Introduction
Diabetic cardiomyopathy (DCM) serves an essential
role in the progression of diabetes, and is one of the major causes
of morbidity and mortality in patients with diabetes. DCM
prevalence is ~12% in the population of patients with diabetes, and
causes heart failure and mortality (1). It has been reported that intracellular
lipid accumulation, altered cell signaling, high glucose-induced
generation of advanced glycation end products and reactive oxygen
species, and altered fuel use in the hearts of patients with
diabetes are considered as risk factors for DCM (2). Fibroblast growth factor 21 (FGF21) is a
secreted protein involved in the regulation of the glucolipid
metabolism, ketogenesis and insulin sensitivity (3,4). A
previous study has detected an increased level of FGF21 in the
plasma of DCM patients (5).
Studies have attempted to investigate the
association between FGF21 and cardiac function, and observed that
the heart was a target and also a source of FGF21, the heart
expresses and releases FGF21, which protects against cardiac
hypertrophy (6). Elevated FGF21
levels have been identified in patients with carotid
atherosclerosis, hypertension and coronary artery disease (7–9).
Furthermore, FGF21 has been observed to serve a role as an
antioxidant factor, preventing pro-oxidative pathways, which were
induced by inflammatory or hypertrophic conditions (10). FGF21 may also be involved in the
development and maintenance of atrial fibrosis in atrial
fibrillation accompanied with rheumatic heart disease (11). In Chinese patients, the serum FGF21
level is independently associated with the presence of acute
myocardial infarction. In addition, high FGF21 levels may be
associated with the incidence of re-infarction within 30 days of
the first myocardial infarction (12).
These aforementioned findings suggest that FGF21 may
be a critical metabolic hormone with an important function in the
cardiovascular system. However, the mechanism underlying the
function of this metabolic hormone in DCM remains unclear; thus,
investigating the role of FGF21 in DCM is of considerable interest.
The present study investigated the hypothesis that FGF21 may affect
the progression of DCM by regulating the lipid metabolism,
myocardial hypertrophy and fibrosis. The metabolic status, cardiac
structure and function of normal and DCM mice treated with or
without FGF21 siRNA for inhibition of FGF21 levels were
examined.
Materials and methods
Animal models
The animal experiments were conducted in accordance
with the guidelines of the National Institutes of Health of China
for the care and use of laboratory animals. Experimental protocols
were approved by the local Animal Ethics Committee affiliated to
the Shanghai Sixth People's Hospital (Shanghai, China). A total of
44 male C57/BL6J mice were provided by the Animal Laboratory of the
Shanghai Sixth People's Hospital. All mice were housed in housing
units with a 12 h light/dark cycle and these units were maintained
at 25°C with ~50% relative humidity. The mice received a normal
diet and water ad libitum.
The mice were randomly assigned into the normal
(n=6), DCM (n=6), normal + scrambled siRNA (n=6), DCM + scrambled
siRNA (n=6), normal + FGF21 siRNA (n=10) and DCM + FGF21 siRNA
(n=10) groups. Type 1 diabetes mellitus was induced to mice in the
DCM, DCM + scrambled siRNA and DCM + FGF21 siRNA groups by
intraperitoneal (IP) injection of streptozotocin (STZ;
Sigma-Aldrich; Merck, Darmstadt, Germany) at the dose of 150 mg/kg.
Saline was administered by IP injection to the mice in the normal,
normal + scrambled siRNA and normal + FGF21siRNA groups. After 72
h, the Type 1 diabetes mellitus mouse model was successfully
established, with mice exhibiting blood glucose levels of >16.9
mmol/l. At week 12 after the DCM model was established, FGF21 siRNA
and scrambled siRNA (Shanghai GenePharma Co., Ltd., Shanghai,
China) were dissolved in a diethyl pyrocarbonate and glucose
solution at 10 µg/µl, and administrated by injection through the
tail vein at a dose of 5 mg/kg in mice of normal + FGF21 siRNA, DCM
+ FGF21 siRNA, normal + scrambled siRNA, DCM + scrambled siRNA
groups. The siRNA sequences were as follows: FGF21 siRNA,
5′-CCAACAACCAGAUGGAACUTT-3′ (sense) and 5′-TTGGUUGUUGGUCUACCUUGA-3′
(antisense); scrambled siRNA, 5′-UUCUCCGAACGUGUCACGUTT-3′ (sense)
and 5′-ACGUAGCACGUUCGGAGAATT-3′ (antisense). In the siRNA absent
groups (the normal and DCM groups) saline was administrated to the
mice by intravenous injection through the tail vein. In mice of the
normal + FGF21 siRNA and the DCM + FGF21 siRNA groups, FGF21
expression was inhibited by FGF21 siRNA injection.
Transthoracic echocardiography (TTE) was performed 1
week after injection to evaluate the cardiac function. Blood
samples were collected and stored at 4°C prior to TTE; following
TTE the mice were sacrifice and their hearts and livers were
extracted. The tissues used for hematoxylin and eosin (H&E) and
Masson's staining, and biochemical tests were stored in liquid
nitrogen. Tissues used for transmission electron microscope (TEM)
analysis were fixed in 2.5% glutaraldehyde overnight at 4°C. The
heart and liver samples were used to examine the FGF21 expression
levels, cardiac morphologic and fibrotic changes, condition of
lipid accumulation and concentration of peroxisome
proliferator-activated receptor γ co-activator 1α (PGC-1α) and
cluster of differentiation (CD)36. Differences in the acquired
parameters among the groups were compared to evaluate the cardiac
function and structure, degree of myocardial hypertrophy and
fibrosis, and the cardiac metabolism state, as well as to deduce
the possible mechanism behind these differences. These results
would assist in clarifying the possible mechanism underlying the
effect of FGF21 on the progression of DCM.
Echocardiography
TTE was performed to evaluate the cardiac function
and structure using the VisualSonics Vevo 2100 high-resolution
imaging system (FUJIFILM VisualSonics, Inc., Toronto, ON, Canada).
Each mouse was placed in a supine position following anesthesia by
1.5% sodium pentobarbital (Tianjin Jinyao Amino Acid Co., Ltd.,
Tianjin, China) at the dose of 75 mg/kg (intraperitoneal
injection). A heating pad was used to warm the body. Subsequently,
two-dimensional TTE and M-mode TTE were applied to acquire the
parameters that indicate the cardiac structure and function,
including ejection fraction (EF), fractional shortening (FS), left
ventricular (LV) mass, interventricular septum during diastole
(IVSd), interventricular septum during systole (IVSs), LV internal
diameter during diastole (LVIDd), LV internal diameter during
systole (LVIDs), posterior wall thickness during diastole (PWTd)
and posterior wall thickness during systole (PWTs). These
indicators were measured three times and the mean value was used in
statistical analysis.
Plasma FGF21 level assessment
In order to observe the effectiveness of FGF21 siRNA
injection, the plasma FGF21 levels were measured. Briefly, 1 week
after siRNA injection, whole blood was collected from the mice and
centrifuged at 1,509 × g for 10 min at 4°C. The plasma was then
collected and the FGF21 concentration was measured by a FGF21
Quantikine ELISA kit (cat. no. MF2100; R&D systems, Inc.,
Minneapolis, MN, USA), according to the manufacturer's
protocol.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RT-qPCR was used to measure the expression levels of
cardiac and liver FGF21 mRNA. In addition, the levels of indicators
of myocardial hypertrophy and fibrosis were determined, including
atrial natriuretic factor (ANF), α-skeletal actin (α-SKA), collagen
type I (Col I), Col III and transforming growth factor-β (TGF-β).
Total RNA was extracted by TRIzol reagent (Takara Bio, Inc., Shiga,
Japan) according to the manufacturer's instructions. A total of 500
ng RNA and the PrimeScript II 1st Strand cDNA Synthesis Kit (cat.
no. 6210A; Takara Bio, Inc.) were used for cDNA synthesis.
PrimeScript™ RT Reagent Kit (Perfect Real Time; cat. no. RR037A;
Takara, Bio, Inc.) and a 7500 HT Fast Real-Time PCR System (Applied
Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA) were
used to perform a qPCR analysis. For the qPCR analysis, Universal
SYBR Green I (Bioteke Corporation, Beijing, China) was used. The
initial denaturation was 95°C for 30 sec, 40 cycles of denaturation
at 95°C for 5 sec, annealing at 60°C for 30 sec and elongation at
72°C for 30 sec, and a final elongation at 72°C for 10 min. Values
were normalized by levels of GAPDH mRNA and presented as an
expression fold change using the 2−ΔΔCq method (13). The primers were synthesized by
Shanghai GenePharma Co., Ltd. The primers used in qPCR are listed
in Table I.
 | Table I.Primer sequences for quantitative
polymerase chain reaction. |
Table I.
Primer sequences for quantitative
polymerase chain reaction.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| ANF |
GAGCAGACCGATGAAGCG |
AGTGGCAATGCGACCAAG |
| α-SKA |
ATCTCACGTTCAGCTGTGGTCA |
ACCACCGGCATCGTGTTGGAT |
| TGF-β |
TGCGCCTGCAGAGATTCAAG |
AGGTAACGCCAGGAATTGTTGCTA |
| Col I |
GACGCATGGCCAAGAAGACA |
GCACCAGGAGGACCAGGAAGT |
| Col III |
AAACTGGTGAAGGTGGCTATG |
TTTTCACCTCCAACTCCAATG |
| FGF21 |
GGTGCTGCCAAGGCTGTGGG |
CCAGGCGGCATGTCAGATCCAC |
| GAPDH |
AATGGATTTGGACGCATTGGT |
TTTGCACTGGTACGTGTTGAT |
H&E and Masson's trichrome
staining
H&E and Masson's trichrome staining were used to
compare the histology of the liver and myocardial tissue in
different groups. Briefly, subsequent to harvesting, the tissues
were fixed in 10% neutral-buffered paraformaldehyde, embedded in
paraffin and sliced into 4-µm-thick sections. Deparaffinization of
the section was then performed, followed by staining with H&E
to determine the morphology of the cardiomyocytes and liver
tissues, while Masson's trichrome staining was applied in order to
assess the degree of myocardial fibrosis. The staining was examined
under a light microscope. The cardiomyocyte areas were assessed by
ImageJ software (version 1.44; National Institutes of Health,
Bethesda, MD, USA).
Measurements of cardiac lipid droplets
under a TEM
Counting of lipid droplets was applied to evaluate
the level of cardiac metabolism. Briefly, following sectioning into
cubes of ~1 mm3 in size, the myocardial tissues were
fixed with 2.5% glutaraldehyde overnight at 4°C, then washed three
times with 0.1 mol/l phosphate-buffered saline (pH=7.4).
Subsequently, the samples were fixed in 1% osmium tetroxide for 2 h
at 4°C, washed three times by phosphate buffered solution,
dehydrated in a graded ethanol series and embedded in epoxy resin.
The cubes were then stained with uranyl acetate and lead citrate,
cut into ultrathin sections (80 nm) with a diamond knife and
collected on 300-mesh copper or nickel grids. Next, the samples
were examined under a TEM to count the cardiac lipid droplets.
Metabolic parameter assessment
The heart tissues were homogenized in the standard
diluted assay reagent (10 µl/mg tissue) and then centrifuged at
10,000 × g for 10 min at 37°C. The resultant supernatant was then
used to measure the triglyceride concentration with a Triglyceride
Colorimetric Assay kit (Cayman Chemicals, Ann Arbor, MI, USA).
Plasma triglyceride and cholesterol levels were also measured by
colorimetric methods using a triglyceride E-test and a cholesterol
E-test (Wako Pure Chemical Industries, Ltd., Japan),
respectively.
Western blot analysis
Western blot analysis was used to measure the
myocardial concentrations of PGC-1α and CD36. Briefly, total
protein was separated by 10% SDS-PAGE and then transferred to a
nitrocellulose membrane, which was blocked by 5% skimmed milk
powder solution for 1 h. Next, the protein membrane was incubated
overnight at 4°C with the primary antibodies, including anti-PGC-1α
(cat no. ab54481; 1:1,000), anti-CD36 (cat no. ab133625; 1;2,000)
and the internal antibody, anti-GAPDH (cat. no. ab181602; 1:10,000)
(all Abcam, Cambridge, MA, USA). Any unbound antibodies in the
membrane were washed using 0.1% Tween 20 in tris-buffered saline,
and then the membrane was incubated with horseradish
peroxidase-conjugated goat anti-rabbit antibodies (cat. no.
ab205718; 1:2,000; Abcam) for 1 h at room temperature.
Subsequently, the antigen-antibody complex was detected with an
enhanced chemiluminescence detection kit (EMD Millipore, Billerica,
MA, USA). The grey levels of blots were quantified by ImageJ
software (version 1.44).
Statistical analysis
SPSS statistical software (version 21; IBM Corp.,
Armonk, NY, USA) was used to conduct all the data analysis. The
data are expressed as the mean ± standard deviation in all groups,
and differences among the results were determined by one-way
analysis of variance and post hoc Turkey's test. P<0.05 was
considered as indication of a statistically significant
difference.
Results
Analyses of body weight and blood
glucose
The blood glucose levels of mice in the DCM, DCM +
scrambled siRNA and DCM + FGF21 siRNA groups increased to >16.9
mmol/l at 72 h after STZ injection (Table II). Significant differences
(P<0.05) were identified between the mice injected with STZ and
non-treated mice in the normal, normal + scrambled siRNA and normal
+ FGF21 siRNA groups, while no significant difference existed
between these three groups, indicating that the diabetes model was
successfully established. Prior to sacrifice, the body weights of
mice in the three DCM groups were significantly lower compared with
those in the three normal groups (P<0.05). Notably, the DCM +
FGF21 siRNA group demonstrated a markedly higher blood glucose
level (P<0.05) compared with the DCM and DCM + scrambled siRNA
groups, indicating that FGF21 inhibition promoted an increase in
the blood glucose level of DCM mice. However, no significant
difference was identified between the normal, normal + scrambled
siRNA and normal + FGF21 siRNA groups in terms of the body weight
and blood glucose (P>0.05), indicating that the regulating
effect of FGF21 only occurred in DCM mice.
 | Table II.Alterations in body weight and blood
glucose in mice. |
Table II.
Alterations in body weight and blood
glucose in mice.
|
| 72 h after STZ
injection | Prior to
sacrifice |
|---|
|
|
|
|
|---|
| Group | Body weight (g) | Blood glucose
(mmol/l) | Body weight (g) | Blood glucose
(mmol/l) |
|---|
| N | 23.55±1.21 | 8.4±1.68 | 29.4±1.59 | 9.75±1.84 |
| N + Scsi | 23.37±1.75 | 8.9±1.20 | 29.2±1.37 | 10.15±1.24 |
| N + FGF21si | 23.26±1.47 | 9.63±0.67 | 29.09±1.46 | 10.48±0.94 |
| DCM | 23.73±2.05 |
20.93±2.03a |
22±1.16a |
24.21±2.65a |
| DCM + Scsi | 22.98±1.12 | 21.67±1.98 | 21.83±1.2 | 23.9±2.18 |
| DCM +FGF21si | 23.11±1.85 |
21.84±2.11a |
21.54±0.96a |
29.79±4.48b |
FGF21 siRNA inhibits the FGF21
expression
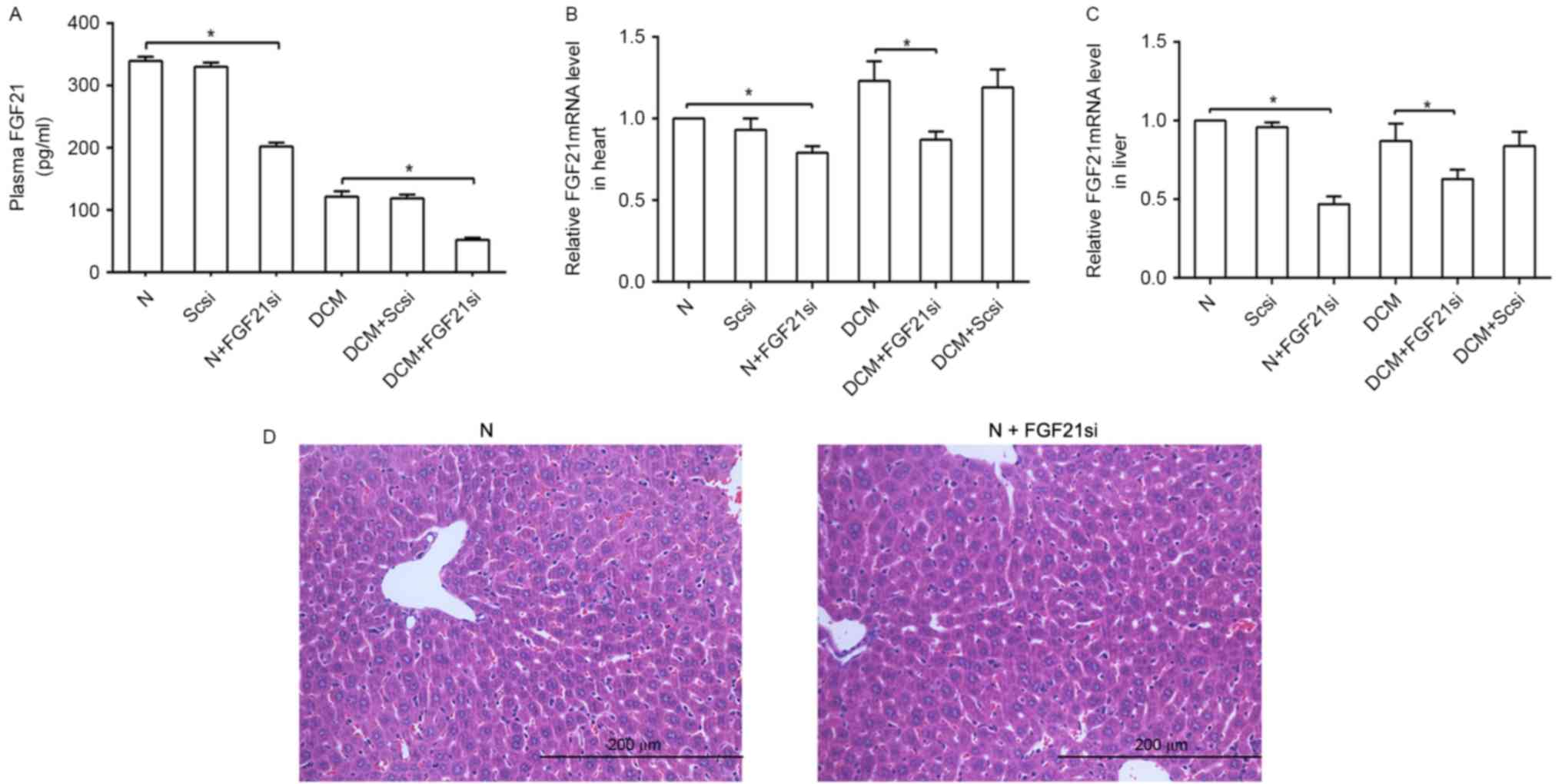
The plasma, cardiac and liver FGF21 expression
levels were evaluated to assess the effectiveness of FGF21 siRNA
(Fig. 1). The liver tissue in the
normal and FGF21 siRNA groups were compared to reveal if FGF21
siRNA itself had any significant effect on the liver. A significant
difference in FGF21 expression levels was observed between the
normal and normal + FGF21 siRNA groups, as well as between the DCM
and DCM + FGF21 siRNA group, indicated that FGF21 inhibition was
successfully performed. By contrast, a non-significant difference
was observed between the normal + scrambled siRNA and normal
groups, as well as between the DCM + scrambled siRNA and DCM
groups, suggesting that the scrambled siRNA was not able to
markedly affect the FGF21 expression and indicating the specific
effect of FGF21 siRNA on mice.
Inhibition of FGF21 aggravates the
diabetes-induced cardiac hypertrophy and dysfunction
The cardiac structure and function were evaluated by
TTE (Table III). Structural
indicators of DCM group were significantly increased in comparison
with those of the normal group, including the LV mass, LVIDd,
LVIDs, IVSd, IVSs, PWTd and PWTs, while functional indicators of
the DCM group, including the EF and FS, were significantly lower as
compared with those of the normal group. In addition, structural
indicators in the DCM + FGF21 siRNA group were significantly
enhanced compared with those in the DCM group, accompanied by
reduced functional indicators. However, no significant difference
was observed between the normal, normal + scrambled siRNA and
normal + FGF21 siRNA groups, as well as between the DCM + scrambled
siRNA and DCM groups in the IVSd, IVSs, PWTd, PWTs, EF and FS
values, indicating that FGF21 inhibition further thickened the
cardiac wall and deteriorated the function of the heart in DCM
mice.
 | Table III.Cardiac structure and function of
mice in each group. |
Table III.
Cardiac structure and function of
mice in each group.
| Parameter | Normal | Normal + Scsi | Normal +
FGF21si | DCM | DCM + Scsi | DCM + FGF21si |
|---|
| EF (%) | 92.44±1.43 | 90.30±1.26 | 89.03±1.97 |
82.20±3.21a | 81.6±2.47 |
71.45±3.62b |
| FS (%) | 65.67±2.74 | 64.20±2.3 | 62.71±2.15 |
50.98±2.67a | 49.89±2.31 |
40.99±2.27b |
| LV mass (mg) | 46.28±8.29 | 54.30±7.69 | 61.28±3.02 |
72.38±1.37a | 71.87±1.25 |
81.90±3.93b |
| LVIDd (mm) | 2.21±0.15 | 2.37±0.13 | 2.48±0.07 |
2.90±0.10a | 2.86±0.12 |
3.15±0.12b |
| LVIDs (mm) | 0.99±0.06 | 0.98±0.06 | 1.09±0.05 |
1.48±0.16a | 1.48±0.13 |
1.82±0.06b |
| IVSd (mm) | 0.75±0.07 | 0.748±0.04 | 0.84±0.03 |
0.93±0.03a | 0.937±0.02 |
1.09±0.08b |
| IVSs (mm) | 1.28±0.04 | 1.31±0.03 | 1.36±0.09 |
1.55±0.03a | 1.52±0.04 |
1.66±0.02b |
| PWTd (mm) | 0.80±0.11 | 0.83±0.07 | 0.87±0.02 |
0.94±0.04a | 0.96±0.03 |
1.07±0.046b |
| PWTs (mm) | 1.09±0.11 | 1.10±0.09 | 1.14±0.13 |
1.45±0.06a | 1.42±0.05 |
1.64±0.06b |
FGF21 inhibition promotes the
myocardial hypertrophy and cardiac fibrosis
The myocardial morphology (Fig. 2A) was observed under a light
microscope, the cardiomyocyte areas were assessed by ImageJ
software (Fig. 2B), while RT-qPCR
was used to measure the expression levels of the hypertrophic
markers ANF and α-SKA (Fig. 2C).
Furthermore, the cardiac fibrosis (Fig.
3A) was examined under the light microscope, and the levels of
fibrosis-associated factors (TGF-β, Col I and Col III; Fig. 3B) were evaluated by RT-qPCR.
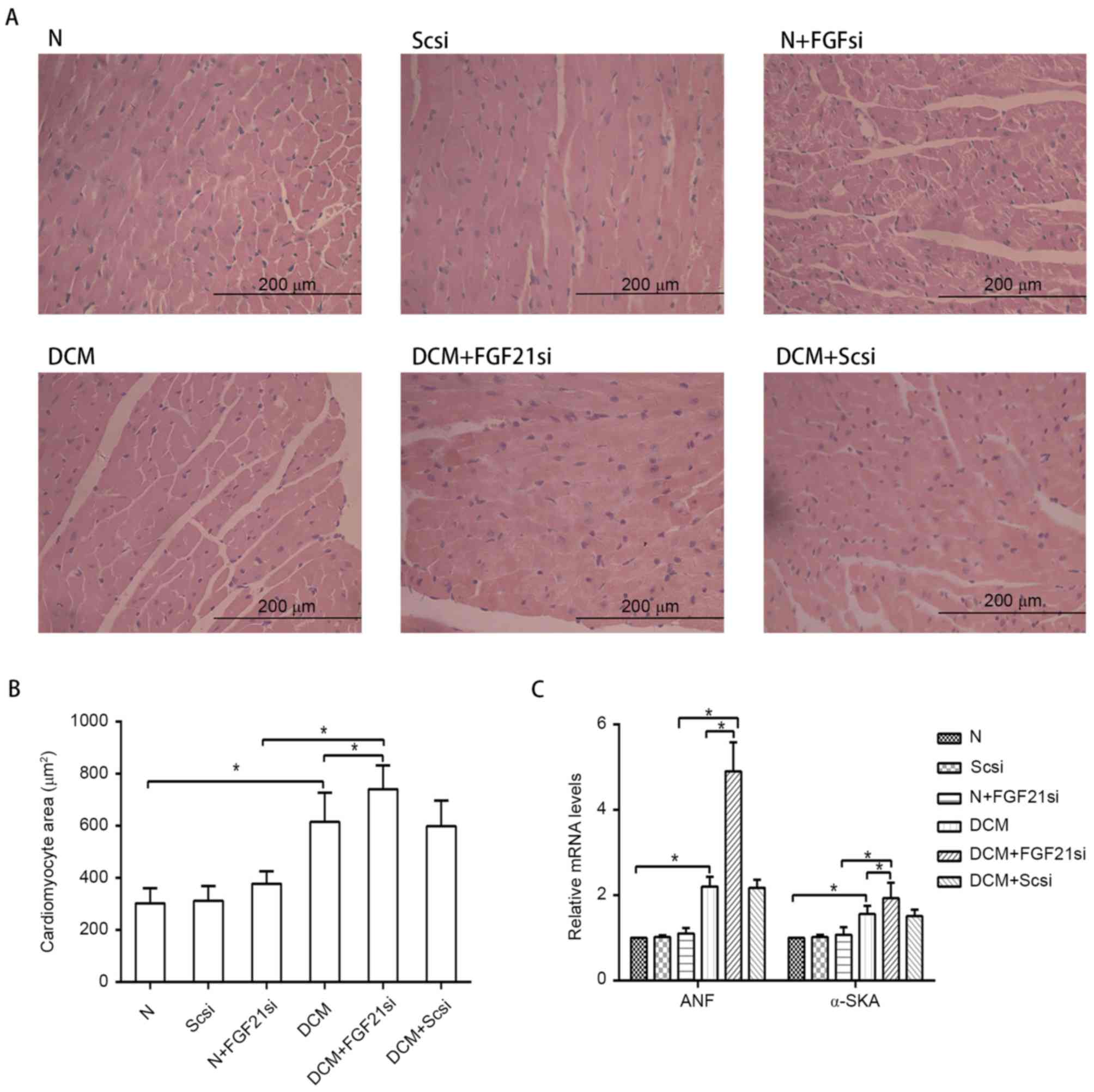 | Figure 2.Morphology of cardiomyocytes and
expression of hypertrophic markers. (A) Hematoxylin and eosin
staining of cardiac tissue (magnification, ×400; scale bar, 200
µm). (B) Quantification of cardiomyocyte cross-section area. (C)
Relative mRNA expression levels of hypertrophic markers, ANF and
α-SKA. *P<0.05. ANF, atrial natriuretic peptide; α-SKA,
α-skeletal actin; FGF21, fibroblast growth factor 21; N, normal;
DCM, diabetic cardiomyopathy; Scsi, scrambled siRNA; FGF21si, FGF21
siRNA. |
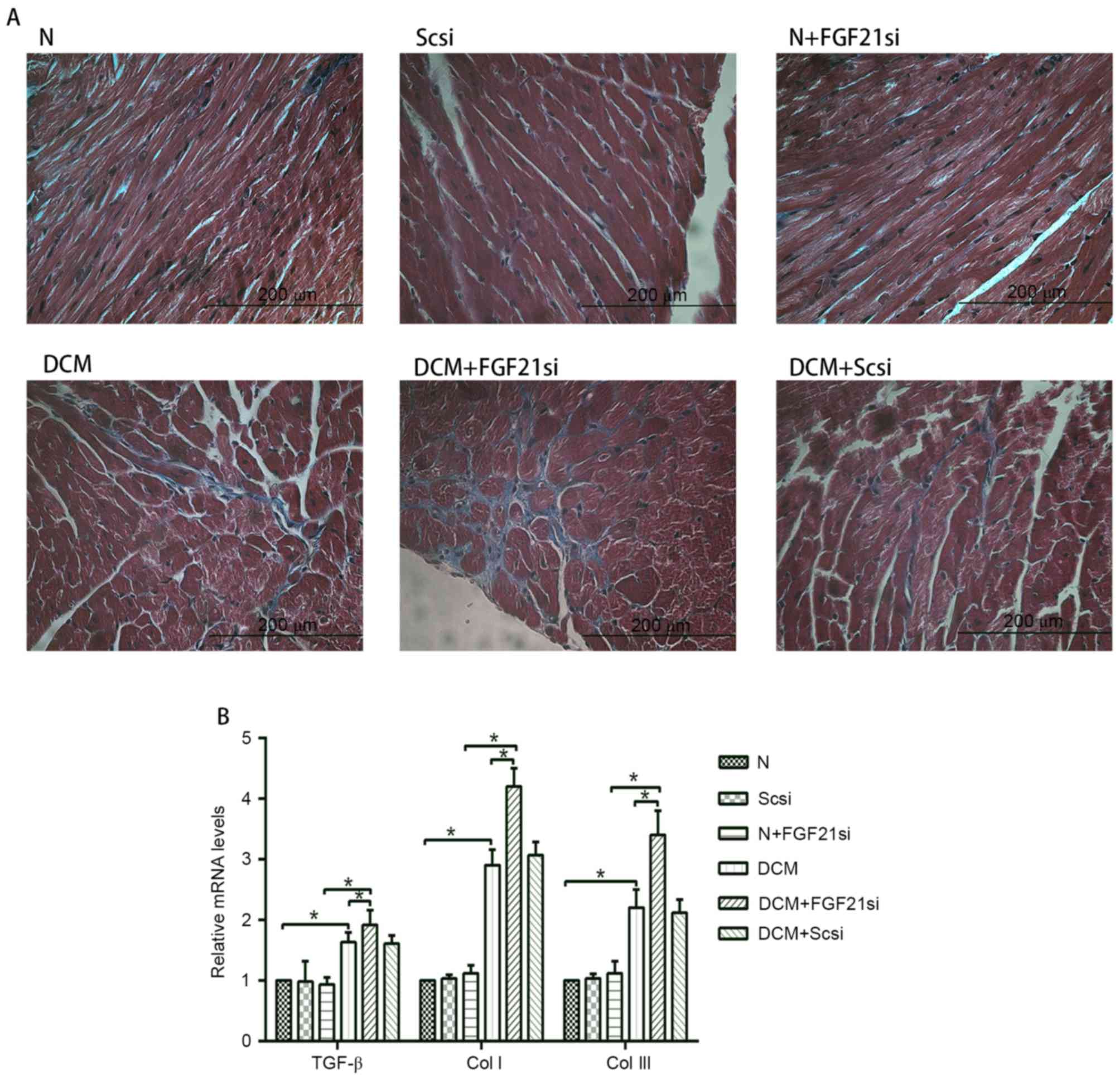 | Figure 3.Cardiac fibrosis and expression levels
of TGF-β, Col I and Col III. (A) Masson's trichrome staining was
performed in cardiac tissues (magnification, ×400; scale bar, 200
µm). (B) Relative mRNA expression levels of fibrosis-associated
factors, including TGF-β, Col I and Col III. *P<0.05. TGF-β,
transforming growth factor-β; Col, collagen; FGF21, fibroblast
growth factor 21; N, normal; DCM, diabetic cardiomyopathy; Scsi,
scrambled siRNA; FGF21si, FGF21 siRNA. |
Light microscopy examination indicated apparent
myocardial hypertrophy and cardiac fibrosis in the DCM and DCM +
scrambled siRNA groups, which deteriorated in the DCM + FGF21 siRNA
group. By contrast, no evident morphologic and fibrotic alterations
were observed in the scramble siRNA and normal + FGF21 siRNA groups
when compared with the normal group. The mRNA expression levels of
hypertrophic markers (α-SKA and ANF) and fibrosis-associated
factors (Col I, Col III and TGF-β) exhibited no significant
alterations among the normal, normal + scrambled siRNA or normal +
FGF21 siRNA groups, as well as between the DCM and DCM + scramble
siRNA groups. However, these indicators were significantly
increased in the DCM group when compared with the normal group.
Furthermore, mRNA levels of these factors in the DCM + FGF21 siRNA
group were markedly enhanced compared with the DCM or the normal +
FGF21 siRNA groups. These findings indicated that FGF21 inhibition
by siRNA injection aggravated the myocardial hypertrophy and
cardiac fibrosis in DCM mice.
FGF21 inhibition elevates lipid
accumulation
The cardiac lipid accumulation status was detected
by counting the lipid droplets under a TEM (Fig. 4A and B) and assessing the
triglyceride content in heart tissue (Fig. 4C). Additionally, the plasma
triglyceride and cholesterol levels were assessed (Fig. 4D and E). The number of lipid drops in
the heart tissue of the DCM group was evidently increased in
comparison with the normal group, although it was lower when
compared with the DCM + FGF21 siRNA group (both P<0.05).
Furthermore, the cardiac triglyceride, plasma triglyceride and
plasma cholesterol concentrations presented no significant
differences among the normal, normal + scrambled siRNA and normal +
FGF21 siRNA groups, as well as between the DCM + scrambled siRNA
and DCM groups. By contrast, these concentrations were
significantly increased in the DCM group compared with the normal
group, as well as in the DCM + FGF21 siRNA group compared with the
DCM and normal groups. All these observations suggested that FGF21
inhibition promoted lipid accumulation in DCM mice.
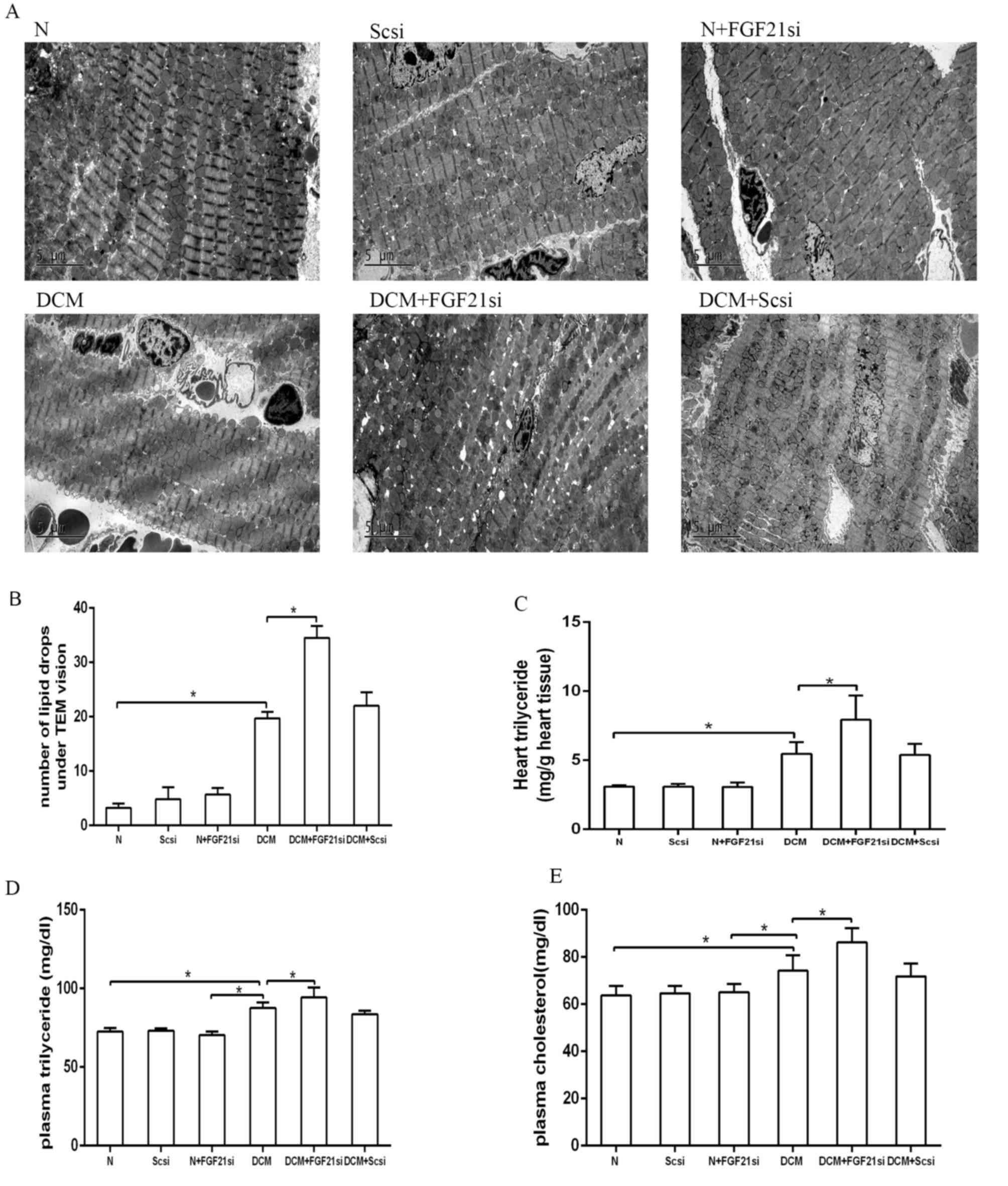 | Figure 4.Quantification of lipid droplets, as
well as heart triglyceride, plasma triglyceride and cholesterol
concentrations in the different groups. (A) Lipid droplets observed
by examination under a transmission electron microscope.
Magnification, ×4,200. (B) Quantitative analysis of lipid droplets.
Analysis of (C) heart triglyceride, (D) plasma triglyceride and (E)
plasma cholesterol levels. *P<0.05. FGF21, fibroblast growth
factor 21; N, normal; DCM, diabetic cardiomyopathy; Scsi, scrambled
siRNA; FGF21si, FGF21 siRNA. |
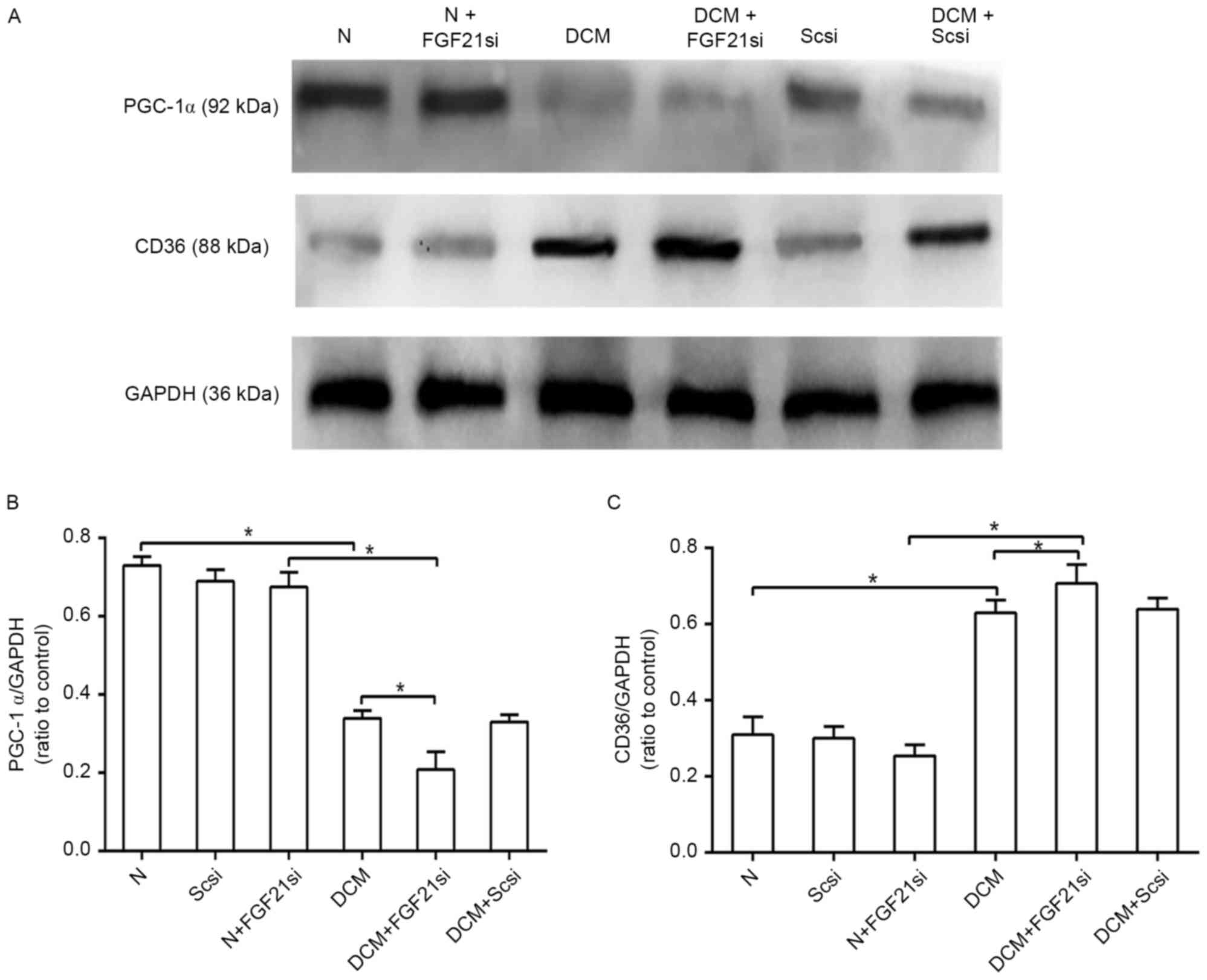
FGF21 inhibition affects the PGC-1α
and CD36 expression levels
CD36 and PGC-1α are two factors associated with
lipid metabolism. As CD36 is a lipid transport protein and serves a
role in mediating the cardiac fatty acid transportation and
utilization (14), increased
expression of this factor in the heart can lead to cardiac lipid
accumulation (15). In addition,
PGC-1α is critical for regulating fatty acid β-oxidation and
mediating the effect of FGF21 on lipid metabolism (16). Therefore, in the present study, the
concentrations of PGC1-α and CD36 were used to estimate the status
of the cardiac lipid metabolism. The protein expression levels of
PGC-1α and CD36 were detected by western blot analysis (Fig. 5A). PGC-1α expression in the DCM group
was inhibited as compared with that of the normal group, and its
expression was further decreased in the DCM + FGF21 siRNA group
when compared with the DCM and normal + FGF21 siRNA groups
(P<0.05). No significant difference was, however, identified
between the three normal groups (Fig.
5B). In contrast to the PGC-1α level, the protein expression of
CD36 in the DCM + FGF21 siRNA group was markedly upregulated when
compared with the DCM and normal + FGF21 siRNA groups, while the
expression level in the DCM group was also significantly increased
in comparison with that of the normal group, while no significant
difference was identified between the normal and normal + FGF21
siRNA group (Fig. 5C). All these
findings indicated that absence of FGF21 decreased the expression
of PGC-1α and promoted the expression of CD36 in the DCM mice.
Discussion
DCM was initially defined by Rubler et al
(16), reporting the cases of four
diabetic patients with congestive heart failure and normal coronary
arteries in 1972. DCM is a common condition in the general
population, with a prevalence of 1.1% (17) characterized with cardiac structural
and functional alterations, including LV hypertrophy, as well as
diastolic and systolic dysfunction (18). At the later stages, systolic
dysfunction characterized by the inability of LV to pump sufficient
volume of blood emerges in DCM patients (18).
It have been reported that hyperglycemia and
hyperlipidemia are major contributors to DCM, while FGF21 exhibited
anti-hyperglycemic and anti-hyperlipidemic abilities in diabetic
rodent and monkey models (19).
FGF21 participates in regulating the lipid homoeostasis in adipose
tissue (20) and in the liver
(3). In addition, FGF21 was also
reported to induce renal protection, partially by lessening the
renal lipid accumulation through enhancing fatty acid oxidation and
lipolysis (21). Therefore, in the
current study, it was hypothesized that FGF21 inhibition may
further elevate the levels of plasma glucose, and plasma and
cardiac lipid accumulation, leading to the accelerated and
aggravated development of DCM.
In the present study, FGF21 was effectively
inhibited by FGF21 siRNA, which was confirmed by the significantly
decreased FGF21 expression in normal and DCM mice treated with
FGF21 siRNA. It was observed that the plasma and liver FGF21
expression levels of DCM mice were markedly reduced compared with
those in normal mice, which may be caused by tissue damage at the
late stages of DCM, resulting in decreased secretion of FGF21. As
there was no significant change in the liver between the normal and
normal + FGF21 siRNA groups, it was suggested that FGF21 siRNA
alone did not cause liver dysfunction; thus it is concluded that
the observations in these experiments were not a result of liver
dysfunction.
Lipid droplets store excess lipids, particularly
triglycerides, when the lipid concentration surpasses the required
amounts for cellular structures and ATP generation. A small number
of droplets can be detected in the normal heart under basic
conditions (22); however,
significantly increased lipid droplets imply that the lipid
metabolism is unbalanced. In the present study, a greater number of
cardiac lipid droplets was identified in the hearts of DCM mice
than in normal mice, while increased droplets were also observed in
the DCM + FGF21 siRNA group compared with the DCM group. These data
suggested that the lipid metabolism in DCM mice was unbalanced and
that the unbalanced status was worse in mice of the DCM + FGF21
siRNA group. In accordance with these findings on lipid metabolism,
increased levels of cardiac triglyceride, plasma triglyceride and
cholesterol levels were detected in DCM mice compared with normal
mice; subsequent to inhibition of FGF21 expression by FGF21 siRNA,
these levels further increased. The aforementioned findings
suggested that a lack of FGF21 in DCM mice caused enhanced lipid
accumulation.
Increased IVSd, IVSs, PWTd, PWTs and LV mass values,
along with the H&E staining of heart tissues, demonstrated that
cardiac hypertrophy existed in DCM mice in the present study.
Furthermore, Masson's trichrome staining, and decreased EF and FS
revealed that cardiac fibrosis and dysfunction were also present in
DCM mice. Long-term cardiac remodeling is known to lead to
aggravated ventricular hypertrophy and cardiac fibrosis, along with
contractile dysfunction (12,23).
Deteriorated cardiac hypertrophy and fibrosis resulted in further
inhibition of EF and FS in mice of the DCM + FGF21 siRNA group,
indicating that these mice suffered more severe cardiac remodeling,
which was not observed in the normal, scrambled siRNA and normal +
FGF21 siRNA groups. According to the aforementioned analyses, mice
in the DCM + FGF21 siRNA group, followed by the DCM mice,
accumulated the highest level of triglycerides and cholesterol,
suffering the highest degree of cardiac hypertrophy and fibrosis,
as well as the lowest cardiac function. These results are in
accordance with earlier studies reporting that excess lipid
accumulation in cardiomyocytes is responsible for diabetic cardiac
dysfunction (19), and that
triglyceride concentration is associated with LV hypertrophy in
patients with diabetes (24),
suggesting the association of the lipid accumulation observed in
the present study with cardiac remodeling in DCM.
CD36, as a lipid transport protein, has been
reported to mediate cardiac fatty acid transport and utilization
(14). It was initially described as
a component of the platelet membrane and later identified as a
receptor for thrombospondin-1 and a class B scavenger receptor B,
which is associated with the binding of modified and native
lipoproteins and anionic phospholipids (25–27). In
murine diabetic models, as well as in mice fed a high-fat diet,
increased CD36 expression in the heart mediated excess fatty acid
uptake, which led to cardiac lipid accumulation, and this
expression was associated with the parenchymal cell lipid
metabolism (15). CD36 deletion was
also reported to improve the heart function in aged mice that were
fed with a diet enriched in medium chain fatty acids (28). In the present study, CD36 expression
was enhanced in the DCM group in comparison with the normal group,
and even higher expression was observed in the DCM + FGF21 siRNA
group compared with the DCM group. Considering that more lipids
were accumulated in DCM mice compared with normal mice and in mice
of the DCM + FGF21 siRNA group compared with DCM mice, it is
concluded that FGF21 inhibition regulated the CD36 upregulation,
resulting in increased lipid concentration and partly contributing
to the significantly elevated lipid accumulation.
PGC-1α is a transcriptional coactivator protein and
its expression can be induced by the nutritional status and
stimuli, such as cold and exercise (29–32).
PGC-1α serves a critical role in regulating the fatty acid
β-oxidation and mediating the effect of FGF21 on lipid metabolism
(33). In the present study, PGC-1α
expressed in DCM mice was downregulated, accompanied by increased
lipid accumulation. Its expression in mice of the DCM + FGF21 siRNA
group was further decreased, along with the significant increase in
accumulated lipids. These findings demonstrated that FGF21
regulated PGC-1α expression, contributing to lipid accumulation.
Thus, the current study revealed that FGF21 inhibition induced
PGC-1α expression downregulation in DCM mice, contributing to the
significantly elevated lipid concentration along with the CD36
expression increase.
In conclusion, the present study observed that FGF21
inhibition caused more significant hyperglycemia and hyperlipidemia
in DCM mice. Furthermore, FGF21 inhibition upregulated CD36
expression and downregulated PGC-1α expression, causing excess
lipid uptake and leading to increased lipid accumulation, which
promoted cardiac remodeling involving cardiac hypertrophy, cardiac
fibrosis and cardiac dysfunction, further accelerating the
development of DCM. Therefore, it is suggested that FGF21 may be a
potential tool in the development of medical strategies for the
prevention of DCM.
References
|
1
|
Lorenzo-Almorós A, Tuñón J, Orejas M,
Cortés M, Egido J and Lorenzo Ó: Diagnostic approaches for diabetic
cardiomyopathy. Cardiovasc Diabetol. 16:282017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang J, Song Y, Wang Q, Kralik PM and
Epstein PN: Causes and characteristics of diabetic cardiomyopathy.
Rev Diabet Stud. 3:108–117. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Badman MK, Pissios P, Kennedy AR, Koukos
G, Flier JS and Maratos-Flier E: Hepatic fibroblast growth factor
21 is regulated by PPARalpha and is a key mediator of hepatic lipid
metabolism in ketotic states. Cell Metab. 5:426–437. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Galman C, Lundåsen T, Kharitonenkov A,
Bina HA, Eriksson M, Hafström I, Dahlin M, Amark P, Angelin B and
Rudling M: The circulating metabolic regulator FGF21 is induced by
prolonged fasting and PPARalpha activation in man. Cell Metab.
8:169–174. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheng P, Zhang F, Yu L, Lin X, He L, Li X,
Lu X, Yan X, Tan Y and Zhang C: Physiological and pharmacological
roles of FGF21 in cardiovascular diseases. J Diabetes Res.
2016:15402672016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Planavila A, Redondo I, Hondares E,
Vinciguerra M, Munts C, Iglesias R, Gabrielli LA, Sitges M, Giralt
M, van Bilsen M and Villarroya F: Fibroblast growth factor 21
protects against cardiac hypertrophy in mice. Nat Commun.
4:20192013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chow WS, Xu A, Woo YC, Tso AW, Cheung SC,
Fong CH, Tse HF, Chau MT, Cheung BM and Lam KS: Serum fibroblast
growth factor-21 levels are associated with carotid atherosclerosis
independent of established cardiovascular risk factors.
Arterioscler Thromb Vasc Biol. 33:2454–2459. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Semba RD, Crasto C, Strait J, Sun K,
Schaumberg DA and Ferrucci L: Elevated serum fibroblast growth
factor 21 is associated with hypertension in community-dwelling
adults. J Hum Hypertens. 27:397–399. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin Z, Wu Z, Yin X, Liu Y, Yan X, Lin S,
Xiao J, Wang X, Feng W and Li X: Serum levels of FGF-21 are
increased in coronary heart disease patients and are independently
associated with adverse lipid profile. PLoS One. 5:e155342010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Planavila A, Redondo-Angulo I, Ribas F,
Garrabou G, Casademont J, Giralt M and Villarroya F: Fibroblast
growth factor 21 protects the heart from oxidative stress.
Cardiovasc Res. 106:19–31. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang R, Yi X, Li X and Jiang X: Fibroblast
growth factor-21 is positively associated with atrial fibrosis in
atrial fibrillation patients with rheumatic heart disease. Int J
Clin Exp Pathol. 8:14901–14908. 2015.PubMed/NCBI
|
|
12
|
Zhang W, Chu S, Ding W and Wang F: Serum
level of fibroblast growth factor 21 is independently associated
with acute myocardial infarction. PLoS One. 10:e01297912015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koonen DP, Glatz JF, Bonen A and Luiken
JJ: Long-chain fatty acid uptake and FAT/CD36 translocation in
heart and skeletal muscle. Biochim Biophys Acta. 1736:163–180.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Greenwalt DE, Scheck SH and
Rhinehart-Jones T: Heart CD36 expression is increased in murine
models of diabetes and in mice fed a high fat diet. J Clin Invest.
96:1382–1388. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rubler S, Dlugash J, Yuceoglu YZ, Kumral
T, Branwood AW and Grishman A: New type of cardiomyopathy
associated with diabetic glomerulosclerosis. Am J Cardiol.
30:595–602. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Dandamudi S, Slusser J, Mahoney DW,
Redfield MM, Rodeheffer RJ and Chen HH: The prevalence of diabetic
cardiomyopathy: A population-based study in Olmsted County,
Minnesota. J Card Fail. 20:304–309. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yilmaz S, Canpolat U, Aydogdu S and Abboud
HE: Diabetic cardiomyopathy; summary of 41 years. Korean Circ J.
45:266–272. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Boudina S and Abel ED: Diabetic
cardiomyopathy revisited. Circulation. 115:3213–3223. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Muise ES, Souza S, Chi A, Tan Y, Zhao X,
Liu F, Dallas-Yang Q, Wu M, Sarr T, Zhu L, et al: Downstream
signaling pathways in mouse adipose tissues following acute in vivo
administration of fibroblast growth factor 21. PLoS One.
8:e730112013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang C, Shao M, Yang H, Chen L, Yu L,
Cong W, Tian H, Zhang F, Cheng P, Jin L, et al: Attenuation of
hyperlipidemia- and diabetes-induced early-stage apoptosis and
late-stage renal dysfunction via administration of fibroblast
growth factor-21 is associated with suppression of renal
inflammation. PLoS One. 8:e822752013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Goldberg IJ, Trent CM and Schulze PC:
Lipid metabolism and toxicity in the heart. Cell Metab. 15:805–812.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Neely JR, Rovetto MJ and Oram JF:
Myocardial utilization of carbohydrate and lipids. Prog Cardiovasc
Dis. 15:289–329. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Herrero P, Peterson LR, McGill JB, Matthew
S, Lesniak D, Dence C and Gropler RJ: Increased myocardial fatty
acid metabolism in patients with type 1 diabetes mellitus. J Am
Coll Cardiol. 47:598–604. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Asch AS, Barnwell J, Silverstein RL and
Nachman RL: Isolation of the thrombospondin membrane receptor. J
Clin Invest. 79:1054–1061. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Silverstein RL, Asch AS and Nachman RL:
Glycoprotein IV mediates thrombospondin-dependent platelet-monocyte
and platelet-U937 cell adhesion. J Clin Invest. 84:546–552. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Endemann G, Stanton LW, Madden KS, Bryant
CM, White RT and Protter AA: CD36 is a receptor for oxidized low
density lipoprotein. J Biol Chem. 268:11811–11816. 1993.PubMed/NCBI
|
|
28
|
Koonen DP, Febbraio M, Bonnet S, Nagendran
J, Young ME, Michelakis ED and Dyck JR: CD36 expression contributes
to age-induced cardiomyopathy in mice. Circulation. 116:2139–2147.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Puigserver P, Rhee J, Donovan J, Walkey
CJ, Yoon JC, Oriente F, Kitamura Y, Altomonte J, Dong H, Accili D
and Spiegelman BM: Insulin-regulated hepatic gluconeogenesis
through FOXO1-PGC-1alpha interaction. Nature. 423:550–555. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lin J, Handschin C and Spiegelman BM:
Metabolic control through the PGC-1 family of transcription
coactivators. Cell Metab. 1:361–370. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Finck BN and Kelly DP: PGC-1 coactivators:
Inducible regulators of energy metabolism in health and disease. J
Clin Invest. 116:615–622. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Handschin C and Spiegelman BM: Peroxisome
proliferator-activated receptor gamma coactivator 1 coactivators,
energy homeostasis, and metabolism. Endocr Rev. 27:728–735. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Potthoff MJ, Inagaki T, Satapati S, Ding
X, He T, Goetz R, Mohammadi M, Finck BN, Mangelsdorf DJ, Kliewer SA
and Burgess SC: FGF21 induces PGC-1alpha and regulates carbohydrate
and fatty acid metabolism during the adaptive starvation response.
Proc Natl Acad Sci USA. 106:pp. 10853–10858. 2009; View Article : Google Scholar : PubMed/NCBI
|