Introduction
Coronary heart disease patients complicated with
diabetes mellitus may present with declined insulin sensitivity,
weakened blood pressure regulating ability, damage to vascular
endothelial cells, dysfunction of the fibrinolytic system and
increased sterile inflammatory responses, all of which are
indicators of disease progression and poor prognosis (1–7).
Vascular endothelial cell injury, insulin resistance and increased
inflammatory markers have attracted increasing attention in the
clinical practice, as core factors for the occurrence and
development of simple coronary heart disease or type 2 diabetes
mellitus.
Telmisartan, an angiotensin II receptor blocker
(ARB), commonly used in the clinic, can improve the function of
endothelial cells, reduce oxidative stress responses in the body
and alleviate superoxide damage (8).
In addition, for patients with diabetes mellitus, telmisartan can
inhibit the peroxisome proliferator-activated receptor-γ and is
used as an agonist to some extent (with clinical effects similar to
those of pioglitazone). Therefore, it can substantially improve
pancreatic islet blood flow (9),
thus reducing the occurrence of insulin resistance.
The focus of the present study was on coronary heart
disease patients complicated with diabetes mellitus who were
treated with telmisartan. Their vascular endothelial functions,
inflammatory factors and insulin resistance markers were obtained
to analyze the effects of the treatment.
Materials and methods
General information
Eighty coronary heart disease patients complicated
with type 2 diabetes mellitus, admitted and treated in the Zhangqui
Hospital from January 2016 to March 2017, participated in the
study. All the patients were diagnosed via coronary angiography;
type 2 diabetes mellitus was confirmed by glucose tolerance test
results. Signed informed consent forms were obtained from all the
patients and the Ethics Committee of the Zhangqiu Hospital
(Shandong, China) approved the study. Patients complicated with
malignant tumors, mental or immune system diseases, as well as
patients who had used glucocorticoid and/or immunodepressants over
a long period of time, were excluded. The patients were randomly
divided into two groups of 40 patients each, using a random number
table. There were 25 men and 15 women in the observation group aged
from 60 to 83 years, averaging 77.1±1.1 years; they had suffered
from diabetes mellitus for 5 to 35 years (23.1±3.1 years on
average) and from coronary heart disease for 5 to 30 years
(19.2±1.1 years on average). In the control group, there were 24
men and 16 women, aged from 60 to 84 years (averaging 77.0±1.0
years); they had diabetes mellitus for 5 to 35 years (23.0±3.0
years on average) and coronary heart disease for 5 to 30 years
(19.1±1.2 years on average). In terms of the general information of
patients, differences in sex, age and length of disease between the
two groups were not statistically significant (p>0.05).
Methods
All the patients received conventional treatments
such as antiplatelet, anticoagulation, lipid lowering, vasodilation
and blood pressure drugs for coronary heart disease; and dietary
control, exercise therapy and oral hypoglycemic drugs for diabetes
mellitus. Moreover, insulin was injected subcutaneously in
selective cases, it was generally recommended to maintain the level
of fasting blood glucose at <7.0 mmol/l and that of blood
glucose 2 h after a meal at <11.1 mmol/l and to adjust the
glycosylated hemoglobin to 7.0% or lower. The patients received
health education to support diabetes mellitus treatment.
Additionally, telmisartan (NMPN H20041082; Shanghai Sine-Tianping
Pharmaceuticals, Shanghai, China) was given to patients in the
observation group (80 mg telmisartan orally once a day each morning
for 12 consecutive weeks).
Observation variables
The two groups were treated for 12 consecutive weeks
and relevant markers were measured at different time-points (before
treatment and 4, 8 and 12 weeks after treatment). The fasting blood
glucose, homeostasis model assessment of insulin resistance
(HOMA-IR), vascular endothelin (ET) and changing trend of brachial
artery diameter in the basal state of the patients in the two
groups in the intervention process were recorded. Additionally, the
levels of blood glucose 2 h after a meal, the fasting serum insulin
(FINS) and the levels of inflammation-associated cytokines of the
patients in the two groups after intervention were compared.
Evaluation methods and criteria
A Hitachi 7080 Automatic Biochemical Tester and
Analyzer was used to examine the fasting blood glucose and the
blood glucose 2 h after a meal (the normal value for fasting blood
glucose was 3.9–6.1 mmol/l and that for blood glucose 2 h after
meal was <7.8 mmol/l).
The double-antibody single-step sandwich method was
used to detect the tumor necrosis factor-α (TNF-α) and
interleukin-6 (IL-6) levels, and the normal reference value for
TNF-α was 5 to 100 ng/l and that for IL-6 56.4 to 150.3 ng/l.
Immunity transmission turbidity was used for the
detection of C-reactive protein (CRP) in plasma (normal ≤10
mg/l).
A Beckman-Coulter Access DxI 800 Type
chemiluminescent analyzer (Beckman-Coulter, Brea, CA, USA) was used
to measure the FINS, and that value was used in a formula to obtain
the HOMA-IR = [fasting blood glucose (mmol/l) × FINS (mU/l)]/22.5.
The normal range for FINS was 3.0–24.9 U/ml and the normal value of
HOMA-IR was 1.
Radioimmunoassay was applied to examine vascular ET
and the normal reference value was 50.8±7.58 pg/l. For detection of
the brachial artery diameter in the basal state, all the patients
were examined at 24°C and a Philips HD11 XE Color Doppler
ultrasonic detector was used. During the detection, patients were
required to lie in the supine position with the abduction of tested
upper limb at 15°, and the ultrasonic probe was placed 5–15 cm
above the elbow to detect the brachial artery and the recorded
parameters included the vertical dimension of the brachial artery
intima (average of 3 continuous measurements at the end of
vasorelaxation).
Statistical analysis
Statistical Product and Service Solutions (SPSS)
19.0 software (SPSS, Inc., Chicago, IL, USA) was used. The
measurement data were presented as mean ± standard deviation (SD).
The t-test was used to compare the means of two groups, and the
χ2 test was applied to compare the rates between groups.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Comparison of changing fasting blood
glucose trends between the two groups in the intervention
process
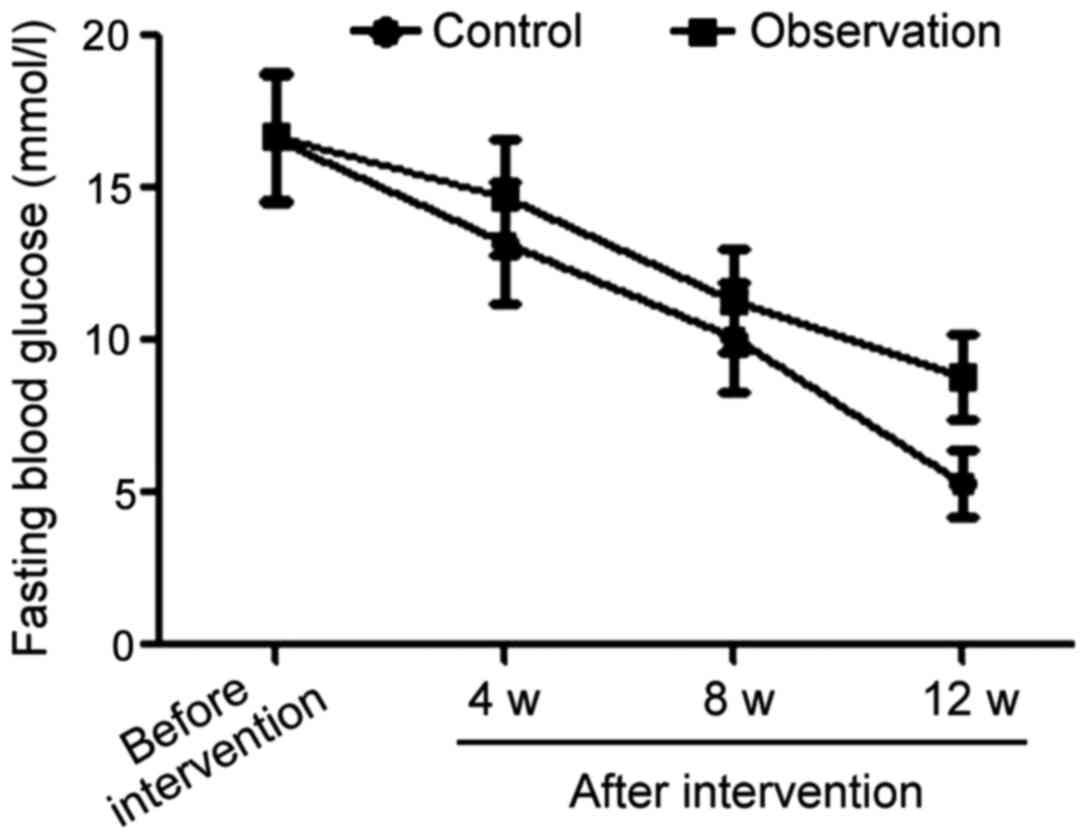
The average level of fasting blood glucose in the
observation group was 16.6±2.1 mmol/l prior to the intervention,
13.2±2.0 mmol/l in the 4th week after intervention, then 10.1±1.8
mmol/l in the 8th week after intervention and 5.2±1.0 mmol/l in the
12th week after intervention. For comparison the average level of
fasting blood glucose in the control group started at 16.7±2.1
mmol/l before intervention, decreased to 14.7±1.9, then to 11.3±1.7
and finally 8.7±1.1 mmol/l for the same time-points after
intervention as for the observation group. The difference between
the average values of the two groups before the intervention was
not significant. However, from the 4th week after intervention, the
levels of fasting blood glucose in the observation group were
always significantly lower than those in the control group at the
same time-points (p<0.05) (Fig.
1).
Comparisons of average fasting blood
glucose and blood glucose 2 h after meal in two groups after the
intervention
The average level of blood glucose 2 h after meal in
the observation group was siginificantly lower than that in the
control group after the intervention (p<0.05). Values of fasting
blood glucose and blood glucose 2 h after a meal are shown in
Table I.
 | Table I.Comparisons of fasting blood glucose
and blood glucose 2 h after meal in the two groups after
intervention (mmol/l, mean ± SD). |
Table I.
Comparisons of fasting blood glucose
and blood glucose 2 h after meal in the two groups after
intervention (mmol/l, mean ± SD).
| Variables | Fasting blood
glucose | Blood glucose 2 h
after meal |
|---|
| Observation
group | 5.2±1.0 | 7.0±1.4 |
| Control group | 8.7±1.1 | 14.0±2.1 |
| t-test | 14.890 | 17.541 |
| P-value | <0.001 | <0.001 |
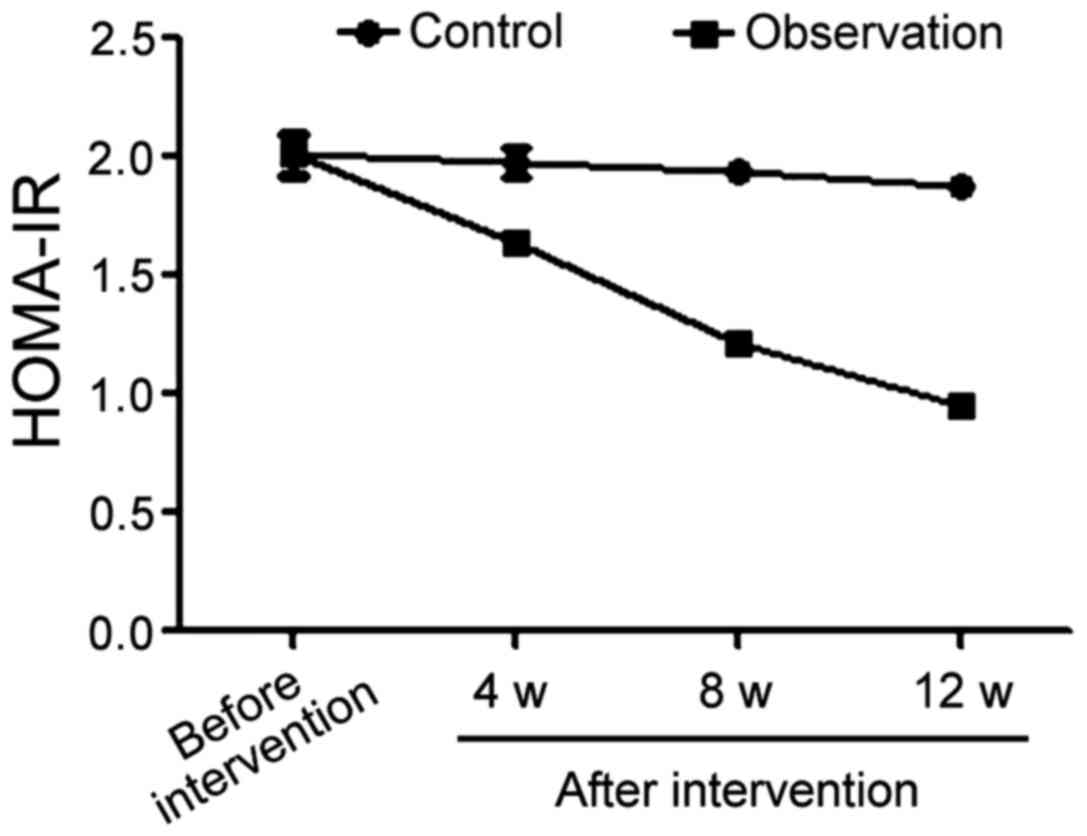
Comparison of trends of HOMA-IR values
in the two groups during the intervention process
Prior to treatment, the average HOMA-IR value in the
control group was 2.01±0.08, 1.98±0.06 in the 4th week after
beginning of the treatment, 1.94±0.04 in the 8th week and 1.88±0.02
in the 12th week. The same HOMA-IR values in the observation group
were 2.01±0.09 before treatment and 1.64±0.03, 1.21±0.02 and
0.95±0.01 in the 4th, 8th and 12th week after intervention,
respectively. The comparison of the HOMA-IR averages before
intervention in the two groups yielded no statistically significant
difference. From the 4th week after the beginning of treatment, the
levels of HOMA-IR of patients in the observation group were
significantly better than those of patients in the control group
during the same period (p<0.05) (Fig.
2).
Comparisons of average FINS and
HOMA-IR values in the two groups after intervention
The levels of FINS and HOMA-IR in the observation
group were significantly improved compared with those in the
control group after the beginning of treatment (p<0.05)
(Table II).
 | Table II.Comparisons of FINS and HOMA-IR after
intervention in the two groups (mean ± SD). |
Table II.
Comparisons of FINS and HOMA-IR after
intervention in the two groups (mean ± SD).
| Variables | HOMA-IR | FINS (mU/l) |
|---|
| Observation
group | 0.95±0.01 | 4.26±1.20 |
| Control group | 1.88±0.02 | 8.35±1.61 |
| t-test | 263.044 | 12.882 |
| P-value | <0.001 | <0.001 |
Comparisons of average levels of
inflammatory factors in the two groups after intervention
Average levels of inflammatory cytokines, including
TNF-α, IL-6 and CRP in the observation group were lower than those
in the control group (p<0.05) (Table III).
 | Table III.Comparisons of inflammatory factors
after intervention in the two groups (mean ± SD). |
Table III.
Comparisons of inflammatory factors
after intervention in the two groups (mean ± SD).
| Variables | TNF-α (ng/l) | IL-6 (ng/l) | CRP (ng/l) |
|---|
| Observation
group | 100.3±5.1 | 79.0±3.1 | 6.1±0.2 |
| Control group | 251.6±11.0 | 125.0±5.0 | 11.1±1.5 |
| t-test | 78.922 | 49.452 | 20.897 |
| P-value | <0.001 | <0.001 | <0.001 |
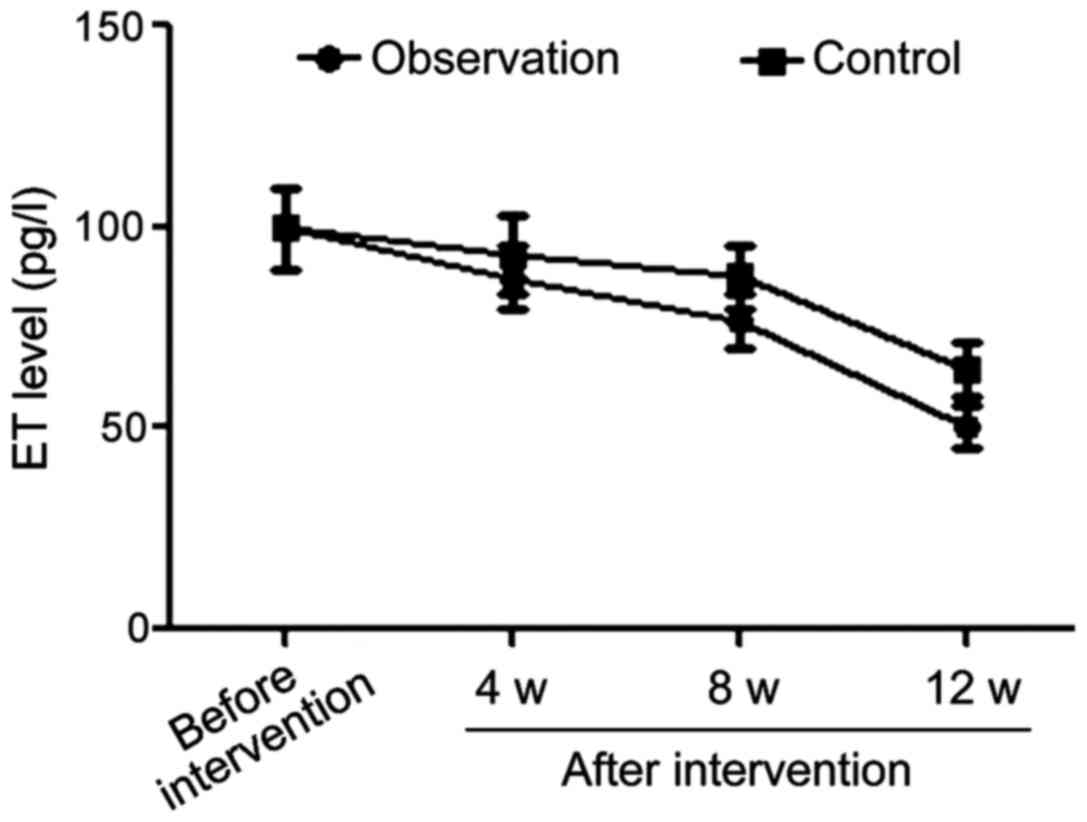
Comparisons of ET changes in the two
groups at different time-points of intervention
The differences in ET levels between the the two
groups before intervention were not statistically significant.
However, once treatment started, the ET levels in the observation
group were significantly lower than those in control group in the
4th, 8th and 12th week after intervention (p<0.05) (Table IV and Fig. 3).
 | Table IV.Comparisons of ET changes in the two
groups at different time-points during treatment (pg/l, mean ±
SD). |
Table IV.
Comparisons of ET changes in the two
groups at different time-points during treatment (pg/l, mean ±
SD).
| Variables | Before treatment
onset | 4 weeks after
treatment onset | 8 weeks after
treatment onset | 12 weeks after
treatment onset |
|---|
| Observation
group | 99.8±10.1 | 87.4±7.8 | 76.4±6.9 | 50.2±5.2 |
| Control group | 99.7±10.0 | 92.9±9.6 | 87.8±7.9 | 64.3±6.8 |
| t-test | 0.044 | 2.812 | 6.874 | 10.417 |
| P-value | 0.965 | 0.006 | <0.001 | <0.001 |
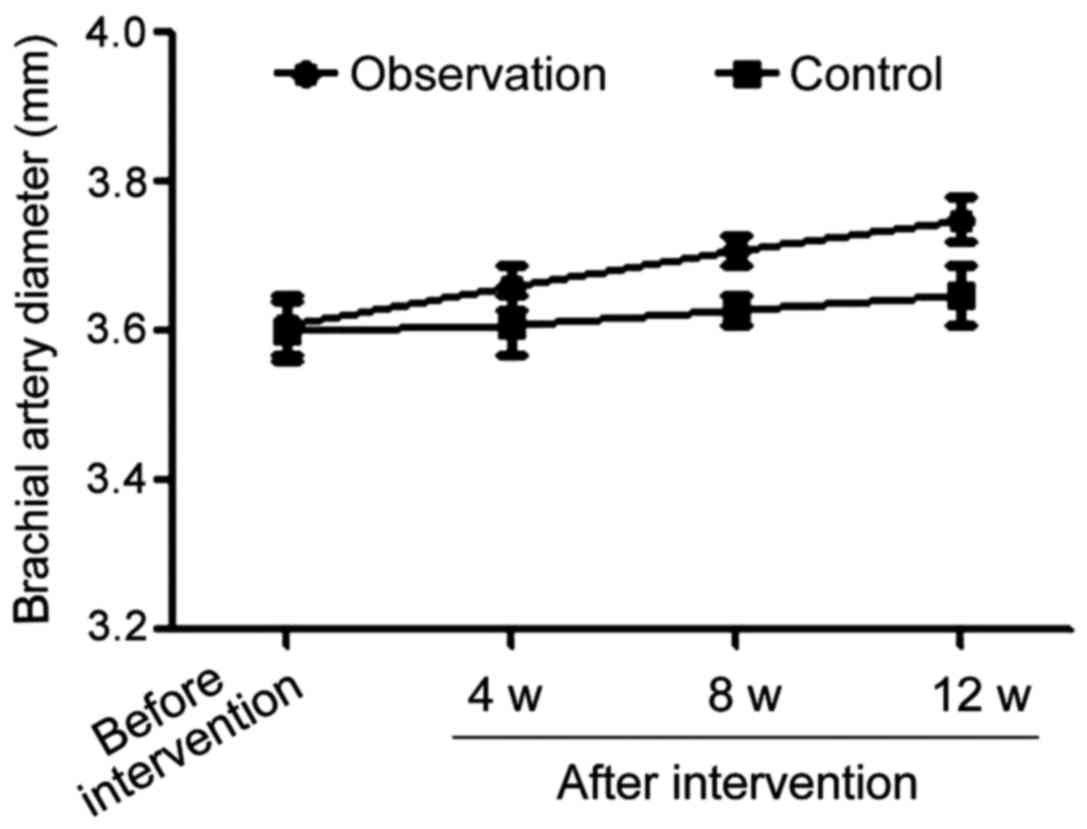
Comparisons of brachial artery
diameters in the basal state in the two groups during the
intervention process
Comparison of the brachial artery diameters in the
basal state before the beginning of treatment in both groups showed
the difference was not statistically significant. However, once
treatment started, the brachial artery diameters in the basal state
in observation group were significantly larger than those in the
control group (p<0.05) at all time-points during treatment
(Table V and Fig. 4).
 | Table V.Comparisons of brachial artery
diameters in the basal state in the two groups during the
intervention process (mm, mean ± SD). |
Table V.
Comparisons of brachial artery
diameters in the basal state in the two groups during the
intervention process (mm, mean ± SD).
| Variables | Before treatment
onset | 4 weeks after
treatment onset | 8 weeks after
treatment onset | 12 weeks after
treatment onset |
|---|
| Observation
group | 3.61±0.04 | 3.66±0.03 | 3.71±0.02 | 3.75±0.03 |
| Control group | 3.60±0.04 | 3.61±0.04 | 3.63±0.02 | 3.65±0.04 |
| t-test | 1.118 | 6.325 | 17.889 | 12.649 |
| P-value | 0.267 | <0.001 | <0.001 | <0.001 |
Discussion
For coronary heart disease patients complicated with
diabetes mellitus, hyperglycemia, hyperlipidemia, insulin
resistance, hyperinsulinemia, increase of inflammatory markers,
injury of vascular endothelial cells and blood pressure surges
constitute risk factors associated with further aggravation of
atherosclerosis (10–12). Telmisartan, which belongs to the
family of terminal angiotensin 1 receptor selective blockers
(13), can regulate vascular tone by
blocking the angiotensin downstream cascade without affecting the
levels of bradykinin and prostacyclin in the body; therefore, it is
suitable for clinical treatment of coronary heart disease
complicated with diabetes mellitus.
This study was carried out among coronary heart
disease patients complicated with diabetes mellitus; conventional
symptomatic and supporting therapies were used in both the control
and observation groups, while treatment combined with telmisartan
was used exclusively in the observation group. By monitoring
different variables during treatment, it was found that, the levels
of fasting blood glucose in the observation group were
significantly lower, and the levels of HOMA-IR clearly improved
compared to those in the control group. This suggests that, for
coronary heart disease patients complicated with diabetes mellitus,
treatment in combination with telmisartan can better regulate their
blood glucose and HOMA-IR levels and is more conducive to the
control of diabetes mellitus. In addition, the level of blood
glucose 2 h after a meal in the observation group was lower than
that in the control group, and the levels of FINS and HOMA-IR in
the observation group were significantly improved compared to those
in the control group. This provides further evidence that the
combined treatment with telmisartan is of great value in
effectively regulating the blood glucose and improving insulin
resistance in these patients. Moreover, our findings showed lower
levels of TNF-α, IL-6 and CRP in the observation group and thus the
efficacy of the approach for reducing the sterile inflammatory
responses in the body that increase the severity of diabetes
mellitus and/or coronary heart disease and carry a poor prognosis
was also revealed. Finally, by comparing the changes of ET at
different time-points and the brachial artery diameters in the
basal state in the two groups, we showed the values for the two
variables were significantly better in the observation group. Our
results indicate the telmisartan combination treatment was
effective for lowering the levels of angiotensin, improving the
vascular endothelial functions and dilating blood vessels as
evidenced by other studies (14,15). The
fact that telmisartan can inhibit the sterile inflammatory
responses in the body may be related to its blocking of the
angiotensin II receptor (15). The
drug is also known to activate the peroxisome
proliferator-activated receptor-γ and reduce vasoconstriction
(16). Furthermore, its effects
regulating the blood glucose level in patients with diabetes
mellitus can be explained by its ability to improve lipid
metabolism in the body, alleviate body oxidative stress responses
(17), reduce damage to blood
vessels caused by oxygen-free radicals (18), lower the ET level, improve
vasodilation functions and relieve insulin resistance (19).
In conclusion, our findings support a conventional
treatment in combination with telmisartan for coronary heart
disease patients complicated with diabetes mellitus, as this
approach can better regulate blood glucose levels, reduce insulin
resistance and body inflammatory responses and improve the vascular
endothelial functions in patients.
References
|
1
|
Park S, Kario K, Park CG, Huang QF, Cheng
HM, Hoshide S, Wang JG and Chen CH; Characteristics On the
ManagEment of Hypertension in Asia-Morning Hypertension Discussion
Group (COME Asia MHDG), : Target blood pressure in patients with
diabetes: Asian perspective. Yonsei Med J. 57:1307–1311. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bays H, Gao P, Völker B, Mattheus M,
Ruilope LM and Zhu D: Efficacy of single-pill combination of
telmisartan 80 mg and hydrochlorothiazide 25 mg in patients with
cardiovascular disease risk factors: A prospective subgroup
analysis of a randomized, double-blind, and controlled trial. Int J
Hypertens. 2013:7498302013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Verdecchia P, Dagenais G, Healey J, Gao P,
Dans AL, Chazova I, Binbrek AS, Iacobellis G, Ferreira R, Holwerda
N, et al Ongoing Telmisartan Alone and in Combination With Ramipril
Global Endpoint TrialTelmisartan Randomized AssessmeNt Study in ACE
iNtolerant subjects with cardiovascular Disease Investigators, :
Blood pressure and other determinants of new-onset atrial
fibrillation in patients at high cardiovascular risk in the Ongoing
Telmisartan Alone and in Combination With Ramipril Global Endpoint
Trial/Telmisartan Randomized Assessment Study in ACE intolerant
subjects with cardiovascular Disease studies. J Hypertens.
30:1004–1014. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takagi H and Umemoto T: Telmisartan
increases adiponectin levels: A meta-analysis and meta-regression
of randomized head-to-head trials. Int J Cardiol. 155:448–451.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nilsson PM: Target blood pressure in
diabetes patients with hypertension - what is the accumulated
evidence in 2011? J Zhejiang Univ Sci B. 12:611–623. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hong SJ, Choi SC, Ahn CM, Park JH, Kim JS
and Lim DS: Telmisartan reduces neointima volume and pulse wave
velocity 8 months after zotarolimus-eluting stent implantation in
hypertensive type 2 diabetic patients. Heart. 97:1425–1432. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Toyama K, Nakamura T, Kataoka K, Yasuda O,
Fukuda M, Tokutomi Y, Dong YF, Ogawa H and Kim-Mitsuyama S:
Telmisartan protects against diabetic vascular complications in a
mouse model of obesity and type 2 diabetes, partially through
peroxisome proliferator activated receptor-γ-dependent activity.
Biochem Biophys Res Commun. 410:508–513. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cowan BR, Young AA, Anderson C, Doughty
RN, Krittayaphong R, Lonn E, Marwick TH, Reid CM, Sanderson JE,
Schmieder RE, et al ONTARGET Investigators, : Left ventricular mass
and volume with telmisartan, ramipril, or combination in patients
with previous atherosclerotic events or with diabetes mellitus
(from the ONgoing Telmisartan Alone and in Combination With
Ramipril Global Endpoint Trial [ONTARGET]). Am J Cardiol.
104:1484–1489. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chang WT, Cheng JT and Chen ZC:
Telmisartan improves cardiac fibrosis in diabetes through
peroxisome proliferator activated receptor δ (PPARδ): From bedside
to bench. Cardiovasc Diabetol. 15:1132016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Toma I, Kim PJ, Dash R, McConnell MV,
Nishimura D, Harnish P and Yang PC: Telmisartan in the diabetic
murine model of acute myocardial infarction: Dual contrast
manganese-enhanced and delayed enhancement MRI evaluation of the
peri-infarct region. Cardiovasc Diabetol. 15:24–25. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen AX, Jerums G, Baqar S, Lambert E,
Somarajah G, Thomas G, O'Callaghan C, MacIsaac RJ and Ekinci EI:
Short-term dietary salt supplementation blunts telmisartan induced
increases in plasma renin activity in hypertensive patients with
type 2 diabetes mellitus. Clin Sci (Lond). 129:415–422. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chaudagar KK and Mehta AA: Effect of
telmisartan on VEGF-induced and VEGF-independent angiogenic
responsiveness of coronary endothelial cells in normal and
streptozotocin (STZ)-induced diabetic rats. Clin Exp Hypertens.
36:557–566. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rizos CV, Liberopoulos EN, Tellis K,
DiNicolantonio JJ, Tselepis AD and Elisaf MS: Combining
rosuvastatin with angiotensin-receptor blockers of different
PPARγ-activating capacity: Effects on high-density lipoprotein
subfractions and associated enzymes. Angiology. 66:36–42. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Antoniou T, Camacho X, Yao Z, Gomes T,
Juurlink DN and Mamdani MM: Comparative effectiveness of
angiotensin-receptor blockers for preventing macrovascular disease
in patients with diabetes: A population-based cohort study. CMAJ.
185:1035–1041. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Michel MC, Foster C, Brunner HR and Liu L:
A systematic comparison of the properties of clinically used
angiotensin II type 1 receptor antagonists. Pharmacol Rev.
65:809–848. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dehghan M, Mente A, Teo KK, Gao P, Sleight
P, Dagenais G, Avezum A, Probstfield JL, Dans T and Yusuf S;
Ongoing Telmisartan Alone and in Combination With Ramipril Global
End Point Trial (ONTARGET)/Telmisartan Randomized Assessment Study
in ACEI Intolerant Subjects With Cardiovascular Disease (TRANSCEND)
Trial Investigators, : Relationship between healthy diet and risk
of cardiovascular disease among patients on drug therapies for
secondary prevention: A prospective cohort study of 31546 high-risk
individuals from 40 countries. Circulation. 126:2705–2712. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Volpe M: Preventing cardiovascular events
with angiotensin II receptor blockers: A closer look at telmisartan
and valsartan. Expert Rev Cardiovasc Ther. 10:1061–1072. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guo Z, Zhang R, Li J and Xu G: Effect of
telmisartan on the expression of adiponectin receptors and
nicotinamide adenine dinucleotide phosphate oxidase in the heart
and aorta in type 2 diabetic rats. Cardiovasc Diabetol. 11:94–111.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kappert K, Böhm M, Schmieder R, Schumacher
H, Teo K, Yusuf S, Sleight P and Unger T; ONTARGET/TRANSCEND
Investigators, : Impact of sex on cardiovascular outcome in
patients at high cardiovascular risk: Analysis of the Telmisartan
Randomized Assessment Study in ACE-Intolerant Subjects With
Cardiovascular Disease (TRANSCEND) and the Ongoing Telmisartan
Alone and in Combination With Ramipril Global End Point Trial
(ONTARGET). Circulation. 126:934–941. 2012. View Article : Google Scholar : PubMed/NCBI
|