Introduction
Gastric cancer is one of the most common human
epithelial malignancies and remains the second leading cause of
cancer-associated mortality for clinical cancer (1). Clinical statistics and investigations
have shown that >70% of new cases of gastric cancer-associated
deaths occur in developing countries (2,3). Gastric
cancer exhibits higher morbidity and mortality rates than other
carcinoma derived from the digestive system as gastric cancer is
more invasive (4,5). A previous study has indicated that the
five-year survival rate for gastric cancer is <80% (6).
Currently, the majority of clinical patients with
gastric cancer are categorized as having an advanced stage once
diagnosed. In addition, although reports have shown that
apoptosis-resistance of gastric cancer is inevitable in the
development of cancer progression, the apoptotic resistance of
gastric cancer cells in patients with gastric cancer has been
indicated as the most important hurdle to overcome in clinical
treatment (7,8). Furthermore, apoptotic resistance has
become the greatest challenge in cancer therapy due to fierce
resistance of tumor cells though various types of molecules
mechanism (9–11). Although several comprehensive
therapies exist that induce apoptosis, which is the most important
component of treatment for patients with gastric cancer, the
survival rate remains low (12).
These previous reports focused on the efficacy of targeted
molecular therapies, and the early diagnosis of gastric cancer is
often overlooked.
In recent years, contrast-enhanced ultrasound,
computed tomography, fl tomography, ont-positron emission, and
tomography (FDG-PET) has been widely used in the diagnosis of human
cancer (13). Although many
advantages of contrast-enhanced ultrasound have been presented, its
relatively reduced resolution compared with CT means that it cannot
confirm the final diagnosis for patients with suspected gastric
cancer (14). However, nanoscale
microbubbles have been used to improve the resolution of
ultrasound, as they resonate when exposed to ultrasound waves
(14,15). In addition, computerized tomography
and chip technology are the most common methods used to diagnose
patients with suspected cancer (16). However, the efficacy of single
computerized tomography is limited to diagnosing patients with
early phase tumors. Furthermore, contrast agent combined with CT
for the analysis of tumor biology has been studied and achieved
adequate efficacy (17). Therefore,
we hypothesized that specific-targeted nanoscale microbubbles may
contribute to the efficacy and resolution of CT in the diagnosis of
patients with suspected gastric cancer.
In the present study, CECT combined with targeting
nanoscale microbubble contrast agent (CECT-TNCA) was introduced to
detect the early stage of patients with suspected gastric cancer.
Notably, CECT in conjunction with ultrasound contrast further
expanded its application in the field of primary diagnosis and
confirmed diagnosis (18). This
clinical analysis demonstrated the potential application of
CECT-TNCA for imaging modality and sensitivity improvements in the
diagnosis of gastric cancer. Our outcomes indicated many advantages
of CECT-TNCA in both early diagnosis and final confirmation of
suspected cases when compared to single CT detection.
Materials and methods
Ethics statement
The design of this clinical study was carried out in
strict accordance with the approval and recommendations in the
Guide for the Care and Use of clinical study of Xianning Central
Hospital (XNCH: 20091108A4). All surgery and euthanasia protocols
were standardized. All patients provided written informed
consent.
Patients
A total of 484 patients with suspected gastric
cancer aged 14.8–65.2 years were recruited for this prospective
analysis, in which the follow-up period was 60 months. The number
of male (222) and female (264) patients was approximately equal.
Furthermore, 236 healthy subjects (male, 124; female, 112) aged
24.0–62.6 years were recruited. Biochemical parameters of patients
with suspected NSCLC and healthy subjects recruited between May
2012 and June 2015 were eligible for further analysis. All patients
were subjected to scanning for the detection of early-stage gastric
cancer by CECT and CECT-TNCA. All healthy subjects had no cancer
history or gastric diseases. Patients with cancer history were
excluded in the present study.
Nanoparticles contrast agent
A novel liposome-encapsulated nanoparticles contrast
agent containing multiple targets was introduced for diagnosing
patients with early-stage gastric cancer. Platelet-derived growth
factor receptor-β (PDGFR-β), Ret, and Kit bound with the
nanoparticles of superparamagnetic iron oxide particles via
covalent bonds described in a previous study (19). Nanoparticles contrast agent and
Optison (GE Healthcare, Chicago, IL, USA) were orally administered
to ensure that they covered each corner of the stomach prior to
CECT and CECT-TNCA (30 min). Following administration with
CECT-TNCA, the TNCA was distributed in the stomach. Microbubbles
contained targeting nanoparticles contrast agent with the capacity
to target travelling tumor cells, which acted as an accurate tracer
for tumor cells (20). TNCA was
located in the lesion following administration with CECT-TNCA.
After 30 min, the TNCA was visualized via CECT. No side effects
were observed in patients exposed to TNCA.
Scan protocol
A CECT diagnosis system was used to analyze CECT
clinical trials using preprogrammed settings. Preprogrammed
settings were optimized to achieve optimal image formation. CECT
was performed on the stomachs of all patients according to
manufacturers instructions (Philips Medical Systems, Inc., Bothell,
WA, USA). Details of the principles and settings of
contrast-enhanced ultrasound were described in a previous study
(21). In addition, CECT-TNCA
imaging was performed in all patients with suspected gastric
cancer.
Data analysis
Data from CECT-TNCA image sets was analyzed using
the ADMIRE CECT system (version 3.10; Siemens Healthineers,
Erlangen, Germany). Volume of tumors was measured by CECT-TNCA
imaging. All patients with suspected early stage gastric cancer
were analyzed by CECT-TNCA and CT. Gastric tumor nodules were
observed and tumor size was automatically calculated using Sante CT
Viewer (version 2.0; Santesoft, Ltd., Athens, Greece).
Treatment of patients with gastric
cancer diagnosed by CECT-TNCA
Patients with early stage gastric cancer diagnosed
by CECT-TNCA received various different treatments including
radiotherapy (n=39), chemotherapy (n=49), Chinese medicine (n=58),
biological therapy (n=35), and comprehensive therapy (n=53). Median
overall survival and median progression-free survival were analyzed
as previously described (22).
Immunofluorescence and histological
staining
Following diagnostic confirmation via CECT-TNCA,
tumor cells from patients with gastric cancer were cultured in
vitro with Dulbecco's modified Eagle's medium supplemented with
10% heat-inactivated FBS (Invitrogen; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). Gastric tumors cells (1×106
cells/ml) were incubated with TNCA for 30 min at 37°C. Cells were
observed under a fluorescence microscope. Immunofluorescence
procedures were previously reported in detail (23). For histological staining, tumor
sections were stained with hematoxylin and eosin staining as
previously reported (24).
Statistical analysis
All data were presented as the mean and standard
deviation of triplicate experiments. Unpaired data was determined
by Student's t-test and comparisons of data between multiple groups
were analyzed by variance. Kaplan-Meier was used to estimate the
survival rate during 60-month long-term follow-up observations.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Plasma concentrations of PDGFR-β, Ret,
and Kit in patients with suspected gastric cancer
In order to analyze the target characteristic of
TNCA, a total of 484 patients with suspected gastric cancer were
voluntarily recruited to investigate the efficacy of CECT-TNCA in
the diagnosis of patients with early-stage gastric cancer.
Characteristics of patients with suspected gastric cancer are
summarized in Table I. The dose of
TNCA to achieve the optimum efficiency was identified as 15 mg/kg
(Table II). In addition, we
investigated the plasma concentration of PDGFR-β, Ret, and Kit in
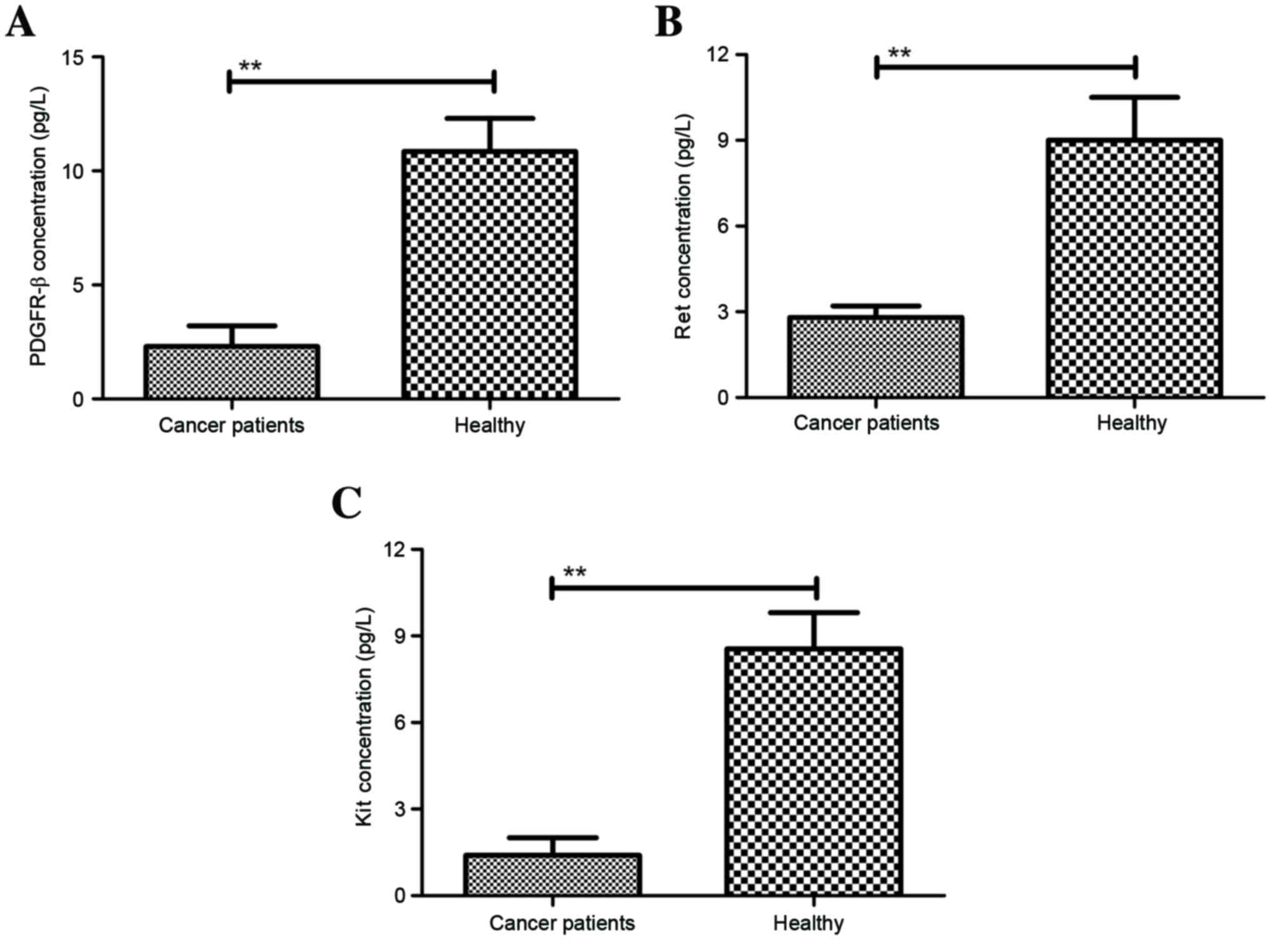
patients with suspected gastric cancer. As shown in Fig. 1A, PDGFR-β plasma concentration was
significantly lower in patients with gastric cancer when compared
with healthy subjects. We also found that plasma concentration of
Ret was significantly downregulated in patients with gastric cancer
when compared with healthy subjects (Fig. 1B). In addition, compared with healthy
subjects, plasma concentration of Kit was significantly lower in
patients with gastric cancer (Fig.
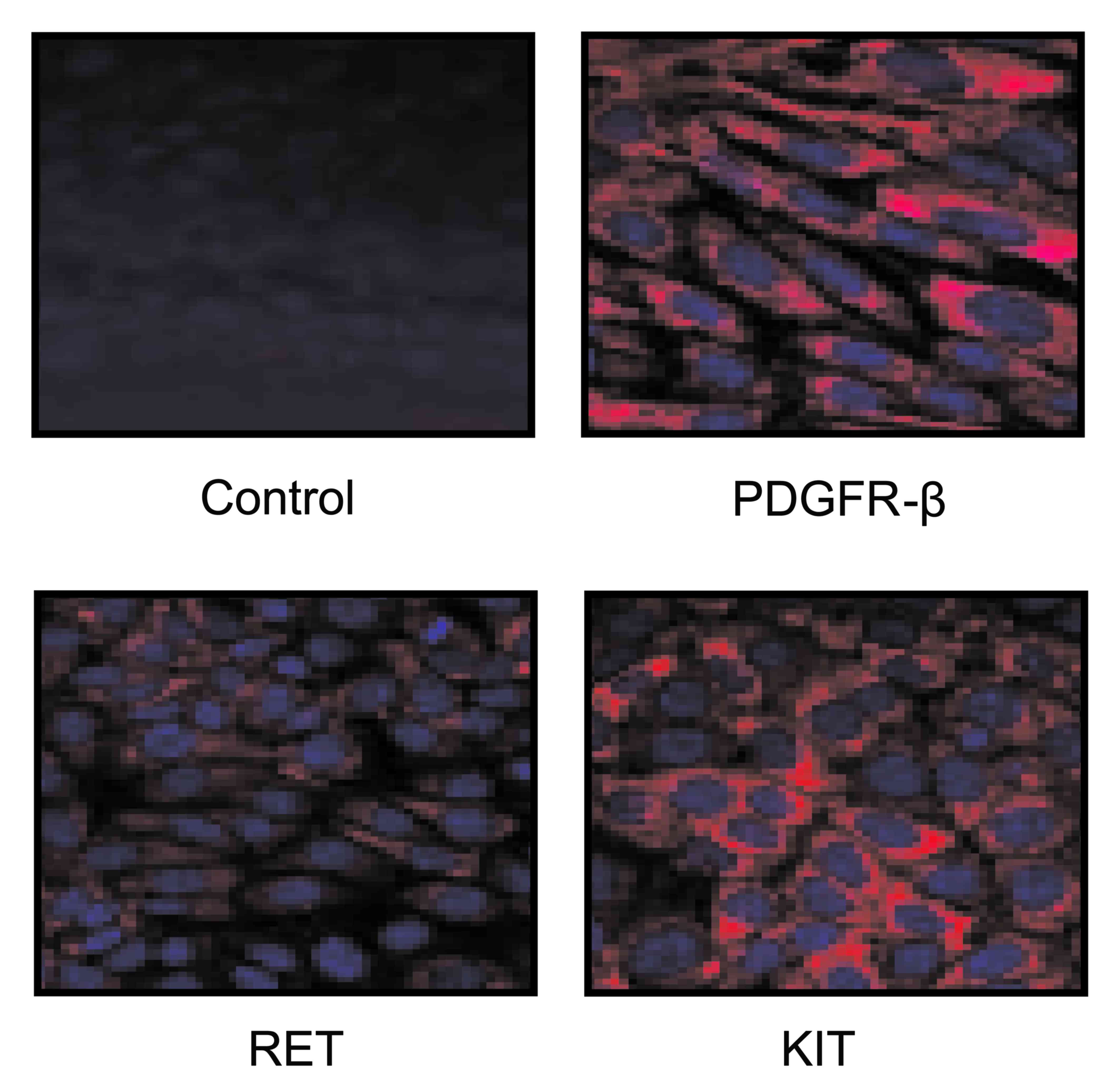
1C). Furthermore, clinical analysis also indicated that our
novel TNCA containing PDGFR-β, Ret, and Kit adhered to gastric
cancer cells, which contributed to the signal strength and
resolution (Fig. 2).
 | Table I.Characteristics of patients with
suspected gastric cancer. |
Table I.
Characteristics of patients with
suspected gastric cancer.
|
Characteristics | Male | Female |
|---|
| Patients (n) | 222 | 264 |
| Age (years) | 14.8–65.2 | 21.6–62.2 |
| Medical history of
cancer (n) | 3 | 5 |
| Blood pressure
(mmHg) | 110.2±12.8 | 113.4±10.3 |
| Blood glucose
(mmol/l) | 7.7±3.6 | 8.2±3.2 |
| Diagnosis (n) |
|
|
|
CECT-TNCA | 222 | 264 |
|
CECT | 222 | 264 |
 | Table II.Confirmation of the dosage of
targeting nanoparticles contrast agent required for diagnosis. |
Table II.
Confirmation of the dosage of
targeting nanoparticles contrast agent required for diagnosis.
| Variable | 1–10mg/kg
(n=16) | 11–20mg/kg
(n=24) | 21–30mg/kg
(n=20) |
|---|
| Signal intensity
(HU) |
76.5±7.2 |
92.5±5.8 |
93.4±6.4 |
| Sensitive (%) |
64.4±17.3 |
86.3±10.4 |
83.8±9.5 |
Efficacy of CECT-TNCA for the early
diagnosis of patients with suspected gastric cancer
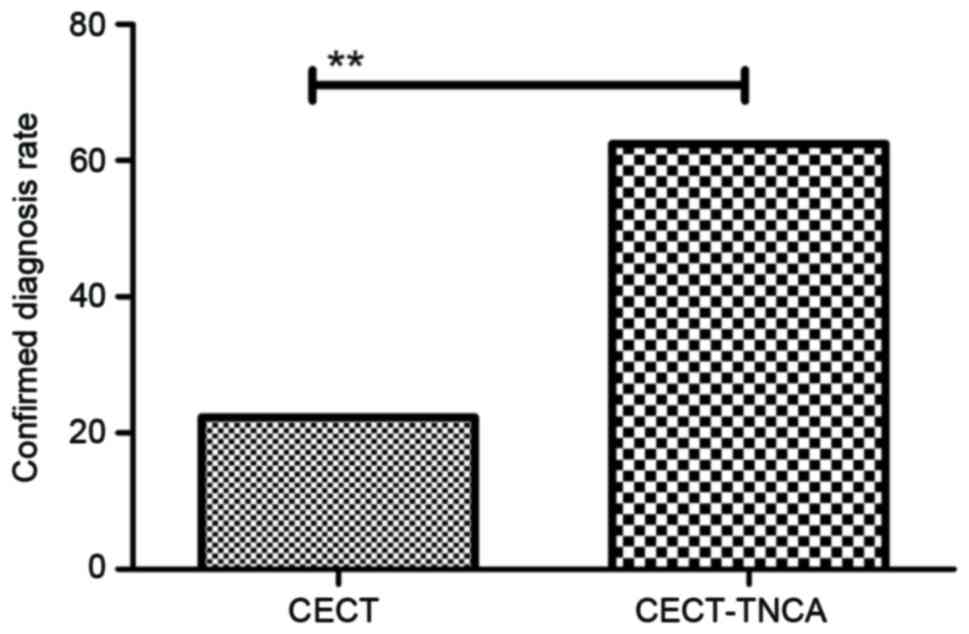
As shown in Fig. 3,
clinical analysis showed that 182 patients (37.6%) were diagnosed
as tumor-free and 302 patients (62.4%) were identified as having
gastric cancer, as determined by CECT-TNCA. However, the positive
rate of patients with gastric cancer was only 22.3% (108 patients)
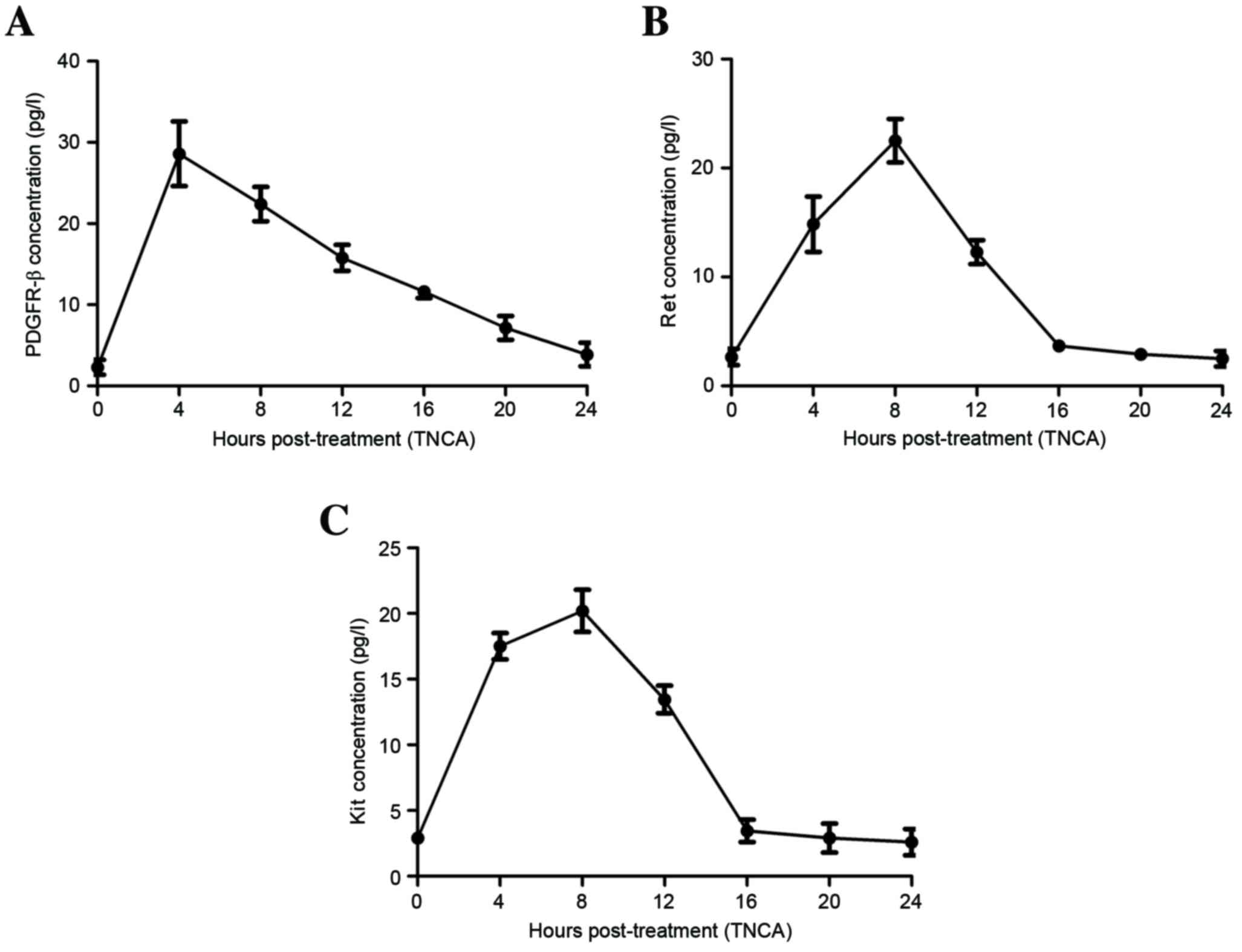
when evaluated via CECT alone. In addition, the investigation found
that TNCA increased the plasma concentration of PDGFR-β and
metabolized within 24 h (Fig. 4A).
Patients who underwent CECT-TNCA exhibited increasing plasma
concentrations of Ret that peaked at 12 h and attenuated by 16 h
(Fig. 4B). Plasma concentrations of
Kit were increased and metabolized within 20 h (Fig. 4C). These clinical data indicated that
CECT-TNCA is an efficient diagnostic strategy for the early
diagnosis of patients with suspected gastric cancer.
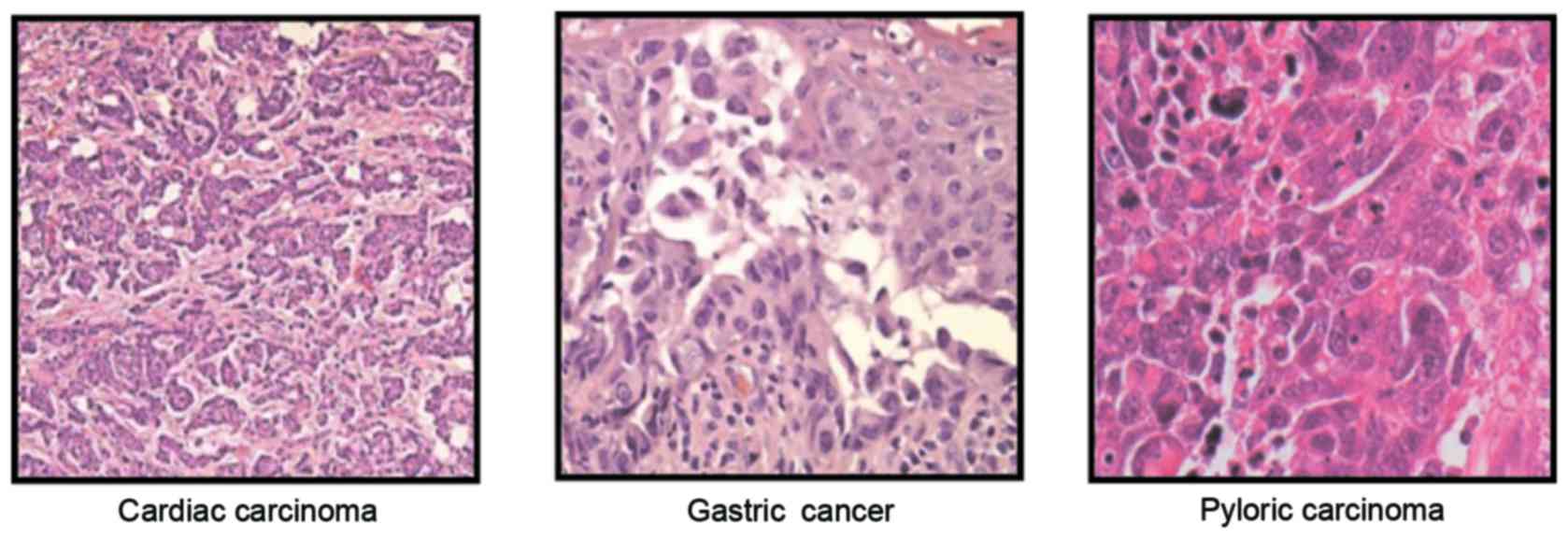
Histopathology analysis of the accuracy of
CECT-TNCA-diagnosis patients with gastric cancer. After diagnosing
patients with suspected gastric cancer, histopathology analysis was
used to further confirm diagnosis. Representative cardiac
carcinoma, gastric cancer and pylorus carcinoma incidence rates
were studied in the patients who had a confirmed diagnosis of
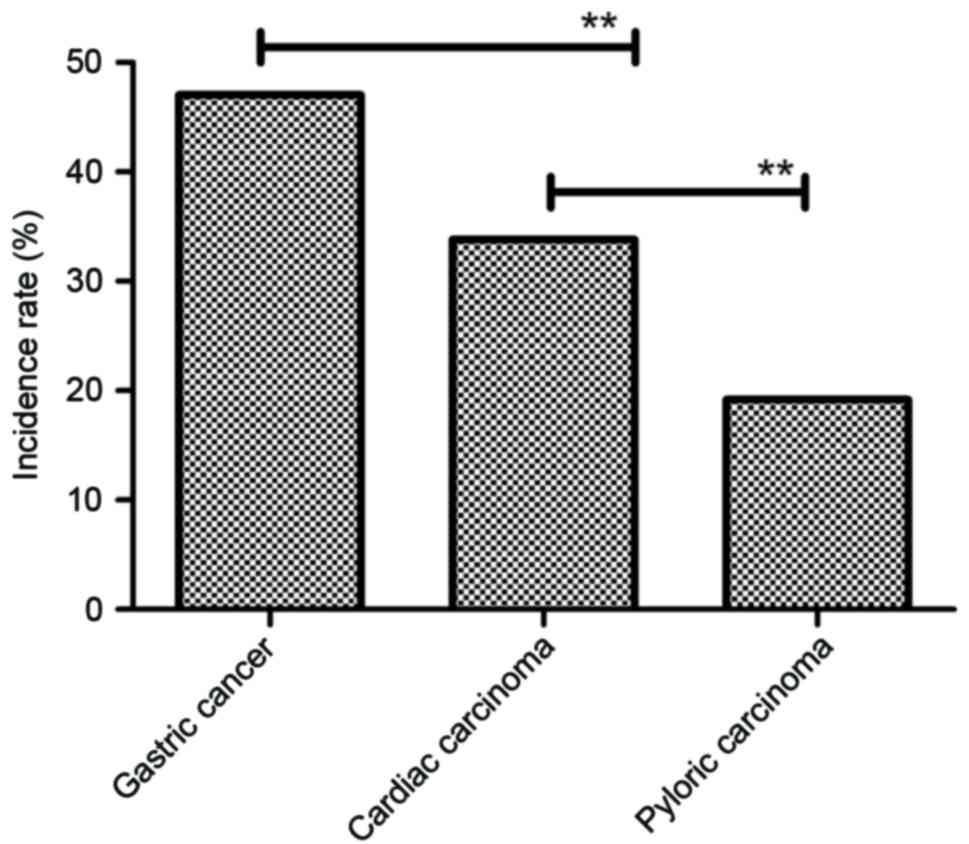
gastric cancer. As shown in Fig. 5,
our data showed that histopathology analysis identified cardiac
carcinoma, gastric cancer and pyloric carcinoma gastric cancer. The
incidence rate of cardiac carcinoma, gastric cancer and pyloric
carcinoma was 33.8% (102 cases), 47.0% (142 cases) and 19.2% (58
cases), respectively, in gastric cancer (Fig. 6). These outcomes suggest that the
CECT-TNCA method is accurate and sensitive for diagnosing patients
with gastric cancer.
Survival rate of patients with gastric
cancer diagnosed by CECT-TNCA
Patients with early-phase gastric cancer received
different treatments to inhibit tumor cell growth or eradicate
gastric cancer. We analyzed the reports of the treatment methods
and survival rates of patients with gastric cancer diagnosed by
CECT-TNCA. Characteristics of 234 patients with early-phase gastric
cancer diagnosed by CECT-TNCA are summarized in Table III. At the 60-month follow-up, we
observed that 132 patients (56.4%) were tumor-free and 88 patients
(37.6%) had survived and exhibited tumors. The mortality rate was
6.0% (14 patients; Table IV).
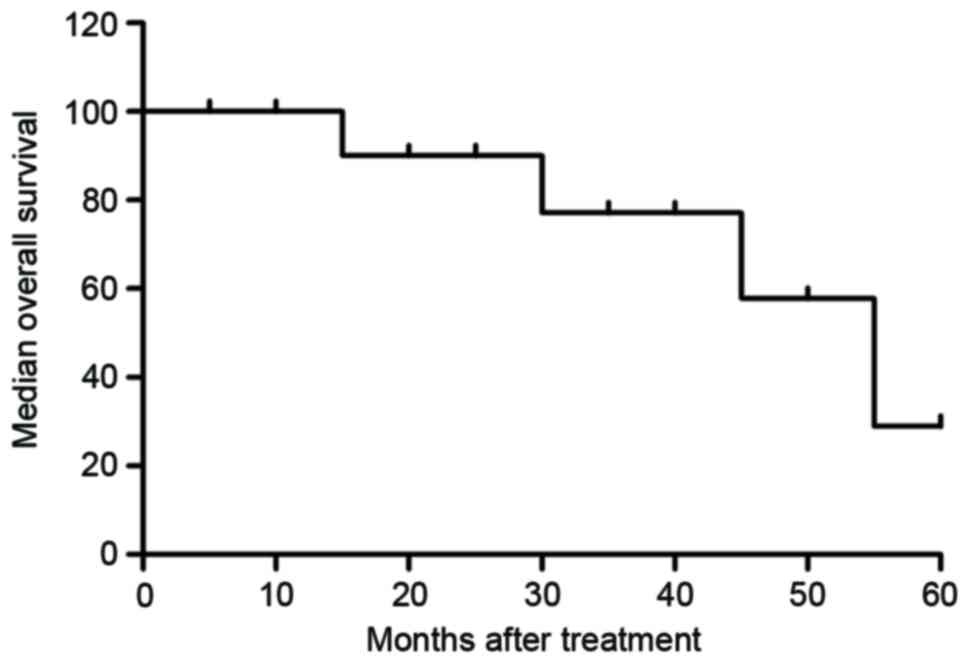
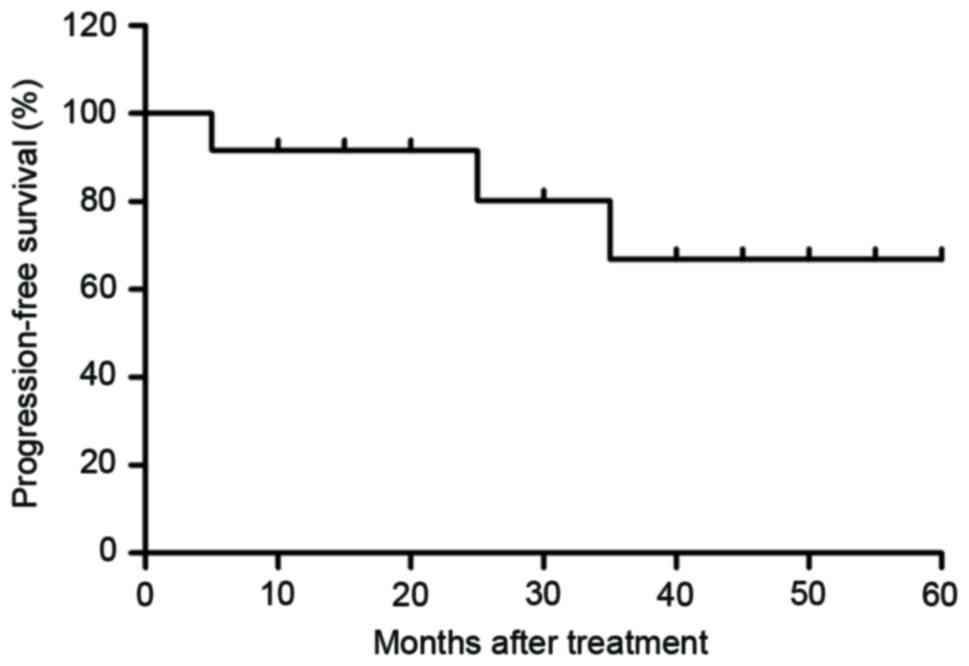
Median overall survival was 45.8 months (Fig. 7; range, 30.4–63.8 months) and median
progression-free survival was 36.8 months (Fig. 8; range, 24.5–52.4 months). These data
indicate that patients with early-phase gastric cancer received
anti-cancer treatments that prolonged the survival and
progression-free survival period, and that that comprehensive
therapy had notable therapeutic effects compared with the others
treatments.
 | Table III.Treatment of patients with gastric
cancer diagnosed by CECT-TNCA. |
Table III.
Treatment of patients with gastric
cancer diagnosed by CECT-TNCA.
|
Characteristics | Male | Female |
|---|
| Patients (n) | 114 | 120 |
| Age (years) | 15.6–56.2 | 22.4–52.8 |
| Medical history of
cancer (n) | 2 | 2 |
| Blood pressure (mm
Hg) | 108.6±8.5 | 107.5±9.8 |
| Blood glucose
(mmol/l) |
8.4±1.5 |
7.6±2.4 |
| Treatments (n) |
|
|
| Radiotherapy | 18 | 21 |
| Chemotherapy | 25 | 24 |
| Chinese
medicine | 32 | 26 |
| Biological
therapy | 18 | 17 |
| Comprehensive
therapy | 21 | 32 |
 | Table IV.Survival of patients diagnosed by
CECT-TNCA after 60-month follow-up. |
Table IV.
Survival of patients diagnosed by
CECT-TNCA after 60-month follow-up.
| Treatment | Tumor-free | Survived with
tumor(s) | DNS |
|---|
| Radiotherapy | 12 | 22 | 5 |
| Chemotherapy | 25 | 20 | 4 |
| Chinese
medicine | 37 | 18 | 3 |
| Biological
therapy | 19 | 15 | 1 |
| Comprehensive
therapy | 39 | 13 | 1 |
Discussion
Cancer early diagnosis is the biggest obstacle in
human cancer treatment (25,26). In recent years, contrast-enhanced
ultrasound, fluorodeoxyglucose-positron emission, tomography
(FDG-PET), CECT and chip technology have been widely used in the
diagnosis of human cancer (27,28). In
particular, CECT and chip technology present more advantages than
other diagnostic methods (29,30).
However, the application of gene chip technology is restricted due
to expensive detection and the professional analysts required
(31,32). Therefore, CECT has been become the
most prevalent diagnostic method in the majority of hospitals
worldwide (33,34).
Though CECT has been widely applied in the diagnosis
of human cancer, the accuracy and sensitivity of CECT is
insufficient for the detection of early-stage tumors (35,36).
Barium sulfate and iodinated contrast media are frequently used for
angiography studies and the diagnosis of tumors in the digestive
system (37,38). In addition, many electropositive iron
and iron oxide nanoparticles are used as contrast media and have
been reported to be useful in the diagnosis of human cancer in
previous clinical trials (39,40).
Furthermore, retroreflective-type Janus microspheres have also been
reported as a novel contrast agent for enhanced optical coherence
tomography (41). However, these
contrast mediums only improve the partial accuracy of CT in a
certain degree. Therefore, elucidating more efficient contrast
mediums with targeting characteristics has attracted increasing
attention from researchers and clinicians in the field of cancer
research and clinical therapy.
In the present study, we introduced a comprehensive
approach of CECT combined with target nanoparticles contrast agent
to improve the accuracy for patients with suspected gastric cancer
in the early stage. Target nanoparticles contrast mediums,
containing PDGFR-β, Ret, and Kit, were encapsulated by liposome.
Our clinical outcomes indicate that liposome-encapsulated TNCA
presents a potential tumor-specific approach that may lead to
improvements in the diagnostic accuracy of patients with
early-stage gastric cancer. Targeted binding of
liposome-encapsulated TNCA with gastric tumor cells enhances signal
intensity in the lesions of the stomach, resulting in an
improvement in the spatial resolution of CECT. Notably,
pharmacokinetic tracer kinetics analysis demonstrated that the
target nanoparticles contrast mediums of PDGFR-β, Ret, and Kit were
metabolized within 24 h. No side effects were noted during the
diagnostic period. Long-term follow-up reports showed that patients
diagnosed by CECT-TNCA at the early stage present higher median
overall survival (30.4–63.8 months) and median progression-free
survival (24.5–52.4 months).
Tyrosine kinase inhibitors of PDGFR-β, Ret, and
Kit-mediated angiogenesis have been identified as key factors in
the development of human cancer (42,43).
PDGFR-β, Ret, and Kit have potent anti-tumor activity against a
number of human tumors through binding with targets (44). Previous reports have suggested that
target receptors of PDGFR-β, Ret, and Kit in gastric tumors are
effective treatments (45–47). Although previous contrast media have
not previously been compared to determine which media is optimal
for the visualization and diagnosis of gastric cancer (48). Indeed, CECT using a different
contrast agent enables non-destructive diagnosis of the biochemical
and biomechanical properties of patients with various diseases
(49). In addition, a previous study
has evaluated dynamic CECT imaging in the differentiation of benign
and malignant tumors observed by tumor vessel and permeability
nodule perfusion (50). However,
conventional contrast agents present lower efficacy for tumor
analysis due to rapid diffusion outside the lungs, which prevents
optimal imaging (51). Furthermore,
previous reports have shown that iodinated contrast agents are less
sensitive to changes in cells morphology (52,53). Our
design showed that liposome-encapsulated target nanoparticles
contrast mediums are potential nanoparticles contrast agents that
may improve the accuracy of diagnosing early-stage gastric tumors.
Targeted binding of target nanoparticles contrast mediums with
gastric tumor cells enhanced signal intensity in lesions in
stomachs diagnosed by CECT-TNCA.
In conclusion, to the best of our knowledge, this is
the first report of liposome-encapsulated targeted nanoparticles
contrast mediums combined with CECT for the diagnosis of patients
with suspected early stage gastric cancer. CECT-TNCA was
administered orally to augment the signal intensity in the stomach,
leading to a reliable and sensitive assessment of the tumor for
clinical diagnosis in patients with gastric cancer.
References
|
1
|
Golpour S, Rafie N, Safavi SM and
Miraghajani M: Dietary isoflavones and gastric cancer: A brief
review of current studies. J Res Med Sci. 20:893–900. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hossain MM and Chen Z: Comments on
‘Application of an adaptive design to a randomized phase II
selection trial in gastric cancer: A report of the study design’ by
Satoshi Morita and Junichi Sakamoto. Pharmaceutical Statistics.
Pharm Stat. 11:267–268. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Khayatzadeh S, Feizi A, Saneei P and
Esmaillzadeh A: Vitamin D intake, serum Vitamin D levels, and risk
of gastric cancer: A systematic review and meta-analysis. J Res Med
Sci. 20:790–796. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Pimenta-Melo AR, Monteiro-Soares M,
Libânio D and Dinis-Ribeiro M: Missing rate for gastric cancer
during upper gastrointestinal endoscopy: A systematic review and
meta-analysis. Eur J Gastroenterol Hepatol. 28:1041–1049. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Veisani Y and Delpisheh A: Survival rate
of gastric cancer in Iran; a systematic review and meta-analysis.
Gastroenterol Hepatol Bed Bench. 9:78–86. 2016.PubMed/NCBI
|
|
6
|
Nakajima T, Inokuchi K, Hattori T, Inoue
K, Taguchi T, Kondou T, Abe O, Kikuchi K, Tanabe T and Ogawa N:
Multi-institutional cooperative study of adjuvant
immunochemotherapy in gastric cancer-five-year survival rate. Gan
To Kagaku Ryoho. 16:799–806. 1989.(Article in Japanese). PubMed/NCBI
|
|
7
|
Jung SA, Park YM, Hong SW, Moon JH, Shin
JS, Lee HR, Ha SH, Lee DH, Kim JH, Kim SM, et al: Cellular
inhibitor of apoptosis protein 1 (cIAP1) stability contributes to
YM155 resistance in human gastric cancer cells. J Biol Chem.
290:9974–9985. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Izawa M, Mori T, Satoh T, Teramachi K and
Sairenji T: Identification of an alternative form of caspase-9 in
human gastric cancer cell lines: A role of a caspase-9 variant in
apoptosis resistance. Apoptosis. 4:321–325. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moody SE, Schinzel AC, Singh S, Izzo F,
Strickland MR, Luo L, Thomas SR, Boehm JS, Kim SY, Wang ZC and Hahn
WC: PRKACA mediates resistance to HER2-targeted therapy in breast
cancer cells and restores anti-apoptotic signaling. Oncogene.
34:2061–2071. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shiota M, Yokomizo A and Naito S:
Pro-survival and anti-apoptotic properties of androgen receptor
signaling by oxidative stress promote treatment resistance in
prostate cancer. Endocr Relat Cancer. 19:R243–R253. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yamato I, Sho M, Shimada K, Hotta K, Ueda
Y, Yasuda S, Shigi N, Konishi N, Tsujikawa K and Nakajima Y:
PCA-1/ALKBH3 contributes to pancreatic cancer by supporting
apoptotic resistance and angiogenesis. Cancer Res. 72:4829–4839.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kanat O, O'Neil B and Shahda S: Targeted
therapy for advanced gastric cancer: A review of current status and
future prospects. World J Gastrointest Oncol. 7:401–410. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ebell MH, Culp MB and Radke TJ: A
systematic review of symptoms for the diagnosis of ovarian cancer.
Am J Prev Med. 50:384–394. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mazzei MA, Guerrini S, Mazzei FG,
Squitieri N Cioffi, Notaro D, de Donato G, Galzerano G, Sacco P,
Setacci F, Volterrani L and Setacci C: Follow-up of endovascular
aortic aneurysm repair: Preliminary validation of digital
tomosynthesis and contrast enhanced ultrasound in detection of
medium- to long-term complications. World J Radiol. 8:530–536.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Schalk SG, Demi L, Bouhouch N, Kuenen MPJ,
Postema AW, de la Rosette JJMCH, Wijkstra H, Tjalkens TJ and Mischi
M: Contrast-enhanced ultrasound angiogenesis imaging by mutual
information analysis for prostate cancer localization. IEEE Trans
Biomed Eng. 64:661–670. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Markic D, Krpina K, Ahel J, et al:
Different presentations of renal cell cancer on ultrasound and
computerized tomography. Urologia. 81:228–232. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shi H, Wang Z, Huang C, Gu X, Jia T, Zhang
A, Wu Z, Zhu L, Luo X, Zhao X, et al: A functional CT contrast
agent for in vivo imaging of tumor hypoxia. Small. 12:3995–4006.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Krylova NS, Demkina AE, Poteshkina NG and
Khashieva FM: Tissue Doppler imaging and ultrasonic methods for
evaluating myocardial deformation in the diagnosis of hypertrophic
cardiomyopathy. Kardiologiia. 54:79–84. 2014.(In Russian).
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen CL, Hu GY, Mei Q, Qiu H, Long GX and
Hu GQ: Epidermal growth factor receptor-targeted ultra-small
superparamagnetic iron oxide particles for magnetic resonance
molecular imaging of lung cancer cells in vitro. Chin Med J (Engl).
125:2322–2328. 2012.PubMed/NCBI
|
|
20
|
Shan L: Polyethylene glycol-coated and
folic acid-conjugated superparamagnetic iron oxide
nanoparticlesMolecular Imaging and Contrast Agent Database (MICAD).
Bethesda (MD): 2004
|
|
21
|
Nakamoto Y, Ishimori T, Sano K, Temma T,
Ueda M, Saji H and Togashi K: Clinical efficacy of dual-phase
scanning using Ga-DOTATOC-PET/CT in the detection of neuroendocrine
tumours. Clin Radiol. 71:1069.e1–5. 2016. View Article : Google Scholar
|
|
22
|
Angelov KG, Vasileva MB, Grozdev KS,
Sokolov MB and Todorov G: Clinical and pathological
characteristics, and prognostic factors for gastric cancer survival
in 155 patients in Bulgaria. Hepatogastroenterology. 61:2421–2424.
2014.PubMed/NCBI
|
|
23
|
Dirani M, Nasreddine W, Abdulla F and
Beydoun A: Seizure control and improvement of neurological
dysfunction in Lafora disease with perampanel. Epilepsy Behav Case
Rep. 2:164–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kargahi N, Razavi SM, Deyhimi P and
Homayouni S: Comparative evaluation of eosinophils in normal
mucosa, dysplastic mucosa and oral squamous cell carcinoma with
hematoxylin-eosin, Congo red, and EMR1 immunohistochemical staining
techniques. Electron Physician. 7:1019–1026. 2015.PubMed/NCBI
|
|
25
|
Soler M, Estevez MC, Villar-Vazquez R,
Casal JI and Lechuga LM: Label-free nanoplasmonic sensing of
tumor-associate autoantibodies for early diagnosis of colorectal
cancer. Anal Chim Acta. 930:31–38. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hopper AD and Campbell JA: Early diagnosis
of oesophageal cancer improves outcomes. Practitioner. 260:23–28,
3. 2016.PubMed/NCBI
|
|
27
|
Shah RB, Leandro G, Romerocaces G, Bentley
J, Yoon J, Mendrinos S, Tadros Y, Tian W and Lash R: Improvement of
Diagnostic Agreement among Pathologists in Resolving an ‘Atypical
Glands Suspicious for Cancer’ diagnosis in prostate biopsies
utilizing a novel ‘Disease-Focused Diagnostic Review’ quality
improvement process. Hum Pathol. 56:155–162. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Haider MA, Yao X, Loblaw A and Finelli A:
Multiparametric magnetic resonance imaging in the diagnosis of
prostate cancer: A Systematic Review. Clin Oncol (R Coll Radiol).
28:550–567. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen WG, Yang CM, Xu LH, Zhang N, Liu XY,
Ma YG, Huo XL, Han YS, Tian DA and Zheng Y: Gene chip technology
used in the detection of HPV infection in esophageal cancer of
Kazakh Chinese in Xinjiang Province. J Huazhong Univ Sci Technol
Med Sci. 34:343–347. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li RZ, Shi JF, Zhou QZ, Wu RF, Li N, Wu
LN, Zhou YQ, Wang Q, Liu ZH, Liu B and Qiao YL: Evaluation of gene
chip technology for high risk type human papillomavirus in cervical
cancer screening. Zhonghua Yi Xue Za Zhi. 86:307–311. 2006.(In
Chinese). PubMed/NCBI
|
|
31
|
Hampton T: Breast cancer gene chip study
under way: Can new technology help predict treatment success? Jama.
291:2927–2930. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Watson RW: Gene-chip technology and
prostate cancer: The identification of new genes regulating tumour
progression. BJU Int. 91:3072003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kooiman J, Sijpkens YW, de Vries JP,
Brulez HF, Hamming JF, van der Molen AJ, Aarts NJ, Cannegieter SC,
Putter H, Swarts R, et al: A randomized comparison of 1-h sodium
bicarbonate hydration versus standard peri-procedural saline
hydration in patients with chronic kidney disease undergoing
intravenous contrast-enhanced computerized tomography. Nephrol Dial
Transplant. 29:1029–1036. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mir MA, Bali BS, Mir RA and Wani H:
Assessment of the severity of acute pancreatitis by
contrast-enhanced computerized tomography in 350 patients. Ulus
Travma Acil Cerrahi Derg. 19:103–108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kakimoto N, Chindasombatjaroen J, Tomita
S, Shimamoto H, Uchiyama Y, Hasegawa Y, Kishino M, Murakami S and
Furukawa S: Contrast-enhanced multidetector computerized tomography
for odontogenic cysts and cystic-appearing tumors of the jaws: Is
it useful? Oral Surg Oral Med Oral Pathol Oral Radiol. 115:104–113.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Freling N, Roele E, Schaefer-Prokop C and
Fokkens W: Prediction of deep neck abscesses by contrast-enhanced
computerized tomography in 76 clinically suspect consecutive
patients. Laryngoscope. 119:1745–1752. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Konduru N, Keller J, Ma-Hock L, Gröters S,
Landsiedel R, Donaghey TC, Brain JD, Wohlleben W and Molina RM:
Biokinetics and effects of barium sulfate nanoparticles. Part Fibre
Toxicol. 11:552014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Liss P, Hansell P, Fasching A and Palm F:
Iodinated contrast media inhibit oxygen consumption in freshly
isolated proximal tubular cells from elderly humans and diabetic
rats: Influence of nitric oxide. Ups J Med Sci. 121:12–16. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Mishra SK, Kumar BS, Khushu S, Tripathi RP
and Gangenahalli G: Increased transverse relaxivity in ultrasmall
superparamagnetic iron oxide nanoparticles used as MRI contrast
agent for biomedical imaging. Contrast Media Mol Imaging.
11:350–361. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kitoh Y: 5. Inspection of hepatocellular
carcinoma 3-Contrast for the diagnosis of hepatocellular carcinoma:
Techniques of image contrast and the choice of MR contrast agent.
Nihon Hoshasen Gijutsu Gakkai Zasshi. 72:441–451. 2016.(In
Japanese).
|
|
41
|
Zhang J, Liu J, Wang LM, Li ZY and Yuan Z:
Retroreflective-type Janus microspheres as a novel contrast agent
for enhanced optical coherence tomography. J Biophotonics.
10:878–886. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Rini BI and Atkins MB: Resistance to
targeted therapy in renal-cell carcinoma. Lancet Oncol.
10:992–1000. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Eichelberg C, Junker K, Ljungberg B and
Moch H: Diagnostic and prognostic molecular markers for renal cell
carcinoma: A critical appraisal of the current state of research
and clinical applicability. Eur Urol. 55:851–863. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hutson TE: Targeted therapies for the
treatment of metastatic renal cell carcinoma: Clinical evidence.
Oncologist. 16 Suppl 2:S14–S22. 2011. View Article : Google Scholar
|
|
45
|
Pinto RP, Lima FK, Kulkzynski JM and
Moreira LF: Expression of P16 and PDGFR-beta in gastric
adenocarcinoma. Rev Col Bras Cir. 36:199–203. 2009.(In English,
Portuguese). View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang F, Tang JM, Wang L, Wu PP and Zhang
M: Immunohistochemical detection of RET proto-oncogene product in
tumoral and nontumoral mucosae of gastric cancer. Anal Quant
Cytopathol Histopathol. 36:128–136. 2014.
|
|
47
|
Bdo N Borges, Eda S Santos, Bastos CE,
Pinto LC, Anselmo NP, Quaresma JA, Calcagno DQ, Burbano RM and
Harada ML: Promoter polymorphisms and methylation of E-cadherin
(CDH1) and KIT in gastric cancer patients from northern Brazil.
Anticancer Res. 30:2225–2233. 2010.PubMed/NCBI
|
|
48
|
Kingston MJ, Perriman DM, Neeman T, Smith
PN and Webb AL: Contrast agent comparison for three-dimensional
micro-CT angiography: A cadaveric study. Contrast Media Mol
Imaging. 11:319–324. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lakin BA, Patel H, Holland C, Freedman JD,
Shelofsky JS, Snyder BD, Stok KS and Grinstaff MW:
Contrast-enhanced CT using a cationic contrast agent enables
non-destructive assessment of the biochemical and biomechanical
properties of mouse tibial plateau cartilage. J Orthop Res.
34:1130–1138. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Sudarski S, Henzler T and Schoenberg SO:
Post-therapeutic positron emission tomography/computed tomography
for early detection of non-small cell lung cancer recurrence.
Transl Lung Cancer Res. 2:295–303. 2013.PubMed/NCBI
|
|
51
|
Hagberg GE, Mamedov I, Power A, Beyerlein
M, Merkle H, Kiselev VG, Dhingra K, Kubìček V, Angelovski G and
Logothetis NK: Diffusion properties of conventional and
calcium-sensitive MRI contrast agents in the rat cerebral cortex.
Contrast Media Mol Imaging. 9:71–82. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Turetschek K, Preda A, Novikov V, Brasch
RC, Weinmann HJ, Wunderbaldinger P and Roberts TP: Tumor
microvascular changes in antiangiogenic treatment: Assessment by
magnetic resonance contrast media of different molecular weights. J
Magn Reson Imaging. 20:138–144. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Samei E, Saunders RS, Badea CT, Ghaghada
KB, Hedlund LW, Qi Y, Yuan H, Bentley RC and Mukundan S Jr:
Micro-CT imaging of breast tumors in rodents using a liposomal,
nanoparticle contrast agent. Int J Nanomedicine. 4:277–282. 2009.
View Article : Google Scholar : PubMed/NCBI
|