Introduction
Common implant materials, including titanium alloys,
stainless steel and cobalt-chromium alloys, have considerable
advantages in terms of their load-bearing capabilities and
resistance to fatigue, wear and corrosion (1–3).
However, these traditional materials also have multiple
disadvantages. For instance, their mechanical properties differ
from those of natural bone, which may cause stress shielding
(4–6). A second surgery is often required to
remove the implant after the tissues have healed, which markedly
increases the risk and costs of healthcare (7,8).
Furthermore, implant materials release cytotoxic ions and may cause
physical irritation due to their rigidity (9,10).
Recently, magnesium and its alloys have attracted
increasing interest as innovative biodegradable materials,
particularly due to their potential use as temporary orthopedic
implants (11,12). The fundamental properties of
magnesium make it relatively suitable for this application
(13); it has low density and
elastic modulus, both of which are close to that of natural bone,
thus preventing stress shielding during fracture consolidation
(14,15). In addition, magnesium is
biocompatible and essential for human metabolism as a cofactor for
many enzymes (16). Notably,
magnesium ions that are produced as a result of implant degradation
have been reported to aid in the growth and healing of tissues
(17,18). Magnesium is well tolerated by the
human body and does not induce systemic inflammatory reactions or
negatively affect the cellular blood composition (19). It also degrades in aqueous solutions,
and is prone to degradation in body fluids (20); thus, follow-up surgery to remove the
implant is not required (21).
Finally, due to its functional roles and presence in bone tissue,
magnesium may exert stimulatory effects on the growth of new bone
tissue (21–23).
Despite these advantageous properties, magnesium and
its alloys have not been widely used as human body implants to
date. A major drawback is that magnesium alloys tend to corrode
rapidly in chloride solutions, including the physiological
environment, leading to a loss in their mechanical integrity before
their expected service life (1,23,24). One
method of altering the surface and degradation properties without
adding a coating material, and thus inducing other potentially
irritating materials, is micro-arc oxidation (25,26).
This method of surface modification, which is based on the
principle of plasma-electrolytic oxidation, produces an increased
oxide layer at the surface (27).
Micro arc-treated magnesium surfaces exhibit improved resistance to
corrosion in various environments (28,29).
In vitro tests in simulated body fluid have repeatedly
confirmed the favorable behavior of surface-treated magnesium
alloys regarding reduced dissolution and enhanced biocompatibility
(30).
There has been much research on degradable implants
for applications in orthopedics (31–34),
such as those made of polylactic-co-glycolic acid and magnesium
alloys. It has been reported that the degradation of implant
material and formation of new bone, and notably, the balance
between these two processes, are important evaluation indices
(35). Traditional methods of
assessing degradation have unavoidable disadvantages, and the
evaluation of new bone formation generally relies on images
obtained from histology and radiography, which are imprecise and
difficult to analyze quantitatively (36,37).
Thus, the present study used images and data from micro-computed
tomography (micro-CT), as a non-traumatic, in vivo,
quantitative and precise process (38,39).
Over the past decade, the number of orthopedic
studies that have used micro-CT imaging has increased (40–49).
Higher spatial and temporal resolution are key technical advances
that have enabled researchers to capture increasingly detailed
anatomical images of small animals and monitor the progression of
orthopedic disease in small animal models (38,50).
Furthermore, a range of data may be derived from micro-CT,
including bone volume (BV), bone mineral content (BMC), bone
mineral density (BMD), bone volume fraction (BVF), bone
volume/tissue volume (BV/TV), bone surface/bone volume (BS/BV),
trabecular thickness (Tb.Th), trabecular number (Tb.N) and
trabecular separation (Tb.Sp). With the development of radiography
methods for the evaluation of implants, measuring the weight loss
of implants and histological analysis, as ‘traumatic’ methods, and
micro-CT imaging, as a ‘non-traumatic’ method, have become
mainstream (51); however,
quantitative analyses and methods that evaluate the association
between implant material degradation and new bone formation are
still lacking (52,53).
In the present study, micro-CT images and data were
used to assess the degradation of micro arc-oxidized AZ31 magnesium
alloy implants in vivo. Changes in the volume of the AZ31
implants were assessed, and the application of the degradable
implant for bone-regeneration was evaluated. By applying micro-arc
surface treatment, the initial intention was to decrease the
degradation rate of the magnesium implants in the initial
post-implantation period and reduce the impact of degradation
products on the postoperatively irritated surrounding tissue, then
to slow dissolution of the implant material once the implant's
function became redundant. Generally, a cylinder of the same size
and position of the pin was selected as the region of interest
(ROI) to analyze the degradation of the material, and a new larger
ROI in the same shape and position was selected to observe new bone
formation and assess the stimulatory effects of magnesium alloys on
bone growth.
Materials and methods
Implants
Micro arc-oxidized cylindrical pins (n=60; diameter
2.0 mm, length 6.0 mm, weight 0.500 g) made of AZ31 material were
used (Trauson Medical Instrument Co., Ltd., Changzhou, China). AZ31
is a fast-degrading alloy of magnesium with 3 wt.% Al, 1 wt.% Zn
and 0.15 wt.% Mn.
Experimental design
All animal experiments were conducted following
ethical guidelines by Ethics Committee of Chinese PLA General
Hospital (Beijing, China), obtained international standard
authentication-SIDCER (54) and were
authorized by the Institutes for Food and Drug Control of China and
KEYU Animal Experiment Center.
A total of 60 male New Zealand white rabbits (body
weight 2.2–2.5 kg, 3 months old) were purchased from KEYU Animal
Experiment Center (Beijing, China; accreditation number SCXK(Jing)
2012-0004, certification no. 11400800001109) for the present study.
Experiments were performed under standard conditions throughout the
study (temperature, 23±2°C; relative humidity, 60±10%; with access
to a 12-h light/dark cycle). Rabbits had been ensured adequate food
and water. Each rabbit had an AZ31 pin implanted into its right
femoral condyle. Rabbits were randomly divided into six groups
(n=10), and were sacrificed after 1, 4, 12, 24, 36 or 48 weeks (1
group per time-point). Each pin was weighed prior to implantation
and following sacrifice. Micro-CT, 3D reconstruction and
histological examinations were performed following sacrifice.
Surgical procedure
Rabbits were fasted for 12 h prior to surgery and
then anesthetized with an intraperitoneal injection of 3% sodium
pentobarbital (Sigma-Aldrich; Merck KGaA; Darmstadt, Germany) with
a dosage of 24 mg/kg. Surgery was performed under sterile
conditions. First, full-thickness lesions were created with 2.0 mm
Kirschner pins (Beijing Fule Science & Technology Development
Co., Ltd., Beijing, China). AZ31 alloy cylindrical pins were
implanted into the defects in the right femoral condyles. All pins
were γ-ray-sterilized with 29 kGy of 60Co radiation
prior to surgery. All rabbits received an intramuscular
anti-inflammatory injection with penicillin (Sigma-Aldrich; Merck
KGaA) with a dosage of 400,000 U/day following surgery and were
housed individually. Postoperatively, the rabbits were allowed to
move freely in their cages without external support and with
unrestricted weight bearing. Daily clinical observations were made
throughout the study period.
Weighing
Prior to implantation, the AZ31 pins were weighed on
an electronic balance (accuracy, ±0.001 g). At 1, 4, 12, 24, 36 or
48 weeks, pins were removed from the femoral condyle and dry
machined with clean tools. After machining, the pins were cleaned
with pure ethanol in an ultrasonic bath and dried in warm air
(23). The pins were weighed again
and the difference between the pre- and post- implantation weights
was calculated. To explore the difference between weight and
Micro-CT methods, the pin weight fraction was calculated using the
following formula: Weight loss of implanted pins/weight before
implantation. Subsequently the pin weight fraction was compared
with the pin volume fraction.
Micro-CT
Micro-CT is an emerging technology that permits
non-invasive, tissue-preserving imaging and quantitative
morphometry of bone structure in three dimensions (55–56).
Scans were performed with an RS-9 micro-CT (GE Healthcare, Chicago,
IL, USA) to assess the condyle before it was placed in 25% formic
acid solution for decalcification. The micro-CT system was operated
at 80 kV tube voltage and 450 µA tube electric current, with a scan
resolution of 45 µm and exposure time of 400 msec. Images were
reconstructed and the pin volume, bone volume, BMD, tissue mineral
density (TMD), BMC, BVF, BV/TV, BS/BV, Tb.Th, Tb.N, and Tb.Sp were
analyzed using the built-in software (Version MicroView Advanced
Bone Analysis Application 2.2; GE Healthcare) of the micro-CT
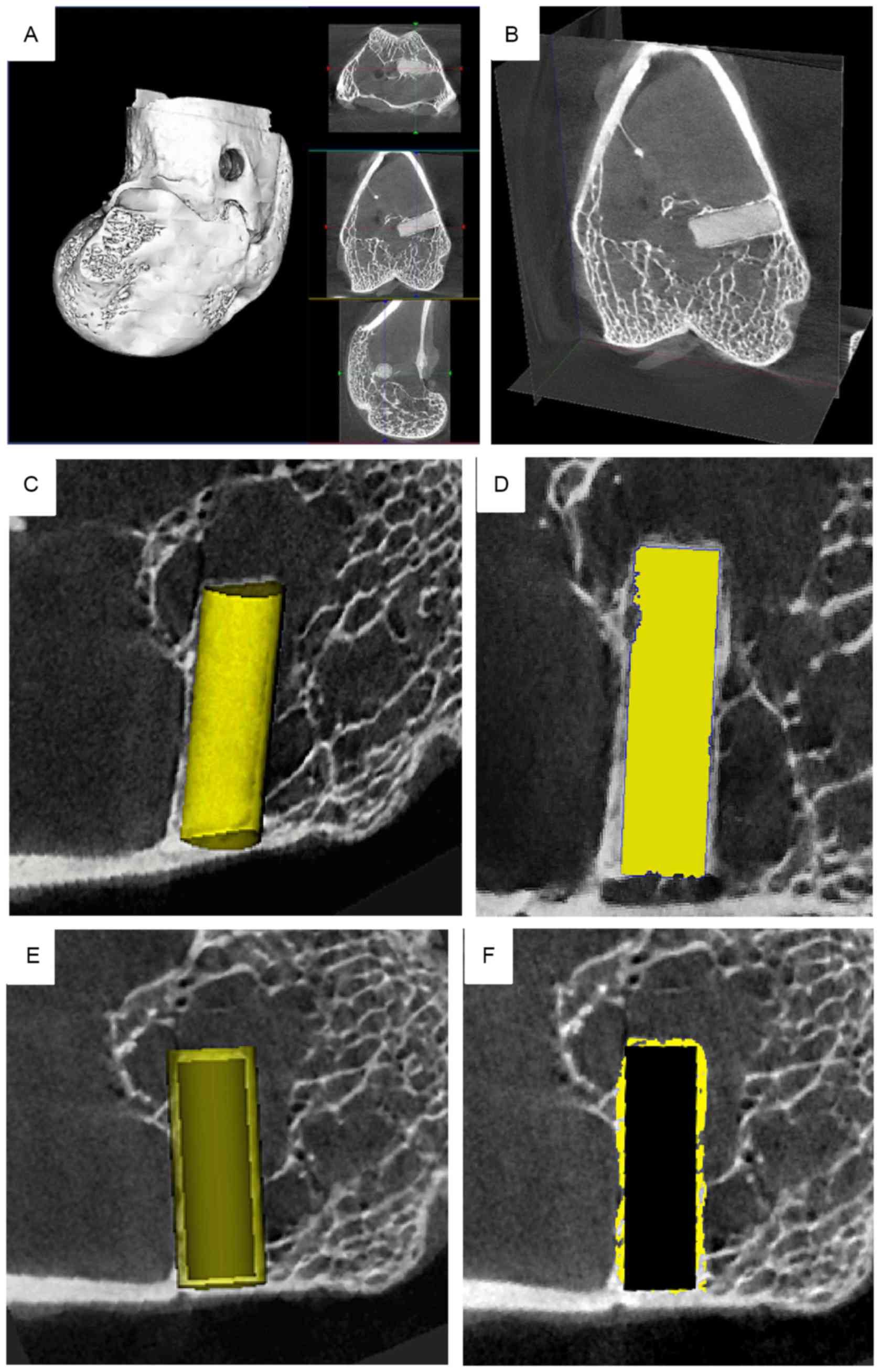
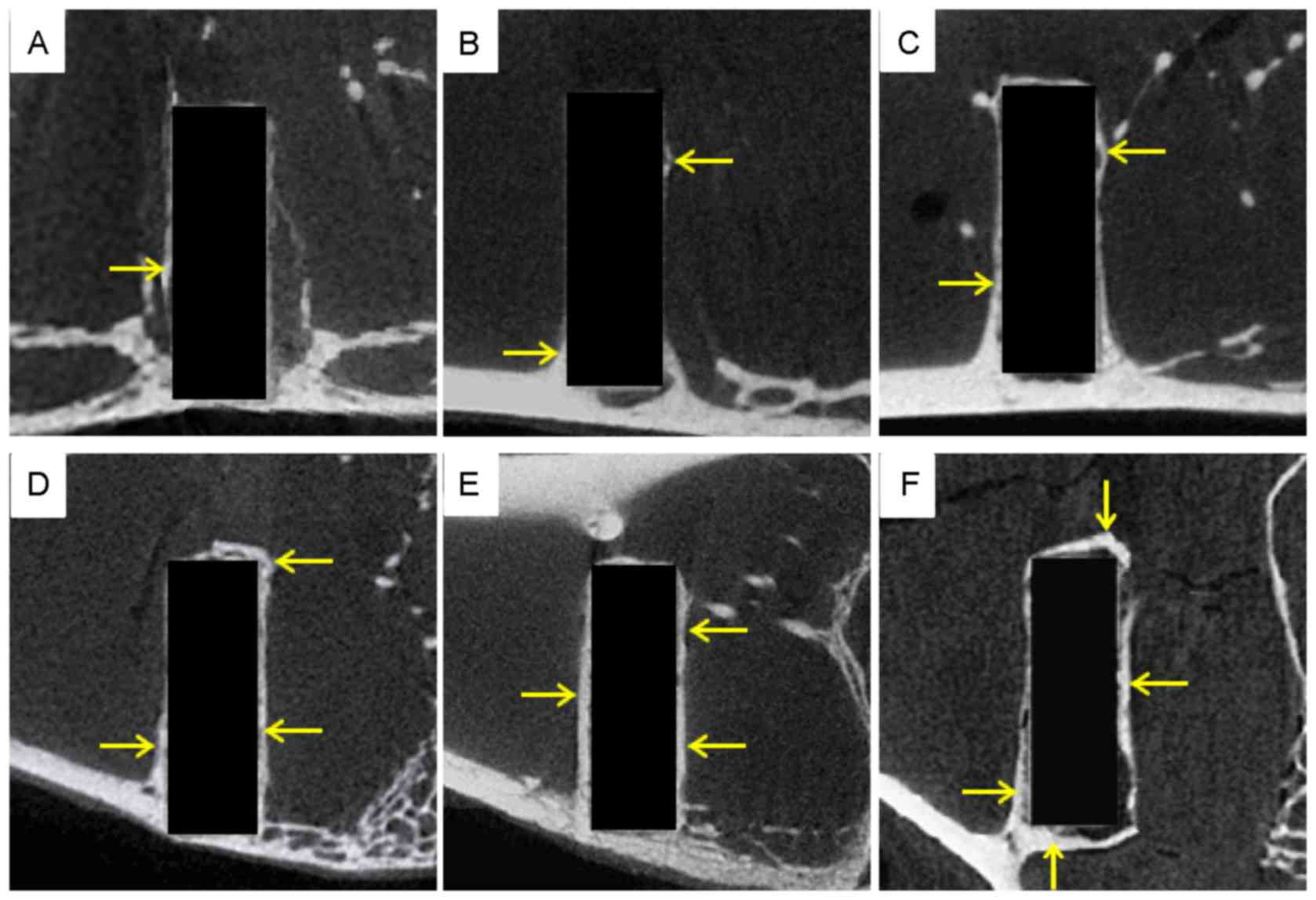
equipment at each time point. A cylinder of the same size was
selected from the corresponding region around the pins of the
femoral condyle (Fig. 1A-F). A
cylindrical ROI was set at each time point, matching the size of
the magnesium alloy pins, 2.0 mm in diameter and 6.0 mm in length,
ensuring that the ROI and pin fully overlapped (Fig. 1C). The threshold was set to 850 and
the ROI was highlighted using the built-in software (Fig. 1D). The magnesium alloy was considered
as bone tissue to assess degradation of the implant. Similar to the
bone volume fraction, the Mg volume fraction was applied as:
Degraded alloy pin volume/pin volume before implantation. The BMD
and TMD reflected the Mg cylinder mineral and CT image densities,
respectively. However, unlike natural bone, the pins had no
trabecular, and thus the trabecular meant the thickness of the pin
was calculated from the radius of its cross-section. To investigate
new bone formation, the previous ROI was replaced with a larger ROI
(diameter 2.5 mm, length 6.5 mm) in the same shape and position
(Fig. 1E), from which new bone
formation and the stimulatory effects on the growth of new tissue
were observed. The threshold was then set at 1,000 and the ROI was
highlighted with the built-in software (Fig. 1F). The maximum inner diameter of the
new bone loop in the cross-section of the magnesium pin was
selected, and the diameter of the pin was measured with the
built-in software at the same position. Magnesium degradation and
bone ingrowth were subsequently investigated.
Histological processing
The femoral condyles were excised and fixed in a 10%
buffered neutral formalin solution for 1 week at room temperature
at weeks 4, 24 and 48. Following decalcification, the samples were
cut in half longitudinally and embedded in paraffin wax. Central
sections (5 µm thick) were cut from the femoral condyle with a
Leitz 1512 microtome (Leica Microsystems GmbH, Wetzlar, Germany)
and stained at room temperature for 10 min with hematoxylin and
eosin (H&E) for histological examination. Microscopic images
were captured using a light microscope (dotSlide Virtual Slide
System; Olympus Corporation, Tokyo, Japan) at ×40, ×100 and ×200
magnification.
Statistical analysis
Statistical analyses were performed using SPSS
software (version 22.0; IBM Corp., Armonk, NY USA). Experimental
values were expressed as the mean ± standard deviation and were
analyzed using an unpaired Student's t-test to determine
differences between the pre-implantation value and the
post-implantation values at each time point. P<0.05 was
considered to indicate a statistically significant difference.
Results
Changes in the weight of implant
material
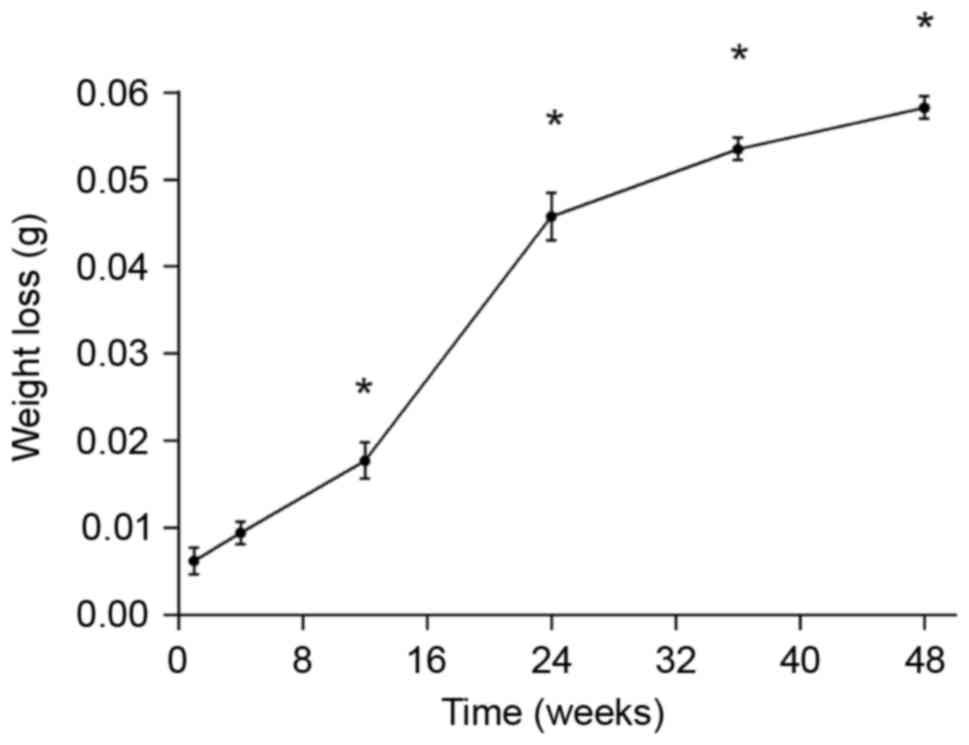
According to the weight change of pins at each time
point (Fig. 2), there was almost no
weight loss of the implants within the first week (−0.007 g).
However, by week 12, significant weight loss of the pins was
observed compared with their pre-implantation weights (−0.017 g;
P<0.05; Fig. 2). During weeks
12–24, the rate of weight loss was markedly increased when compared
with weeks 1–4 and 4–12. After week 24, the rate of weight loss
gradually decreased. This may be due to the production of new bone
during corrosion of the micro arc-oxidized surface at the later
time points.
Micro-CT evaluation of implant
degradation
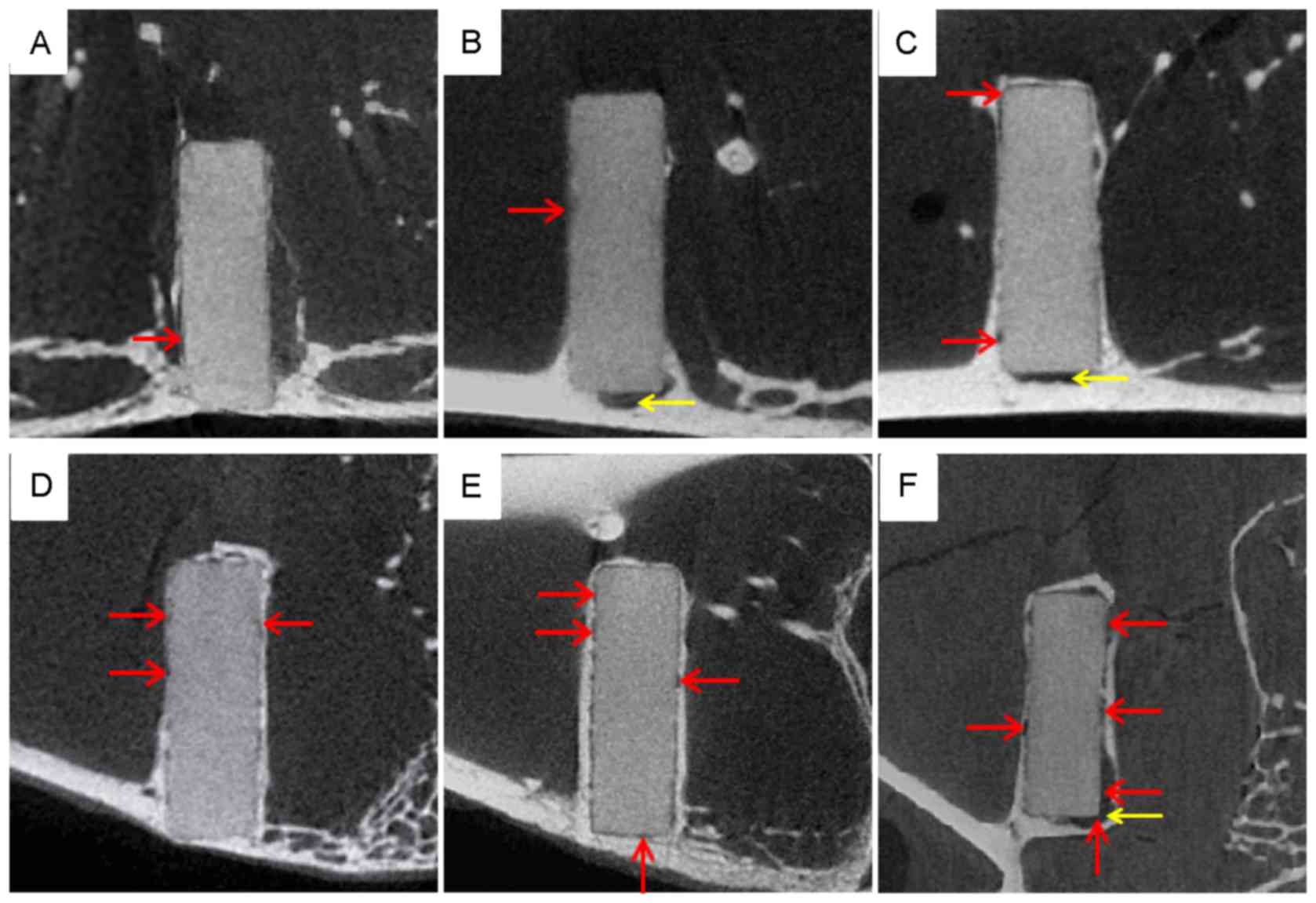
The micro-CT images in Fig. 3 provide examples of the degradation
process of an AZ31 implant at each time point. In the first week of
the first month, little corrosion was observed, and the boundary of
the pin was continuous and smooth (Fig.
3A). By week 4, the surface of the pin appeared blurred
(Fig. 3B), indicating that the rate
of degradation had increased compared with week 1. Corrosion
pitting became evident at week 12 (Fig.
3C), and during weeks 12–24 the surfaces of the pins were
surrounded by new bone tissue (Fig. 3C
and D). These results suggest that degradation of the implants
accelerated between weeks 4–24 and was markedly faster than that in
the first 4 weeks. From week 36, corrosion pitting became more
obvious, the boundary of the pin was inconclusive and the corners
of the pins became indistinct (Fig. 3E
and F). The images at this point suggested that degradation was
still proceeding and the area of corrosion pitting was expanding.
The acceleration of the degradation rate was observed at the
preexisting corrosion points, and there did not appear to be
corrosion at new positions on the surface of the pins. As indicated
in Fig. 3F, corrosion pitting was
evident on all surfaces and degradation was clear by week 48.
However, the micro-CT images also identified a small amount of
hydrogen gas around the pins at weeks 4, 12 and 48 only (Fig. 3B, C and F), however, no apparent
trend was observed.
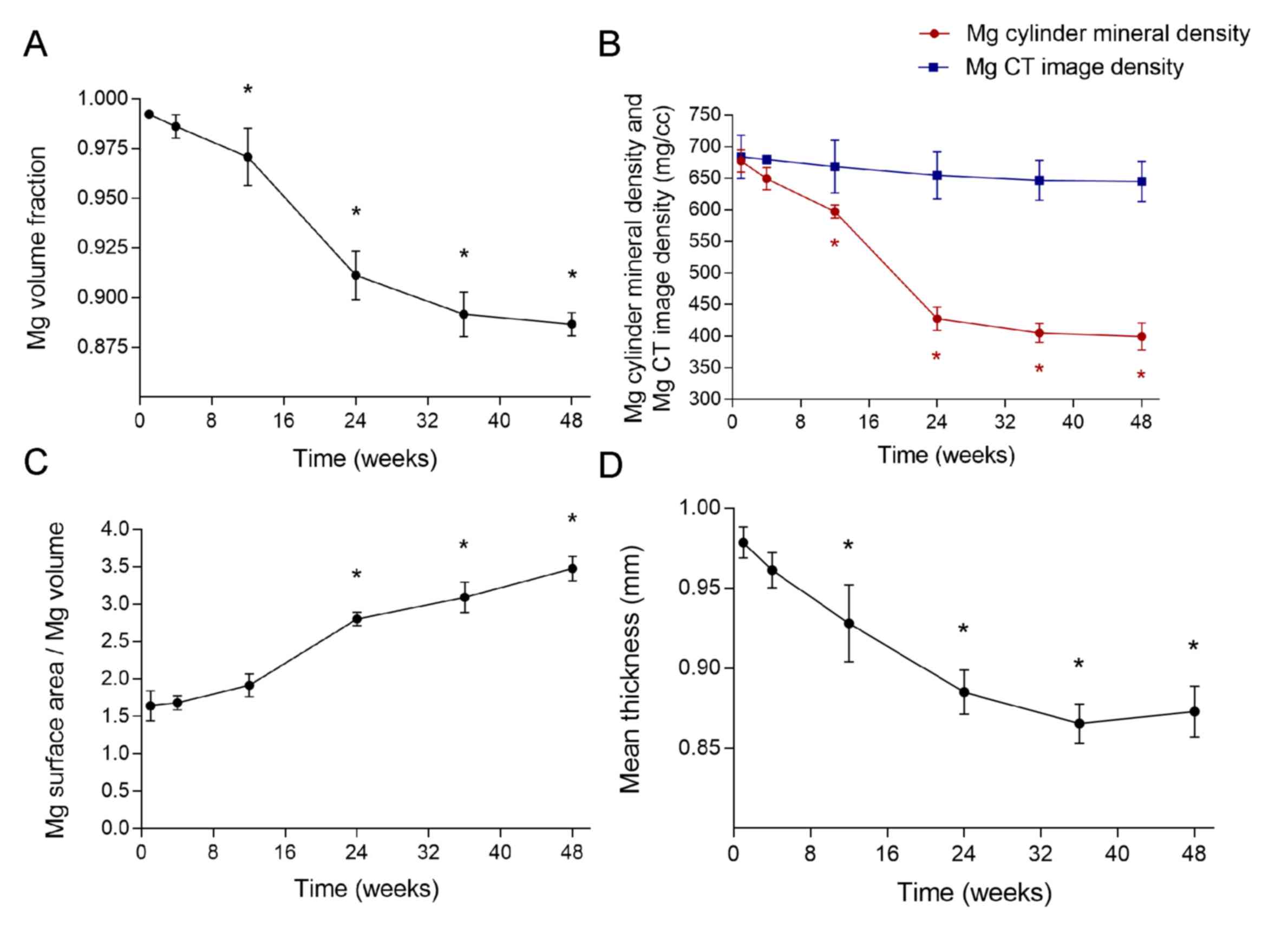
Similar to the micro-CT images, the data exported
from the built-in software of the micro-CT equipment provided
precise results (Fig. 4). The pin
volume fraction was ≥99.0% during the first month of the study,
indicating that degradation was slow and minor, and remained above
96.5% until week 12, after which the volume fractions were
significantly lower when compared with the pre-implantation value
(P<0.05; Fig. 4A). From week 12
to 24, the pin volume fraction decreased markedly faster than in
the first 3 months, and reached 91.2% by week 24. During this
period, the micro arc-oxidized surface was being degraded and new
bone tissue had not yet been sufficiently produced (Fig. 3A-D). After week 24, the rate of
decrease slowed, and during the final 24 weeks, pin volume fraction
only decreased by 2.25% (Fig. 4A).
This may have been due to enclosure of the residual pin by new bone
tissue, thus reducing contact between the metal and tissue
fluid.
The AZ31 magnesium alloy pin was regarded as bone to
analyze the BMD and TMD, which reflected the Mg cylinder mineral
and CT image densities, respectively. The Mg cylinder mineral
density continuously decreased throughout the study period,
degrading most rapidly between weeks 12 and 24. From week 12, the
Mg cylinder material densities were significantly decreased
compared with the pre-implantation value (P<0.05; Fig. 4B), and by week 48 it had reached
403.1424 mg/cc. By contrast, no significant change was observed in
the pin CT image density, which started at 683.6439 (week 1) and
ended at 644.9468 mg/cc (week 48; Fig.
4B). These results indicate that Mg content decreased as the
implant degraded, while the density of the material underwent
little change.
During degradation, the volume of the pin decreased,
most notably during weeks 12–24 (Fig.
4A). Furthermore, with corrosion pitting, the surface of the
pins became rough (Fig. 3C-F), which
may increase the superficial area. Thus, the ratio between the
surface area and volume of the pins increased throughout the study
period, and were significantly higher compared with the
pre-implantation value from week 24 (P<0.05; Fig. 4C). However, the surface area of the
pins was preserved during the first 4 weeks (Fig. 4C). Unlike natural bone, the pins had
no trabecular, and thus the trabecular mean thickness of the pin
was calculated from the radius of its cross-section, and
theoretically should be 1.0 mm. The computer creates a theoretical
line that automatically runs through the longitudinal axis of the
AZ31 pin, and the radius of its cross-section represents the pin's
mean thickness. During the period investigated, degradation of the
pins and increased corrosion pitting on the surface resulted in a
decrease in the pin radius. The thickness of the pre-implantation
pin was 1.0 mm and remained above 0.925 mm up to week 12, from
which point it was significantly decreased compared with the
pre-implantation value (P<0.05; Fig.
4D). During weeks 24–36, the thickness decreased more slowly
than in 1–24 weeks, and during weeks 36–48, a slight increase in
pin thickness was observed. This may have been due to the
stimulatory effects of magnesium on the growth of new bone tissue
at the metal corrosion loci.
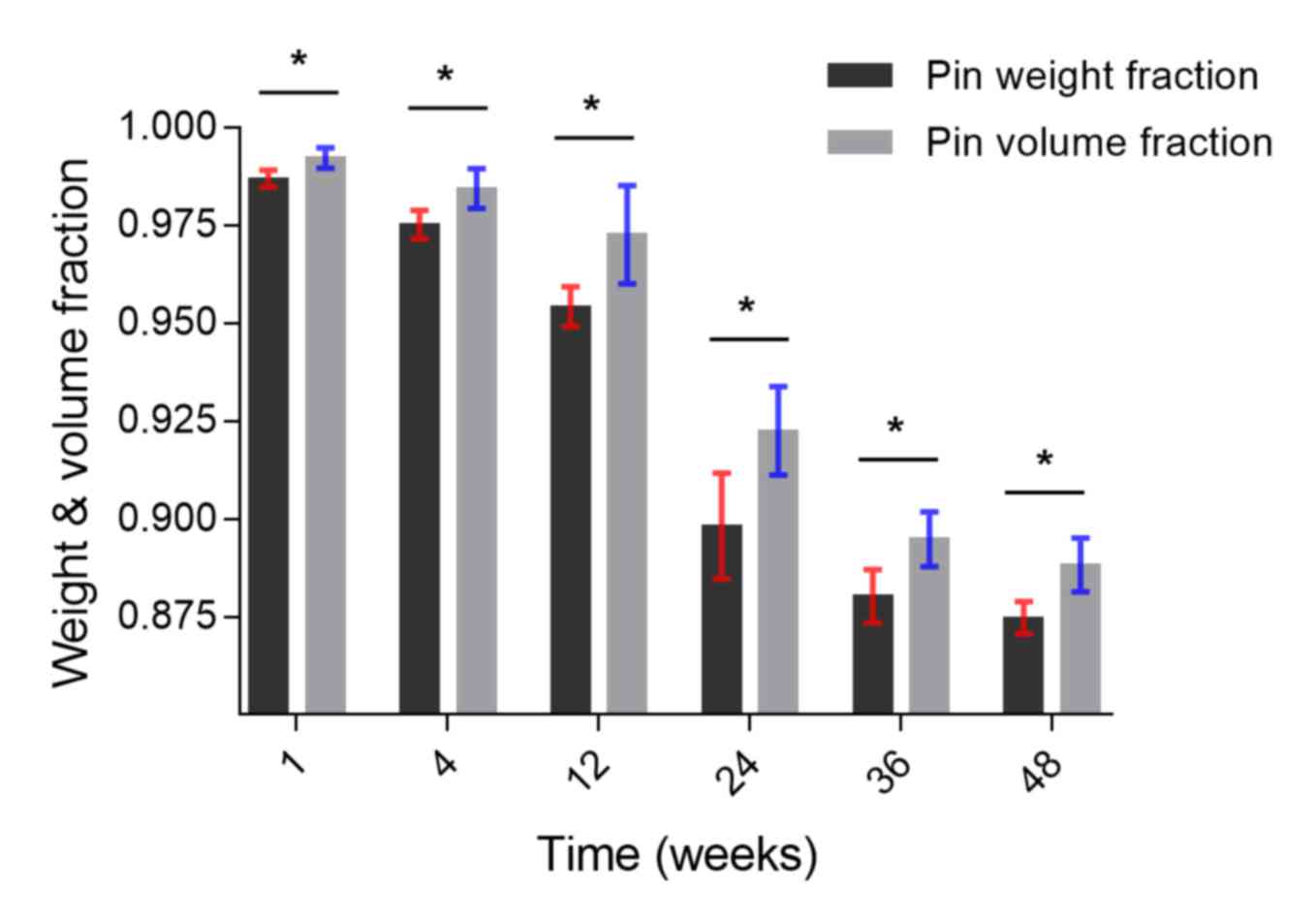
To analyze pin degradation, the changes in pin
weight fraction and volume fraction over the 48 weeks was plotted
(Fig. 5). The results of two methods
used (weighing and Micro-CT) to calculate amount of remaining pin
were similar: both the weight and volume of the pins decreased over
the 48 weeks, and during weeks 12–24, the decrease of the weight
and volume of the pin was markedly fast. However, the data were not
wholly consistent: at each time point, the quantity of magnesium
corrosion by weight was significantly greater than by volume
(P<0.05; Fig. 5).
Micro-CT evaluation of bone
formation
A new, larger ROI was subsequently selected in the
same shape and position as the original ROI (Fig. 1E), from which new bone formation and
the stimulatory effects on bone tissue growth were observed. The
threshold was set at 1,000, and the new ROI was highlighted with
the built-in software (Fig. 1F).
Before week 12, the majority of new bone formation occurred
longitudinally on the surface of the metal, and the new bone
appeared fragmentary and small (Fig. 6A
and B). From week 4, a small amount of new bone began to grow
transversely, and at the junction of the longitudinal and
transverse profiles (Fig. 6B).
Furthermore, the majority of new bone growth started at the bottom
of the pin, which was in contact with the host cortical bone
(Fig. 6B and C). From week 12, more
new bone was formed, and by week 48, the pin was almost surrounded
(Fig. 6C-E). The new bone growth was
progressive and increased in all directions.
The data exported from the built-in software of the
micro-CT equipment provided further precise results (Fig. 7). As indicated in Fig. 7A, from week 12, BVF values were
significantly increased compared with the pre-implantation value
(P<0.05). In addition, it was observed that osteogenesis
increased slowly before week 24, while from week 24 to 36, the rate
of new bone formation markedly increased (Fig. 7A). This may have been due to a faster
corrosion rate and the stimulatory effects of released magnesium on
the growth of new bone tissue. From week 36 to 48, the increase in
BVF gradually slowed (Fig. 7A). This
may be explained, to some extent, by the new bone preventing the
magnesium alloy from degrading, to the disadvantage of further new
bone formation. As new bone was produced, the superficial area of
the ROI increased, and the ratio between the superficial area and
volume of new bone (BS/BV) decreased, with significantly lower
values of BS/BV observed from week 24 (P<0.05; Fig. 7B). This indicates that the new bone
was gradually transforming from cribrate to compact. Fig. 7C-E illustrates the thickness,
separation, and number of trabecular in the new bone over time. As
the pin degraded, trabecular thickness and number significantly
increased and separation significantly decreased from week 12
onwards (P<0.05; Fig. 7C-E).
Concurrently to these changes, the TMD of new bone surrounding the
pin significantly increased (Fig.
7F; P<0.05), indicating that the number of bones and new
bone density increased as magnesium degraded.
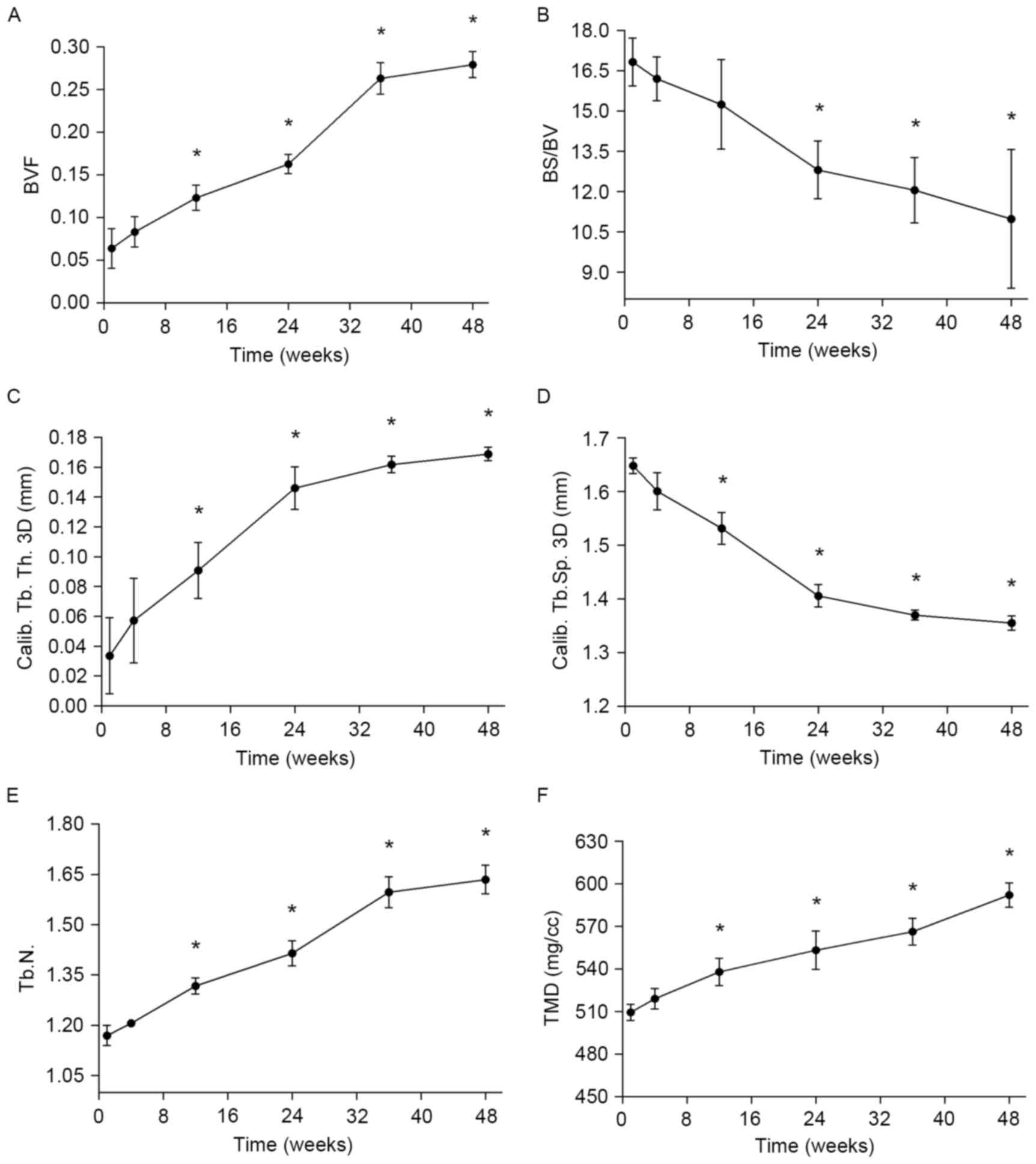 | Figure 7.In vivo bone formation around the
pins during the study period, assessed via micro-CT measurements.
(A) BVF, (B) BS/BV. (C) Calib. Tb.Th., (D) Calib. Tb.Sp., (E) Tb.N
and (F) TMD of new bone. *P<0.05 vs. prior to implantation. BVF,
bone volume fraction; BS/BV, bone surface/bone volume; Calib,
calibrated; Tb., trabecular; Th., thickness; Sp., separation; N.,
number; TMD, tissue mineral density. |
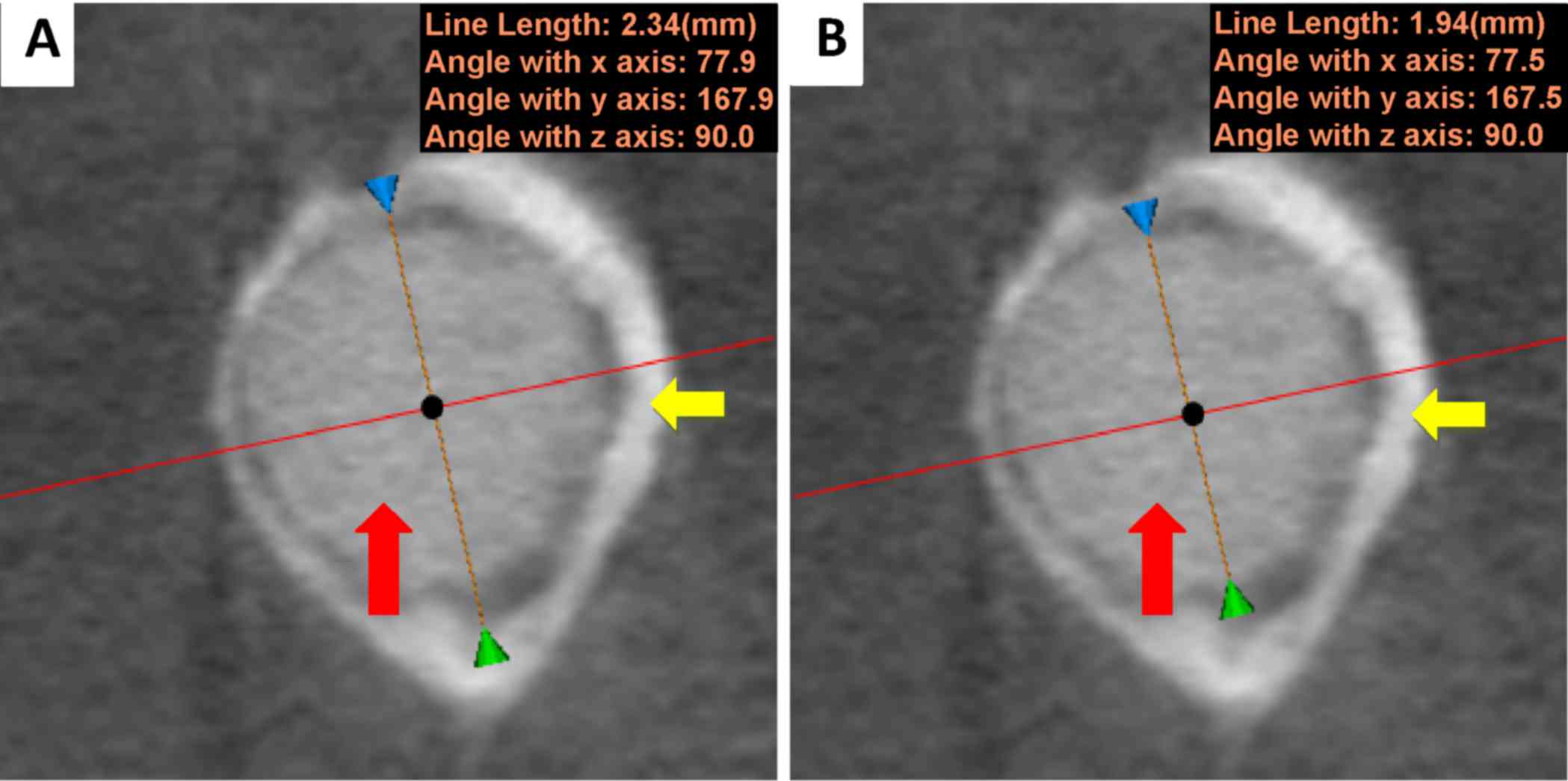
In the cross-section of the magnesium pin, the
maximum inner diameter of the new bone loop (Fig. 8A) and the diameter of the pin in the
same position (Fig. 8B) were
measured. During degradation, the diameter of the magnesium pin
decreased. Theoretically, a firm attachment between the pin and new
bone would cause the maximum inner diameter of the new bone loop to
diminish, possibly faster than the diameter of the pin. As depicted
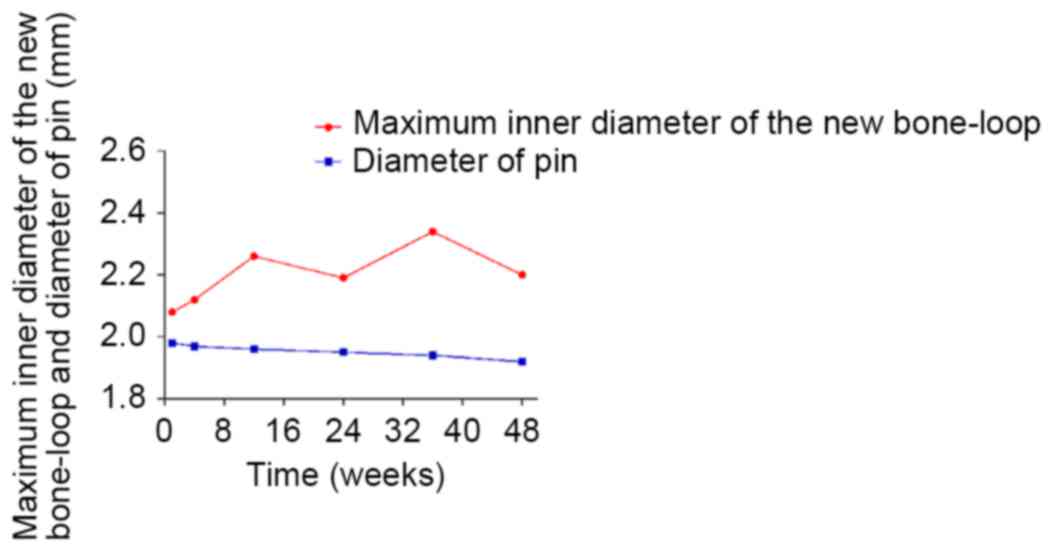
in Fig. 9, the diameter of the
magnesium pin decreased with degradation, from 1.98 mm in week 1 to
1.92 mm in week 48; however, no trend was observed in the maximum
inner diameter of the new bone loop (max: 2.34 mm, week 36; min:
2.08 mm, week 1), instead of the expected downtrend. The two curves
indicated that bridges had not been not adequately created by bone
formation between the pin and surrounding tissues, although some
bone and tissue contacts had appeared. It is probable that the
biomaterials were not firmly attached to the surrounding tissues
due to inadequate holding forces. This suggests that the magnesium
alloy was not capable of creating sufficient bridges between the
bones and biomaterials when there were preexisting gaps.
To investigate the association between material
degradation and novel bone formation, the changes in pin volume
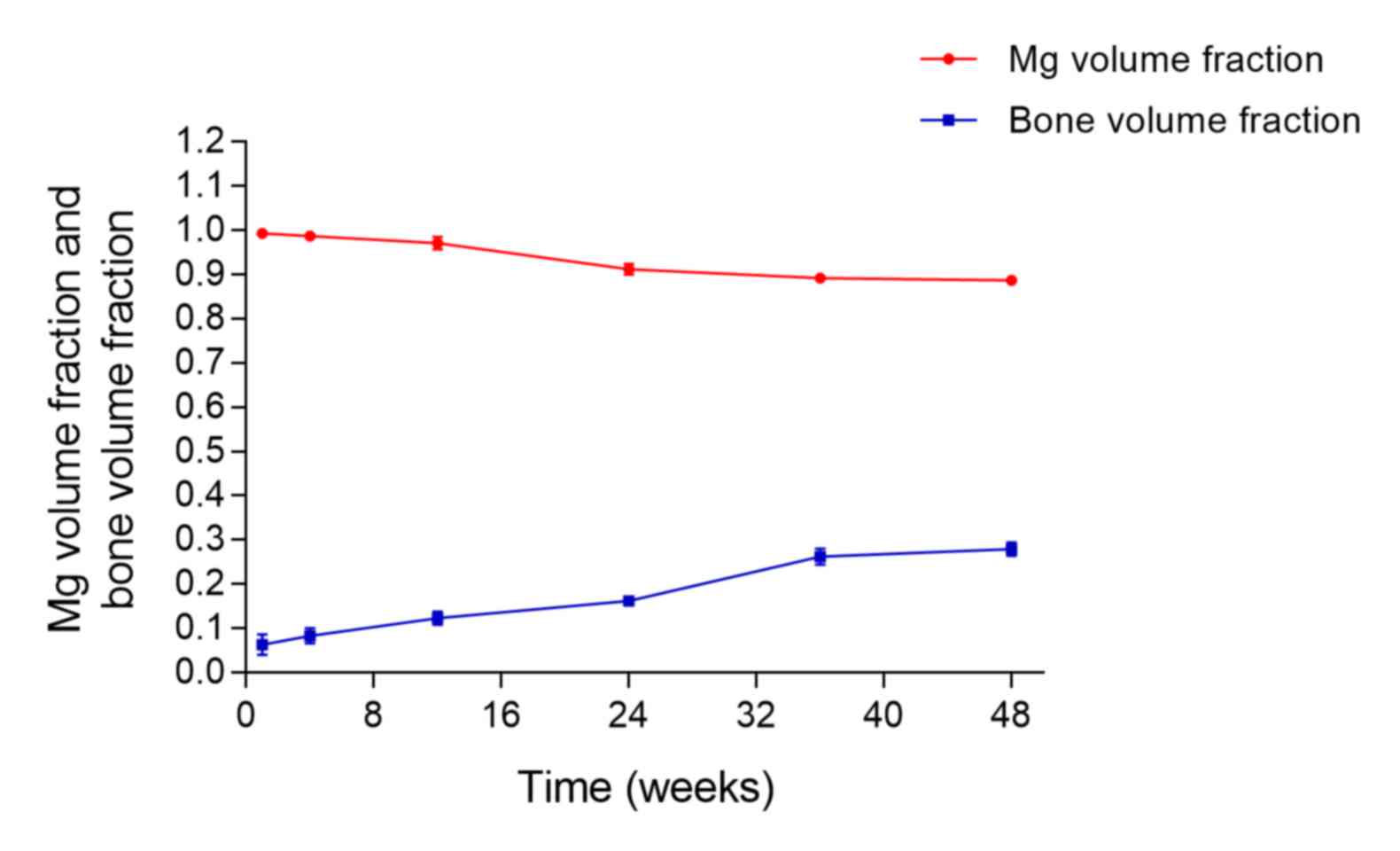
fraction and bone volume fraction with time were plotted (Fig. 10). During the experiment, both rates
were slow, and from week 1 to week 48, the volume of the magnesium
alloy at each time point decreased by 10.58% (from 99.23 to
88.65%), whereas the volume of novel bone had increased by 21.54%
(from 6.39 to 27.93%). By the end of the study, there was no
intersection of the two curves (Fig.
10).
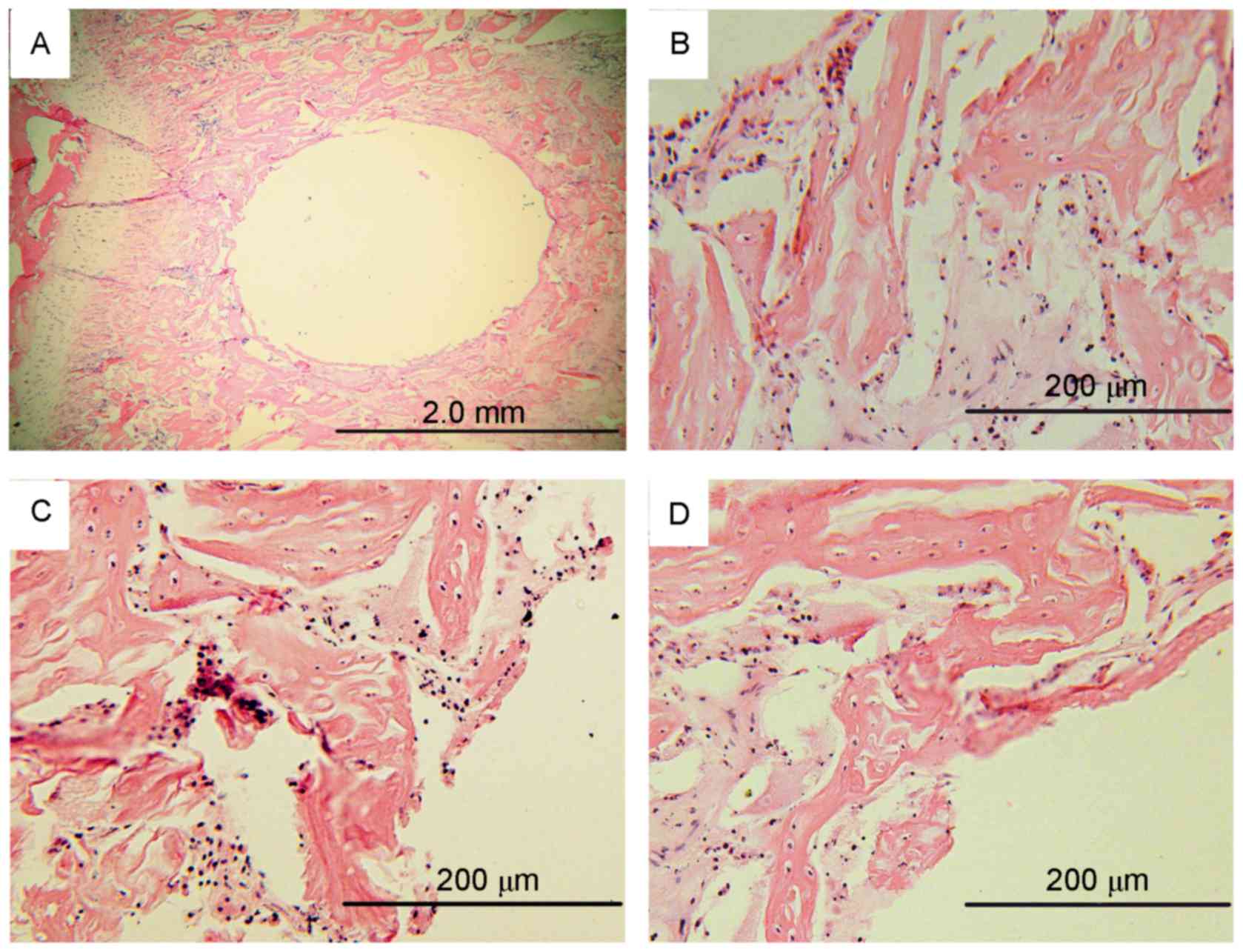
Histological evaluation
H&E staining was performed at weeks 4, 24 and
48. There were no apparent inflammatory cells surrounding the
implants; however, chondrocytes and osteocytes were observed. No
changes in the morphology of these cells between samples at 4, 24
and 48 weeks was observed (Fig.
11). Thus, there were no apparent inflammatory response around
the implants. These findings suggested that the AZ31 magnesium
alloys may be safe in human body.
Discussion
Metallic materials serve an essential role as
biomaterials for the repair or replacement of diseased bone.
Magnesium and its alloys have been investigated in recent years due
to their advantages over other materials, such as titanium alloys
and stainless steel (57,58). However, due to the electrochemical
activity of magnesium and its alloys in physiological solutions, it
is necessary to control the rate of corrosion for safe application
in the human body (59). Thus, the
development of suitable strategies to enhance the corrosion
resistance of magnesium alloys is important. A number of
preventative measures have been proposed and are being adopted to
overcome corrosion problems. While many previous studies have
focused on surface-modified magnesium alloys, protective coatings
must be nontoxic for orthopedic applications, and should ideally
improve the biocompatibility/bioactivity of the implant (1,3,59). In the present study, AZ31 magnesium
(alloyed with 3 wt.% Al, 1 wt.% Zn and 0.15 wt.% Mn) with a
micro-arc surface treatment was evaluated in vivo.
The degradation of the AZ31 magnesium alloy was
examined. It was demonstrated that, at 48 weeks, the total weight
loss of the magnesium was only 0.058 g, and the pin volume fraction
remained at 88.66%. This may have been due to the formation of new
bone around the pins, decreasing the rate of degradation at later
time points. In the first 4 weeks corrosion was slow, probably due
to protection by the micro arc-oxidized surface, whereas during
weeks 12 to 24, the surface of the magnesium alloy may have become
damaged, as indicated by an increase in corrosion. However, as the
alloy degraded, it is possible that high levels of released
magnesium stimulated the formation of new bone. After week 36, new
bone tissue around the pins formed an enclosed space, which may
have reduced contact between the pins and interstitial fluid and
again slowed degradation.
The weight fraction was calculated based on the
weight loss of the pins at each time point and compared with the
volume fraction. The two methods used to calculate the amount of
remaining pin were not wholly consistent; at each time point, the
quantity of magnesium corrosion by weight was greater than by
volume. Micro-CT is non-traumatic, which should improve the
accuracy of the results; however, the same precision cannot be
guaranteed with measurements based on weight loss. Prior to
weighing, pins were isolated from the femoral condyle, dry-machined
with cleaning tools, cleaned in ethanol in an ultrasonic bath and
dried in warm air (23). Each of
these steps may lead to a loss of material. Therefore, micro-CT was
selected as the gold standard in the present study. To investigate
the association between material degradation and new bone
formation, the changes in pin volume fraction and bone volume
fraction with time were plotted. During the experiment, both rates
were slow, and at the end of week 48, the volume of the magnesium
alloy had decreased by 10.58%, whereas the volume of new bone had
increased by 21.54%. However, by the end of the study, there was no
intersection of the two curves.
Magnesium exposed to a typical atmosphere will
develop a gray oxide film of magnesium hydroxide
[Mg(OH)2], which slows further corrosion (60,61).
Mg(OH)2 films are slightly soluble in water; however,
severe corrosion occurs in aqueous physiological environments where
chloride ions are present at levels (on the order of 150 mmol/l),
as Mg(OH)2 reacts with Cl− to form highly
soluble magnesium chloride and hydrogen gas (62). The following reactions summarize the
corrosion of magnesium (1,63):
Mg(s)+2H2O(aq) ↔
Mg(OH)2(s)+H2(g) (i)
Equations (ii)-(iv) show the partial reactions:
Mg(s) ↔
Mg2+(aq)+2e− (anodic reaction;
ii)
2H2O(aq)+2e− ↔
H2(g)+2 OH− (aq) (cathodic
reaction; iii)
Mg2+(aq)+2OH−(aq) ↔
Mg(OH)2(s) (product formation; iv)
This hydrogenation results in an alkaline
environment that raises the pH, which is harmful to cells and
decreases cell viability (64).
In the present study, implant degradation occurred
at a slower rate than expected. This potentially reduced the rate
of hydrogen gas production, and enabled the hydrogen to be absorbed
before it accumulated (65). The
micro-CT images identified a small amount of hydrogen gas around
the pins at weeks 4, 12 and 48 only, and no apparent trend was
observed.
The micro-CT results included both images and
numerical data. The images indicated the shape of the pins and the
corrosion pitting on the surface (black spots on the white surface
with high signals) at each time point, and new bone formation
around the pins. The numerical data indicated that the degradation
of magnesium pins had undergone a ‘slow-quick-slow’ process, and
that magnesium had stimulatory effects on the growth of new bone
tissue.
Micro-CT imaging and data analysis were used to
assess the consequences of degradation of micro-arc-oxidized AZ31
magnesium alloy used in bone implants. It also identified
increasing amounts of new bone around the alloy during the
experiment, and as the magnesium degraded, both the number of bones
and new bone density increased. The micro-CT data demonstrated
decreases in pin volume, mineral density, mean ‘pin thickness’,
BS/BV, Tb.Sp, and increases in pin surface area/pin volume, BVF,
Tb.Th, Tb.N and TMD, indicating a positive effect of magnesium on
osteogenesis. However, the data indicated that the magnesium alloy
was not capable of creating sufficient bridges between the bones
and biomaterials when there were preexisting gaps. In terms of the
biological safety, there were no apparent inflammatory responses
around the implants.
Overall, the results of the present study suggest
that the AZ31 magnesium had a long degradation period. Further
experiments should be performed to explore the degradation of AZ31
pins lacking prior surface treatment and the biomechanics of
magnesium alloys.
Acknowledgements
The present study was supported by the National
Science Foundation of China (grant nos. 81572148 and 51361130034),
the National Key Basic Research Development Program (973 Program;
grant no. 2012CB518106), the People's Liberation Army 12th
Five-Year Plan Period (grant no. BWS11J025), the People's
Liberation Army Key Scientific Research Program (grant no.
BWS13C029) and the Beijing Science and Technology Development
Foundation (grant no. Z141107004414044). The authors would like to
thank the Institutes for Food and Drug Control of China, the AVIC
Beijing Institute of Aeronautical Materials, Beijing Fule Science
& Technology Development Co., Ltd. and Beijing KEYU Animal
Experiment Center.
References
|
1
|
Staiger M, Pietak AM, Huadmai J and Dias
G: Magnesium and its alloys as orthopedic biomaterials: A review.
Biomaterials. 27:1728–1734. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mantovani D and Witte F: The attraction of
a lightweight metal with mechanical properties suitable for many
applications brought a renewed focus on magnesium alloys in the
automotive and aerospace industries. Acta Biomater. 6:16792010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gordon LM and Joester D: Mapping residual
organics and carbonate at grain boundaries and the amorphous
interphase in mouse incisor enamel. Front Physiol. 6:572015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vladimirov BV, Krit BL, Lyudin VB,
Morozova NV, Rossiiskaya AD, Suminov IV and Epel'feld AV: Microarc
oxidation of magnesium alloys: A review. Surface Eng Applied
Electrochemistry. 50:195–232. 2014. View Article : Google Scholar
|
|
5
|
Windhagen H, Radtke K, Weizbauer A,
Diekmann J, Noll Y, Kreimeyer U, Schavan R, Stukenborg-Colsman C
and Waizy H: Biodegradable magnesium-based screw clinically
equivalent to titanium screw in hallux valgus surgery: Short term
results of the first prospective, randomized, controlled clinical
pilot study. Biomed Eng Online. 12:622013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yamako G, Chosa E, Totoribe K, Watanabe S
and Sakamoto T: Trade-off between stress shielding and initial
stability on an anatomical cementless stem shortening: In-vitro
biomechanical study. Med Eng Phys. 37:820–825. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zielinski SM, Heetveld MJ, Bhandari M,
Patka P and Van Lieshout EM: FAITH Trial Investigators: Implant
removal after internal fixation of a femoral neck fracture: Effects
on physical functioning. J Orthop Trauma. 29:e285–e292. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wong K, Yeung K, Lam J, Chu P, Luk K and
Cheung K: Reduction of corrosion behavior of magnesium alloy by PCL
surface treatment. Ors Ann Meeting. 2009;
|
|
9
|
Liu GY, Hu J, Ding ZK and Wang C:
Bioactive calcium phosphate coating formed on micro-arc oxidized
magnesium by chemical deposition. App Surface Sci. 257:2051–2057.
2011. View Article : Google Scholar
|
|
10
|
Kraus T, Fischerauer SF, Hänzi AC,
Uggowitzer PJ, Löffler JF and Weinberg AM: Magnesium alloys for
temporary implants in osteosynthesis: In vivo studies of their
degradation and interaction with bone. Acta Biomater. 8:1230–1238.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang E: Phosphate treatment of magnesium
alloy implants forbiomedical applicationsSurface Modification of
Magnesium and its Alloys for Biomedical Applications. Narayanan
TSNS, Park IS and Lee MH: Woodhead Publishing; Waltham, MA: pp.
23–57. 2015, View Article : Google Scholar
|
|
12
|
Feng H, Wang G, Jin W, Zhang X, Huang Y,
Gao A, Wu H, Wu G and Chu PK: Systematic study of inherent
anti-bacterial properties of magnesium-based biomaterials. Acs App
Mater Interfaces. 8:9662–9673. 2016. View Article : Google Scholar
|
|
13
|
Warwick ME and Binions R: Advances in
thermochromic vanadium dioxide films. J Mat Chem A. 2:3275–3292.
2014. View Article : Google Scholar
|
|
14
|
Ko YM, Lee K and Kim BH: Effect of
functional groups on biodegradation and pre-osteoblastic cell
response on the plasma-polymerized magnesium surface. Jap J App
Phy. 52:20–21. 2013.
|
|
15
|
Sealy MP, Guo YB, Caslaru RC, Sharkins J
and Feldman D: Fatigue performance of biodegradable
magnesium-calcium alloy processed by laser shock peening for
orthopedic implants. Int J Fatigue. 82:428–436. 2016. View Article : Google Scholar
|
|
16
|
Sandip S, Asha K, Paulin G, Hiren S,
Gagandeep S and Amit V: A comparative study of serum uric acid,
calcium and magnesium in preeclampsia and normal pregnancy. J Adv
Res Biol Sci. 5:55–58. 2013.
|
|
17
|
Yoshizawa S, Brown A, Barchowsky A and
Sfeir C: Magnesium ion stimulation of bone marrow stromal cells
enhances osteogenic activity, simulating the effect of magnesium
alloy degradation. Acta Biomater. 10:2834–2842. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoshizawa S, Brown A, Barchowsky A and
Sfeir C: Role of magnesium ions on osteogenic response in bone
marrow stromal cells. Connect Tissue Res. 55 Suppl 1:S155–S159.
2014. View Article : Google Scholar
|
|
19
|
Castellani C, Lindtner RA, Hausbrandt P,
Tschegg E, Stanzl-Tschegg SE, Zanoni G, Beck S and Weinberg AM:
Bone-implant interface strength and osseointegration: Biodegradable
magnesium alloy versus standard titanium control. Acta Biomater.
7:432–440. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Johnson I, Akari K and Liu H:
Nanostructured hydroxyapatite/poly(lactic-co-glycolic acid)
composite coating for controlling magnesium degradation in
simulated body fluid. Nanotechnology. 24:3751032013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Barfield WR, Colbath G, Desjardins JD, An
Yuehuei H, Hartsock and Langdon A: The potential of magnesium alloy
use in orthopaedic surgery. Curr Orthopaedic Practice. 23:146–150.
2012. View Article : Google Scholar
|
|
22
|
Seyedraoufi ZS and Sh M: Synthesis,
microstructure and mechanical properties of porous Mg-Zn scaffolds.
J Mech Behav Biomed Mater. 21:1–8. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fischerauer SF, Kraus T, Wu X, Tangl S,
Sorantin E, Hänzi AC, Löffler JF, Uggowitzer PJ and Weinberg AM: In
vivo degradation performance of micro-arc-oxidized magnesium
implants: A micro-CT study in rats. Acta Biomater. 9:5411–5420.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Song G: Control of biodegradation of
biocompatable magnesium alloys. Corrosion Sci. 49:1696–1701. 2007.
View Article : Google Scholar
|
|
25
|
Chaya A, Yoshizawa S, Verdelis K, Myers N,
Costello BJ, Chou DT, Pal S, Maiti S, Kumta PN and Sfeir C: In vivo
study of magnesium plate and screw degradation and bone fracture
healing. Acta Biomater. 18:262–269. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li H, Pan H, Ning C, Tan G, Liao J and Ni
G: Magnesium with micro-arc oxidation coating and polymeric
membrane: An in vitro study on microenvironment. J Mater Sci Mater
Med. 26:1472015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang YS and Liu HW: TEM Analysis on
Micro-Arc Oxide Coating on the Surface of Magnesium Alloy. J Mat
Eng Perf. 20:463–467. 2011. View Article : Google Scholar
|
|
28
|
Ma WH, Liu YJ, Wang W and Zhang YZ:
Improved biological performance of magnesium by micro-arc
oxidation. Braz J Med Biol Res. 48:214–225. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Pan YK, Chen CZ, Wang DG and Yu X:
Microstructure and biological properties of micro-arc oxidation
coatings on ZK60 magnesium alloy. J Biomed Mater Res B Appl
Biomater. 100:1574–1586. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Han P, Tan M, Zhang S, Ji W, Li J, Zhang
X, Zhao C, Zheng Y and Chai Y: Shape and Site dependent in vivo
degradation of Mg-Zn pins in rabbit femoral condyle. Int J Mol Sci.
15:2959–2970. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Marukawa E, Tamai M, Takahashi Y,
Hatakeyama I, Sato M, Higuchi Y, Kakidachi H, Taniguchi H, Sakamoto
T, Honda J, et al: Comparison of magnesium alloys and
poly-l-lactide screws as degradable implants in a canine fracture
model. J Biomed Mater Res B Appl Biomater. 104:1282–1289. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Victor SP and Muthu J: Bioactive,
mechanically favorable, and biodegradable copolymer nanocomposites
for orthopedic applications. Mater Sci Eng C Mater Biol Appl.
39:150–160. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Walker J, Shadanbaz S, Woodfield TB,
Staiger MP and Dias GJ: Magnesium biomaterials for orthopedic
application: A review from a biological perspective. J Biomed Mater
Res B Appl Biomater. 102:1316–1331. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yeung KW and Wong KH: Biodegradable
metallic materials for orthopaedic implantations: A review. Technol
Health Care. Sep 6–2012.(Epub ahead of print). PubMed/NCBI
|
|
35
|
Niu Y, Dong W, Guo H, Deng Y, Guo L, An X,
He D, Wei J and Li M: Mesoporous magnesium silicate-incorporated
poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone)
bioactive composite beneficial to osteoblast behaviors. Int J
Nanomedicine. 9:2665–2675. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hofstetter J, Martinelli E, Weinberg AM,
Becker M, Mingler B, Peter J, Uggowitzera J and Löffler JF:
Assessing the degradation performance of ultrahigh-purity magnesium
in vitro, and in vivo. Corrosion Sci. 91:29–36. 2015. View Article : Google Scholar
|
|
37
|
Witkowski M, Hubert J and Mazur A: Methods
of assessment of magnesium status in humans: A systematic review.
Magnes Res. 24:1632011.PubMed/NCBI
|
|
38
|
Schambach SJ, Bag S, Schilling L, Groden C
and Brockmann MA: Application of micro-CT in small animal imaging.
Methods. 50:2–13. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Vanderoost J and van Lenthe GH: From
histology to micro-CT: Measuring and modeling resorption cavities
and their relation to bone competence. World J Radiol. 6:643–656.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Karl-Göran Thorngren: Proceedings of the
Swedish Orthopedic Society Helsingborg, June 1–2, 1987. Acta Orth.
59:77–100. 1988. View Article : Google Scholar
|
|
41
|
Wang ZL, Yu S, Sether LA and Haughton VM:
Incidence of unfused ossicles in the lumbar facet joints: CT, MR,
and cryomicrotomy study. J Comput Assist Tomogr. 13:594–597. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kapadia RD, Stroup GB, Badger AM, Koller
B, Levin JM, Coatney RW, Dodds RA, Liang X, Lark MW and Gowen M:
Applications of micro-CT and MR microscopy to study pre-clinical
models of osteoporosis and osteoarthritis. Technol Health Care.
6:361–372. 1998.PubMed/NCBI
|
|
43
|
Ding M, Odgaard A and Hvid I: Accuracy of
cancellous bone volume fraction measured by micro-CT scanning. J
Biomech. 32:323–326. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Salmon P: Micro-CT 3D Image Analysis
Techniques for Orthopedic Applications: Metal Implant-to-Bone
Contact Surface and Porosity of BiomaterialsA Practical Manual For
Musculoskeletal Research. World Scientific Publishing Co. Pte.
Ltd.; Hackensack, NJ: pp. 583–603. 2008, View Article : Google Scholar
|
|
45
|
Rhee Y, Hur JH, Won YY, Lim SK, Beak MH,
Cui WQ, Kim KG and Kim YE: Assessment of bone quality using finite
element analysis based upon micro-CT images. Clin Orthop Surg.
1:40–47. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Agholme F, Li X, Isaksson H, Ke HZ and
Aspenberg P: Sclerostin antibody treatment enhances metaphyseal
bone healing in rats. J Bone Miner Res. 25:2412–2418. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang J, Bi L, Bai JP, et al: Comparative
study of micro-CT and histological section in bone morphometry.
Orth J Chin. 381–384. 009.(In Chinese).
|
|
48
|
Park CH, Abramson ZR, Taba M Jr, Jin Q,
Chang J, Kreider JM, Goldstein SA and Giannobile WV:
Three-dimensional micro-computed tomographic imaging of alveolar
bone in experimental bone loss or repair. J Periodontol.
78:273–281. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Buie HR, Campbell GM, Klinck RJ, MacNeil
JA and Boyd SK: Automatic segmentation of cortical and trabecular
compartments based on a dual threshold technique for in vivo
micro-CT bone analysis. Bone. 41:505–515. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Clark DP and Badea CT: Micro-CT of
rodents: State-of-the-art and future perspectives. Phys Med.
30:619–634. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Chang CY, Huang AJ and Palmer WE:
Radiographic evaluation of hip implants. Semin Musculoskeletal
Radiol. 19:12–20. 2015. View Article : Google Scholar
|
|
52
|
Ding W: Opportunities and challenges for
the biodegradable magnesium alloys as next-generation biomaterials.
Regen Biomater. 3:79–86. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Liu YJ, Yang ZY, Tan LL, Li H and Zhang
YZ: An animal experimental study of porous magnesium scaffold
degradation andosteogenesis. Braz J Med Biol Res. 47:715–720. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
World Health Organization (WHO), .
Strategic Initiative for Developing Capacity in Ethical Review.
WHO; Geneva: 2005
|
|
55
|
Wong RW, Rabie B, Bendeus M and Hägg U:
The effects of Rhizoma Curculiginis and Rhizoma Drynariae, extracts
on bones. Chin Med. 2:132007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Feldkamp LA, Goldstein SA, Parfitt AM,
Jesion G and Kleerekoper M: The direct examination of
three-dimensional bone architecture in vitro by computed
tomography. J Bone Miner Res. 4:3–11. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wang J, Witte F, Xi T, Zheng Y, Yang K,
Yang Y, Zhao D, Meng J, Li Y and Li W: Recommendation for modifying
current cytotoxicity testing standards for biodegradable
magnesium-based materials. Acta Biomater. 21:237–249. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Chen X, Geng Y and Pan F: Research
progress in magnesium alloys as functional materials. Rare Metal
Mat Eng. 45:2269–2274. 2016. View Article : Google Scholar
|
|
59
|
Chen Y, Xu Z, Smith C and Sankar J: Recent
advances on the development of magnesium alloys for biodegradable
implants. Acta Biomater. 10:4561–4573. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Weizbauer A, Kieke M, Rahim MI, Angrisani
GL, Willbold E, Diekmann J, Flörkemeier T, Windhagen H, Müller PP,
Behrens P and Budde S: Magnesium-containing layered double
hydroxides as orthopaedic implant coating materials An in vitro and
in vivo study. J Biomed Mater Res B Appl Biomater. 104:525–531.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Janning C, Willbold E, Vogt C, Nellesen J,
Meyer-Lindenberg A, Windhagen H, Thorey F and Witte F: Magnesium
hydroxide temporarily enhancing osteoblast activity and decreasing
the osteoclast number in peri-implant bone remodeling. Acta
Biomater. 6:1861–1868. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Salahshoor M and Guo Y: Biodegradable
orthopedic magnesium-calcium (MgCa) alloys, processing, and
corrosion performance. Materials (Basel). 5:135–155. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Weng L and Webster TJ: Nanostructured
magnesium has fewer detrimental effects on osteoblast function. Int
J Nanomedicine. 8:1773–1781. 2013.PubMed/NCBI
|
|
64
|
Hampp C, Ullmann B, Reifenrath J, Nina A,
Dina D, Dirk B, Jan-Marten S and Andrea ML: Research on the
biocompatibility of the new magnesium alloy LANd442-An in vivo
study in the Rabbit Tibia over 26 weeks. Advanced Engineering
Materials. 14:B28–B37. 2012. View Article : Google Scholar
|
|
65
|
Kuhlmann J, Bartsch I, Willbold E,
Schuchardt S, Holz O, Hort N, Höche D, Heineman WR and Witte F:
Fast escape of hydrogen from gas cavities around corroding
magnesium implants. Acta Biomater. 9:8714–8721. 2013. View Article : Google Scholar : PubMed/NCBI
|