Introduction
Osteosarcoma is the most common cancer in the bone
and mainly occurs in regions undergoing active bone growth and
repair (1,2). Despite improvements in the efficacy of
surgery combined with radiotherapy and/or chemotherapy, the 5-year
survival rate of patients with osteosarcoma remains poor, primarily
due to recurrence and metastasis (3,4).
Therefore, identifying the molecular mechanisms underlying
osteosarcoma growth and metastasis is important to facilitate the
development of effective therapeutic strategies to treat
osteosarcoma.
MicroRNAs (miRs) are a class of small non-coding
RNAs that regulate gene expression by directly binding to the
3′-untranslated region (UTR) of their target mRNAs, resulting in
either mRNA degradation or the inhibition of translation (5–7). It has
been demonstrated that miRs are involved in a variety of biological
processes by regulating the expression of their targets, including
mRNAs involved in cell survival, differentiation, proliferation,
apoptosis, migration and angiogenesis (6,8–10). Furthermore, deregulated miRs have
been identified in different types of cancer, such as osteosarcoma
and miRs may be used as potential diagnostic and therapeutic
targets for osteosarcoma (11–14).
miR-133b and miR-503 are markedly downregulated in osteosarcoma
tissues compared with adjacent non-tumor tissues and their
downregulation is associated with the malignant progression of
osteosarcoma and poor overall survival of patients (15). Furthermore, it has been determined
that miR-199a-3p is significantly downregulated in osteosarcoma and
negatively regulates the proliferation and migration of
osteosarcoma cells (16).
Among these miRs, it has been observed that miR-92b
serves a role in certain types of human cancer (17,18).
miR-92b promotes the proliferation, migration and invasion of
glioma cells, and induces apoptosis via regulation of the
phosphatase and tensin homolog (PTEN)/protein kinase B signaling
pathway (17). Additionally, miR-92b
represses the invasion and metastasis of esophageal squamous cell
carcinoma (ESCC) in vitro and in vivo, and higher
expression of miR-92b in ESCC tissue is inversely correlated with
lymph node metastasis and indicates better patient prognosis
(18). Recently, it has been
demonstrated that miR-92b promotes the malignant phenotype of
osteosarcoma cells by inhibiting the expression of
reversion-inducing, cysteine-rich protein with kazal motifs (RECK)
(19). As each miR may have multiple
target genes, it remains to be clarified whether there are other
targets of miR-92b in osteosarcoma cells.
The present study aimed to investigate the clinical
significance and regulatory mechanism of miR-92b in osteosarcoma.
The expression of miR-92b in osteosarcoma tissues and cell lines
was examined, and the potential target gene of miR-92b was
explored.
Materials and methods
Tissue collection
A total of 58 primary osteosarcoma tissues and
matched adjacent non-tumor tissues were collected from patients (35
males and 23 females; mean age, 31.4 years) in the Second
Affiliated Hospital of Nanchang from April 2008 to September 2015.
Prior to surgical resection, no patients received radio- or
chemotherapy. Tissues were immediately snap-frozen in liquid
nitrogen following surgical resection and stored in liquid
nitrogen. The clinicopathological characteristics of patients
included in the present study are summarized in Table I. The current study was approved by
the Ethics Committee of the Second Affiliated Hospital of Nanchang
University (Nanchang, China) and informed consent was obtained from
all participants.
 | Table I.Association between miR-92b expression
and clinicopathological characteristics in osteosarcoma. |
Table I.
Association between miR-92b expression
and clinicopathological characteristics in osteosarcoma.
| Variables | Cases (n=58) | Low miR-92b
(n=28) | High miR-92b
(n=30) | P-value |
|---|
| Age, years |
|
|
| 0.435 |
|
<20 | 24 | 10 | 14 |
|
| ≥20 | 34 | 18 | 16 |
|
| Sex |
|
|
| 0.789 |
| Male | 35 | 16 | 19 |
|
|
Female | 23 | 12 | 11 |
|
| Tumor size, cm |
|
|
| 0.199 |
|
<8 | 26 | 10 | 16 |
|
| ≥8 | 32 | 18 | 14 |
|
| Location |
|
|
| 0.781 |
| Femur
or tibia | 39 | 18 | 21 |
|
|
Other | 19 | 10 | 9 |
|
| Lung
metastasis |
|
|
| 0.011 |
| No | 40 | 24 | 16 |
|
|
Yes | 18 | 4 | 14 |
|
| TNM stage |
|
|
| 0.013 |
|
I/IIA | 21 | 15 | 6 |
|
|
IIB/III | 37 | 13 | 24 |
|
| Serum lactate
dehydrogenase |
|
|
| 0.184 |
|
Normal | 22 | 8 | 14 |
|
|
Elevated | 36 | 20 | 16 |
|
| Serum alkaline
phosphatase |
|
|
| 0.795 |
|
Normal | 24 | 11 | 13 |
|
|
Elevated | 34 | 17 | 17 |
|
Cell culture
The human osteoblast cell line hFOB and the
osteosarcoma cell lines U2OS, Saos-2, MG63 and SW1353 were
purchased from the Cell bank of the Chinese Academy of Sciences
(Shanghai, China). All cell lines were cultured in Dulbecco's
Modified Eagle's medium (DMEM; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum (Thermo
Fisher Scientific, Inc.) in a 37°C humidified atmosphere of 5%
CO2.
Cell transfection
U2OS cells were transfected with miR-92b inhibitor
or negative control (NC) inhibitor; miR-92b mimic or scramble miR
mimic (miR-NC); or pcDNA3.1-DKK3 expression plasmid using
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. Following 48 h
transfection, levels of miR-92b or DKK3 expression were
determined.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIzol®
Reagent (Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. To detect miR expression, RT-qPCR was
performed using the All-in-One™ miRNA qRT-PCR Detection
kit (GeneCopoeia, Inc., Rockville, MD, USA) and an ABI 7500
thermocycler (Applied Biosystems; Thermo Fisher Scientific, Inc.)
according to the manufacturer's instructions. The thermocycling
conditions were as follows: 95°C for 10 min, and 45 cycles of
denaturation at 95°C for 15 sec and annealing/elongation at 60°C
for 15 sec. The U6 gene was used as an internal control. The
primers for miR-92b (cat. no. HmiRQP0834) and U6 (cat. no.
HmiRQP9001) were purchased from Guangzhou Fulengen Co., Ltd.
(Guangzhou, China). To detect mRNA expression, total RNA was
converted to cDNA using the PrimeScript 1st Strand cDNA Synthesis
kit (Takara Bio, Inc., Tokyo, Japan) according to the
manufacturer's instructions. A SYBR Green I Real-Time PCR kit
(Biomics Biotechnologies, Co., Ltd., Nantong, China) was then used
to perform qPCR according to the manufacturer's instructions. GAPDH
was used as the internal reference for mRNA. The primers used were
as follows: DKK3, forward, 5′-AGGACACGCAGCACAAATTG-3′ and reverse,
5′-CCAGTCTGGTTGTTGGTTATCTT-3′; GAPDH, forward,
5′-ACAACTTTGGTATCGTGGAAGG-3′ and reverse;
5′-GCCATCACGCCACAGTTTC-3′. The thermocycling conditions were 95°C
for 3 min, followed by 40 cycles of 95°C for 15 sec and 60°C for 15
sec. Relative expression was analyzed using the 2−ΔΔCq
method (20).
Western blot analysis
Cells were solubilized in cold
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology, Shanghai, China). Protein concentration was
determined using the BCA assay kit (Thermo Fisher Scientific, Inc.)
and proteins (50 µg) were separated with 10% SDS-PAGE and
transferred onto a polyvinylidene difluoride membrane (Thermo
Fisher Scientific, Inc.). The membrane was blocked with PBS
containing 5% milk (Yili Group, Beijing, China) for 3 h at room
temperature. Following 3 washes with PBS (Beyotime Institute of
Biotechnology), the membrane was incubated with rabbit polyclonal
anti-DKK3 antibody (1:100; ab187532; Abcam, Cambridge, MA, USA) or
rabbit polyclonal anti-GAPDH antibody (1:50; ab37168; Abcam) at
room temperature for 3 h. Following 3 washes with PBS, the membrane
was incubated with goat anti-rabbit immunoglobulin G (1:5,000;
ab6721; Abcam) at room temperature for 40 min. An ECL kit (Thermo
Fisher Scientific, Inc.) was then used to perform enhanced
chemiluminent detection. Relative protein expression was presented
as the density ratio vs. GAPDH using Image-Pro Plus software 6.0
(Media Cybernetics, Inc., Rockville, MD, USA).
MTT assay
U2OS cell suspension (5×104 cells/well)
was plated in a 96-well plate, and cultured for 0, 24, 48 or 72 h.
Subsequently, MTT (10 µl, 5 mg/ml) was added to each well and then
incubated at 37°C for 4 h. The supernatant was removed and 100 µl
dimethyl sulfoxide (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
was added to each well to dissolve the purple formazan. The
absorbance at 570 nm was measured using the Model 680 Microplate
Reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Transwell assay
U2OS cell suspension (1×106 cells/ml) was
prepared in DMEM, 300 µl of which was added to the upper transwell
chamber (BD Biosciences, Franklin Lakes, NJ, USA) pre-coated with
Matrigel (BD Biosciences). Subsequently, 300 µl DMEM with 10% FBS
was added to the lower chamber. Following 24 h culture at 37°C,
cells that did not invade through the membrane in the filter were
lightly wiped using a cotton-tipped swab (BD Biosciences). The
filter was then fixed in 90% alcohol at room temperature for 10 min
and cells were stained at room temperature for 10 min using 0.1%
crystal violet (Beyotime Institute of Biotechnology). Invading
cells were observed under an inverted microscope and images were
captured.
Bioinformatics predication and
luciferase reporter assay
Targetscan (http://www.targetscan.org) was used to predicate the
potential targets of miR-92b, according to the manufacturer's
instructions. ‘Human’ was selected as the species and ‘miR-92b’ was
entered. The mutant type (MT) of DKK3 3′UTR lacking complementarity
with the miR-92b seed sequence was generated using the QuickChange
Site-Directed Mutagenesis kit (Agilent Technologies Inc., Santa
Clara, CA, USA), according to the manufacturer's instructions. The
wild-type (WT) or MT of DKK3 3′UTR was then cloned downstream of
the firefly luciferase coding region of the pMIR-GLOTM Luciferase
vector (Promega Corporation, Madison, WI, USA). U2OS cells were
co-transfected with WT-DKK3-3′UTR or MUT-DKK3-3′UTR plasmid, and
miR-92b mimic or miR-NC, respectively, using Lipofectamine 2000
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. Following 48 h transfection, luciferase activity was
determined using the dual-Luciferase Reporter assay system (Promega
Corporation) according to the manufacturer's instruction and
normalized to Renilla luciferase activity.
Statistical analysis
The results are expressed as the mean ± standard
deviation of three independent experiments. Student's t test was
used to analyze the difference between two groups. One-way analysis
of variance with the Tukey post hoc test was used to analyze the
differences between more than two groups, and Pearson's correlation
analysis was used to look for associations between groups. SPSS 19
(IBM Corp., Armonk, NY, USA) was used to perform statistical
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
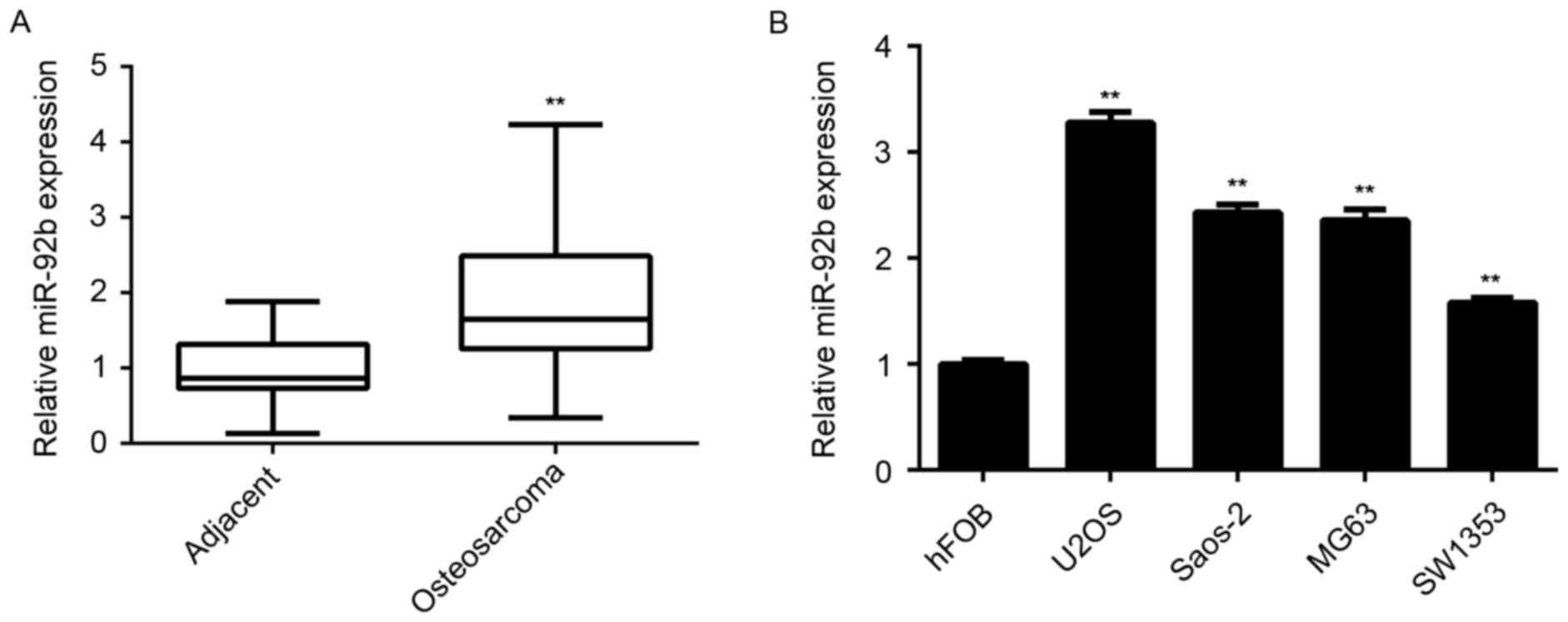
miR-92b is upregulated in
osteosarcoma
RT-qPCR was performed to measure miR-92b expression
in a total of 58 osteosarcoma tissues as well as matched adjacent
normal tissues from patients with osteosarcoma. The results
indicated that miR-92b levels were significantly increased in
osteosarcoma tissue compared with adjacent normal tissue
(P<0.01; Fig. 1A). Furthermore,
it was significantly upregulated in the osteosarcoma cell lines
U2OS, Saos-2, MG63 and SW1353, compared with normal osteoblast hFOB
cells (P<0.01; Fig. 1B).
Upregulation of miR-92b is associated
with osteosarcoma progression
The association between miR-92b expression and
clinical characteristics in osteosarcoma was investigated. Mean
miR-92b levels were used as the cutoff point and this cutoff point
was used to divide patients with osteosarcoma into a high miR-92b
expression group and a low miR-92b expression group. A total of 30
patients were in the high miR-92b expression group, whereas 28
patients were in the low miR-92b expression group (Table I). High miR-92b levels were
significantly associated with lung metastasis and an advanced
clinical stage of osteosarcoma (P<0.05; Table I). However, no significant
associations were identified between miR-92b expression and age,
sex, tumor size, location, serum lactate dehydrogenase or serum
alkaline phosphatase in osteosarcoma (Table I). The results demonstrated that
upregulation of miR-92b may be associated with osteosarcoma
progression.
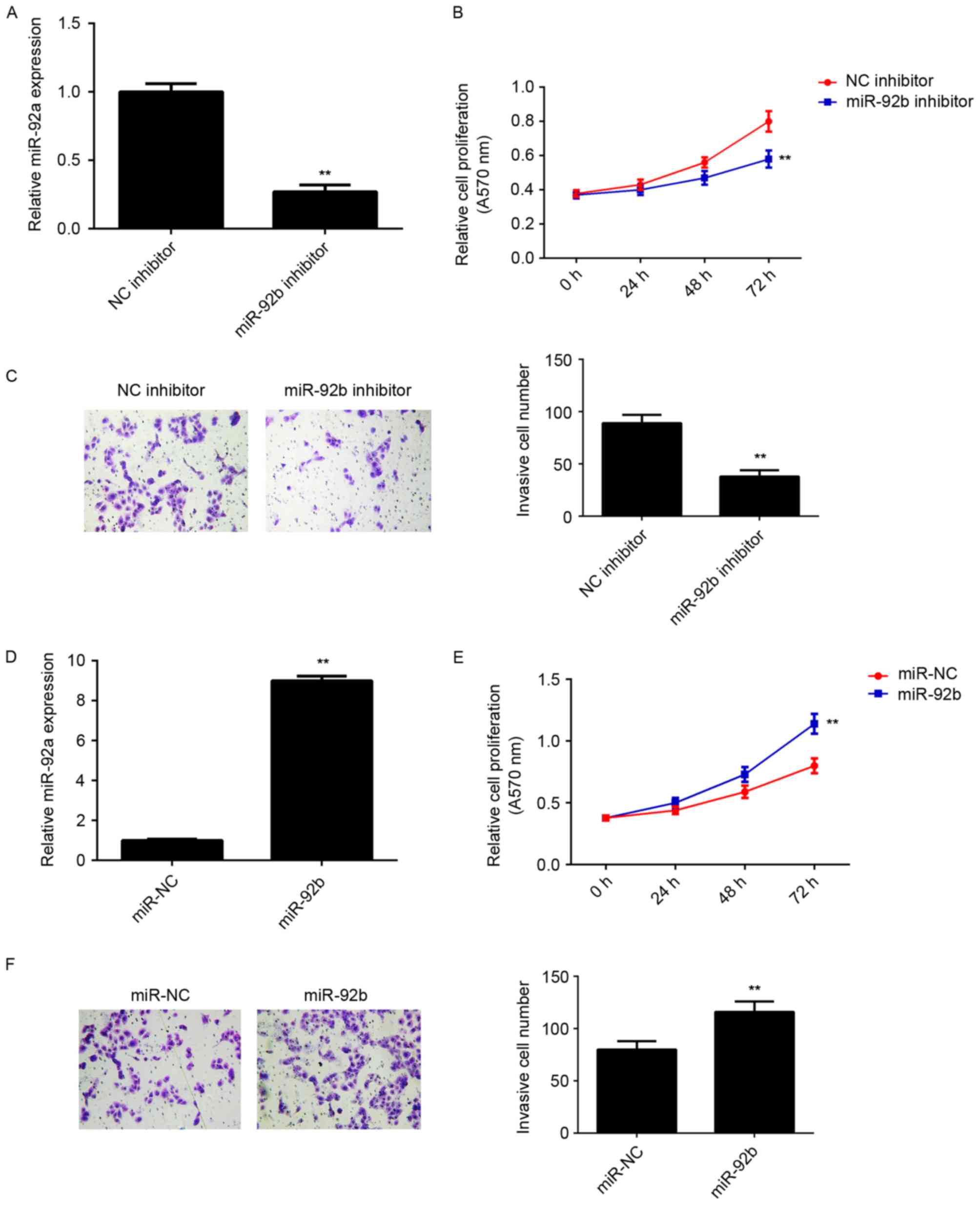
miR-92b promotes the proliferation and
invasion of osteosarcoma cells
The regulatory role of miR-92b in osteosarcoma in
vitro was determined using U2OS cells, as miR-92b expression
was highest in U2OS cells compared with the other osteosarcoma cell
lines. miR-92b expression was upregulated in U2OS cells, thus they
were transfected with either miR-92b inhibitor or NC inhibitor.
Transfection with miR-92b inhibitor significantly decreased miR-92b
expression compared with the NC inhibitor group (P<0.01;
Fig. 2A). MTT and Transwell assays
were further conducted to examine cell proliferation and invasion,
respectively. miR-92b knockdown significantly decreased U2OS cell
proliferation and invasion compared with the NC inhibitor group
(P<0.01; Fig. 2B and C). This
suggests that miR-92b may promote the proliferation and invasion of
osteosarcoma cells. To further confirm these results, U2OS cells
were transfected with miR-92b mimic or miR-NC. Transfection with
miR-92b mimic significantly upregulated miR-92b levels compared
with miR-NC transfection (P<0.01; Fig. 2D). Overexpression of miR-92b
significantly increased the proliferation and invasion of U2OS
cells (P<0.01; Fig. 2E and F),
indicating that miR-92b promotes the proliferation and invasion of
osteosarcoma cells.
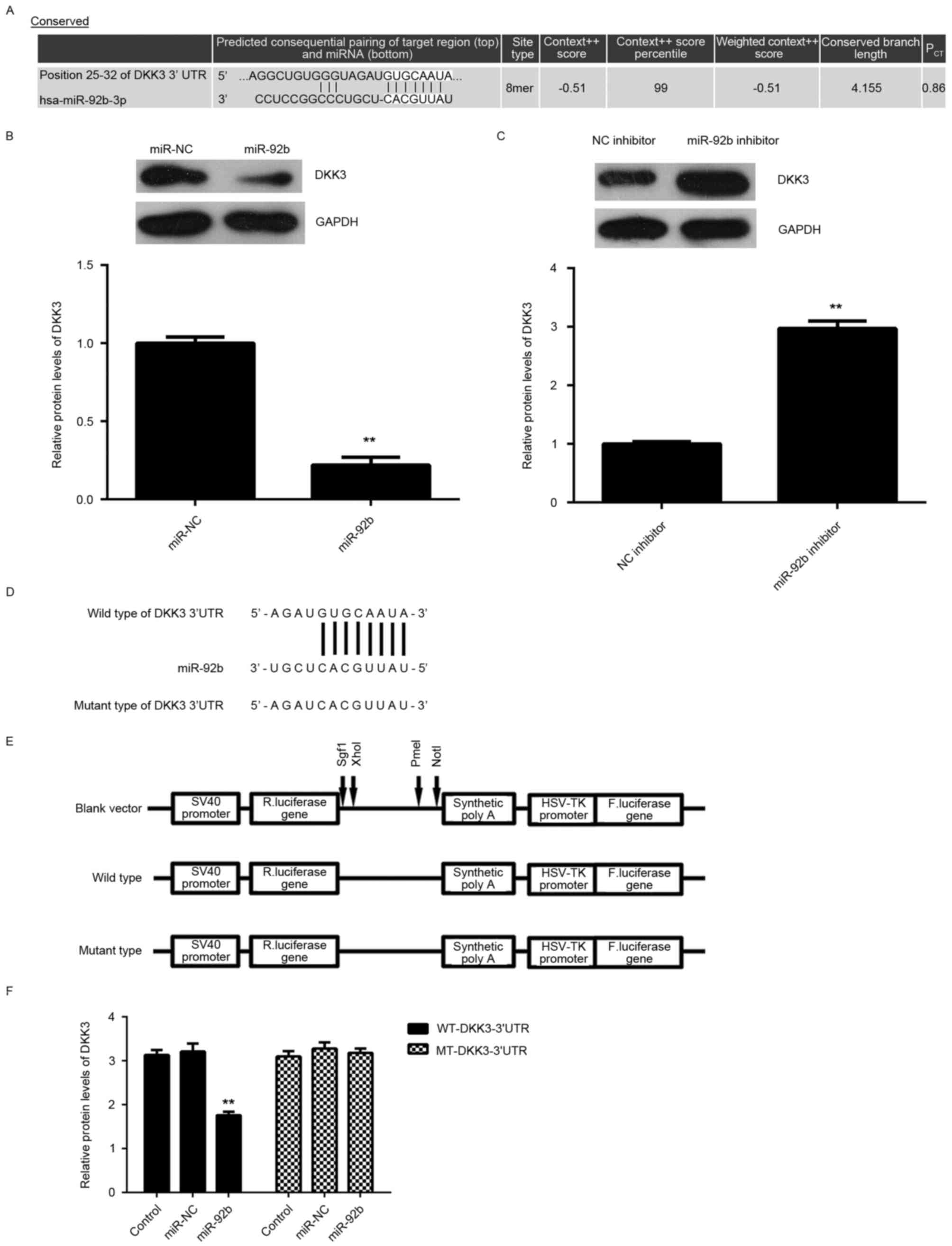
DKK3 is a target gene of miR-92b in
U2OS cells
The potential target of miR-29b in osteosarcoma
cells was investigated. Bioinformatics predication indicated that
DKK3 was a putative target of miR-92b (Fig. 3A). Furthermore, overexpression of
miR-92b significantly reduced DKK3 expression (P<0.01; Fig. 3B), whereas knockdown of miR-92b
significantly increased DKK3 expression in U2OS cells (P<0.01;
Fig. 3C), indicating that the
expression of DKK3 is negatively regulated by miR-92b.
To further investigate the association between
miR-92b and DKK3, WT-DKK3-3′UTR and MUT-DKK3-3′UTR luciferase
reporter plasmids were generated (Fig.
3D and E). Subsequently, U2OS cells were co-transfected with
WT-DKK3-3′UTR or MUT-DKK3-3′UTR luciferase reporter plasmid and
miR-92b mimic or miR-NC, respectively. Luciferase activity was
significantly decreased in U2OS cells co-transfected with miR-92b
mimic and WT-DKK3-3′UTR luciferase reporter plasmid compared with
the control group (P<0.01; Fig.
3F). However, luciferase activity was unchanged in cells
co-transfected with miR-92b mimic and MUT-DKK3-3′UTR luciferase
reporter plasmid compared with the control group (Fig. 3F). Taken together, the aforementioned
results demonstrate that DKK3 is a target gene of miR-92b in U2OS
cells.
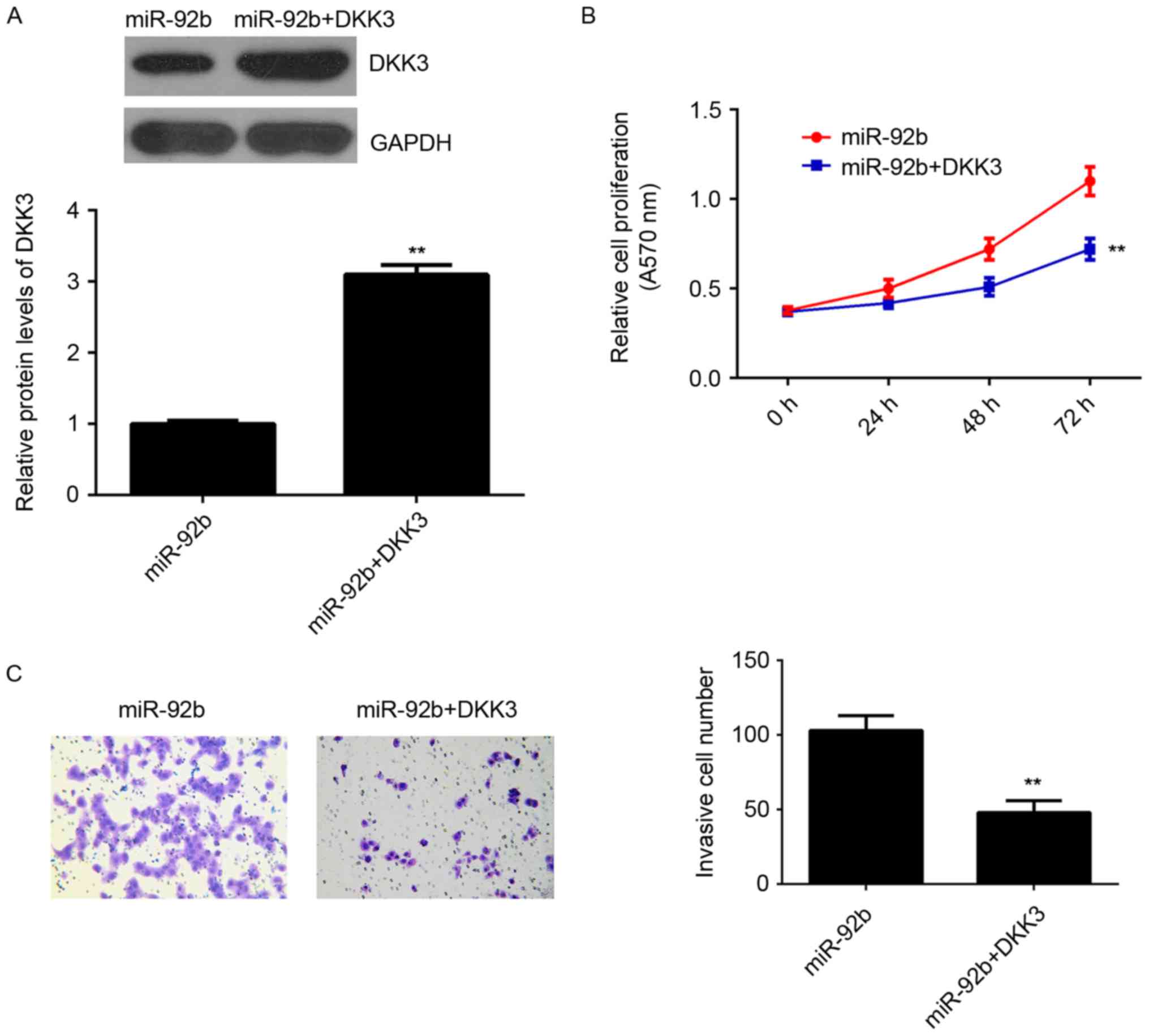
Restoration of DKK3 reverses the
increased proliferation and invasion of osteosarcoma cells induced
by miR-92b overexpression
It was speculated that DKK3 may be involved in the
miR-92b-induced proliferation and invasion of U2OS cells. To
clarify this, miR-92b-overexpressing osteosarcoma cells were
transfected with pcDNA3.1-DKK3 expression plasmid to restore DKK3
expression. Following transfection, DKK3 expression was
significantly higher in the miR-92b+DKK3 group compared with the
miR-92b group (P<0.01; Fig. 4A).
MTT and Transwell assays indicated that the proliferation and
invasion of U2OS cells was significantly decreased in the
miR-92b+DKK3 group compared with the miR-92b group (P<0.01;
Fig. 4B and C). This suggests that
restoration of DKK3 expression reverses the increased proliferation
and invasion of osteosarcoma cells induced by miR-92b
overexpression, suggesting that miR-92b stimulates the
proliferation and invasion of osteosarcoma cells, at least partly,
by targeting DKK3.
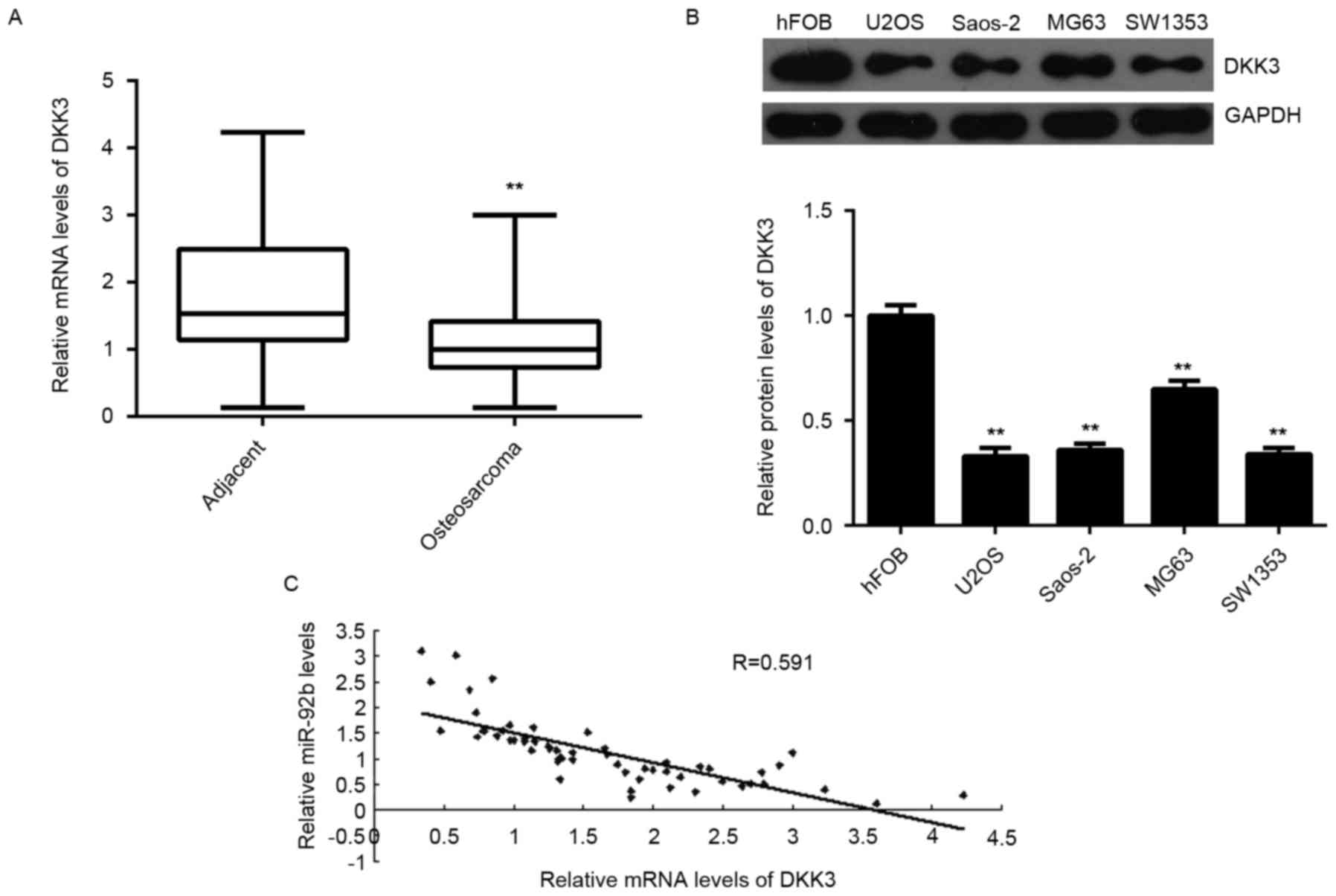
DKK3 expression is downregulated in
sarcoma and inversely correlated with miR-92b expression
DKK3 expression was measured in osteosarcoma tissues
and cell lines. Levels of DKK3 mRNA were significantly decreased in
osteosarcoma tissues compared with adjacent normal tissues
(P<0.01; Fig. 5A). DKK3 mRNA was
also significantly downregulated in the osteosarcoma cell lines
compared with normal osteoblast hFOB cells (P<0.01; Fig. 5B). Furthermore, an inverse
correlation was detected between miR-92b expression and DKK3 mRNA
levels in osteosarcoma tissues (P<0.01; Fig. 5C). These results suggest that the
decreased expression of DKK3 may be caused by the upregulation of
miR-92b in osteosarcoma.
Discussion
In the present study, the expression, clinical
significance and regulatory mechanism of miR-92b in osteosarcoma
were measured. The results of the current study demonstrated that
miR-92b was significantly upregulated in osteosarcoma tissues and
cell lines and that the increased expression of miR-92b was
associated with the malignant progression of osteosarcoma. It was
also indicated that miR-92b may promote the proliferation and
invasion of osteosarcoma cells by directly targeting DKK3. In
addition, it was observed that DKK3 was significantly downregulated
in osteosarcoma tissues and cell lines, and was inversely
correlated with miR-92b levels in osteosarcoma tissues.
The deregulation of miR-92b has been implicated in
several different types of human cancer and serves an oncogenic
role (21–23). For instance, miR-92b directly targets
PTEN, promotes cell growth and induces cisplatin chemosensitivity
in non-small cell lung cancer (NSCLC) cells (21). Inhibition of miR-92b suppresses NSCLC
cell growth and motility by targeting RECK (22). Additionally, miR-92b functions as a
potential oncogene in glioblastomas by targeting SMAD3 (23). However, the underlying regulatory
mechanism of miR-92b in osteosarcoma growth and metastasis remains
largely unclear. The results of the current study demonstrated that
miR-92b is upregulated in osteosarcoma tissues compared with
matched adjacent non-tumor tissues, and in osteosarcoma cell lines
compared with normal osteoblasts. These results are consistent with
those of another study by Zhou et al (19), which reported that miR-92b was
upregulated in osteosarcoma cell lines and tissues, and that its
upregulation was correlated with poor prognosis in osteosarcoma.
The current study determined that increased miR-92b expression was
significantly associated with advanced clinical stage and lung
metastasis, suggesting that upregulation of miR-92b may contribute
to the malignant progression of osteosarcoma. Furthermore, it was
demonstrated that miR-92b knockdown significantly inhibited U2OS
cell proliferation and invasion, whereas overexpression of miR-92b
enhanced these cellular events. Similarly, Zhou et al
(19) identified that overexpression
of miR-92b promotes osteosarcoma cell proliferation, migration and
invasion, which were abrogated following miR-92b knockdown. Taken
together, the results of the current study and those of previous
studies suggest that miR-92b may be used as a therapeutic target to
attenuate the growth and metastasis of osteosarcoma.
DKK3 belongs to a member of the dickkopf family and
contains two cysteine-rich regions (24). DKK3 interacts with and suppresses the
Wnt signaling pathway and participates in embryonic development as
well as tumorigenesis (25,26). It has been demonstrated that DKK3
expression is decreased in human cancer and DKK3 also acts as a
tumor suppressor (27,28). DKK3 is downregulated in uterine
cervical squamous cell carcinoma and this decreased expression is
associated with adverse clinical outcomes (27). Lee et al (28) determined that DKK3 expression was
downregulated in cervical cancer tissues and cell lines and
suppressed the colony formation and cell growth of cervical cancer
cells by inhibiting β-catenin signaling. In the present study, DKK3
was identified as a direct target gene of miR-92b in U2OS cells and
DKK3 was negatively regulated by miR-92b at the
post-transcriptional level. Furthermore, restoration of DKK3
expression significantly eliminated the promoting effects of
miR-92b on U2OS cell proliferation and invasion. These results
suggest that miR-92b promotes the proliferation and invasion of
osteosarcoma cells by inhibiting DKK3. This association between
miR-92b and DKK3 was also identified in glioma (29). Therefore, the results of the current
study expand understanding regarding the importance of the
miR-92b/DKK3 axis in human cancer.
The current study demonstrated that DKK3 expression
was significantly reduced in osteosarcoma tissues compared with
adjacent non-tumor tissues, and in osteosarcoma cell lines compared
with normal osteoblast cells. It has previously been demonstrated
that DKK3 functions as a tumor suppressor in osteosarcoma and
suppresses the invasion and motility of osteosarcoma cells by
inhibiting the Wnt-β-catenin pathway (30). Furthermore, an inverse correlation
between miR-92b and DKK3 expression in osteosarcoma tissue was
detected in the current study, suggesting that the decreased
expression of DKK3 may be due to the increased expression of
miR-92b in osteosarcoma tissues. The primary limitation of the
current study was a lack of information regarding the survival time
of patients. In the future, the role of the miR-92b/DKK3 axis in
vivo, as well as the molecular mechanism downstream of the
miR-92b/DKK3 axis should be studied further.
Taken together, the results of the current study
demonstrate that miR-92b expression is upregulated in osteosarcoma
and promotes the proliferation and invasion of osteosarcoma cells
by directly targeting DKK3. This suggests that miR-92b may be a
potential target for the treatment of osteosarcoma.
References
|
1
|
Maximov VV and Aqeilan RI: Genetic factors
conferring metastasis in osteosarcoma. Future Oncol. 12:1623–1644.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang J, Yu XH, Yan YG, Wang C and Wang
WJ: PI3K/Akt signaling in osteosarcoma. Clin Chim Acta.
444:182–192. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
John B, Enright AJ, Aravin A, Tuschl T,
Sander C and Marks DS: Human MicroRNA targets. PLoS Biol.
2:e3632004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mannavola F, Tucci M, Felici C, Stucci S
and Silvestris F: miRNAs in melanoma: A defined role in tumor
progression and metastasis. Expert Rev Clin Immunol. 12:79–89.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bostjancic E and Glavac D: Importance of
microRNAs in skin morphogenesis and diseases. Acta Dermatovenerol
Alp Pannonica Adriat. 17:95–102. 2008.PubMed/NCBI
|
|
11
|
Lee JW, Choi CH, Choi JJ, Park YA, Kim SJ,
Hwang SY, Kim WY, Kim TJ, Lee JH, Kim BG and Bae DS: Altered
MicroRNA expression in cervical carcinomas. Clin Cancer Res.
14:2535–2542. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Negrini M and Calin GA: Breast cancer
metastasis: A microRNA story. Breast Cancer Res. 10:2032008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thompson LD: Osteosarcoma. Ear Nose Throat
J. 92:288–290. 2013.PubMed/NCBI
|
|
14
|
Xu H, Mei Q, Xiong C and Zhao J:
Tumor-suppressing effects of miR-141 in human osteosarcoma. Cell
Biochem Biophys. 69:319–325. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bassampour SA, Abdi R, Bahador R, Shakeri
M, Torkaman A, Yahaghi E and Taheriazam A: RETRACTED ARTICLE:
Downregulation of miR-133b/miR-503 acts as efficient prognostic and
diagnostic factors in patients with osteosarcoma and these
predictor biomarkers are correlated with overall survival. Tumour
Biol. Aug 16–2015.(Epub ahead of print). PubMed/NCBI
|
|
16
|
Duan Z, Choy E, Harmon D, Liu X, Susa M,
Mankin H and Hornicek F: MicroRNA-199a-3p is downregulated in human
osteosarcoma and regulates cell proliferation and migration. Mol
Cancer Ther. 10:1337–1345. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Song H, Zhang Y, Liu N, Wan C, Zhang D,
Zhao S, Kong Y and Yuan L: miR-92b regulates glioma cells
proliferation, migration, invasion, and apoptosis via PTEN/Akt
signaling pathway. J Physiol Biochem. 72:201–211. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ma G, Jing C, Li L, Huang F, Ding F, Wang
B, Lin D, Luo A and Liu Z: MicroRNA-92b represses
invasion-metastasis cascade of esophageal squamous cell carcinoma.
Oncotarget. 7:20209–20222. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou Z, Wang Z, Wei H, Wu S, Wang X and
Xiao J: Promotion of tumour proliferation, migration and invasion
by miR-92b in targeting RECK in osteosarcoma. Clin Sci (Lond).
130:921–930. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Soltani S, Mokarian F and Panjehpour M:
The expression of CK-19 gene in circulating tumor cells of blood
samples of metastatic breast cancer women. Res Pharm Sci.
10:485–496. 2015.PubMed/NCBI
|
|
21
|
Li Y, Li L, Guan Y, Liu X, Meng Q and Guo
Q: MiR-92b regulates the cell growth, cisplatin chemosensitivity of
U2OS non small cell lung cancer cell line and target PTEN. Biochem
Biophys Res Commun. 440:604–610. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lei L, Huang Y and Gong W: Inhibition of
miR-92b suppresses nonsmall cell lung cancer cells growth and
motility by targeting RECK. Mol Cell Biochem. 387:171–176. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wu ZB, Cai L, Lin SJ, Lu JL, Yao Y and
Zhou LF: The miR-92b functions as a potential oncogene by targeting
on Smad3 in glioblastomas. Brain Res. 1529:16–25. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Niehrs C: Function and biological roles of
the Dickkopf family of Wnt modulators. Oncogene. 25:7469–7481.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fukusumi Y, Meier F, Götz S, Matheus F,
Irmler M, Beckervordersandforth R, Faus-Kessler T, Minina E, Rauser
B, Zhang J, et al: Dickkopf 3 promotes the differentiation of a
rostrolateral midbrain dopaminergic neuronal subset in vivo and
from pluripotent stem cells in vitro in the mouse. J Neurosci.
35:13385–13401. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Z, Lin L, Thomas DG, Nadal E, Chang
AC, Beer DG and Lin J: The role of Dickkopf-3 overexpression in
esophageal adenocarcinoma. J Thorac Cardiovasc Surg.
150:377–385.e2. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ryu SW, Kim JH, Kim MK, Lee YJ, Park JS,
Park HM, Kim DH, Lee SH and Lee EJ: Reduced expression of DKK3 is
associated with adverse clinical outcomes of uterine cervical
squamous cell carcinoma. Int J Gynecol Cancer. 23:134–140. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lee EJ, Jo M, Rho SB, Park K, Yoo YN, Park
J, Chae M, Zhang W and Lee JH: Dkk3, downregulated in cervical
cancer, functions as a negative regulator of beta-catenin. Int J
Cancer. 124:287–297. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Q, Shen K, Zhao Y, Ma C, Liu J and Ma
J: MiR-92b inhibitor promoted glioma cell apoptosis via targeting
DKK3 and blocking the Wnt/beta-catenin signaling pathway. J Transl
Med. 11:3022013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hoang BH, Kubo T, Healey JH, Yang R,
Nathan SS, Kolb EA, Mazza B, Meyers PA and Gorlick R: Dickkopf 3
inhibits invasion and motility of Saos-2 osteosarcoma cells by
modulating the Wnt-beta-catenin pathway. Cancer Res. 64:2734–2739.
2004. View Article : Google Scholar : PubMed/NCBI
|