Introduction
Osteonecrosis of the femoral head (ONFH) is caused
by the interruption of the blood supply to the femoral head or the
pathological process of osteoblast death (1,2).
Steroid-induced avascular necrosis of the femoral head (SANFH) is a
type of non-traumatic ANFH and the most common type of femoral head
necrosis. It occurs following the long-term use of
adrenocorticotropic hormones (ACTH) or the use of high doses of
ACTHs (3–5). The major clinical characteristics of
ANFH include hip pain, limited mobility, lameness and a high
disability rate, which negatively impact patient health and quality
of life (6–8). It is estimated that ~50% of all ONFH
cases are induced by ACTH (5,9,10). In China, there are 5–7.5 million
patients with ONFH requiring treatment and the number of new ONFH
cases was 150,000–200,000 in 1998, with the majority of cases
diagnosed in young adults (11).
Corticosteroids are widely used to treat several
diseases, including asthma (12),
nephritic syndrome (13) and
leukemia (14), as they suppress
inflammation, allergic reactions and the immune response. However,
in the process of producing marked improvements in the symptoms of
these diseases, corticosteroids induce certain adverse effects,
including growth retardation (15),
obesity (16), osteoporosis
(17), hyperglycemia (18), osteonecrosis (19), cataracts (20) and Cushing's syndrome (21), which limits their use clinically.
Therefore, increasing attention has been given to identifying
methods of treating corticosteroid-induced ANFH (22–25).
Currently, patients with advanced ANFH receive hip
arthroplasty, whereas those at the pre-collapse stage receive
salvage surgery; however, for patients with non-traumatic ANFH, as
well as those for whom surgery would be high-risk, non-surgical
treatment is more appropriate (26).
Numerous studies have been conducted to investigate the potential
of novel drugs, including statins (27) and alendronate (28) to treat ANFH without surgery; however,
to date, their therapeutic effects have been unsatisfactory. For
many years, Traditional Chinese Medicine (TCM) has been widely used
to treat different diseases involving the bone, and deer antlers
are often used in TCM (29,30). The outside of the antlers consist of
skin covered with hair, while the inner antler is made up of
connective tissue, cartilaginous tissue, blood vessels and abundant
nerves (31). It has been reported
that antlers may have preventive and therapeutic effects on
osteoporosis (32) and may also help
with sperm production and strengthen the muscles and bones
(33). However, there have been few
papers investigating the therapeutic effect of deer antler extracts
on femoral head necrosis.
The 11β-hydroxysteroid dehydrogenase (11β-HSD)
family of enzymes, which includes 11β-HSD1 and 11β-HSD2, catalyze
the interconversion of active glucocorticoids (34). Previous studies have demonstrated
that 11β-HSD1 and 11β-HSD2 influence the function of adipocytes and
endotheliocytes (35,36). Adipocyte and endotheliocyte
dysfunction may cause blood flow in the terminal vessels to become
abnormal and induce complications, including intravascular
coagulation, microcirculation disturbance and vascular
embolization, which in turn may lead to femoral head necrosis
(37). The phenomenon indicates that
11β-HSD may be important in the development of SANFH.
The current study used antler extract to treats
rabbit with SANFH and measured the expression of 11β-HSD to confirm
that 11β-HSD was one of the targets in the treatment of SANFH using
antler extract.
Materials and methods
Antler preparation
Mature deer antlers were obtained from Beijing Tong
Ren Tang Chinese Medicine Co., Ltd., (Beijing, China). A total of
200 g antler was dried, milled and sliced, and then extracted 4
times by dissolving in 1 l distilled water and boiling for 5 h.
Following filtration with 3 levels of sterile gauze (TZSB; Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China), the
liquid containing the antler extract was concentrated at 20% by
boiling for 5 h and stored at 4°C for 3 days.
Animals
A total of 30 male New Zealand rabbits, 4 months old
and weighing 2.6–3.2 kg, were obtained from the Experimental Animal
Center of the Affiliated Hospital of Inner Mongolia Medical
University (Hohot, China). Rabbits were housed in an animal chamber
maintained at 22±2°C with a relative humidity of 50±5% and a 12/12
h light/dark cycle. Access to food and water was ad libitum. All
animal experiments in the present study were approved by the Animal
Care and Use Committee of the Affiliated Hospital of Inner Mongolia
Medical University (Hohhot, China) and followed the ethical
guidelines set by the European Community guidelines (38).
Establishment of SAMFH rabbit
model
SANFH rabbit models were established following a
previously described method (39,40). The
30 healthy New Zealand rabbits were randomly divided into 5 groups
(n=6): A control, ANFH, ANFH + antler (250 mg/kg), ANFH + antler
(500 mg/kg) and ANFH + antler (1,000 mg/kg) group. All rabbits
received an injection of 10 ml/kg horse serum (100%; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) via the ear vein for 2 weeks.
Subsequently, 6 ml/kg/day horse serum was injected for 2 days.
Rabbits in the experimental groups received an
intraperitoneal injection of 20 mg/kg methylprednisolone
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) twice a week, over
a 2-week period. Rabbits in the control group were
intraperitoneally injected with the same volume of saline.
Subsequently, 200,000 U penicillin was injected into the buttock of
each rabbit. After 2 weeks, 1 rabbit in each group was randomly
selected to confirm whether the SANFH model was successfully
established by performing a femoral head histopathological
examination. The femoral head was separated and fixed with 2%
glutaraldehyde at 4°C overnight (Beijing Solarbio Science &
Technology Co., Ltd.), decalcified with 5% nitric acid (Beijing
Solarbio Science & Technology Co., Ltd.), dehydrated with a
graded series of ethanol (70, 80, 90, 95 and 100%) embedded in
paraffin and cut into 5 µm slices using a microtome (HM 355S;
Thermo Fisher Scientific, Inc.). Sections were subsequently dewaxed
with xylene, rehydrated with a graded series of ethanol (100, 95,
90, 80 and 70%) and stained with hematoxylin at 25°C for 10 min and
eosin at 25°C for 1 min. Stained sections were observed under a
light microscope (magnification, ×200).
Following confirmation of successful establishment
of the SANFH model, the antler extract was dissolved in distilled
water to form final concentrations of 250 mg/kg, 500 mg/kg and
1,000 mg/kg. The different concentrations of antler extracts were
administered to rabbits in the ANFH + antler (250 mg/kg), ANFH +
antler (500 mg/kg) and ANFH + antler (1,000 mg/kg) groups,
respectively, every day for 60 days by intraperitoneal injection.
Rabbits in the control and ANFH groups were intraperitoneally
injected with the same volume of saline.
CT and triglyceride determination
Following treatments, all rabbits were anesthetized
with a combination of xylazine (6 mg/kg) and ketamine (40 mg/kg;
both Seebio Biotech Co., Ltd., Shanghai, China) according to
previous report and sacrificed by cervical dislocation (41). Blood was collected from the ear vein
in EDTA tubes and centrifuged at 4°C and 5,000 × g to separate
serum. CT and triglyceride concentrations in the blood samples of
all rabbits were measured using an automatic biochemistry analyzer
(Siemens AG, Munich, Germany).
Histopathological examination
Femoral heads, including the metaphyses and
thighbones, of all the rabbits were collected and cut into
sections. Sections were fixed in 2% glutaraldehyde (Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China) at
25°C for 24 h. Sections were then embedded in paraffin and cut into
slices 5 µm thick. Following deparaffinization and rehydration with
graded ethanol, sections were stained with hematoxylin and eosin
(Thermo Fisher Scientific, Inc.). Histopathological changes of the
femoral heads were observed under a light microscope (Olympus
Corporation, Tokyo, Japan).
Osteoblast culture
A total of 2 male newborn New Zealand rabbits, 10
days old, weight 20.17±1.03 kg, were obtained from the Experimental
Animal Center of the Affiliated Hospital of Inner Mongolia Medical
University and sacrificed as described above. Rabbits were housed
in an animal chamber maintained at 22±2°C with a relative humidity
of 50±5% and a 12 h light/dark cycle with access to food and water
ad libitum. Primary osteoblasts were obtained from newborn rabbit
calvarias. Tissues were digested with trypsin 3 times and incubated
with type I collagenase and 0.25% trypsin-EDTA in Hank's buffer
solution (all Thermo Fisher Scientific, Inc.). The tissues were
then cut into pieces <0.5 mm3 and placed on the inner
surface of the dish to release the cells. Cells were cultured with
Dulbecco's Modified Eagle medium (DMEM; Thermo Fisher Scientific,
Inc.) containing 10% fetal bovine serum (Thermo Fisher Scientific,
Inc.), 100 U/ml penicillin and 100 U/ml streptomycin
(Sigma-Aldrich; Merck KGaA) at 37°C in 5% CO2 for 24 h.
The tissues were then washed with 1X PBS to discard unattached
cells. The medium was replenished every 2–3 days and cells were
passaged until 90% confluence was reached.
Antler serum treatment
Osteoblasts were seeded at 1×106/ml and
cultured in a 96-well plate. Cells from rabbits in the 5 groups
including the control, ANFH, ANFH + antler (250 mg/kg), ANFH +
antler (500 mg/kg) and ANFH + antler (1,000 mg/kg) groups and there
were 3 replicates from each group. A total of 40 µl
methylprednisolone solution (1 µmol/l) was added to the ANFH, ANFH
+ antler (250 mg/kg), ANFH + antler (500 mg/kg) and ANFH + antler
(1,000 mg/kg) groups, while 40 µg/DMEM was added to the cells in
the control group. Following incubation for 24 h, medium was
replenished and serum samples collected from the rabbits (1:40)
were added to the corresponding group of cells for 24 h.
Western blotting
Total proteins from the femoral head tissue or the
cells treated with antler serum were extracted using
radioimmunoprecipitation assay buffer (Thermo Fisher Scientific,
Inc.) and measured using the BCA method (Thermo Fisher Scientific,
Inc.). A total of 75 µg protein was analyzed using sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) with a 10%
separating gel and 5% stocking gel. Following SDS-PAGE, proteins
were transferred to a polyvinylidene difluoride membrane (Merck
KGaA). The membrane was blocked with 5% skimmed milk at 25°C for 2
h. Rabbit anti-11β-HSD1 and rabbit anti-11β-HSD2 primary antibodies
(1:1,000, Wuhan Sanyang Biotechnology, Wuhan, China) were incubated
with the membrane at 4°C overnight. Membranes were washed with PBS
3 times for 7 min. Membranes were incubated with goat anti-rabbit
secondary antibodies (Jackson ImmunoResearch Laboratories, Inc.,
West Grove PA, USA) at 25°C for 1 h. β-actin was used as an
internal control. An electrochemiluminescence system (Thermo Fisher
Scientific, Inc.) was used for exposure.
Alkaline phosphatase (ALP)
detection
Following treatment with antler serum for 72 h, 20
µl 0.05% Triton X-100 was added to each well and incubated at 25°C
for 5 min to induce cell lysis. An ALP assay kit (AP0100-1KT;
Sigma-Aldrich; Merck KGaA) was used to detect ALP levels in the
blood serum and osteoblasts. A total of 980 µl (blank) and 960 µl
(test and control) of reaction buffer was pipetted into cuvettes
and 20 µl of 0.67 M pNPP solution was added to each cuvette and
equilibrated at 37°C. Subsequently, 20 µl of sample was added to
each test cuvette, and 20 µl of diluted ALP solution was added to
the enzyme control cuvette. Cuvettes were mixed by inversion and
the A405 nm was recorded (SpectraMax i3X plant reader;
Molecular Devices, LLC, Sunnyvale, CA, USA). Obtained the maximum
linear rate (∆A405 nm/minute).
Measurement of cell proliferation
Following treatment with antler serum for 72 h, 10
µl cell counting kit-8 (Thermo Fisher Scientific, Inc.) was added
to each well and incubated at 37°C for 20 min. An automatic
biochemistry analyzer was used to measure the optical density (OD)
value at 450 nm.
Cell cycle analysis
Following treatment with antler serum for 72 h,
cells were collected and fixed in pre-cooled 70% ethanol at 4°C
overnight. A Cell Cycle and Apoptosis Analysis kit (C1052, Beyotime
Institute of Biotechnology) was used to assess the cell cycle
according to the manufacturer's protocol. A flow cytometer
(Cytomics FC 500; Beckman Coulter, Inc., Brea, CA, USA) was used to
detect and determine the proportion of cells in each phase of the
cell cycle.
Statistical analysis
All data were analyzed using SPSS 19.0 software (IBM
Corp, Armonk, NY, USA) and presented as the mean ± standard
deviations. One-way ANOVA followed by Dunnett's test was used for
comparison and analysis between groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
CT and triglyceride concentration
CT and triglyceride levels in the blood serum of the
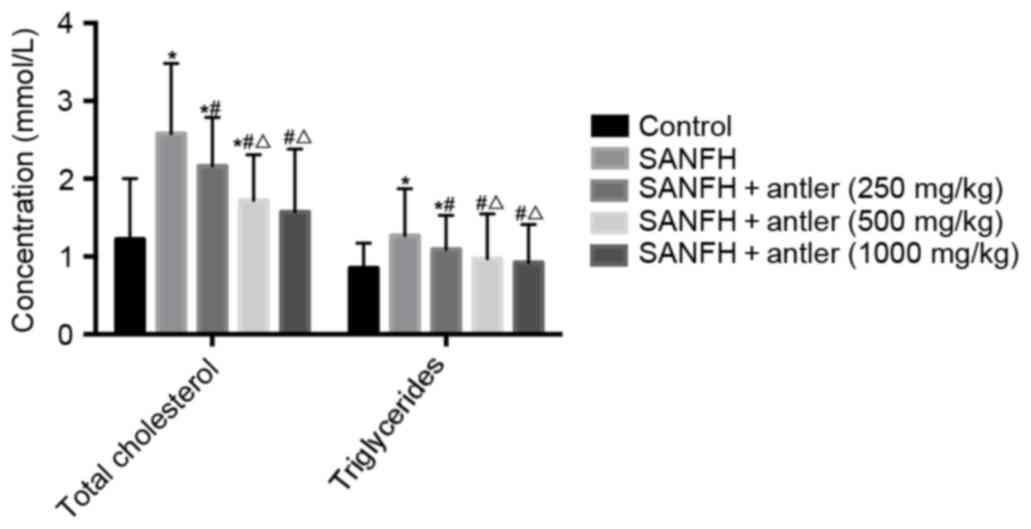
rabbits with or without SAFHN are presented in Table I and Fig.
1. The serum CT levels of the rabbits in the SANFH, SANFH +
antler (250 mg/kg) and SANFH + antler (500 mg/kg) groups (2.58±0.90
mmol/l, 2.16±0.63 mmol/l and 1.72±0.59 mmol/l) were significantly
higher (P<0.05) than that of the control (1.22±0.78 mmol/).
Serum CT levels in the SANFH + antler (1,000 mg/kg) group
(1.57±0.81) mmol/l were higher than that of the control, but not
significantly. Furthermore, serum CT levels in the SANFH + antler
(250 mg/kg), SANFH + antler (500 mg/kg) and SANFH + antler (1,000
mg/kg) groups were all significantly lower than that of the SANFH
group (all P<0.05) and serum CT levels in the SANFH + antler
(500 mg/kg) and SANFH + antler (1,000 mg/kg) groups were
significantly lower than that of the SANFH + antler (250 mg/kg)
group (P<0.05). Serum CT levels did not differ significantly
between the SANFH + antler (1,000 mg/kg) and SANFH + antler (500
mg/kg) groups (Fig. 1 and Table I).
 | Table I.Total cholesterol and triglycerides
in the serum of rabbits with or without SANFH. |
Table I.
Total cholesterol and triglycerides
in the serum of rabbits with or without SANFH.
| Groups | Total cholesterol
(mmol/l) | Triglycerides
(mmol/l) |
|---|
| Control |
1.22±0.78 |
0.85±0.32 |
| SANFH |
2.58±0.90a |
1.27±0.60a |
| SANFH + antler (250
mg/kg) |
2.16±0.63a,b |
1.09±0.44a,b |
| SANFH + antler (500
mg/kg) |
1.72±0.59a–c |
0.97±0.58b,c |
| SANFH + antler
(1,000 mg/kg) |
1.57±0.81b,c |
0.92±0.49b,c |
Serum triglyceride levels of the rabbits in the
SANFH and SANFH + antler (250 mg/kg) groups (1.27±0.60 mmol/l and
1.09±0.44 mmol/l, respectively) were significantly higher
(P<0.05) than that of the control (0.85±0.32 mmol/l). However,
serum triglyceride levels in the SANFH + antler (500 mg/kg) and
SANFH + antler (1,000 mg/kg) groups (0.97±0.58 mmol/l and 0.92±0.49
mmol/l, respectively) did not differ significantly from those in
the control. Serum triglyceride levels in the SANFH + antler (250
mg/kg), SANFH + antler (500 mg/kg) and SANFH + antler (1,000 mg/kg)
groups were all significantly lower than those in the SANFH group
(P<0.05). Furthermore, serum triglyceride levels in the SANFH +
antler (500 mg/kg) and SANFH + antler (1,000 mg/kg) groups were
significantly lower than those in the SANFH + antler (250 mg/kg)
group (P<0.05). Levels of serum triglycerides did not differ
significantly between the SANFH + antler (1,000 mg/kg) and SANFH +
antler (500 mg/kg) group.
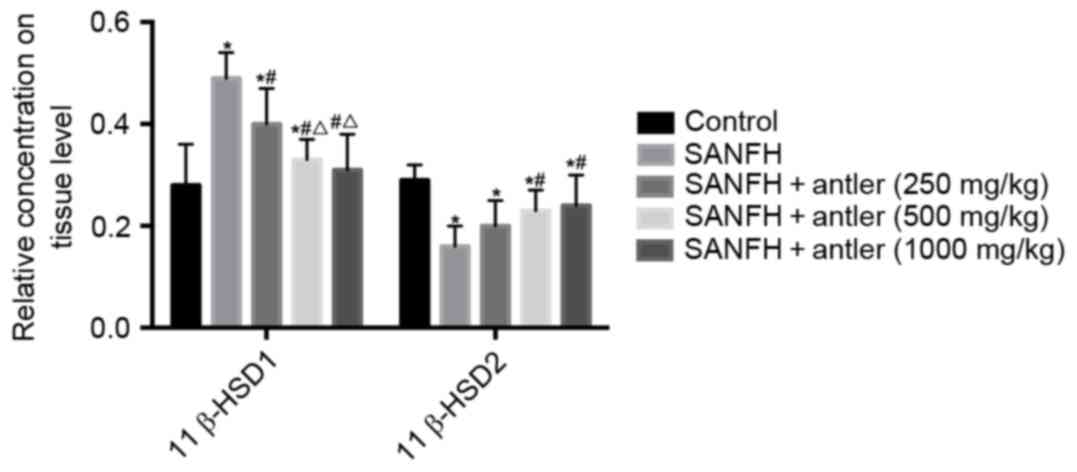
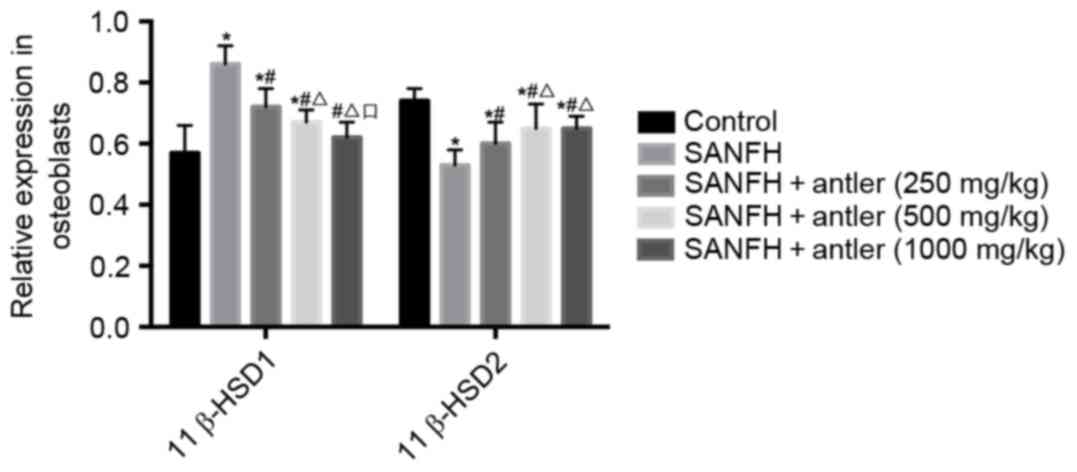
11β-HSD expression
The expression of 11β-HSD1 and 11β-HSD2 in the
rabbit tissues (Fig. 2) and
osteoblasts (Fig. 3) are also
presented in Table II.
 | Table II.Relative expression of 11β-HSD1 and
11β-HSD2 in the rabbit femoral heads and osteoblasts. |
Table II.
Relative expression of 11β-HSD1 and
11β-HSD2 in the rabbit femoral heads and osteoblasts.
|
| Tissues | Osteoblasts |
|---|
|
|
|
|
|---|
| Groups | 11β-HSD1 | 11β-HSD2 | 11β-HSD1 | 11β-HSD2 |
|---|
| Control |
0.28±0.08 |
0.29±0.03 |
0.57±0.09 |
0.74±0.04 |
| SANFH |
0.49±0.05a |
0.16±0.04a |
0.86±0.06a |
0.53±0.05a |
| SANFH + antler (250
mg/kg) |
0.40±0.07a,b |
0.20±0.05a |
0.72±0.06a,b |
0.60±0.07a,b |
| SANFH + antler (500
mg/kg) |
0.33±0.04a–c |
0.23±0.04a,b |
0.67±0.04a–c |
0.65±0.08a–c |
| SANFH + antler
(1000 mg/kg) |
0.31±0.07b,c |
0.24±0.06a,b |
0.62±0.05b–d |
0.65±0.04a–c |
In the rabbit tissues, relative 11β-HSD (P<0.05),
SANFH + antler (250 mg/kg) and SANFH + antler (500 mg/kg) groups
(0.49±0.05, 0.40±0.07 and 0.33±0.04, respectively) were all
significantly higher than that of the control (0.28±0.08;
P<0.05). However, 11β-HSD1 expression in the SANFH + antler
(1,000 mg/kg) group (0.31±0.07) did not differ significantly from
that of the control. Furthermore, the expression of 11β-HSD1 in the
SANFH + antler (250 mg/kg), SANFH + antler (500 mg/kg) and SANFH +
antler (1,000 mg/kg) groups were all significantly lower than that
of the SANFH group (P<0.05). Levels of 11β-HSD1 in the SANFH +
antler (500 mg/kg) and SANFH + antler (1,000 mg/kg) groups were
also significantly lower than in the SANFH + antler (250 mg/kg)
group (P<0.05). There were no significant difference in the
expression of 11β-HSD1 in SANFH + antler (500 mg/kg) and SANFH +
antler (1,000 mg/kg) groups (Fig. 2;
Table II).
In the rabbit tissue, 11β-HSD2 in both experimental
groups was significantly lower than in the control group
(0.29±0.03; P<0.05). Levels of 11β-HSD2 in the SANFH + antler
(500 mg/kg) and SANFH + antler (1,000 mg/kg) groups (0.23±0.04;
0.24±0.06, respectively) were significantly higher than in the
SANFH group (0.16±0.04; P<0.05). There were no significant
differences in 11β-HSD2 between the SANFH and SANFH + antler (250
mg/kg) groups, as well as between in SANFH + antler (500 mg/kg) and
in SANFH + antler (1,000 mg/kg) groups (Fig. 2 and Table
II).
In osteoblasts, 11β-HSD1 levels in the SANFH, SANFH
+ antler (250 mg/kg) and SANFH + antler (500 mg/kg) groups
(0.86±0.06, 0.72±0.06 and 0.67±0.04, respectively) were all
significantly higher than those of the control (0.57±0.09;
P<0.05). However, 11β-HSD1 levels in the SANFH + antler (250
mg/kg), SANFH + antler (500 mg/kg) and SANFH + antler (1,000 mg/kg)
groups were all significantly lower than that of the SANFH group
(P<0.05). Furthermore, 11β-HSD1 levels in the SANFH + antler
(1,000 mg/kg) group were significantly lower than those of the
SANFH + antler (500 mg/kg) group (P<0.05; Fig. 3).
In osteoblasts, levels of 11β-HSD2 in all
experimental groups were significantly lower than that of the
control (0.74±0.04; P<0.05). 11β-HSD2 levels in the SANFH +
antler (250 mg/kg), SANFH + antler (500 mg/kg) and SANFH + antler
(1,000 mg/kg) groups (0.60±0.07, 0.65±0.08 and 0.65±0.04,
respectively) were all significantly higher than that of the SANFH
(0.53±0.05) group (P<0.05). 11β-HSD2 levels in the SANFH +
antler (500 mg/kg) and SANFH + antler (1,000 mg/kg) groups were
significantly higher than of the SANFH + antler (250 mg/kg) group
(P<0.05). However, 11β-HSD2 levels did not differ significantly
between the SANFH + antler (500 mg/kg) and SANFH + antler (1,000
mg/kg) groups (Fig. 3; Table II).
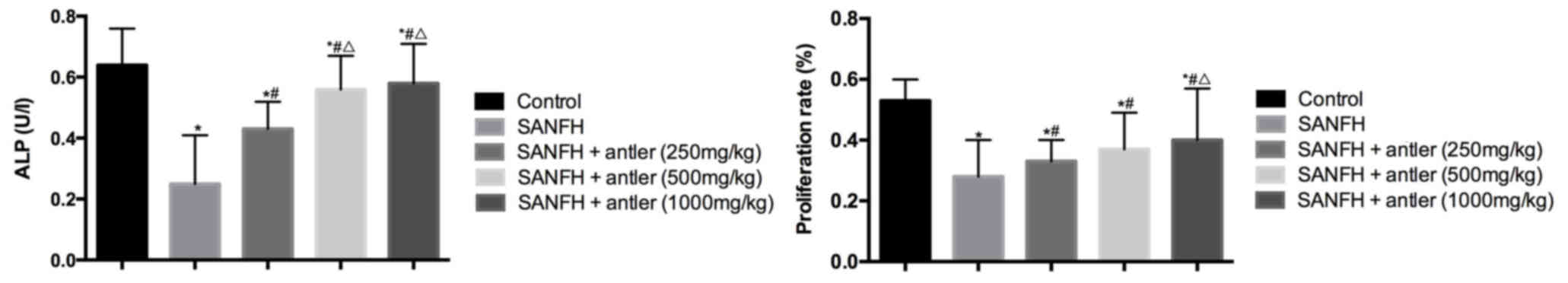
ALP levels and osteoblast
proliferation
The ALP concentration (U/l) and osteoblast
proliferation rate (%) are presented in Table III and Fig. 4. The proliferation rates of the
osteoblasts in all experimental groups were significantly lower
than that of the control group (0.53±0.07; P<0.05). The
proliferation rate of the SANFH + antler (250 mg/kg), SANFH +
antler (500 mg/kg) and SANFH + antler (1,000 mg/kg) groups
(0.33±0.07, 0.37±0.12 and 0.40±0.17, respectively) were all
significantly higher than that of the SANFH (0.28±0.12) group
(P<0.05). The proliferation rate of the SANFH + antler (1,000
mg/kg) group was significantly higher than that of the SANFH +
antler (250 mg/kg) group (P<0.05) but did not differ
significantly from that of the SANFH + antler (500 mg/kg) group
(Fig. 4; Table III).
 | Table III.Effect of antler-containing serum on
ALP levels, proliferation and the cell cycle in osteoblasts. |
Table III.
Effect of antler-containing serum on
ALP levels, proliferation and the cell cycle in osteoblasts.
|
|
|
| Cell cycle (%) |
|---|
|
|
|
|
|
|---|
| Groups | Proliferation
(%) | ALP (U/l) | G0/G1 | S | G2/M |
|---|
| Control |
0.53±0.07 |
0.64±0.12 |
63.39±4.51 |
28.04±4.50 |
8.57±0.95 |
| SANFH |
0.28±0.12a |
0.25±0.16a |
75.60±3.88a | 22.05±3.63 |
2.35±1.14 |
| SANFH + antler (250
mg/kg) |
0.33±0.07a,b |
0.43±0.09a,b |
70.25±4.06a,b | 25.37±3.84 |
4.38±0.87 |
| SANFH + antler (500
mg/kg) |
0.37±0.12a,b |
0.56±0.11a–c |
68.77±2.96a,b | 26.29±2.17 |
4.94±0.93 |
| SANFH + antler
(1,000 mg/kg) |
0.40±0.17a–c |
0.58±0.13a–c |
67.12±3.58b |
26.96±4.11 |
5.92±1.06 |
ALP levels in all experimental groups were
significantly lower than in the control (0.64±0.12; P<0.05). ALP
in the SANFH + antler (250 mg/kg), SANFH + antler (500 mg/kg) and
SANFH + antler (1,000 mg/kg) groups (0.43±0.09, 0.56±0.11 and
0.58±0.13, respectively) were all significantly higher than in the
SANFH group (0.25±0.16; P<0.05). ALP in the SANFH + antler (500
mg/kg) and SANFH + antler (1,000 mg/kg) groups were significantly
higher than that of the SANFH + antler (250 mg/kg) group
(P<0.05), however there were no significant differences in the
ALP between the SANFH + antler (500 mg/kg) and SANFH + antler
(1,000 mg/kg) groups.
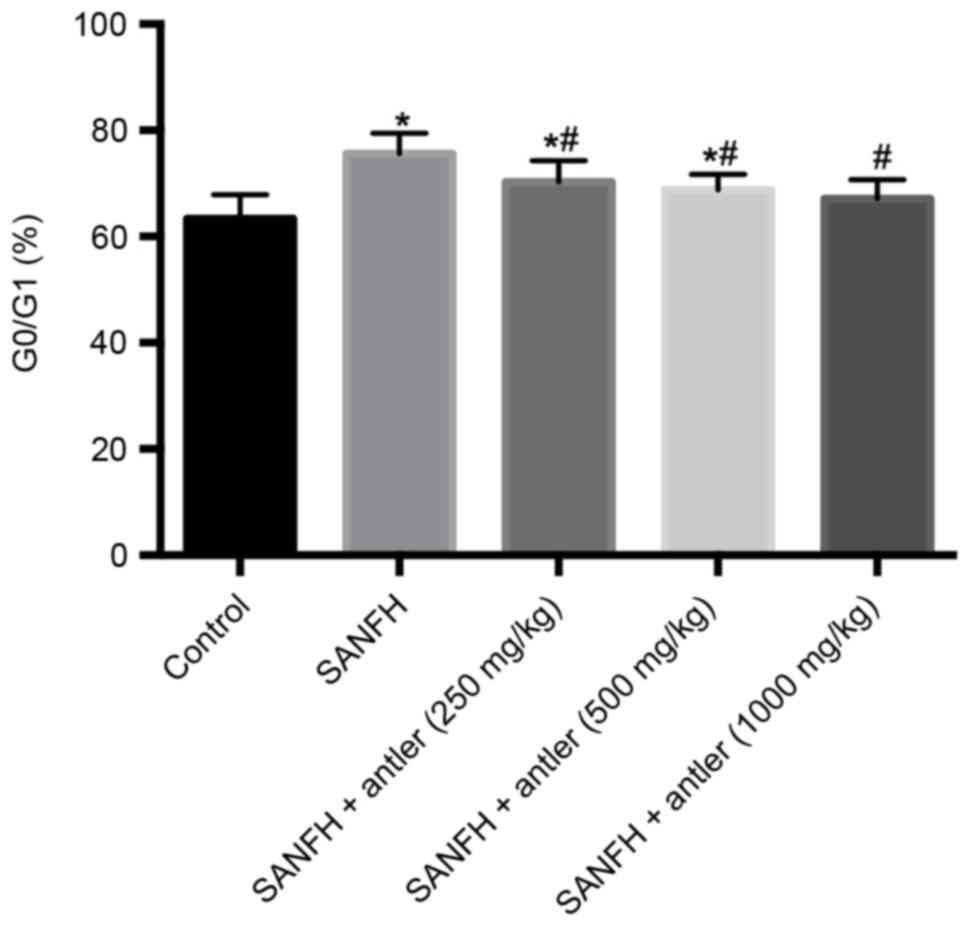
Osteoblast cell cycle
As presented in Table
III and Fig. 5, the proportion
of osteoblasts in G0/G1 in the SANFH, SANFH + antler (250 mg/kg)
and SANFH + antler (500 mg/kg) groups (75.60±3.88, 70.25±4.06 and
68.77±2.96, respectively) were significantly higher than that of
the control (63.39±4.51; P<0.05), whereas the proportion of
osteoblasts in G0/G1 in the SANFH + antler (1,000 mg/kg) group
(67.12±3.58) did not differ significantly from that of the control.
Furthermore, the proportion of osteoblasts in G0/G1 in the SANFH +
antler (250 mg/kg), SANFH + antler (500 mg/kg) and SANFH + antler
(1,000 mg/kg) groups were significantly lower than that of the
SANFH group (P<0.05; Fig. 5).
Discussion
Steroids are clinically used to inhibit
inflammation, allergic and immune responses in a number of diseases
(42–44). For example, steroids were widely used
to treat SARS during the 2003 outbreak (45,46).
However, the chronic use of steroids may induce severe side
effects, including the onset of ANFH. Previous studies have
demonstrated that the number of cases of ANFH that occur following
the use of steroids is increasing (47–49).
Without therapy or intervention, femoral head necrosis may become
an irreversible process (50,51).
Previously, the majority of treatments for hip arthroplasty
(52), prosthesis and reconstructive
surgery (53) were surgical.
However, few cases could not undergo surgical treatment.
The current study used the steroid
methylprednisolone to induce and establish a model of SANFH in
rabbits. Following successful establishment of the model, different
concentrations of antler extract were used to treat rabbits to
determine the therapeutic effect of antler extract on SANFH.
Subsequently, serum CT and triglyceride levels in the rabbits were
measured and it was observed that CT and triglyceride levels
increased following the establishment of SANFH. However, CT and
triglyceride levels decreased in the serum following treatment with
antler extract. Serum CT levels of the rabbits in the SANFH, SANFH
+ antler (250 mg/kg), SANFH + antler (500 mg/kg) and SANFH + antler
(1,000 mg/kg) groups were all higher than that of the control,
indicating the successful establishment of the SANFH model and the
presence of metabolic disorder. Serum CT levels in the SANFH +
antler (250 mg/kg), SANFH + antler (500 mg/kg) and SANFH + antler
(1,000 mg/kg) groups were lower than those of the SANFH group.
Furthermore, serum CT levels in the SANFH + antler (500 mg/kg) and
SANFH + antler (1,000 mg/kg) groups were significantly lower than
in the SANFH + antler (250 mg/kg) group (P<0.05). Similar
results were observed regarding triglyceride levels. This may be
explained by the fact that SANFH induces fat embolism in the
peripheral vessels, leading to intravascular coagulation (54–56). The
results of the current study therefore indicate that antler extract
induces a therapeutic effect on metabolic disorders in SANFH.
11β-HSD1 are the critical enzymes that regulate
glucocorticoids in bone tissue, which are expressed in osteoblasts
and osteoclasts (57) and are able
to participate in oxidation and reduction reactions (58). However, the mechanisms by which
steroids induce ANFH remain unclear. The current study detected and
compared 11β-HSD1 and 11β-HSD2 expression in femoral head tissue
and osteoblasts with or without treatment with different
concentrations of antler extract or antler-containing serum. The
results demonstrated that in the femoral head tissues of SANFH
model rabbits and osteoblasts treated with different concentrations
of antler extract, 11β-HSD1 levels significantly decreased, whereas
levels of 11β-HSD2 significantly increased (P<0.05). In the
femoral head tissue, 11β-HSD1 levels in the experimental groups,
apart from in the SANFH + antler (1,000 mg/kg) group, were
significantly higher than in the control (P<0.05). Levels of
11β-HSD1 in the SANFH + antler (250 mg/kg), SANFH + antler (500
mg/kg) and SANFH + antler (1,000 mg/kg) groups were all
significantly lower than that in SANFH group (P<0.05). 11β-HSD2
levels in the experimental groups were lower compared with the
control, significantly (P<0.05). Levels of 11/kHSD2 in the SANFH
+ antler (250 mg/kg), SANFH + antler (500 mg/kg) and SANFH + antler
(1,000 mg/kg) groups were significantly higher than in the SANFH
groups (P<0.05). Similar results were found regarding in
osteoblasts treated with different concentrations of antler
extract, with decreasing 11β-HSD1 and increasing 11β-HSD2 levels.
This phenomenon indicates that 11β-HSD1 is upregulated, whereas
11β-HSD2 is downregulated in SANFH. Antler induced therapeutic
effects in SANFH by downregulating 11β-HSD1 and upregulating
11β-HSD2. Following treatment with higher concentrations of antler
extract, this therapeutic effect became more marked.
In order to evaluate the therapeutic effect of
antler extract on SANFH, serum extracted from rabbits with SANFH
that had or had not received treatment with different
concentrations of antler, were used to treat osteoblasts extracted
from rabbits. As well as the detection of 11β-HSD expression,
levels of ALP, which is an indicator of early osteoblastic
differentiation (59), were measured
and cell proliferation and the proportion of cells in each phase of
the cell cycle were determined. The proliferation rates of the
osteoblasts in all experimental groups were all significantly lower
than that of the control (P<0.05), indicating that SANFH
inhibits the proliferation of osteoblasts. The proliferation rate
in the SANFH + antler (250 mg/kg), SANFH + antler (500 mg/kg) and
SANFH + antler (1,000 mg/kg) groups were significantly higher than
that of the SANFH group (P<0.05) and increased as the
concentration of antler extract increased, indicating that antler
treatment may promote the proliferation of osteoblasts. ALP levels
in all experimental groups were significantly lower than that of
the control (P<0.05), indicating that SANFH decreases ALP
levels. However, ALP levels in the SANFH + antler (250 mg/kg),
SANFH + antler (500 mg/kg) and SANFH + antler (1,000 mg/kg) groups
were all significantly higher than that of the SANFH group
(P<0.05) and increased as the concentration of antler extract
increased, indicating that treatment with antler extract may
increase ALP levels following SANFH. The proportion of osteoblasts
in the G0/G1 phase (%) in the experimental groups were higher than
that in the control and the proportion of osteoblasts in the SANFH
+ antler (250 mg/kg), SANFH + antler (500 mg/kg) and SANFH + antler
(1,000 mg/kg) groups were all significantly lower than that of the
SANFH group (P<0.05). This phenomenon demonstrates that SANFH
may inhibit the proliferation of osteoblasts proliferation and
attenuate the cell cycle at the G0/G1 phase. Antler extract, as a
therapeutic drug, may promote the proliferation of osteoblasts and
induce cellular differentiation.
In conclusion, the current study demonstrated that
antler has a therapeutic effect on ANFH induced by steroids, which
may promote biochemical metabolism, as well as promoting the
proliferation and differentiation of osteoblasts. 11β-HSDs may
serve important roles in the development of SANFH and may be
targeted by antler extract in order to prevent and treat SANFH.
References
|
1
|
Rodriguez-Merchan EC, Martinez-Lloreda A,
Sanjurjo MJ and Jimenez-Yuste V: Osteonecrosis of the femoral
headMusculoskeletal Aspects of Haemophilia. Rodriguez-Merchan EC,
Goddard NJ and Lee CA: Springer; Berlin, Heidelberg: pp. 153–158.
2014
|
|
2
|
Liakakos T, Thomakos N, Fine PM, Dervenis
C and Young RL: Peritoneal adhesions: Etiology, pathophysiology and
clinical significance. recent advances in prevention and
management. Dig Surg. 18:260–273. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fisher DE and Bickel WH:
Corticosteroid-induced avascular necrosis. a clinical study of
seventy-seven patients. J Bone Joint Surg Am. 53:859–873. 1971.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bozic KJ, Zurakowski D and Thornhill TS:
Survivorship analysis of hips treated with core decompression for
nontraumatic osteonecrosis of the femoral head. J Bone Joint Surg
Am. 81:200–209. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zaidi M, Sun L, Robinson LJ, Tourkova IL,
Liu L, Wang Y, Zhu LL, Liu X, Li J, Peng Y, et al: ACTH protects
against glucocorticoid-induced osteonecrosis of bone. Proc Natl
Acad Sci USA. 107:pp. 8782–8787. 2010; View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Su P, Li R, Liu S, Zhou Y, Wang X, Patil
N, Mow CS, Mason JC, Huang D and Wang Y: Age at onset-dependent
presentations of premature hip osteoarthritis, avascular necrosis
of the femoral head, or legg-calvé-perthes disease in a single
family, consequent upon a p. Gly1170Ser mutation of COL2A1.
Arthritis Rheum. 58:1701–1706. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu J, Chen H, Zhang H, et al: Clinical
characteristics and risk factors of avascular necrosis of the
femoral head in patients with lupus nephritis. Chin J Nephrol Dial
Transplant. 2013.
|
|
8
|
Zeng C, Song YC, Cai DZ and Liu B:
Clinical characteristics and complication prevention of total hip
arthroplasty for the patients of femoral head necrosis with
systemic lupus erythematosus. Chin J Joint Surg. 454–458. 2009.(In
Chinese).
|
|
9
|
Kawate K, Yajima H, Ohgushi H, Kotobuki N,
Sugimoto K, Ohmura T, Kobata Y, Shigematsu K, Kawamura K, Tamai K
and Takakura Y: Tissue-engineered approach for the treatment of
steroid-induced osteonecrosis of the femoral head: Transplantation
of autologous mesenchymal stem cells cultured with beta-tricalcium
phosphate ceramics and free vascularized fibula. Artif Organs.
30:960–962. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhao FC, Li ZR and Guo KJ: Clinical
analysis of osteonecrosis of the femoral head induced by steroids.
Orthop Surg. 4:28–34. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
LaPorte DM, Mont MA, Mohan V, Jones LC and
Hungerford DS: Multifocal osteonecrosis. J Rheumatol. 25:1968–1974.
1998.PubMed/NCBI
|
|
12
|
Barnes PJ and Adcock IM: How do
corticosteroids work in asthma? Ann Intern Med. 139:359–370. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu X, Zhang Y and Huo L: A study on
application of protocol nursing in corticosteroids treatment
compliance of nephritic syndrome patients. Chin Nurs Res.
45:539–545. 2008.
|
|
14
|
Lee AC and Wong LG: Facial palsy,
corticosteroids, and acute leukemia. Pediatr Neurol. 36:137–138.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Steelman J and Kappy M: Adrenal
suppression and growth retardation from ocular corticosteroids. J
Pediatr Ophthalmol Strabismus. 38:177–178. 2001.PubMed/NCBI
|
|
16
|
Morton NM: Obesity and corticosteroids:
11beta-hydroxysteroid type 1 as a cause and therapeutic target in
metabolic disease. Mol Cell Endocrinol. 316:154–164. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Need AG: Corticosteroids and osteoporosis.
Aust N Z J Med. 17:267–272. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hengge UR, Ruzicka T, Schwartz RA and Cork
MJ: Adverse effects of topical glucocorticosteroids. J Am Acad
Dermatol. 54:1–18. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gebhard KL and Maibach HI: Relationship
between systemic corticosteroids and osteonecrosis. Am J Clin
Dermatol. 2:377–388. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Skalka HW and Prchal JT: Effect of
corticosteroids on cataract formation. Arch Ophthalmol.
98:1773–1777. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lavin PJ and Workman R: Cushing syndrome
induced by serial occipital nerve blocks containing
corticosteroids. Headache. 41:902–904. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cranney A, Welch V, Adachi J, Homik J,
Shea B, Suarez-Almazor ME, Tugwell P and Wells GA: Calcitonin for
preventing and treating corticosteroid-induced osteoporosis
(Review). Cochrane Database Syst Rev. CD0019832000.PubMed/NCBI
|
|
23
|
Kerachian MA, Cournoyer D, Harvey EJ, Chow
TY, Bégin LR, Nahal A and Séguin C: New insights into the
pathogenesis of glucocorticoid-induced avascular necrosis:
Microarray analysis of gene expression in a rat model. Arthritis
Res Ther. 12:R1242010. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
Baktursun P, Peng H and Li BB: Effects of
antler powder on treatment of corticosteroid-induced avascular
necrosis of the femoral head in rats. J Clin Rehabilitative Tiss
Eng Res. 346:1030–1031. 2011.
|
|
25
|
Tian L, Tian XY, Fan NN, Liang XP and YU
XM: Treatment of avascular necrosis of the femoral head (ANFH) with
induced differentiation VEGF-165 gene modified mesenchymal stem
cells in rabbits. Journal of Shenyang Medical College. 1–144.
2009.
|
|
26
|
Sen RK: Management of avascular necrosis
of femoral head at pre-collapse stage. Indian J Orthop. 43:6–16.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pritchett JW: Statin therapy decreases the
risk of osteonecrosis in patients receiving steroids. Clin Orthop
Relat Res. 1–178. 2001.
|
|
28
|
Lai KA, Shen WJ, Yang CY, Shao CJ, Hsu JT
and Lin RM: The use of alendronate to prevent early collapse of the
femoral head in patients with nontraumatic osteonecrosis. J Bone
Joint Surg Am. 87:2155–2159. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kruuk EB, Slate J, Pemberton JM,
Brotherstone S, Guinness F and Clutton-Brock T: Antler size in red
deer: Heritability and selection but no evolution. Evolution.
56:1683–1695. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gould SJ: The origin and function of
‘BIZARRE’ structures: Antler size and skull size in the ‘IRISHELK,’
megaloceros giganteus. Evolution. 28:191–220. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chapman DI: Antler structure and
function-a hypothesis. J Biomech. 14:195–197. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li Y, Zhao Y, Tang R and Qu X: Preventive
and therapeutic effects of antler collagen on osteoporosis in
ovariectomized rats. Afr J Biotechnol. 9:6437–6441. 2010.
|
|
33
|
Baktursun P, Peng H and Li BB: Effects of
antler powder on treatment of corticosteroid-induced avascular
necrosis of the femoral head in rats[J]. Journal of Clinical
Rehabilitative Tissue Engineering Research. 346:1030–1031.
2011.
|
|
34
|
Seckl JR and Walker BR: Minireview:
11beta-hydroxysteroid dehydrogenase type 1-a tissue-specific
amplifier of glucocorticoid action. Endocrinology. 142:1371–1376.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nakano D and Nishiyama A: Programmed
11β-hydroxysteroid dehydrogenase type 2 reduction: A possible cause
of adult-onset disease? J hypertens. 29:201–203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kaur K, Hardy R, Ahasan MM, Eijken M, van
Leeuwen JP, Filer A, Thomas AM, Raza K, Buckley CD, Stewart PM, et
al: Synergistic induction of local glucocorticoid generation by
inflammatory cytokines and glucocorticoids: Implications for
inflammation associated bone loss. Ann Rheum Dis. 69:1185–1190.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Kerachian MA, Séguin C and Harvey EJ:
Glucocorticoids in osteonecrosis of the femoral head: A new
understanding of the mechanisms of action. J Steroid Biochem Mol
Biol. 114:121–128. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Combes R and Balls M: Comments on the
sub-group reports of the EU technical expert working group on the
revision of directive 86/609/EEC on the protection of animals used
for experimental and other scientific purposes. Altern Lab Anim.
35:155–175. 2007.PubMed/NCBI
|
|
39
|
Wen Q, Ma L, Chen YP, Yang L, Luo W and
Wang XN: A rabbit model of hormone-induced early avascular necrosis
of the femoral head. Biomed Environ Sci. 21:398–403. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mont MA, Jones LC, Einhorn TA, Hungerford
DS and Reddi AH: Osteonecrosis of the femoral head. Potential
treatment with growth and differentiation factors. Clin Orthop
Relat Res. 355 Suppl:S314–S335. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Baneux PJ, Garner D, Mcintyre HB and
Holshuh HJ: Euthanasia of rabbits by intravenous administration of
ketamine. J Am Vet Med Assoc. 189:1038–1039. 1986.PubMed/NCBI
|
|
42
|
Glenn EM: Steroids, nonsteroids,
intermediary metabolism, inflammation and their probable
interrelationships. Hormonal Steroids Biochem Pharmacol Ther.
1:319–349. 1964. View Article : Google Scholar
|
|
43
|
Hemphill N and Morgan DR: Speaker: For
inflammation, steroids should be used boldly. Primary Care
Optometry News. April. 2013, https://www.healio.com/footer/healio-dot-com/about-the-wyanoke-group
|
|
44
|
Denso P: Free communications-session
1-inflammation & steroidsfree. Clin Experiment Aller. 23:75–76.
1993. View Article : Google Scholar
|
|
45
|
Griffith JF, Antonio GE, Kumta SM, Hui DS,
Wong JK, Joynt GM, Wu AK, Cheung AY, Chiu KH, Chan KM, et al:
Osteonecrosis of hip and knee in patients with severe acute
respiratory syndrome treated with steroids. Radiology. 235:168–175.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen RC, Tang XP, Tan SY, Liang BL, Wan
ZY, Fang JQ and Zhong N: Treatment of severe acute respiratory
syndrome with glucosteroids: The guangzhou experience. Chest.
129:1441–1452. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Rapała K, Walczak P and Truszczyńska A:
Orthopedic diagnostic and therapeutic problems in avascular
poststeroid necrosis of femur head in the course acute
lymphoblastic leukemia-description of 3 cases. Chir Narzadow Ruchu
Ortop Pol. 75:121–125. 2010.PubMed/NCBI
|
|
48
|
Wu D, Song D, Ni J and Dai R: Avascular
necrosis of the femoral head due to the bilateral injection of
heroin into the femoral vein: A case report. Exp Ther Med.
6:1041–1043. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Iwata H, Hasegawa Y, Mizuno M, Genda E,
Kataoka Y and Kada A: Progression of avascular necrosis of femoral
head and choice of treatment. Nagoya J Med Sc. 54:27–39. 1992.
|
|
50
|
Jones LC and Hungerford DS: Osteonecrosis:
Etiology, diagnosis, and treatment. Curr Opin Rheumatol.
16:443–449. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Hungerford DS: Treatment of osteonecrosis
of the femoral head: Everything's new. J Arthroplasty. 22 4 Suppl
1:S91–S94. 2007. View Article : Google Scholar
|
|
52
|
Fritsch EW and Gleitz M: Ceramic femoral
head fractures in total hip arthroplasty. Clin Orthop Relat Res.
1–136. 1996.PubMed/NCBI
|
|
53
|
Cabanela ME: The bipolar prosthesis in
avascular necrosis of the femoral head. Semin Arthroplasty.
2:228–233. 1991.PubMed/NCBI
|
|
54
|
Kawai K, Tamaki A and Hirohata K:
Steroid-induced accumulation of lipid in the osteocytes of the
rabbit femoral head. A histochemical and electron microscopic
study. J Bone Joint Surg Am. 67:755–763. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Jones JP Jr: Fat embolism and
osteonecrosis. Orthop Clin North Am. 16:595–633. 1985.PubMed/NCBI
|
|
56
|
Jones JP Jr and Sakovich L: Fat embolism
of bone. A roentgenographic and histological investigation, with
use of intra-arterial lipiodol, in rabbits. J Bone Joint Surg Am.
48:149–164. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Cooper MS, Walker EA, Bland R, Fraser WD,
Hewison M and Stewart PM: Expression and functional consequences of
11beta-hydroxysteroid dehydrogenase activity in human bone. Bone.
27:375–381. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cooper MS, Rabbitt EH, Goddard PE,
Bartlett WA, Hewison M and Stewart PM: Osteoblastic
11beta-hydroxysteroid dehydrogenase type 1 activity increases with
age and glucocorticoid exposure. J Bone Miner Res. 17:979–986.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chen XP, Qian H, Wu JJ, Ma XW, Gu ZX, Sun
HY, Duan YZ and Jin ZL: Expression of vascular endothelial growth
factor in cultured human dental follicle cells and its biological
roles. Acta Pharmacol Sin. 28:985–993. 2007. View Article : Google Scholar : PubMed/NCBI
|