cells. Exp Mol
Med. 39:483–490. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hijioka M, Matsushita H, Hisatsune A,
Isohama Y and Katsuki H: Therapeutic effect of nicotine in a mouse
model of intracerebral hemorrhage. J Pharmacol Exp Ther.
338:741–749. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tong LS, Shao AW, Ou YB, Guo ZN, Manaenko
A, Dixon BJ, Tang J, Lou M and Zhang JH: Recombinant Gas6 augments
Axl and facilitates immune restoration in an intracerebral
hemorrhage mouse model. J Cereb Blood Flow Metab. 37:1971–1981.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hsieh TH, Kang JW, Lai JH, Huang YZ,
Rotenberg A, Chen KY, Wang JY, Chan SY, Chen SC, Chiang YH and Peng
CW: Relationship of mechanical impact magnitude to neurologic
dysfunction severity in a rat traumatic brain injury model. PLoS
One. 12:e01781862017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
McGirt MJ, Pradilla G, Legnani FG, Thai
QA, Recinos PF, Tamargo RJ and Clatterbuck RE: Systemic
administration of simvastatin after the onset of experimental
subarachnoid hemorrhage attenuates cerebral vasospasm.
Neurosurgery. 58:945–951. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sugawara T, Jadhav V, Ayer R and Zhang J:
Simvastatin attenuates cerebral vasospasm and improves outcomes by
upregulation of PI3K/Akt pathway in a rat model of subarachnoid
hemorrhage. Acta Neurochir Suppl. 102:391–394. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brown RJ, Epling BP, Staff I, Fortunato G,
Grady JJ and McCullough LD: Polyuria and cerebral vasospasm after
aneurysmal subarachnoid hemorrhage. BMC Neurol. 15:2012015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jenson AV, Rodriguez GJ, Alvarado LA,
Cruz-Flores S and Maud A: Higher rate of intracerebral hemorrhage
in hispanic patients with cerebral cavernous malformation. J Vasc
Interv Neurol. 8:1–4. 2015.PubMed/NCBI
|
|
24
|
Sugawara T, Ayer R, Jadhav V, Chen W,
Tsubokawa T and Zhang JH: Simvastatin attenuation of cerebral
vasospasm after subarachnoid hemorrhage in rats via increased
phosphorylation of Akt and endothelial nitric oxide synthase. J
Neurosci Res. 86:3635–3643. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lin C, Zhao Y, Wan G, Zhu A and Wang H:
Effects of simvastatin and taurine on delayed cerebral vasospasm
following subarachnoid hemorrhage in rabbits. Exp Ther Med.
11:1355–1360. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhou HX, Gao LH, Meng LL, Zhang YX, Wei ZF
and Si DW: Preventive and therapeutic effect of simvastatin on
secondary inflammatory damage of rats with cerebral hemorrhage.
Asian Pac J Trop Med. 10:152–156. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang Z, Liu Y, Huang Q, Su Y, Zhang Y,
Wang G and Li F: NF-kB activation and cell death after
intracerebral hemorrhage in patients. Neurol Sci. 35:1097–1102.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Shen X, Ma L, Dong W, Wu Q, Gao Y, Luo C,
Zhang M, Chen X and Tao L: Autophagy regulates intracerebral
hemorrhage induced neural damage via apoptosis and NF-kB pathway.
Neurochem Int. 96:100–112. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mikael LG and Rozen R: Homocysteine
modulates the effect of simvastatin on expression of ApoA-I and
NF-kappaB/iNOS. Cardiovasc Res. 80:151–158. 2008. View Article : Google Scholar : PubMed/NCBI
|