Introduction
Diabetic cataracts are an eye disease featured by
visual deficits and blindness. While diabetes itself is a common
medical condition, the elevated blood glucose levels that patients
are prone to are a major cause of complications, such as cataracts
(1). Disease data indicate a high
incidence rate of cataracts and a rapid disease progression, making
advanced diabetic patients susceptible to the threat of blindness
(2). Diabetic cataracts are similar
to senile cataracts in regards to the abnormal metabolism within
the lens, but the specific biomolecular mechanism of their
pathogenesis is not clear. There are presently three popular
theories on its pathogenesis (3), of
which oxidative stress is the most recognized by scholars. The
theory holds that there are a variety of antioxidant enzyme systems
in a normal functioning lens, including the well-documented
superoxide dismutase, glutathione peroxidase and others (4,5). On the
one hand, the high glucose environment in diabetic patients can
promote increased production of reactive oxygen species. On the
other hand, the regulation of key antioxidant factors can be
impaired in diabetics, leading to an imbalance between oxidation
and reduction and resulting in oxidative damage within the lens. It
has been confirmed that Nrf2 is a key factor in regulating the
balance of oxidation in vivo, through mediation of the
synthesis and expression of a series of downstream antioxidants via
the Nrf2/Keap1 signaling pathway (6).
Calcium dobesilate is a vascular protective agent
that is effective against microvascular circulatory disorders
caused by a variety of diseases, through improving the biosynthesis
of collagen in the basement membrane by inhibiting the high
permeability caused by vasoactive substances (histamine, serotonin
and bradykinin). Studies have shown that calcium dobesilate has a
certain controlling effect on cataracts (7). Clinical research on calcium dobesilate
for the treatment of diabetic cataract has been reported, but its
focus was on therapeutic effects rather than the underlying
mechanisms. The aim of this study was to investigate the effects of
calcium dobesilate on the expression of Nrf2, Keap1 and HO-1 in
rats with D-galactose-induced cataracts, in order to explore the
mechanisms of calcium dobesilate on the treatment of diabetic
cataracts.
Materials and methods
A LYL-I slit lamp micrometer was purchased from
Suzhou QILE Electronic Technology Co., Ltd. (Jiansu, China). A
−80°C ultra-low temperature refrigerator was obtained from Haier
Co., Ltd. (Qingdao, China). A QuantStudio® type 3
real-time fluorescence quantitative PCR system was purchased from
Thermo Fisher Scientific Co., Ltd. (Waltham, MA, USA). ECL
Chemiluminescence kit and PVDF Membrane were both purchased from
Invitrogen (Carlsbad, CA, USA). Thirty Sprague-Dawley (SD) male
rats were provided from Nanjing Qinglongshan Animal Technology Co.,
Ltd. (Nanjing, China). A tissue mRNA extraction kit was obtained
from EMD Millipore (Darmstadt, Germany). A reverse transcription
kit was purchased from Beijing Zhijie Fangyuan Technology Co., Ltd.
(Beijing, China). The primary antibodies of Nrf2, Keap1, HO-1 and
the secondary antibody were all purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA). The pairs of primers used
for the study are as follows: Nrf2 (163 bp) sense,
5′-CCTTCCTCTGCTGCCATTAGT-3′ and antisense,
5′-CCTTCCTCTGCTGCCATTAGT-3′; Keap1 (154 bp) sense,
5′-GGAATGCTATGACCCAGACA-3′ and antisense,
5′-TGCTCAGGTAGTCCAAGTGC-3′; HO-1 (144 bp) sense,
5′-AGCATGTCCCAGGATTTGTC-3′ and antisense,
5′-GTACAAGGAGGCCATCACCA-3′. All PCR primers were synthesized by
Shanghai Bioengineering Co., Ltd. (Shanghai, China).
Experimental groups
The experiment lasted for 21 days. Thirty male SD
rats were randomly divided into three groups: A blank control
group, a model control group, and a model administration group,
with 10 rats in each group. Group assignment was randomized using a
random number table grouping. Rats in the blank control group were
fed a normal diet with water and ordinary feed (nutrient ratio of
protein:fat:carbohydrate=2:1.5:4) over the course of the
experiment. Rats in the model groups were fed 12.5% D-galactose for
7 days from the beginning of the experiment and with 10%
D-galactose from day 8 until the end of the experiment. The rats in
the model administration group were also given calcium dobesilate
daily at a dose of 150 mg/kg/day by intragastric administration
from the first day of the study. The specific dosage for each rat
was calculated according to body weight and the administration
continued until the end of the study.
Specimen collection
The lens samples of the rats were collected for the
expression analysis of Nrf2, Keap1 and HO-1. Twenty-one days after
the study, rats in each group were fasted for 24 h. The rats were
sacrificed by intraperitoneal injection of 10% chloral hydrate and
the eyeball was removed. The lens was then rapidly separated and
homogenized under ice bath to make the lens homogenate, and then
stored at −80°C.
Degree of lens opacity
The degree of lens opacity was examined and
classified on the 21st day of the study using a slit lamp
microscope. The grading criteria for glucose-induced cataracts was
based on literature published by Suryanarayana et al
(8), which graded opacity into six
levels, with grade 0 showing no turbidity of the lens and grade V
indicating that the lens was completely turbid.
The expression of Nrf2, Keap1 and HO-1
mRNA in lens
The expression of Nrf2, Keap1 and HO-1 mRNA in lens
tissue was detected by real-time quantitative PCR. The target mRNA
in the rat lens homogenate was extracted using the RNA extraction
kit under stringent RNase-free and sterile conditions according to
the kit procedure. The RNA extraction process was kept at a low
temperature to prevent RNA degradation. The purity of the extracted
RNA was determined by spectrophotometer and the ratio of the
absorbance at 260 nm to the absorbance at 280 nm was between 1.8
and 2.0. The required samples were stored at −80°C under sterile
and RNase-free conditions. Reverse transcription was conducted
using the reverse transcription kit to synthesize the first strand
of cDNA in strict sterile conditions, with addition of the
reagents, followed by incubation at 40°C for 45 min and then
inactivation of reverse transcriptase at 90°C. Real-time
quantitative PCR was used to amplify and quantitatively detect the
expression of the three mRNAs in the lens samples. The mRNA
expression of the blank control group was taken as a control and
quantified as 1 to obtain the relative values of the other two
groups. PCR amplification cycle parameters were set as follows:
55°C for 2 min, 90°C for 10 min, 95°C for 15 sec, 58°C for 30 sec
and 72°C for 30 sec, repeated over 50 cycles. Amplification was
performed with the internal reference group and the blank control
group (DEPC water).
The expression of Nrf2, Keap1 and HO-1
protein in lens
The total protein of rat lens samples was extracted
and the standard curve of protein concentration was drawn by BCA
method to calculate the expression levels of protein to be
measured. The protein was loaded into SDS-PAGE gel for
electrophoresis and the protein was separated. After
electrophoresis, the protein was transferred to the membrane and
the membrane was taken out, washed and incubated with the primary
antibody and then the secondary antibody. The protein bands on the
membrane were detected by enhanced chemiluminescence and the gray
scale was scanned by an automatic gel imaging analyzer. The
relative gray value was then calculated as the expression levels of
the protein.
Statistical analysis
SPSS 17.0 statistical analysis software (IBM SPSS,
Armonk, NY, USA) was used to evaluate the differences of the
indices before and after treatment. The F-test was used for
comparison between groups and the rank data were analyzed by the
rank sum test. P<0.05 was considered to indicate a statistically
significant difference.
Results
Degree of lens opacity
A comparison of grading levels of lens opacity at 21
days for all three groups of rats is shown in Table I. All the lenses in the blank control
group rats were clarified and clear, with no turbidity, and the
degree of lens opacity for all was graded as 0. In the model
control group, the lenses in rats showed white turbidity, with no
boundary between the core and the cortex of the lens, and the
degree of lens opacity for most rats was graded as V, with a
minority of the rats graded as IV. The lenses of rats in the model
administration group showed turbidity, but the degree was
significantly lighter than that of the model control group. The
rats in the model administration group had mild white opacities in
the lens cortex, but the core was transparent. According to the
rank sum test, there was a significant difference in the degree of
lens opacity among the three groups (P<0.05).
 | Table I.Comparison of the opacity of lens
samples between the 3 groups (n=60). |
Table I.
Comparison of the opacity of lens
samples between the 3 groups (n=60).
| Groups | No. of eyes | 0 | I | II | III | IV | V |
|---|
| Blank control | 20 | 20 | 0 | 0 | 0 | 0 | 0 |
| Model control | 20 | 0 | 0 | 0 | 0 | 6 | 14 |
| Model
administration | 20 | 0 | 0 | 2 | 6 | 8 | 4 |
| F-value |
|
|
|
| 23.870a |
|
|
| P-value |
|
|
|
| <0.001 |
|
|
Expression of Nrf2, Keap1 and HO-1
mRNA in lens samples
The mRNA expression levels of Nrf2, Keap1 and HO-1
in the rat lens samples are shown in Table II. The mRNA expression levels of
Nrf2, Keap1 and HO-1 in the lenses of the three groups was
significantly different (P<0.05). The mRNA expression levels of
Nrf2 and HO-1 was the highest in the model control group, followed
by the model administration group, and the lowest in the blank
control group. The expression levels of Keap1 mRNA were the lowest
in the model control group, followed by the model administration
group, and the highest in the blank control group.
 | Table II.Comparison of expression of Nrf2,
Keap1 and HO-1 mRNA in lens samples among the 3 groups (n=30). |
Table II.
Comparison of expression of Nrf2,
Keap1 and HO-1 mRNA in lens samples among the 3 groups (n=30).
| Groups | n | Nrf2 | Keap1 | HO-1 |
|---|
| Blank control | 10 | 1 | 1 | 1 |
| Model control | 10 | 0.36±0.25 | 0.26±0.17 | 0.41±0.15 |
| Model
administration | 10 | 0.79±0.23 | 0.68±0.13 | 0.87±0.09 |
| F-value |
| 10.808a | 13.415a | 12.874a |
| P-value |
| <0.001 | <0.001 | <0.001 |
The expression of Nrf2, Keap1 and HO-1
protein in the lens
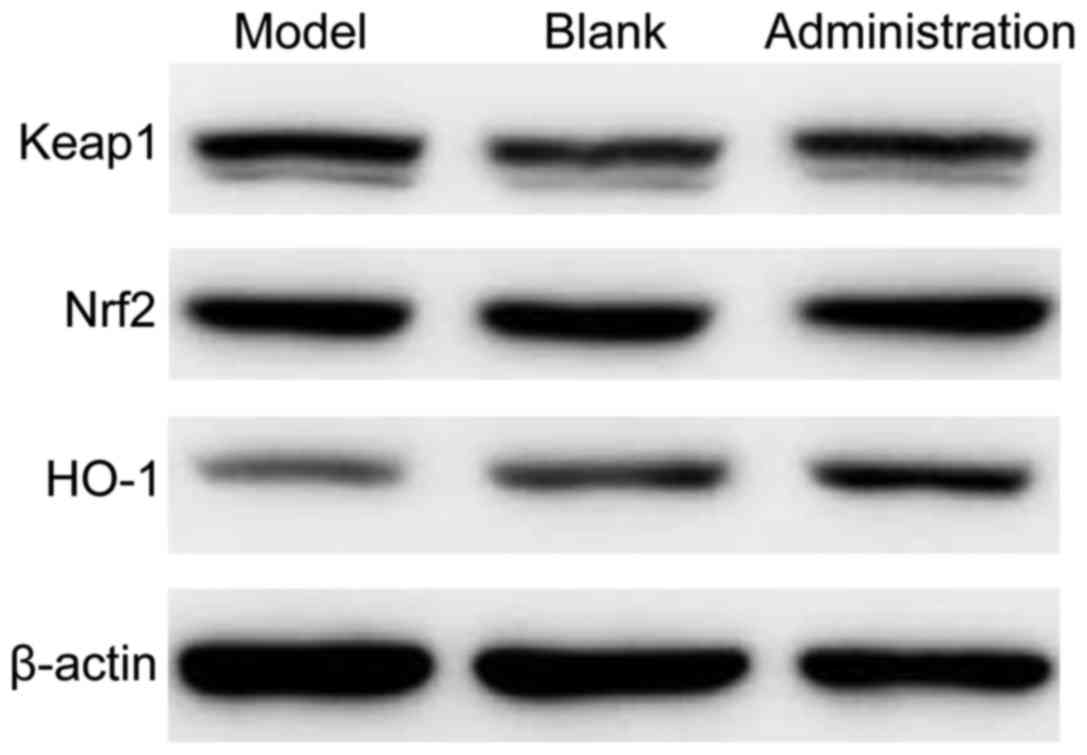
The protein expression levels of Nrf2, Keap1 and
HO-1 in the lenses of rats are shown in Fig. 1 and Table III. The protein expression levels
of Nrf2, Keap1 and HO-1 in the lens samples among the three groups
was significantly different (P<0.05). The protein expression
levels of Nrf2 and HO-1 was the highest in the model control group,
followed by the model administration group, and the lowest in the
blank control group. The protein expression level of Keap1 was the
highest in the model control group, followed by the model
administration group, and the lowest in the blank control
group.
 | Table III.Comparison of expression of Nrf2,
Keap1 and HO-1 protein in lens samples among the blank control and
model administration groups (n=20). |
Table III.
Comparison of expression of Nrf2,
Keap1 and HO-1 protein in lens samples among the blank control and
model administration groups (n=20).
| Groups | n | Nrf2 | Keap1 | HO-1 |
|---|
| Blank control | 10 | 0.79±0.13 | 1.46±0.17 | 0.27±0.04 |
| Model
administration | 10 | 0.43±0.06 | 1.94±0.14 | 0.12±0.03 |
| F-value |
| 15.325a | 17.436a | 16.301a |
| P-value |
| <0.001 | <0.001 | <0.001 |
Discussion
Diabetic cataracts are a complication of diabetes
that are caused by persistently elevated blood glucose levels and
can result in a loss of vision and blindness. While there are
similarities in the etiology of diabetic and senile cataracts, such
as the abnormal metabolism of the lens, the specific biomolecular
mechanism behind its pathogenesis has not been elucidated. Data
have shown that the incidence of diabetic cataracts in both types
of diabetes is close to 15%. Given the increase in diabetes
prevalence and the duration of the disease, this figure is likely
to rise (9). The progression of
diabetic cataracts is fast and poses a serious threat for blindness
among advanced diabetic patients. At present, the management of
diabetic cataracts is still dominated by prevention and early
control.
Previous clinical perspectives held that as long as
control of blood glucose levels is maintained, diabetic cataracts
can be effectively prevented. However, in practice, the truth has
proven to be not so simple. More and more studies have suggested
that controlling blood glucose levels within acceptable target
ranges for diabetic treatment is not enough to control the
progression of diabetic cataract pathology (10). These studies have shown that high
blood glucose is only an enabling factor, rather than the
fundamental causative factor. Currently, the biomolecular
mechanisms of the pathogenesis of diabetic cataracts are still
under study, but increasing evidence suggests that oxidative stress
in the body plays a fundamental role in the disease (11). Therefore, three evaluation indices of
Nrf2, Keap1 and HO-1 were identified as molecular targets in this
study. Nrf2 is a key cytokine that regulates the expression of
antioxidant substances in vivo. Keap1 is the key protein
that regulates the expression of Nrf2. HO-1, along with the other
glutathione peroxidase, quinone oxidoreductase, constitute the
Nrf2-Keap1 signaling pathway downstream of the effector protein
(12). The normal expression of Nrf2
is critical for maintaining oxidant-antioxidant balance in the body
and the Nrf2-Keap1 signaling pathway also plays an important role
in many diseases, including atherosclerosis, diabetes, heart
disease and cataracts. Further study on the Nrf2-Keap1 signaling
pathway may provide a new approach to the treatment of these
diseases (13).
Nrf2 is the most active transcriptional regulator in
the leucine zipper transcription factor family and its biological
activity is mainly exerted through an inhibitory control mechanism
against Keap1. In a healthy state, Nrf2 is mainly present in the
cytoplasm and binds to the Keap1 protein, while being anchored to
the cytoskeletal protein. If Nrf2 is not anchored in the cytoplasm,
free Nrf2 protein is degraded by the ubiquitination-dependent
protein; therefore, the expression levels of Nrf2 protein is
usually low. However, when the cells are exposed to a hyperoxic
environment, the conformation of Keap1 protein changes, preventing
it from binding to Nrf2. Nrf2 is then released into the nucleus,
expressed extensively, and promotes the expression of downstream
antioxidant HO-1, imparting resistance to oxidative damage
(14). Research has further shown
that the expression levels of Nrf2, Keap1 and HO-1 are all
decreased in the lenses of cataract patients, which indicates that
the mechanism of the signaling pathway for antioxidant protection
is damaged. On the one hand, oxidative stress in lens epithelial
cells of cataract patients produces a large amount of reactive
oxygen species, leading to abnormal aggregation of lens protein and
damaging the antioxidant function of Nrf2-Keap1. On the other hand,
the expression of Keap1 protein in lens epithelial cells of
cataract patients is abnormally increased, decreasing the Nrf2
content by the negative regulatory mechanism. Nrf2 content is
further decreased in the disease state by the expression of
protease and its degradation of Nrf2. Due to the decrease in Nrf2
expression, the expression of downstream HO-1 protein is also
decreased and the body enters a highly oxidative state which
further aggravates the cataract.
Calcium dobesilate is used as a traditional
therapeutic drug for diabetic retinopathy, and the pharmacological
effects include reducing capillary permeability and decreasing
blood pressure (15). In this study,
the expression levels of Nrf2, Keap1 and HO-1 in the model
administration group were closer to the blank control group than
the model control group. This suggests that calcium dobesilate may
be able to achieve therapeutic effects by adjusting the
oxidant-antioxidant balance of the lens. The degree of lens opacity
in the model administration group was better than in the model
control group, which may be the result of inhibition of
inflammatory substances by calcium dobesilate. Most inflammatory
substances are vasoactive substances, and their presence in
sufficient concentrations can cause increased vascular
permeability. Calcium dobesilate acts by inhibiting the secretion
of these substances to achieve the effect of reducing vascular
permeability.
In conclusion, our study found that calcium
dobesilate can effectively increase the levels of Nrf2 and HO-1 in
the lens of diabetic cataracts in rats and inhibit the levels of
Keap1. These results help to define the underlying mechanism for
its effects on cataracts through the improvement of Nrf2-Keap1
signaling pathway and stabilization of oxidant-antioxidant balance
in the lens. Altogether, this study indicates the therapeutic
potential for calcium dobesilate against the development and
progression of diabetic cataracts.
References
|
1
|
Saraswat M, Suryanarayana P, Reddy PY,
Patil MA, Balakrishna N and Reddy GB: Antiglycating potential of
Zingiber officinalis and delay of diabetic cataract in rats. Mol
Vis. 16:1525–1537. 2010.PubMed/NCBI
|
|
2
|
Pollreisz A and Schmidt-Erfurth U:
Diabetic cataract-pathogenesis, epidemiology and treatment. J
Ophthalmol. 2010:6087512010.PubMed/NCBI
|
|
3
|
Moreau KL and King JA: Protein misfolding
and aggregation in cataract disease and prospects for prevention.
Trends Mol Med. 18:273–282. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lupachyk S, Stavniichuk R, Komissarenko
JI, Drel VR, Obrosov AA, El-Remessy AB, Pacher P and Obrosova IG:
Na+/H+-exchanger-1 inhibition counteracts
diabetic cataract formation and retinal oxidative-nitrative stress
and apoptosis. Int J Mol Med. 29:989–998. 2012.PubMed/NCBI
|
|
5
|
Styskal J, Van Remmen H, Richardson A and
Salmon AB: Oxidative stress and diabetes: What can we learn about
insulin resistance from antioxidant mutant mouse models? Free Radic
Biol Med. 52:46–58. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tebay LE, Robertson H, Durant ST, Vitale
SR, Penning TM, Dinkova-Kostova AT and Hayes JD: Mechanisms of
activation of the transcription factor Nrf2 by redox stressors,
nutrient cues, and energy status and the pathways through which it
attenuates degenerative disease. Free Radic Biol Med. 88:108–146.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kowluru RA, Mishra M, Kowluru A and Kumar
B: Hyperlipidemia and the development of diabetic retinopathy:
Comparison between type 1 and type 2 animal models. Metabolism.
65:1570–1581. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Suryanarayana P, Saraswat M, Mrudula T,
Krishna TP, Krishnaswamy K and Reddy GB: Curcumin and turmeric
delay streptozotocin-induced diabetic cataract in rats. Invest
Ophthalmol Vis Sci. 46:2092–2099. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ooley C, Jun W, Le K, Kim A, Rock N,
Cardenal M, Kline R, Aldrich D and Hayes J: Correlational study of
diabeticretinopathy and hearing loss. Optom Vis Sci. 94:339–344.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kwon JW, Choi JA and Jee D: Matrix
metalloproteinase-1 and matrix metalloproteinase-9 in the aqueous
humor of diabetic macular edema patients. PLoS One.
11:01597202106.
|
|
11
|
Wojnar W, Kaczmarczyk-Sedlak I and Zych M:
Diosmin ameliorates the effects of oxidative stress in lenses of
streptozotocin-induced type 1 diabetic rats. Pharmacol Rep.
69:995–1000. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu
Y and Dong W: ROS and ROS-mediated cellular signaling. Oxid Med
Cell Longev. 2016:43509652016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bresciani A, Missineo A, Gallo M,
Cerretani M, Fezzardi P, Tomei L, Cicero DO, Altamura S, Santoprete
A, Ingenito R, et al: Nuclear factor (erythroid-derived 2)-like 2
(NRF2) drug discovery: Biochemical toolbox to develop NRF2
activators by reversible binding of Kelch-like ECH-associated
protein 1 (KEAP1). Arch Biochem Biophys. 631:31–41. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Danielli NM, Trevisan R, Mello DF, Fischer
K, Deconto VS, da Silva Acosta D, Bianchini A, Bainy AC and Dafre
AL: Upregulating Nrf2-dependent antioxidant defenses in Pacific
oysters Crassostrea gigas: Investigating the Nrf2/Keap1 pathway in
bivalves. Comp Biochem Physiol C Toxicol Pharmacol. 195:16–26.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Singh RP, Staurenghi G, Pollack A, Adewale
A, Walker TM, Sager D and Lehmann R: Efficacy of nepafenac
ophthalmic suspension 0.1% in improving clinical outcomes following
cataract surgery in patients with diabetes: An analysis of two
randomized studies. Clin Ophthalmol. 11:1021–1029. 2017. View Article : Google Scholar : PubMed/NCBI
|