Introduction
Systemic lupus erythematosus (SLE) is a
multisystemic, chronic inflammatory autoimmune disease of unknown
etiology. It is characterized by various pathogenic autoantibodies
and immune complexes as well as damage to multiple organ systems,
and the course of SLE is long followed by remission and acute
attack. The prevalence varies from 31–70/100,000 individuals in
China and from 20–70/100,000 individuals worldwide. It is
sex-associated, occurring nine times more often in women than in
men, particularly in women of child-bearing age (15–35 years)
(1–3). The interaction between certain genetic
and environmental factors, including chemical factors, viruses and
drugs, damages normal immune tolerance and contributes to SLE.
Dysfunctional T cells interact with new antigens constantly,
leading to a persistent autoimmune reaction (4,5).
Previous studies have confirmed that the abnormal activation and
proliferation of self-reactive cluster of differentiation
(CD)4+ T lymphocytes have a central role in SLE.
CD4+ T cells interact with antigen-specific B cells, to
make the latter ones become more effective and produce
autoantibodies. In addition, CD4+ T cells produce
various cytokines when they are activated, which may engender
inflammatory reactions (6,7).
Long non-coding RNAs (lncRNAs) are a class of
non-protein-coding RNA with transcripts longer than 200
nucleotides. They have numerous important biological functions
through different molecular mechanisms, and are closely associated
with a variety of clinical diseases, including tumors, metabolic
disease and autoimmune diseases. Growth arrest-specific 5 (GAS5), a
non-coding gene that hosts a number of small nucleolar RNAs, has
been suggested to have numerous important roles in apoptosis and
cell growth inhibition. Previous studies have reported that human
GAS5 was upregulated in osteoarthritis (OA) patients (8) and downregulated in certain cancer
types, including breast cancer (9),
renal cell carcinoma (10) and
hepatocellular cancer (11). The
GAS5 gene locus in the mouse BXSB strain has been linked to
increased susceptibility to SLE (12). GAS5 competes with glucocorticoid (GC)
response elements (GRE) by interacting with the DNA binding domain
of glucocorticoid receptors (GRs). As GCs are potent
immunosuppressants, increased lncRNA GAS5 expression and activity
in immune or immune-accessory cells may suppress GC action and
contribute to the development of autoimmune diseases (13,14).
MicroRNAs (miRNAs) are endogenous non-coding
single-stranded RNAs of ~22 nucleotides in length, which regulate
gene expression by targeting mRNA for cleavage or translational
repression. miRNAs are involved in diverse biological processes,
including cell growth, differentiation, apoptosis and the stability
of the immune system. miR-21 was initially known as an ‘oncomiR’,
as it was identified to be tightly associated with oncogenesis.
Multiple studies have identified that miR-21 is overexpressed in
numerous diseases, including breast (15), brain (16), esophageal (17) and gastric cancers (18), cardiovascular diseases (19) and autoimmunity diseases (20). However, to the best of our knowledge,
studies relevant to GAS5 in CD4+ T cells of SLE patients
are still lacking. The present study aimed to investigate whether
the expression levels of GAS5 and miR-21 in CD4+ T cells
were abnormal in SLE patients, and the association of their levels
with clinical manifestations was assessed in an attempt to identify
novel molecular biomarkers involved in the pathogenesis of SLE.
Materials and methods
Patients and healthy controls
A total of 45 SLE patients (41 females, 4 males;
age, 34.1±1.2 years; disease duration, 4.2±0.6 years) were
recruited from the Rheumatology Department of Yijishan Hospital
(Wuhu, China). All patients met the 1982 American College of
Rheumatology classification criteria for SLE. SLE activity was
assessed using the SLE Disease Activity Index (SLEDAI-2K) (21). Furthermore, 30 control subjects (27
females, 3 males; age, 35.9±1.5 years) were frequency-matched with
the patients for age and sex. All participants were from of Han
Chinese ethnicity. Clinical information on the patients is listed
in Table I. The present study was
approved by the Research Ethics Board of Yijishan Hospital
Affiliated to Wannan Medical College (Wuhu, China). Written
informed consent was obtained from all study participants.
 | Table I.Clinical features of patients with
SLE. |
Table I.
Clinical features of patients with
SLE.
| Parameter | SLE patients
(n=45) | Control (n=30) |
|---|
| Age (years) | 34.1±1.2 | 35.9±1.5 |
| Sex (n) |
|
Female | 41 | 27 |
|
Male | 4 | 3 |
| Anti-dsDNA
(P/N)a | 20/23 | – |
| LN (P/N) | 23/22 | – |
| C3
levela |
| <80
mg/dl | 25 | – |
| ≥80
mg/dl | 17 | – |
| Disease duration
(years) | 4.2±0.6 | – |
| SLEDAI-2K
score | 11.4±1.1 | – |
| Medical
therapy |
|
|
|
Prednisone dose ≥30
mg/day | 21 | – |
|
Prednisone dose <30
mg/day | 24 | – |
|
Immunosuppressants
(P/N)b | 19/26 | – |
Isolation of CD4+ T cells
from peripheral blood and RNA processing
Peripheral blood samples were obtained from each
subject. The samples were collected in tubes containing Heparin
sodium. Peripheral blood mononuclear cells (PBMCs) were isolated
from anticoagulated whole blood by use of Ficoll density gradient
centrifugation. CD4+ T cells were purified from PBMCs by
magnetic-activated cell sorting, according to the manufacturer's
instructions. PBMCs were successively incubated with fluorescein
isothiocyanate (FITC) mouse anti-human CD4 antibody (BD Pharmingen,
Franklin Lakes, NJ, USA) and anti-FITC MicroBeads antibody
(Miltenyi Biotec, Bergisch Gladbach, Germany). Cell suspension was
applied onto a magnetic separation column (Miltenyi Biotec);
CD4+ T cells remained in the column and were collected
in buffer. The purity rate of the CD4+ T cells
(typically 92%) was detected using flow cytometry. Total RNA was
then extracted from CD4+ T cells using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The
concentration and purity of RNA were measured by SmartSpec™. Plus
spectrophotometry (A260:A280, >1.8) and the integrity of RNA was
checked by agarose gel electrophoresis with ethidium bromide
staining. The total RNA samples were kept at −80°C prior to
use.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
The RT reaction of GAS5 and miR-21 was performed
using a Thermo Scientific RevertAid First Strand cDNA Synthesis kit
(cat. no. k1622; Thermo Fisher Scientific, Inc.) and a miScript II
RT kit (cat. no. 218161; Qiagen, Hilden, Germany). The RT reaction
conditions for GAS5 were as follows: Initial incubation at 65°C for
5 min, then at 42°C for 50 min and 70°C for 15 min. The RT
conditions for miR-21 were 37°C for 60 min, 95°C for 5 min and a
holding step on ice. The total complementary (c)DNA samples were
kept at −20°C before use.
PCR amplification of cDNA of GAS5 and miR-21 was
performed using the CFX96 real-time system-C1000 thermal cycler
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Real-time PCR of
GAS5 and miR-21 was performed in duplicate or triplicate using a
QuantiNova SYBR-Green PCR kit (cat. no. 208052), and a miScript
SYBR-Green PCR kit (cat. no. 218073) (both from Qiagen),
respectively. The following primers were used: GAS5 forward,
5′-AGCTGGAAGTTGAAATGG-3′ and reverse, 5′-CAAGCCGACTCTCCATACC-3′;
β-actin forward, 5′-TGACGTGGACATCCGCAAAG-3′ and reverse,
5′-CTGGAAGGTGGACAGCAGGG-3′. The catalogue numbers for the miR-21
and U6 primers were MS00009079 and MS00033740 (both from Qiagen),
respectively. The reaction conditions for GAS5 contained an initial
heat activation step at 95°C for 5 min, followed by 40 cycles of
95°C for 15 sec and 60°C for 30 sec. Conditions for qPCR of miR-21
were as follows: Initial heat activation at 95°C for 15 min,
followed by 40 cycles of 95°C for 15 sec, 55°C for 30 sec and 70°C
for 30 sec. The dissociation curves of the used primer pairs were
generated to confirm a single peak. The mean of the quantification
cycle (Cq) was calculated for the reactions. The expression of GAS5
was compared between patients and control subjects by normalizing
to β-actin, and miR-21 was normalized to U6. Relative
quantification was performed using the 2−ΔΔCq method
(22).
Statistical analysis
All statistical analyses were performed using
GraphPad Prism version 5.0 software (GraphPad Software, La Jolla,
CA, USA). Values are expressed as the mean ± standard error of the
mean. Differences in gene expression between two groups were
assessed using a Mann-Whitney U test. A two-tailed P<0.05 was
considered to indicate a statistically significant difference.
Results
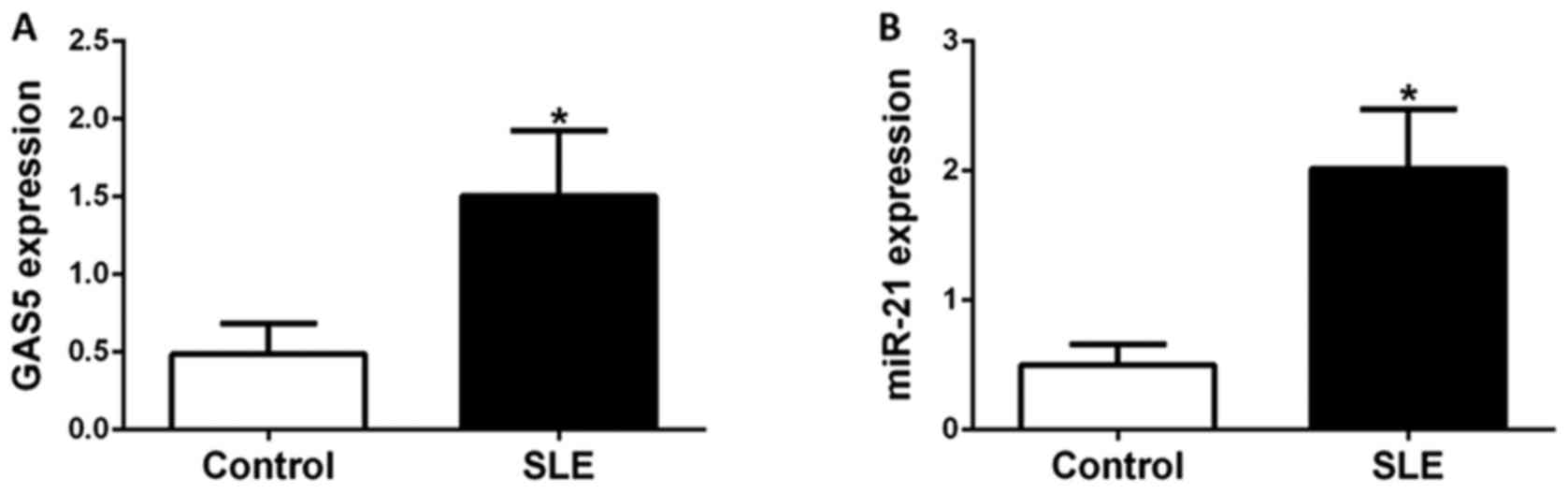
GAS5 and miR-21 expression levels in
CD4+ T cells of controls and SLE patients
CD4+ T cells obtained from 30 healthy
donors and 45 SLE patients were isolated for gene expression
analyses. The expression levels of GAS5 and miR-21 in
CD4+ T cells were evaluated by RT-qPCR. Patients and
control subjects were sex and age-matched. The average disease
duration of patients with SLE enrolled in the present study was 4.2
years, with a mean SLEDAI-2K score of 11.4. Anti-double-stranded
(ds)DNA, lupus nephritis (LN) and complement C3 levels are
important indicators of SLE disease activity and assessed by
SLEDAI-2K. In the present study, 20 patients had anti-dsDNA, 23
patients had LN and 25 had low levels of complement C3. GCs and
immunosuppressants are the two main types of drug for treating SLE.
In the present study, 21 patients were treated with prednisone
(dose, ≥30 mg/day) and 19 were treated with immunosuppressants
(Table I). GAS5 and miR-21
expression was significantly higher in patients with SLE than in
healthy donors (P<0.05). These results indicated that higher
expression of GAS5 and miR-21 was specific for SLE and that GAS5
and miR-21 may contribute to the pathogenesis of SLE (Fig. 1).
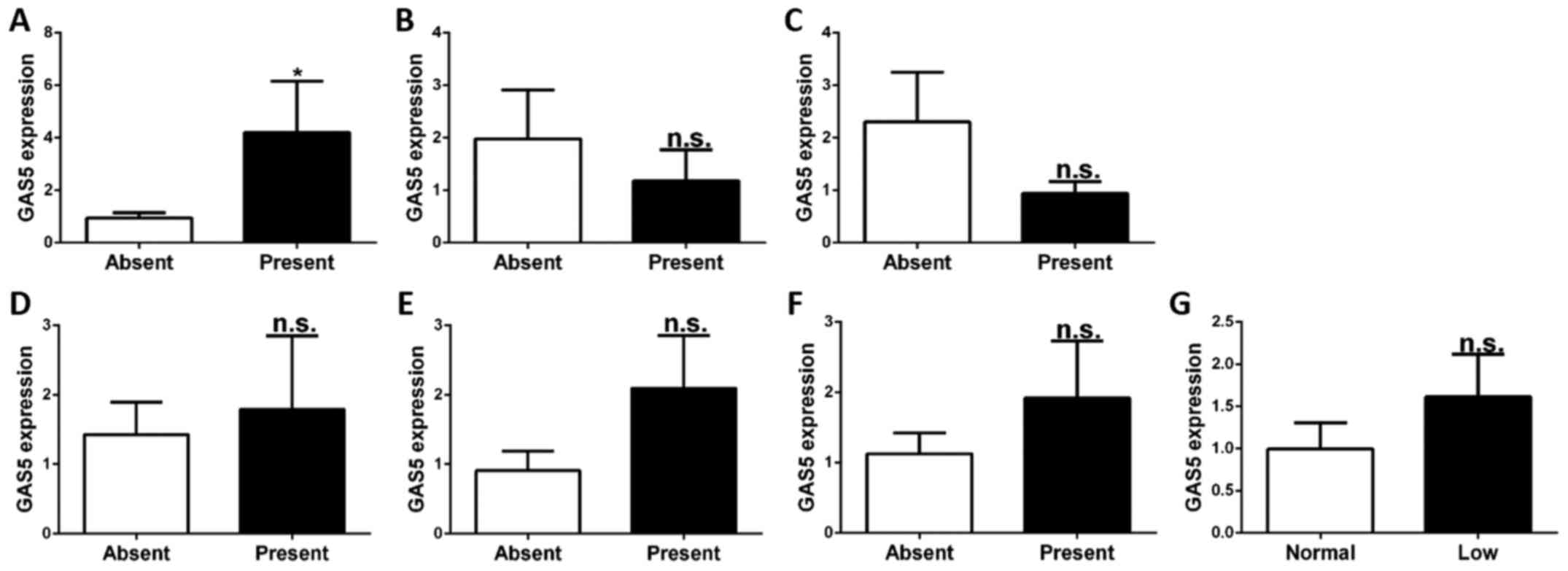
Association of GAS5 expression in
CD4+ T cells and clinical features
SLE is a systemic autoimmune disease with various
clinical features affecting various tissues. Certain clinical
features are correlated with disease activity and progression. To
investigate whether the expression of GAS5 in CD4+ T
cells is associated with these clinical features (nephritis,
arthritis, ulceration, pleurisy, rash, anti-dsDNA and complement
C3), SLE patients were divided into sets of two groups according to
the presence or absence of these respective clinical features.
Regarding GAS5 expression in each of these group pair sets, the
levels of GAS5 were higher in patients with ulceration than in
those without ulceration (P<0.05). However, there were no
significant differences in GAS5 expression regarding other clinical
features (nephritis, arthritis, pleurisy, rash, anti-dsDNA and
complement C3) (P>0.05; Fig.
2).
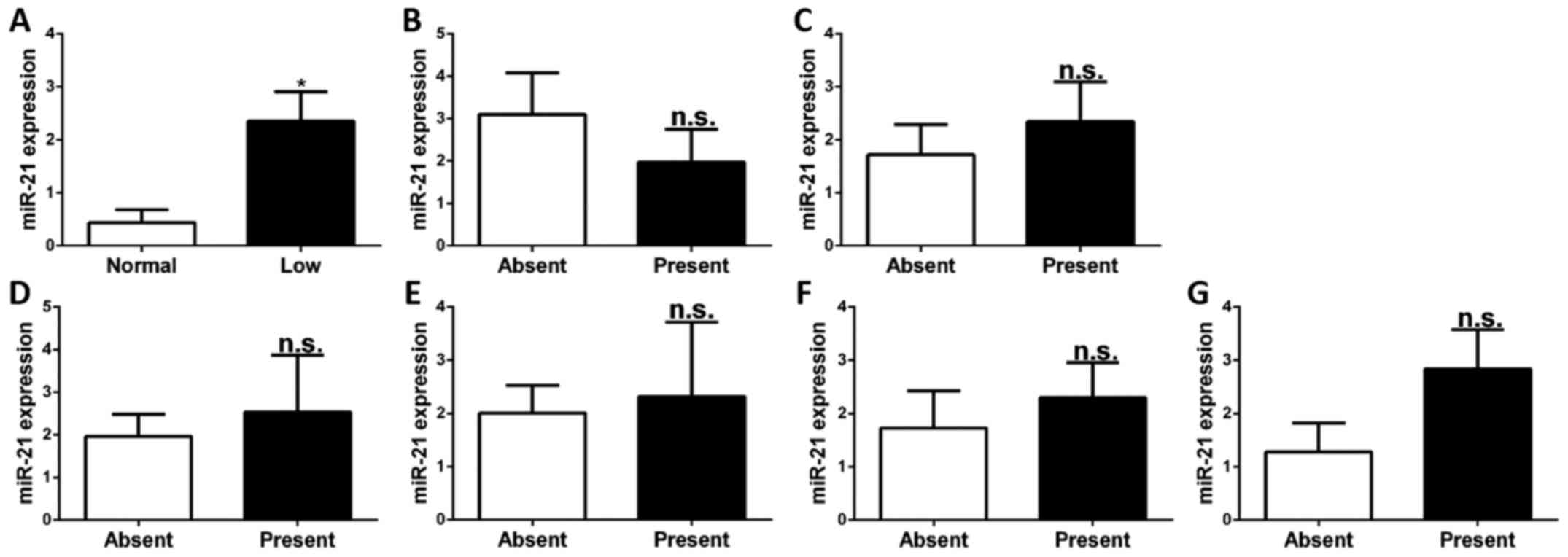
Association of miR-21 expression in
CD4+ T cells and clinical features
To investigate whether the expression of miR-21 in
CD4+ T cells is associated with the abovementioned
clinical features, the relative expression levels of miR-21 in
CD4+ T cells of SLE patients stratified by the presence
or absence of these clinical features were compared. It was
identified that the levels of miR-21 in CD4+ T cells of
patients with low levels of complement C3 were higher than in those
with normal levels of complement C3 (P<0.05). There was no
association between miR-21 expression and any of the other clinical
features, namely nephritis, arthritis, ulceration, pleurisy, rash
and anti-dsDNA (P>0.05; Fig.
3).
Discussion
SLE is an autoimmune disease with a complex and
unpredictable course. It is known that GCs are still the first-line
drugs for SLE. However, chronic high-dose hormone therapy may give
rise to adverse events and GC resistance. Previous studies have
demonstrated that aberrant expression and binding of the GR may be
associated with GC resistance in SLE patients, and that it may be
considered as a biomarker to personalize therapy (23,24).
Little is known about the influence of GAS5 on the susceptibility
for SLE and its prevention. The present study detected for lncRNA
GAS5 and miR-21, and investigated the association between their
expression levels and specific clinical features of SLE. The
results revealed that GAS5 and miR-21 levels were significantly
elevated in patients with SLE compared with those in control
subjects. The results regarding miR-21 were consistent with those
of a previous study (20). The
results of the present study indicated that GAS5 and miR-21
expressed in CD4+ T cells were specific for SLE and may
contribute to its pathogenesis. Among the clinical features of SLE,
ulceration and complement C3 levels are two indicators of disease
activity according to the SLE Disease Activity Index (SLEDAI-2K).
GAS5 levels in CD4+ T cells were identified to be higher
in patients with ulceration than in those without ulceration, and
miR-21 levels in CD4+ T cells were higher in patients
with low levels of complement C3 than in those with normal levels
of complement C3.
To the best of our knowledge, the present study was
the first to report an association of GAS5 and miR-21 in
CD4+ T cells with ulceration and complement C3 levels,
respectively, in patients with SLE. Although the detailed
mechanisms remain to be fully elucidated, GAS5 and miR-21 levels in
CD4+ T cells may be two key indicators of disease
activity in patients with SLE. The levels of GAS5 in
CD4+ T cells had an increasing trend in patients with
pleurisy, rash, anti-dsDNA and low complement C3, and the levels of
miR-21 in CD4+ T cells had an increasing trend in
patients with arthritis, ulceration, pleurisy, rash and anti-dsDNA;
however, there was no significance. All of these results suggested
that GAS5 and miR-21 levels in CD4+ T cells may be
useful for predicting the progression of SLE. It has been reported
that lncRNA GAS5 was negatively regulated by miR-21 in breast
tumors, hepatocellular carcinoma and osteoarthritis. GAS5 was also
capable of suppressing miR-21 through an lncRNA/miRNA interaction,
implying a feedback loop between GAS5 and miR-21 (8,25–27).
However, in the present study, GAS5 as well as miR-21 were
identified to be upregulated in the CD4+ T cells of SLE
patients, which may be associated with different types of diseases
and cells (8,28,29). In
the CD4+ T cells of SLE patients, the function of GAS5
may be primarily dependent on glucocorticoids signaling pathways
(13). Future study is required to
clarify the association between GAS5 and miR-21 in SLE.
GAS5 has been reported to be closely associated with
various human diseases (8,28,30).
GAS5 functions as a potential tumor suppressor and is downregulated
in several types of cancer (28).
GAS5 is also involved in the regulation of mammalian cell apoptosis
and cell population growth (31–33). The
downregulation of GAS5 protects T cell lines as well as
untransformed human T-lymphocytes (34). GAS5, a 5′-terminal oligopyrimidine
RNA, whose translation is specifically controlled by the mammalian
target of rapamycin pathway, is required for the inhibition of
human T cell proliferation by rapamycin and its analogues (35). The function of GAS5 is dependent on
its direct association with the GR protein; GAS5 binds to the GR
through mimicking GRE and acts as a decoy GRE, thus blocking the
upregulation of gene transcription. GR target genes are involved in
apoptosis suppression, such as cellular inhibitor of apoptosis 2
and serum/GC-regulated kinase 1, and inhibit the cell-death
executioners caspase-3, −7 and −9. As GCs are powerful
immunosuppressants, most of the known biological actions of GCs are
mediated by the GR. The present study identified that the GAS5
levels in SLE patients were higher than those in the control group,
indicating that increased lncRNA GAS5 expression in CD4+
T cells suppressed GC action and contributed to the development of
SLE (13,14).
Previous studies have suggested that miR-21
functions as an anti-apoptotic and pro-survival factor in numerous
cell types, and miR-21 was the only miRNA upregulated in all of the
tumor types analyzed (29,36). Programmed cell death protein 4
(PDCD4), novel tumor suppressor gene, is a direct target gene of
miR-21. A previous study supported that the miR-21/PDCD4 controlled
pathway has a central role in SLE (37). Aberrant DNA methylation was also
reported to be involved in the progression of SLE. The present
study found that miR-21 was overexpressed in CD4+ T
cells from patients with SLE, which promoted cell hypomethylation
by repressing DNA methyltransferase 1 expression, induced the
overexpression of autoimmune-associated methylation-sensitive genes
and mediated the pathogenesis of SLE (20).
In conclusion, the present study revealed that GAS5
and miR-21 levels in CD4+ T cells were significantly
elevated in patients with SLE compared with those in control
subjects. Regarding the clinical features of SLE, the expression of
GAS5 and miR-21 in CD4+ T cells was associated with
ulceration and low complement C3, respectively. GAS5 and miR-21 in
CD4+ T cells may serve as two potential biomarkers for
the diagnosis and prediction of the progression of SLE.
Acknowledgements
The present study was supported by the key research
grant of Wannan Medical College (grant no. WK20142F04).
References
|
1
|
Squatrito D, Emmi G, Silvestri E,
Ciucciarelli L, D'Elios MM, Prisco D and Emmi L: Pathogenesis and
potential therapeutic targets in systemic lupus erythematosus: From
bench to bedside. Auto Immun Highlights. 5:33–45. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu Y, Zhang F, Ma J, Zhang X, Wu L, Qu B,
Xia S, Chen S, Tang Y and Shen N: Association of large intergenic
noncoding RNA expression with disease activity and organ damage in
systemic lupus erythematosus. Arthritis Res Ther. 17:1312015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Y, Wu Z, Zhang S, Chen S, Li P, Li J,
Cao C, Liu B, Zhang F and Li Y: Genetic variants of IkappaB kinase
β (IKBKB) and polymerase β (POLB) were not associated with systemic
lupus erythematosus risk in a Chinese Han population. PLoS One.
10:e01325562015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kosalka J, Jakiela B and Musial J: Changes
of memory B- and T-cell subsets in lupus nephritis patients. Folia
Histochem Cytobiol. 54:32–41. 2016.PubMed/NCBI
|
|
5
|
Liu Y, Liao J, Zhao M, Wu H, Yung S, Chan
TM, Yoshimura A and Lu Q: Increased expression of TLR2 in CD4(+) T
cells from SLE patients enhances immune reactivity and promotes
IL-17 expression through histone modifications. Eur J Immunol.
45:2683–2693. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rottman JB and Willis CR: Mouse models of
systemic lupus erythematosus reveal a complex pathogenesis. Vet
Pathol. 47:664–676. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bakshi J, Ismajli M and Rahman A: New
therapeutic avenues in SLE. Best Pract Res Clin Rheumatol.
29:794–809. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Song J, Ahn C, Chun CH and Jin EJ: A long
non-coding RNA, GAS5, plays a critical role in the regulation of
miR-21 during osteoarthritis. J Orthop Res. 32:1628–1635. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pickard MR and Williams GT: The hormone
response element mimic sequence of GAS5 lncRNA is sufficient to
induce apoptosis in breast cancer cells. Oncotarget. 7:10104–10116.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Qiao HP, Gao WS, Huo JX and Yang ZS: Long
non-coding RNA GAS5 functions as a tumor suppressor in renal cell
carcinoma. Asian Pac J Cancer Prev. 14:1077–1082. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chang L, Li C, Lan T, Wu L, Yuan Y, Liu Q
and Liu Z: Decreased expression of long non-coding RNA GAS5
indicates a poor prognosis and promotes cell proliferation and
invasion in hepatocellular carcinoma by regulating vimentin. Mol
Med Rep. 13:1541–1550. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Haywood ME, Rose SJ, Horswell S, Lees MJ,
Fu G, Walport MJ and Morley BJ: Overlapping BXSB congenic
intervals, in combination with microarray gene expression, reveal
novel lupus candidate genes. Genes Immun. 7:250–263. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wapinski O and Chang HY: Long noncoding
RNAs and human disease. Trends Cell Biol. 21:354–361. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kino T, Hurt DE, Ichijo T, Nader N and
Chrousos GP: Noncoding RNA gas5 is a growth arrest- and
starvation-associated repressor of the glucocorticoid receptor. Sci
Signal. 3:ra82010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao Y, Cai Q, Huang Y, Li S, Yang H, Sun
L, Chen K and Wang Y: MicroRNA-21 as a potential diagnostic
biomarker for breast cancer patients: A pooled analysis of
individual studies. Oncotarget. 7:34498–34506. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Qu K, Lin T, Pang Q, Liu T, Wang Z, Tai M,
Meng F, Zhang J, Wan Y, Mao P, et al: Extracellular miRNA-21 as a
novel biomarker in glioma: Evidence from meta-analysis, clinical
validation and experimental investigations. Oncotarget.
7:33994–34010. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wu YR, Qi HJ, Deng DF, Luo YY and Yang SL:
MicroRNA-21 promotes cell proliferation, migration, and resistance
to apoptosis through PTEN/PI3K/AKT signaling pathway in esophageal
cancer. Tumour Biol. 37:12061–12070. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sekar D, Krishnan R, Thirugnanasambantham
K, Rajasekaran B, Islam VI and Sekar P: Significance of microRNA 21
in gastric cancer. Clin Res Hepatol Gastroenterol. 40:538–545.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jazbutyte V and Thum T: MicroRNA-21: From
cancer to cardiovascular disease. Current Drug Targets. 11:926–935.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Pan W, Zhu S, Yuan M, Cui H, Wang L, Luo
X, Li J, Zhou H, Tang Y and Shen N: MicroRNA-21 and microRNA-148a
contribute to DNA hypomethylation in lupus CD4+ T cells by directly
and indirectly targeting DNA methyltransferase 1. J Immunol.
184:6773–6781. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gladman DD, Ibañez D and Urowitz MB:
Systemic lupus erythematosus disease activity index 2000. J
Rheumatol. 29:288–291. 2002.PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lucafò M, Bravin V, Tommasini A,
Martelossi S, Rabach I, Ventura A, Decorti G and De Iudicibus S:
Differential expression of GAS5 in rapamycin-induced reversion of
glucocorticoid resistance. Clin Exp Pharmacol Physiol. 43:602–605.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Du J, Li M, Zhang D, Zhu X, Zhang W, Gu W,
Feng Y, Zhai X and Ling C: Flow cytometry analysis of
glucocorticoid receptor expression and binding in steroid-sensitive
and steroid-resistant patients with systemic lupus erythematosus.
Arthritis Res Ther. 11:R1082009. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hu L, Ye H, Huang G, Luo F, Liu Y, Liu Y,
Yang X, Shen J, Liu Q and Zhang J: Long noncoding RNA GAS5
suppresses the migration and invasion of hepatocellular carcinoma
cells via miR-21. Tumour Biol. 37:2691–2702. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pickard MR and Williams GT: Molecular and
cellular mechanisms of action of tumour suppressor GAS5 lncRNA.
Genes (Besel). 6:484–499. 2015. View Article : Google Scholar
|
|
27
|
Zhang Z, Zhu Z, Watabe K, Zhang X, Bai C,
Xu M, Wu F and Mo YY: Negative regulation of lncRNA GAS5 by miR-21.
Cell Death Differ. 20:1558–1568. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma C, Shi X, Zhu Q, Li Q, Liu Y, Yao Y and
Song Y: The growth arrest-specific transcript 5 (GAS5): A pivotal
tumor suppressor long noncoding RNA in human cancers. Tumour Biol.
37:1437–1444. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gao Y, Dai M, Liu H, He W, Lin S, Yuan T,
Chen H and Dai S: Diagnostic value of circulating miR-21: An update
meta-analysis in various cancers and validation in endometrial
cancer. Oncotarget. 7:68894–68908. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Carter G, Miladinovic B, Patel AA, Deland
L, Mastorides S and Patel NA: Circulating long noncoding RNA GAS5
levels are correlated to prevalence of type 2 diabetes mellitus.
BBA Clin. 4:102–107. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lu X, Fang Y, Wang Z, Xie J, Zhan Q, Deng
X, Chen H, Jin J, Peng C, Li H and Shen B: Downregulation of gas5
increases pancreatic cancer cell proliferation by regulating CDK6.
Cell Tissue Res. 354:891–896. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu Z, Wang W, Jiang J, Bao E, Xu D, Zeng
Y, Tao L and Qiu J: Downregulation of GAS5 promotes bladder cancer
cell proliferation, partly by regulating CDK6. PLoS One.
8:e739912013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mourtada-Maarabouni M and Williams GT:
Growth arrest on inhibition of nonsense-mediated decay is mediated
by noncoding RNA GAS5. Biomed Res Int. 2013:3580152013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hu G, Lou Z and Gupta M: The long
non-coding RNA GAS5 cooperates with the eukaryotic translation
initiation factor 4E to regulate c-Myc translation. PLoS One.
9:e1070162014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Williams GT, Mourtada-Maarabouni M and
Farzaneh F: A critical role for non-coding RNA GAS5 in growth
arrest and rapamycin inhibition in human T-lymphocytes. Biochem Soc
Trans. 39:482–486. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bertoli G, Cava C and Castiglioni I:
MicroRNAs: New biomarkers for diagnosis, prognosis, therapy
prediction and therapeutic tools for breast cancer. Theranostics.
5:1122–1143. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Pratheeshkumar P, Son YO, Divya SP, Wang
L, Turcios L, Roy RV, Hitron JA, Kim D, Dai J, Asha P, et al:
Quercetin inhibits Cr(VI)-induced malignant cell transformation by
targeting miR-21-PDCD4 signaling pathway. Oncotarget.
8:52118–52131. 2016.PubMed/NCBI
|