Introduction
The increasingly aging population in China has led
to increases in the incidence of osteoporosis. Although the hip
joint replacements can significantly promote the functional
recovery of patients, the treatment can cause severe trauma and
carries the inherent risks of surgery and anesthesia (1). New clinical studies that assess the
safety of elderly patients during anesthesia are needed (2). Previous studies have demonstrated that
effective and comprehensive postoperative analgesia does not only
reduce postoperative pain in patients, but can also reduce the
incidence of postoperative complications (3). The administration of nalbuphine, which
does not affect circulatory function or increase the cardiac load
(4,5), is commonly used for patients with
hypertension and cardiac dysfunction (6,7). In the
present study, we report the use of nalbuphine in the postoperative
analgesic treatment of older patients with lower limb fractures
after open reduction and internal fixation.
Materials and methods
General information
Eighty patients undergoing fracture reduction
surgeries to treat inferior limb fractures in the First People's
Hospital of Jingzhou were selected from January 2015 to December
2015. All the patients were diagnosed with inferior limb bone
fractures using imaging techniques (X-ray, CT or MRI). The Ethics
Committee of the First People's Hospital of Jingzhou approved this
study, and all the patients signed informed consent forms. Ages of
patients ranged from 50 to 80 years. Patients presenting with bone
and joint motor system diseases, diabetes, severe cardiopulmonary,
liver, coagulation and kidney dysfunctions, mental disorders,
systemic malignancies and cancer cachexia were excluded from the
study. In addition, patients who were allergic to the drugs in this
study, who refused to use analgesic devices and analgesic drugs
after operation, or who refused to sign the consent forms were not
included. The participants were randomly divided into 2 groups with
40 patients in each. In observation group, there were 25 males and
15 females, and the ages ranged from 60 to 93 years (with an
average of 79.6±1.8 years). In the same group there were 21 femoral
fractures, 11 tibial and fibular fractures and 8 ankle fractures.
In control group, there were 21 males and 19 females, and the ages
ranged from 60 to 90 years with an average of 75.5±2.6 years. The
control group cases included 20 femoral fractures, 10 tibia and
fibula fractures and 10 ankle fractures. No significant differences
were found in sex, age and surgical positions between the patients
in the two groups (P>0.05).
Methods
All patients underwent open fracture reductions and
internal fixation under combined spinal-epidural anesthesia. After
surgery, an intravenous controlled analgesia system (TUORen,
CBI+PCA type controlled analgesia pump) was used for analgesic
treatment. Nalbuphine hydrochloride injection (2 mg/kg, Yichang
Humanwell Pharmaceutical, Yichang, China; SFDA approval number:
H20130127, batch number: 15010211) and granisetron (6 mg, Yangzi
River Pharmaceutical Group, Taizhou, China; SFDA approval number:
H20020718, batch number: 150101H11) were used for the patients in
the observation group. By contrast, fentanyl citrate injection (2.5
µg/kg, Yichang Humanwell Pharmaceutical; SFDA approval number:
H20054171, batch number: 1530103) and granisetron (6 mg, Yangzi
River Pharmaceutical Group; SFDA approval number: H20020718, batch
number: 150101H11) were used for the patients in control group. The
capacity of each analgesia pump was 100 ml, the standard flow rate
was 2 ml/h, each self-administered dose was 0.5 ml, and the
interval of automatic administration was set to 15 min. Analgesic
treatment in both groups was continued for 48 h after surgery.
Clinical observation variables
The conditions of all patients during the
perioperative period were evaluated and data between groups were
statistically compared. The levels of inflammatory factors were
compared between groups after the interventions. Additionally, the
changes in the levels of cortisol, epinephrine and norepinephrine
were recorded during the intervention. The pain score (VAS score),
sedation score (Ramsay score), the number of times analgesia pump
was used (PCIA) and the life and sleep qualities were all recorded.
In addition, adverse reactions were also noted.
Methods
Elbow venous blood was used for the detection of
inflammation factors, including TNF-α (normal reference value
between 1 and 10 ng/ml), IL-1 (normal reference value between 60
and 250 ng/ml) and hs-CRP (normal reference value <10 mg/l).
Inflammation-related factors were measured by enzyme-linked
immunosorbent assay (ELISA). The levels of serum cortisol (normal
reference value 80–550 nmol/l), AD (normal reference value <480
pmol/l) and NE (normal reference value 615 to 3240 pmol/l) were
measured by sandwich ELISA. The Ramsay score is divided into six
levels, higher levels indicate better sedation, lower levels
represent lower level of sedation, and the best sedation levels are
levels 3 and 4. Life quality was evaluated using the Nottingham
Health Profile questionnaire to get the information of 6 items
including mental energy, pain, emotional changes, sleep conditions,
social life and physical fitness from the patients. The total score
is 100 points and lower scores indicate higher life quality. The
sleep quality was assessed using the Pittsburgh Sleep Quality
Index. The total score is 21 points and lower scores indicate
higher sleep quality. The pain VAS scoring was obtained using a
visual analog scale, the total score is 10 points and the lowest
score is 0 point, higher scores indicate higher degrees of
pain.
Statistical analysis
The SPSS 19.0 software (IBM, Armonk, NY, USA) was
used for statistical analyses. The measurement data were expressed
as mean ± standard deviation, and the comparisons between the two
groups were performed by t-tests. The comparisons of rates between
the two groups were performed by χ2 test. A P<0.05
was considered to be statistically significant.
Results
Comparison of average levels of
inflammatory factors between the 2 groups 48 h after
intervention
The levels of IL-6, TNF-α, IL-1 and hs-CRP in the
observation group were significantly lower than those in the
control group (P<0.05) at 48 h after intervention (Table I).
 | Table I.Comparison of average levels of
inflammatory factors between the two groups (mean ± SD). |
Table I.
Comparison of average levels of
inflammatory factors between the two groups (mean ± SD).
| Groups | IL-6 (ng/ml) | TNF-α (ng/ml) | IL-1 (µg/ml) | hs-CRP (mg/ml) |
|---|
| Observation
group | 34.6±6.1 | 12.1±0.2 | 0.61±0.1 | 10.5±1.0 |
| Control group | 153.2±14.1 | 18.3±0.5 | 0.93±0.2 | 31.1±2.0 |
| t-test | 48.825 | 72.815 | 9.051 | 58.266 |
| P-value | <0.05 | <0.05 | <0.05 | <0.05 |
Comparison of average levels of
cortisol, AD and NE between the 2 groups 48 h after
intervention
Forty-eight hours after intervention, the levels of
cortisol, AD and NE were significantly decreased in the observation
group compared with those in the control group (P<0.05)
(Table II).
 | Table II.Comparison of the levels of cortisol,
AD and NE between the two groups 48 h after intervention (mean ±
SD). |
Table II.
Comparison of the levels of cortisol,
AD and NE between the two groups 48 h after intervention (mean ±
SD).
| Group | Cortisol
(nmol/l) | AD (pmol/l) | NE (pmol/l) |
|---|
| Observation | 608.9±13.1 | 58.1±2.0 | 130.6±4.5 |
| Control | 878.6±53.2 | 81.5±6.5 | 251.1±13.3 |
| t-test | 31.133 | 21.762 | 54.278 |
| P-value | <0.05 | <0.05 | <0.05 |
Comparison of pain VAS scores between
the 2 groups at different time-points
The pain VAS scores in the observation group were
significantly lower than those in the control group at 6, 12, 24
and 48 h after operation (P<0.05) (Table III).
 | Table III.Comparison of pain VAS scores between
the two groups at different time-points (points, mean ± SD). |
Table III.
Comparison of pain VAS scores between
the two groups at different time-points (points, mean ± SD).
| Groups | 6 h after
operation | 12 h after
operation | 24 h after
operation | 48 h after
operation |
|---|
| Observation | 4.1±0.3 | 4.0±0.2 | 3.0±0.3 | 2.3±0.1 |
| Control | 5.1±0.2 | 4.2±0.3 | 3.6±0.4 | 3.3±0.2 |
| t-test | 17.541 | 3.508 | 7.589 | 28.284 |
| P-value | <0.05 | <0.05 | <0.05 | <0.05 |
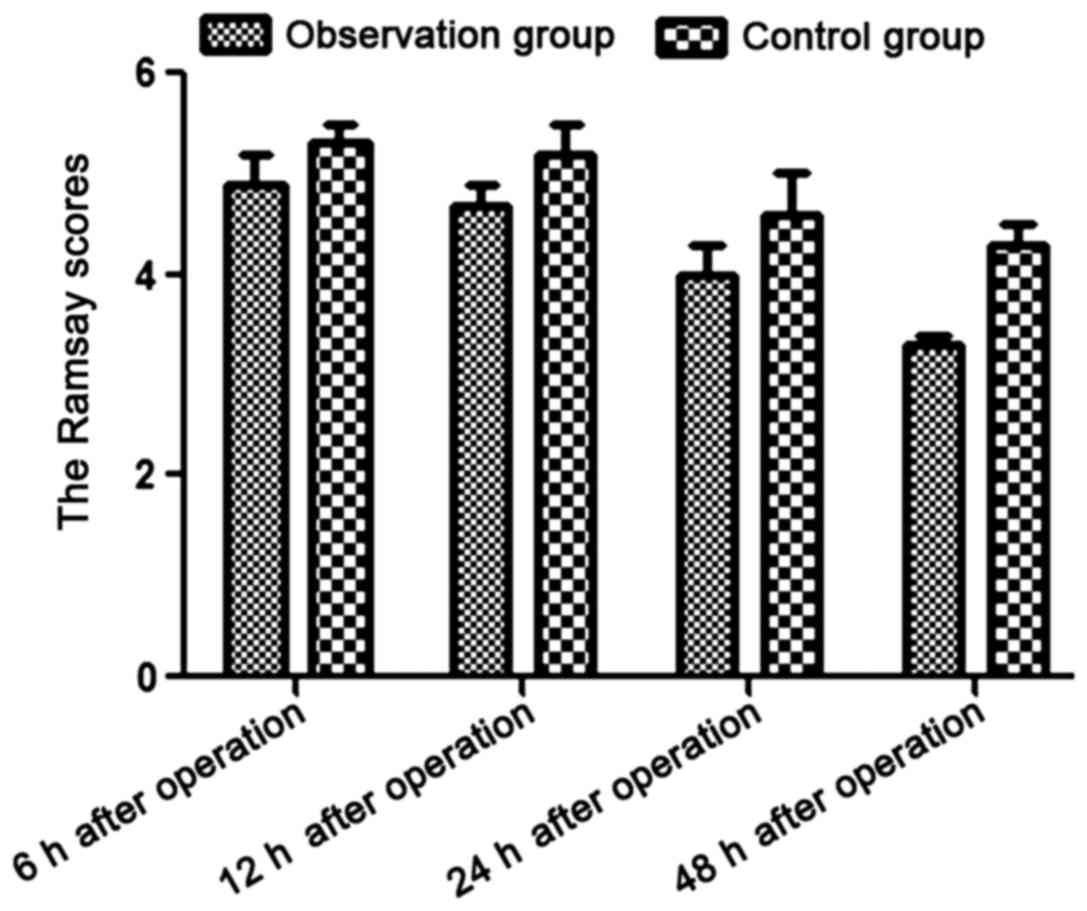
Comparison of Ramsay scores between
the 2 groups at different time-points
The Ramsay scores in the observation group were
significantly lower than those in the control group at 6, 12, 24
and 48 h after operation (P<0.05) (Table IV and Fig. 1).
 | Table IV.Comparison of Ramsay scores between
the two groups at different time-points (points, mean ± SD). |
Table IV.
Comparison of Ramsay scores between
the two groups at different time-points (points, mean ± SD).
| Groups | 6 h after
operation | 12 h after
operation | 24 h after
operation | 48 h after
operation |
|---|
| Observation | 4.9±0.3 | 4.7±0.2 | 4.0±0.3 | 3.3±0.1 |
| Control | 5.3±0.2 | 5.2±0.3 | 4.6±0.4 | 4.3±0.2 |
| t | 7.016 | 8.771 | 8.485 | 28.284 |
| P-value | <0.05 | <0.05 | <0.05 | <0.05 |
The Ramsay scores in the observation group were
significantly lower than those in the control group at 6, 12, 24
and 48 h after operation (P<0.05).
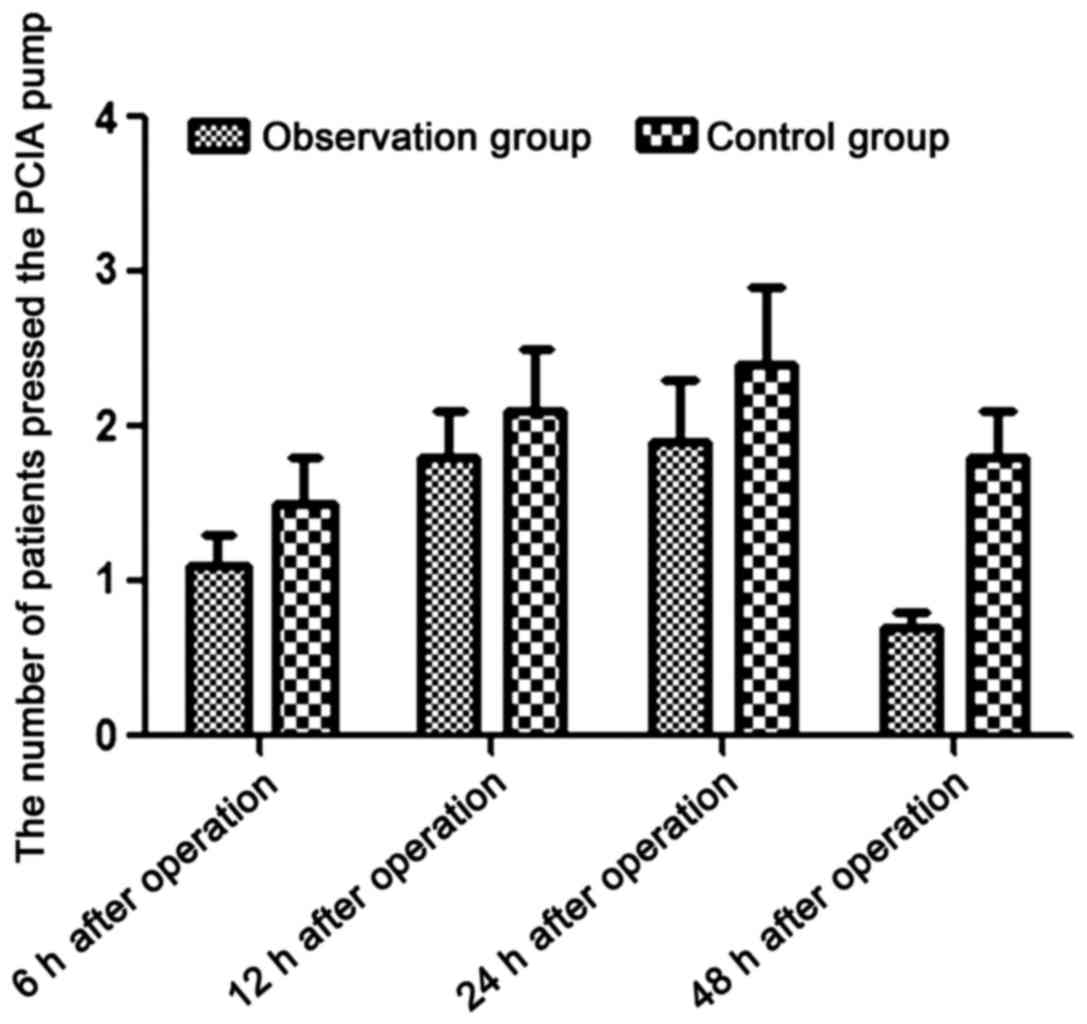
Comparison of the number of presses
(PCIA) between the 2 groups at different time-points
The number of times the patients used the pump for
analgesia (PCIA) in the observation group was significantly lower
than the number of times the patients in the control group used the
pump, at 6, 12, 24 and 48 h after operation (P<0.05) (Table V and Fig.
2).
 | Table V.Comparison of the number of times
patients pressed on the PCIA pump between the two groups at
different time-points (time, mean ± SD). |
Table V.
Comparison of the number of times
patients pressed on the PCIA pump between the two groups at
different time-points (time, mean ± SD).
| Groups | 6 h after
operation | 12 h after
operation | 24 h after
operation | 48 h after
operation |
|---|
| Observation | 1.1±0.2 | 1.8±0.3 | 1.9±0.4 | 0.7±0.1 |
| Control | 1.5±0.3 | 2.1±0.4 | 2.4±0.5 | 1.8±0.3 |
| t-test | 7.016 | 3.795 | 4.939 | 22.000 |
| P-value | <0.05 | <0.05 | <0.05 | <0.05 |
Comparison of life and sleep qualities
between the 2 groups 48 h after intervention
The life and sleep qualities of patients in the
observation group were significantly better than those of patients
in the control group (P<0.05) (Table
VI).
 | Table VI.Comparison life and sleep qualities
between the two groups 48 h after intervention (points, mean ±
SD). |
Table VI.
Comparison life and sleep qualities
between the two groups 48 h after intervention (points, mean ±
SD).
| Groups | Life quality | Sleep quality |
|---|
| Observation | 43.9±5.6 | 8.1±1.1 |
| Control | 61.8±9.1 | 15.2±2.5 |
| t-test | 10.595 | 16.441 |
| P-value | <0.05 | <0.05 |
Comparison of adverse reactions
between the two groups
The rates of nausea, vomiting, dizziness,
drowsiness, urinary retention, skin itch and constipation were
significantly lower in the observation group than in the control
group (P<0.05) (Table VII).
 | Table VII.Comparison of adverse reactions
between the two groups (cases, %). |
Table VII.
Comparison of adverse reactions
between the two groups (cases, %).
| Groups | Nausea, vomiting | Dizziness,
drowsiness | Urinary
retention | Skin itch | Constipation |
|---|
| Observation | 1 (2.5%) | 1 (2.5%) | 1 (2.5%) | 1 (2.5%) | 1 (2.5%) |
| Control | 9 (22.5%) | 9 (22.5%) | 10 (25.0%) | 9 (22.5%) | 10 (25.0%) |
| χ2 | 5.600 | 5.600 | 6.746 | 5.600 | 6.746 |
| P-value | 0.018 | 0.018 | 0.009 | 0.018 | 0.009 |
Discussion
Pain is a negative factor that seriously affects a
patient's postoperative recovery. Surgical trauma can activate
peripheral and visceral nociceptors (8) to induce central pain sensory nerve
sensitization and peripheral sensory nerve conduction enhancement
(9), leading to a decreased
threshold for those receptors and an over-threshold response
enhancement, which is called hyperalgesia (10). At the same time, tissue damage caused
by surgical trauma can further lead to the generation and
aggregation of inflammatory mediators and pain-related factors,
further aggravating pain (11). In
this study, the subjects were elderly lower limb fracture patients
who received open reduction and internal fixation. Severe
postoperative pain leads to increased blood pressure in patients
and it can even induce angina, atelectasis and other complications,
seriously affecting the prognosis of patients (12).
All the patients in this study received
patient-controlled intravenous analgesia. The main analgesic drug
used in the observation group was nalbuphine, while sufentanil was
used in the control group. The levels of inflammatory cytokines
(IL-6, TNF-α, IL-1 and hs-CRP) and catecholamine hormones
(cortisol, AD and NE) 48 h after intervention were significantly
decreased in patients treated with nalbuphine, suggesting the drug
was responsible for the observed effects. Pain and sedation scores
of the observation group were significantly better than those of
the control group after surgery. In addition, the number of times
the patients pressed the PCIA pump was reduced in the observation
group, indicating that postoperative analgesia with nalbuphine,
compared to sufentanil, can lead to more pain relief. Moreover, the
use of nalbuphine helped patients maintain appropriate sedation,
compared of sufentanil, the analgesic effect was more satisfactory.
The comparison of life and sleep qualities between the two groups
48 h after surgery showed that both parameters were significantly
better in the observation group when compared to the control group.
Finally, the comparison of adverse reactions between the two
studied groups showed that the rate of complications was lower in
the observation group than in the control group, indicating that
postoperative analgesia with nalbuphine provides increased
safety.
Opioid receptors can be divided into κ, µ and δ
types, and excitement of any one of them can produce a certain
analgesic effect (12). However, the
stimulation of the latter two can lead to respiratory depression,
gastrointestinal discomfort, dizziness and headaches (13). Nalbuphine can activate the κ receptor
to achieve analgesia at the spinal cord level. Nalbuphine also acts
by blocking central sensitization caused by surgical trauma or
nociceptive stimulation (14), thus,
reducing the occurrence of postoperative analgesic adverse
reactions that can be caused by the use of opioids given the
intraoperative inflammatory response (15). Furthermore, nalbuphine can partially
antagonize the activation of the µ receptor. Thus, nalbuphine
administration can, not only achieve analgesia but also inhibits
adverse reactions caused by activated µ receptors (16). Finally, nalbuphine can also increase
opioid receptor density and activity (17,18),
which in turn improves the analgesic effect and induces sedation
(19,20). Our results, demonstrating the
superiority of the nalbuphine analgesia over that of sufentanil can
all be explained by these reported characteristics.
In conclusion, analgesia with nalbuphine after
fracture reduction surgery in the elderly can reduce the levels of
inflammatory cytokines, improve analgesic effects, induce a certain
level of sedation and reduce the occurrence of adverse
reactions.
References
|
1
|
Nallam SR, Chiruvella S and Reddy A:
Monitored anaesthesia care - Comparison of
nalbuphine/dexmedetomidine versus nalbuphine/propofol for middle
ear surgeries: A double-blind randomised trial. Indian J Anaesth.
61:61–67. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Moustafa AA, Baaror AS and Abdelazim IA:
Comparative study between nalbuphine and ondansetron in prevention
of intrathecal morphine-induced pruritus in women undergoing
cesarean section. Anesth Essays Res. 10:238–244. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tiwari AK, Tomar GS and Agrawal J:
Intrathecal bupivacaine in comparison with a combination of
nalbuphine and bupivacaine for subarachnoid block: A randomized
prospective double-blind clinical study. Am J Ther. 20:592–595.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Keller DL, Sanchez-Migallon Guzman D,
Klauer JM, KuKanich B, Barker SA, Rodríguez-Ramos Fernández J and
Paul-Murphy JR: Pharmacokinetics of nalbuphine hydrochloride after
intravenous and intramuscular administration to Hispaniolan Amazon
parrots (Amazona ventralis). Am J Vet Res. 72:741–745. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Haw AJ, Meyer LC and Fuller A: Nalbuphine
and butorphanol reverse opioid-induced respiratory depression but
increase arousal in etorphine-immobilized goats (Capra hircus). Vet
Anaesth Analg. 43:539–548. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mateshuk-Vatseba L, Pidvalna U and Kost A:
Peculiarities of vascular tunic microstructure of the white rat
eyeball under the effect of opioid. Rom J Morphol Embryol.
56:1057–1062. 2015.PubMed/NCBI
|
|
7
|
Altarifi AA, Rice KC and Negus SS: Effects
of µ-opioid receptor agonists in assays of acute pain-stimulated
and pain-depressed behavior in male rats: Role of μ-agonist
efficacy and noxious stimulus intensity. J Pharmacol Exp Ther.
352:208–217. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen MK, Chau SW, Shen YC, Sun YN, Tseng
KY, Long CY, Feng YT and Cheng KI: Dose-dependent attenuation of
intravenous nalbuphine on epidural morphine-induced pruritus and
analgesia after cesarean delivery. Kaohsiung J Med Sci. 30:248–253.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang HJ, Hsiong CH, Ho ST, Lin MJ, Shih
TY, Huang PW and Hu OY: Commonly used excipients modulate
UDP-glucuro-nosyltransferase 2b7 activity to improve nalbuphine
oral bioavailability in humans. Pharm Res. 31:1676–1688. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liao CC, Chang CS, Tseng CH, Sheen MJ,
Tsai SC, Chang YL and Wong SY: Efficacy of intramuscular nalbuphine
versus diphenhydramine for the prevention of epidural
morphine-induced pruritus after cesarean delivery. Chang Gung Med
J. 34:172–178. 2011.PubMed/NCBI
|
|
11
|
Prasartritha T, Kunakornsawat S,
Tungsiripat R, Jampa J and Throngnumchai R: A prospective
randomized trial comparing epidural morphine through
intraoperatively placed epidural catheter and intravenous morphine
in major lumbar spinal surgery. J Spinal Disord Tech. 23:e43–e46.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Anderson C, Boehme S, Ouellette J, Stidham
C and Mackay M: Physical and chemical compatibility of injectable
acetaminophen during simulated y-site administration. Hosp Pharm.
49:42–47. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Coetzee JF, Lechtenberg KF, Stock ML and
Kukanich B: Pharmacokinetics and effect of intravenous nalbuphine
in weaned Holstein calves after surgical castration. J Vet
Pharmacol Ther. 37:169–177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rao ZA, Choudhri A, Naqvi S and
Ehsan-Ul-Haq: Walking epidural with low dose bupivacaine plus
tramadol on normal labour in primipara. J Coll Physicians Surg Pak.
20:295–298. 2010.PubMed/NCBI
|
|
15
|
Schmitz A, Salgo B, Weiss M, Dillier CM,
Frotzler A and Gerber AC: Intrathecal opioid medication for
perioperative analgesia in severely handicapped children undergoing
spinal operations. Anaesthesist. 59:614–620. 2010.(In German).
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sanchez-Migallon Guzman D, Braun JM,
Steagall PV, Keuler NS, Heath TD, Krugner-Higby LA, Brown CS and
Paul-Murphy JR: Antinociceptive effects of long-acting nalbuphine
decanoate after intramuscular administration to Hispaniolan Amazon
parrots (Amazona ventralis). Am J Vet Res. 74:196–200. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sanchez-Migallon Guzman D, KuKanich B,
Heath TD, Krugner-Higby LA, Barker SA, Brown CS and Paul-Murphy JR:
Pharmacokinetics of long-acting nalbuphine decanoate after
intramuscular administration to Hispaniolan Amazon parrots (Amazona
ventralis). Am J Vet Res. 74:191–195. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang FY, Shen YC, Chen MK, Chau SW, Ku CL,
Feng YT and Cheng KI: Equal volumes of undiluted nalbuphine and
lidocaine and normal diluted saline prevents nalbuphine-induced
injection pain. Acta Anaesthesiol Taiwan. 49:125–129. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim TH, Kim JM, Lee HH, Chung SH and Hong
YP: Effect of nalbuphine hydrochloride on the active phase during
first stage of labour: A pilot study. J Obstet Gynaecol.
31:724–727. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Larance B, Ambekar A, Azim T, Murthy P,
Panda S, Degenhardt L and Mathers B: The availability, diversion
and injection of pharmaceutical opioids in South Asia. Drug Alcohol
Rev. 30:246–254. 2011. View Article : Google Scholar : PubMed/NCBI
|