Introduction
Postoperative acute kidney injury (AKI) is frequent
after cardiac surgery in patients undergoing cardiopulmonary bypass
(CPB) with a prevalence of around 20–30% in the adult population.
It is associated with perioperative mortality as high as 20–80%
(1–3). We (4,5), as well
as others (1) have previously
demonstrated that a reduction in renal perfusion might be a
contributing factor to postoperative kidney injury, however, there
is a lack of an effective remedy to prevent the CPB-associated AKI.
Goldberg and Dennen (6) found that
AKI in the early stage can be fully recovered if early diagnosis
and proper treatment are applied. Leonard et al (7) demonstrated that the reversal of renal
dysfunction by targeted administration of vascular endothelial
growth factor (VEGF) could be a novel potential therapeutic
approach in ischemic renal disease. We hypothesize that VEGF has a
role in protecting against CPB-associated AKI by improving renal
microperfusion.
Materials and methods
The animal protocol of the present study was
approved by the Animal Care and Use Committee at West China
Hospital (Chengdu, China) in accordance with the requirements of
the Chinese Animal Care Committee.
All of the animals used in this study were treated
in compliance with the Guide for the Care and Use of Laboratory
Animals published by the US National Institutes of Health (NIH
Publication, no. 85-23, revised 1996).
CPB procedure
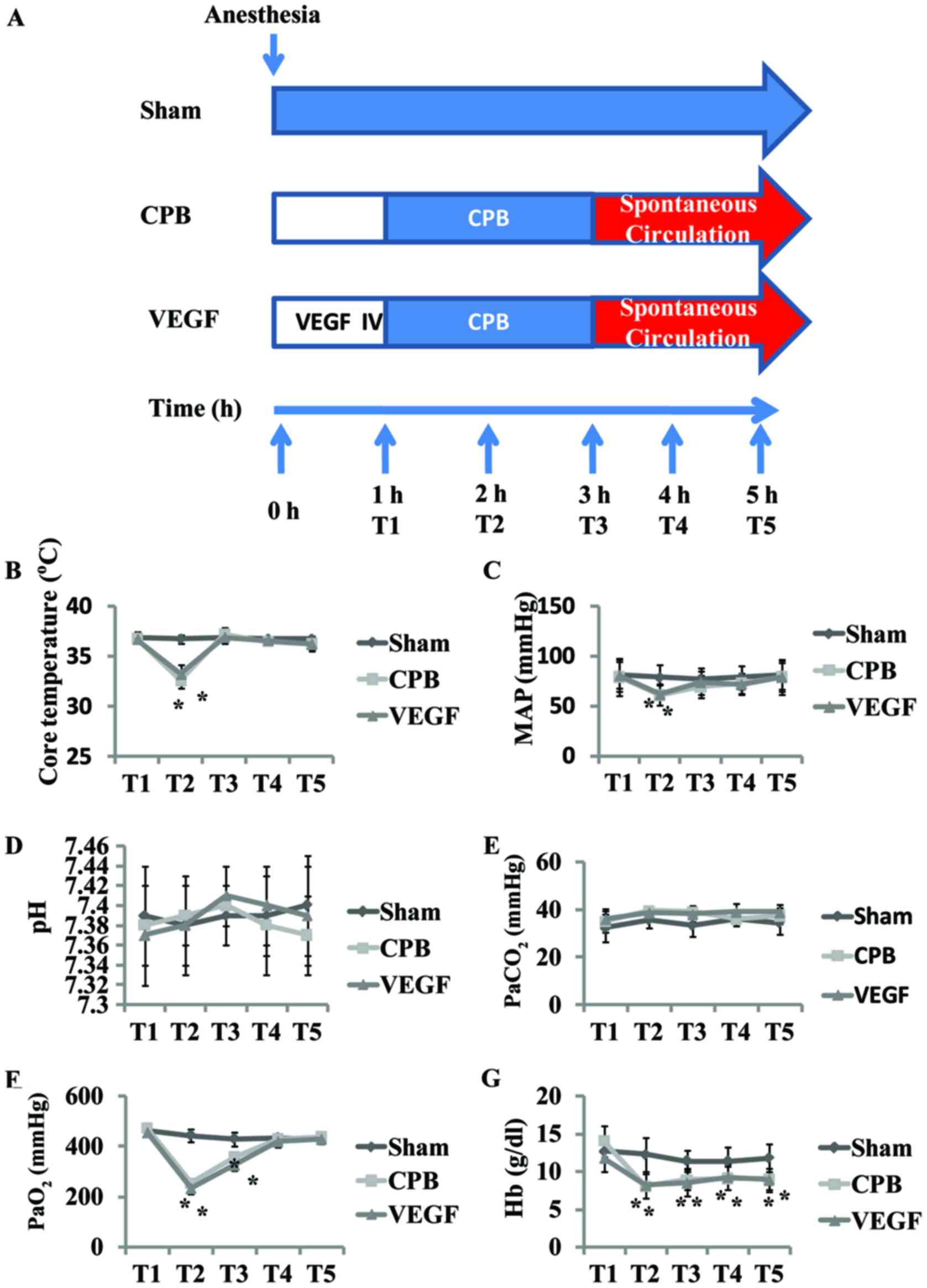
A total of 18 beagles, aged 2–4 years old, 10–15 kg,
were randomized into 3 groups. Sham group, received sternotomy and
under general anesthesia for 5 h; CPB group, 2 h of CPB, 2 h after
CPB; VEGF group, 2 h of CPB, 2 h after CPB plus VEGF 0.1 mg/kg
(8) intravenous administration in 1
h before CPB.
A systemic CPB flow was established via aortic and
venous cannulation in the right atrial appendage undergoing median
sternotomy. CPB was performed for 2 h (32–34°C, 70–100 ml/kg/min)
in the 2 h aortic cross clamping. The beagles were weaned from CPB,
and received another 2 h observation. Core temperature, mean
arterial pressure (MAP), arterial blood sample and ultrasound data
were collected at five defined time-points (T1–5)
(Fig. 1). T1 was defined
as initiation of CPB, T2 was defined as 1 h after
initiation of CPB, T3 was defined as end of CPB,
T4 was defined as 1 h after CPB, and T5 was
defined as 2 h after CPB. Kidneys were harvested at the end of the
procedures for measurements.
Measurement of blood gas values
Arterial blood samples were collected in a
polyethylene catheter from the left femoral artery. The blood gas
parameters, including pH, PaO2, PaCO2 and Hb,
were measured immediately with a blood gas analyzer (i-stat300G;
Abbott Pharmaceutical Co. Ltd., Lake Bluff, IL, USA).
Contrast-enhanced ultrasound
(CEU)
After intravenous administration of stabilized
sulfur hexafluoride microbubbles (SonoVue®; Bracco,
Milan, Italy), renal microcirculation was assessed by CEU (IU22;
Philips Healthcare, Amsterdam, The Netherlands). Time-intensity
curve (TIC) was derived from three region of interest (ROI) using
QLAB post-analysis software. Parameters were calculated, including
ascending slope rate (ASR), area under curve (AUC), peak intensity
(DPI) and time to peak intensity (TTP). We averaged values from
upper, middle and lower segments of kidney for anatomical
factors.
Definition of AKI
AKI was defined by increase in serum creatinine
(SCr) to ≥1.5 times baseline according to Kidney Disease Improving
Global Outcomes (KDIGO) guidelines (9). SCr and blood urea nitrogen (BUN) were
evaluated before CPB and 2 h after CPB.
Pathology scoring and determination of
apoptosis rate
Specimens of right kidneys were collected at 2 h
after CPB, and were stored in formalin 4% for 24 h. After washing,
alcohol cleaning, and paraffin embedding slice, the slides were
stained with hematoxylin and eosin (H&E). The histological
examination was blindly performed in three groups and 10 view
fields per slide were examined. The severity of tissue damage was
scored by two experienced doctors in accordance with the proportion
of necrosis of renal tubule. Criteria: 0, normal morphology; 1, a
small amount of renal tubular necrosis (≤10%); 2, mild renal
tubular necrosis (10–25%); 3, moderate renal tubular necrosis
(26–75%); and 4, severe renal tubular necrosis (≥75%) (10).
Paraffin sections were regularly dewaxed with
xylene, prior to determination of the apoptosis rate by
TdT-mediated dUTP nick end labeling (TUNEL).
Enzyme-linked immunosorbent assay
(ELISA) and western blot analysis
Left kidneys were harvested at the end of the
procedures, and immediately stored at −80°C until assayed. Tissues
were ground with a mortar and pestle and then underwent repeated
freeze-thaw cycles in lysis buffer (Promega, Madison, WI, USA).
Tumor necrosis factor (TNF)-α, MDA, SOD and interleukin (IL)-6 were
measured via ELISA, and B-cell lymphoma 2 (Bcl-2), cluster of
differentiation (CD)95, cleaved caspase-3, HIF-1α, VEGF,
phosphorylated (p-)Akt and p-endothelial nitric oxide synthase
(eNOS) protein levels were measured via western blot analysis.
Statistical analysis
Statistical analysis was performed using the SPSS
15.0 (SPSS, Inc., Chicago, IL, USA). Physiologic data were assessed
for time effect and treatment using two-way repeated measurement
ANOVA with Turkey post hoc tests, TIC Parameter, SCr and BUN
levels, ELISA, western blot analysis were compared using a t-test.
Values are shown as mean ± standard deviation and P<0.05 was
considered significant.
Results
Basic characteristics
There were no differences in baseline values of pH,
PaCO2, PaO2 and Hb among groups. Core
temperature (T) and MAP decreased T2 in comparison with
baseline (P<0.05), with no significant difference after CPB
(Fig. 1). PaO2 and Hb
decreased significantly at T2–3, with Hb continually
decreased at T4–5 (P<0.05; Fig. 1).
TIC analysis of CEU
There were no differences in baseline values in the
three groups. Compared with group sham, ASR decreased significantly
at T2–5, AUC and TTP increased significantly at
T2–5 (P<0.05) in CPB and VEGF group, with DPI
decreased significantly at T3–5 in CPB group
(P<0.05). Compared with the T1, ASR decreased and
AUC, TTP increased significantly at T2–5 in CPB and VEGF
group (P<0.05), with DPI decreased at T3–5
(P<0.05) in CPB group. Compared with group CPB, ASR increased at
T2–5, AUC and TTP decreased at T2–5 in VEGF
group (P<0.05; Fig. 2).
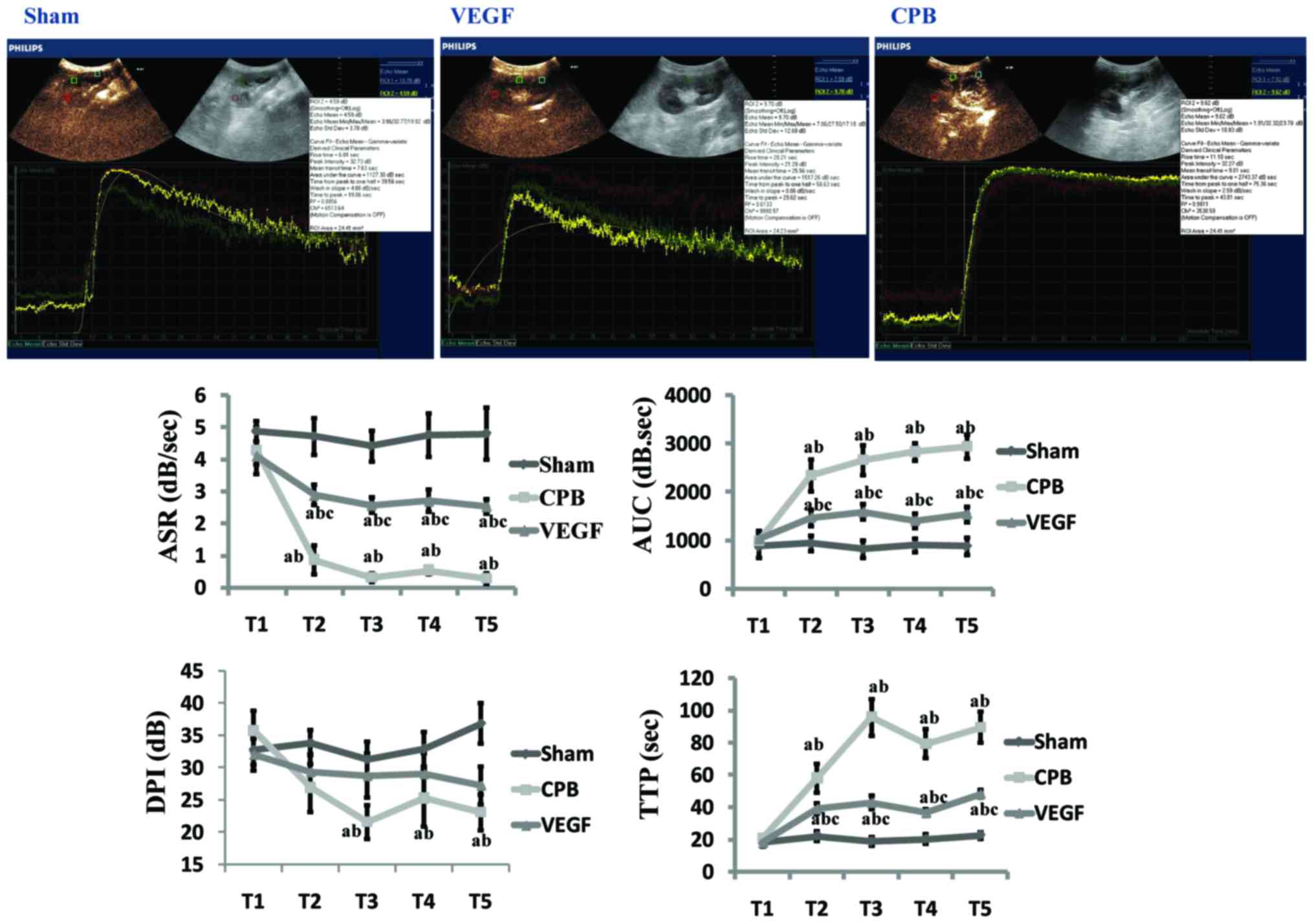 | Figure 2.Image of time-intensity curve 2 h
after CPB (T5), and time-intensity curve parameter
analysis (ASR, AUC, DPI, and TTP). aP<0.05 vs. sham;
bP<0.05 vs. T1; cP<0.05 vs.
CPB. CPB, cardiopulmonary bypass; VEGF, vascular endothelial growth
factor; ASR, ascending slope rate; AUC, area under curve; DPI, peak
intensity; TTP, time to peak intensity. |
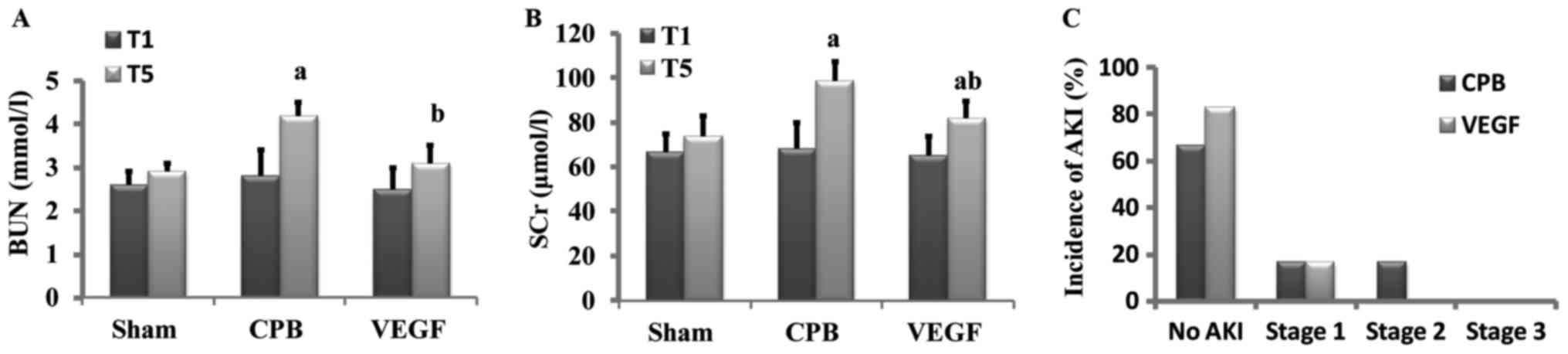
Renal function
Baseline values of SCr and BUN did not differ
significantly among groups. After 2 h CPB, SCr and BUN increased
significantly in CPB group in comparison with baseline, SCr and BUN
in VEGF group were lower than that of CPB group. AKI occurred in 2
beagles in CPB group (1 beagle in stage 1, 1 beagle in stage 2) and
1 beagle in VEGF group (in stage 1; Fig.
3).
Histological analysis
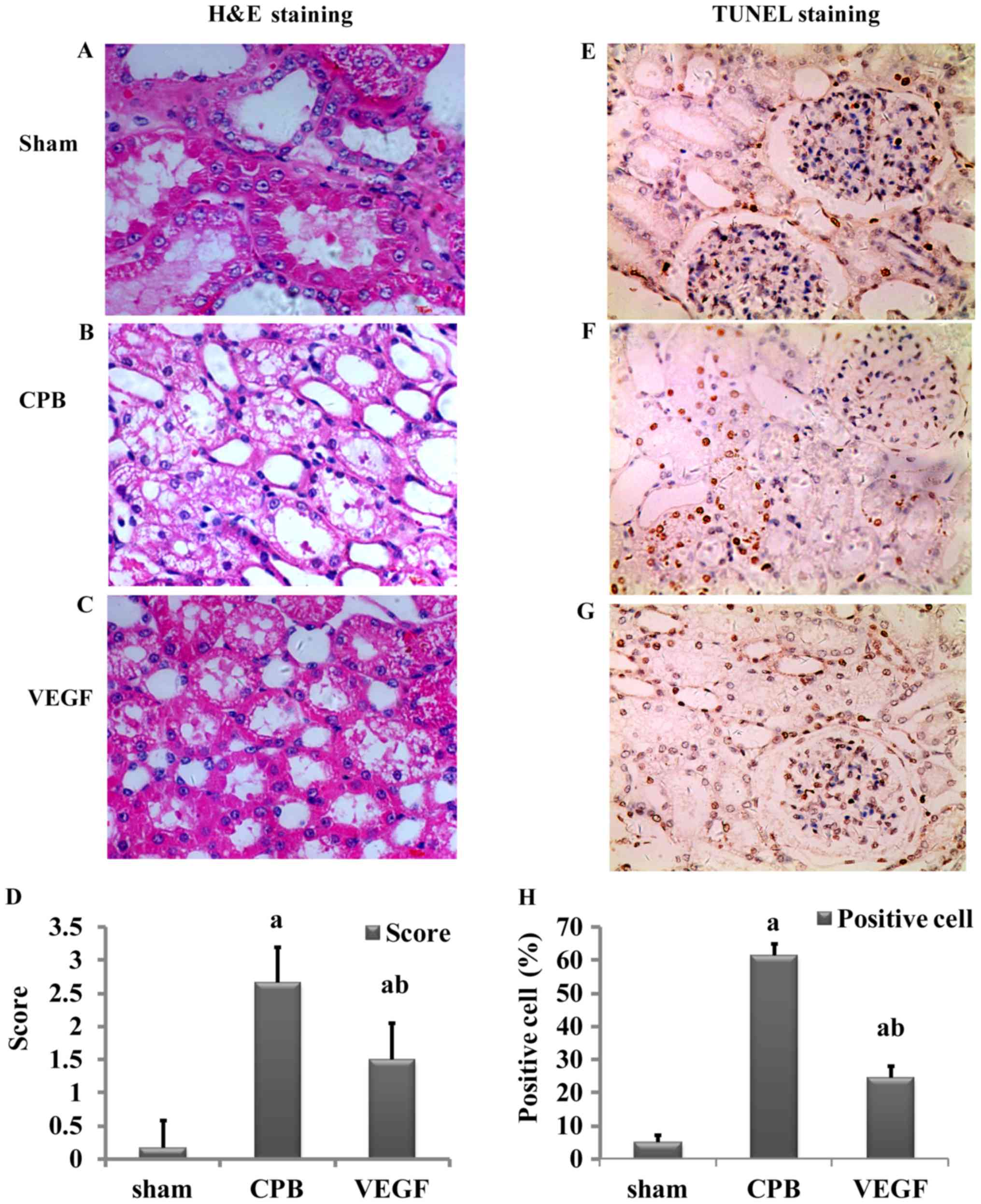
H&E staining
Damage scores of renal tubular epithelial cells in
the CPB and VEGF groups were significantly higher than those in the
sham group (P<0.05), and the score of the CPB group was higher
than that of the VEGF group (P<0.05; Fig. 4).
TUNEL staining
There were few apoptotic cells in the sham group.
Apoptotic index in the CPB group was significantly higher than that
in the VEGF group (P<0.05; Fig.
4).
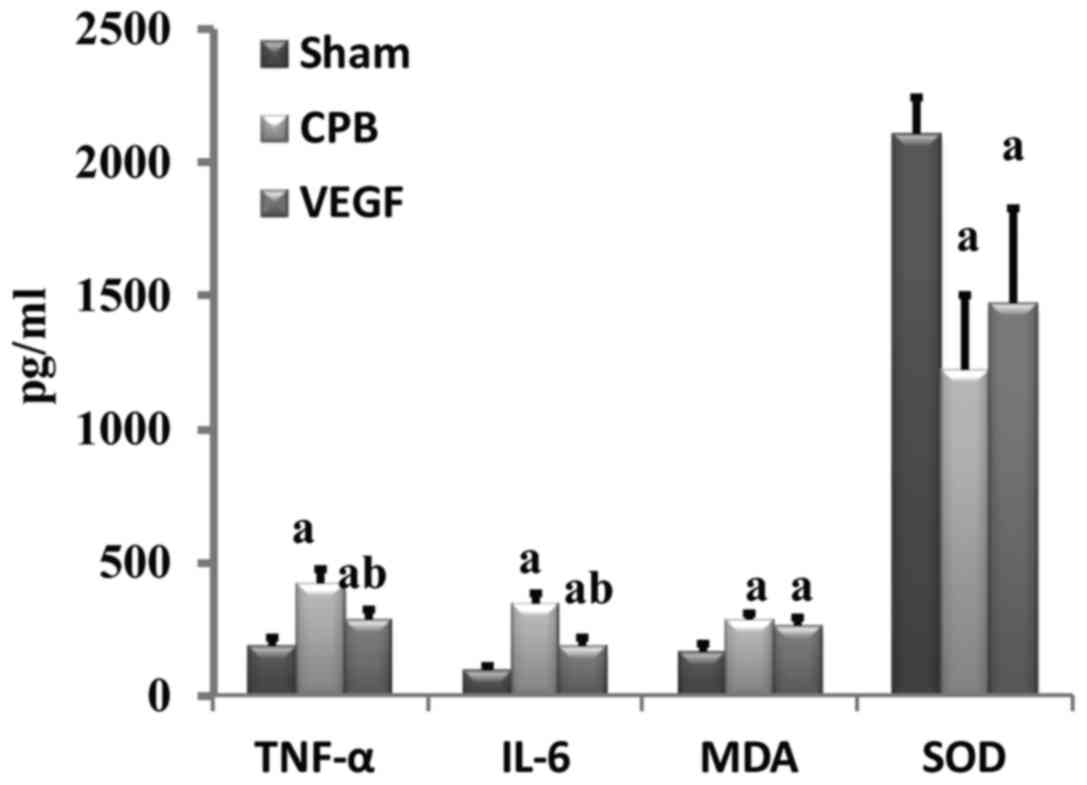
Expression of TNF-α, IL-6, MDA and SOD
Levels of TNF-α, IL-6 and MDA increased
significantly in the CPB and VEGF groups compared with sham group,
whereas SOD decreased significantly (P<0.05). TNF-α and IL-6 in
the VEGF group decreased significantly compared with the CPB group
(Fig. 5).
Western blot analysis
Compared with the sham group, cleaved caspase-3,
HIF-1α, VEGF, p-Akt and p-eNOS increased significantly in the CPB
and VEGF groups, and Bcl-2 decreased obviously (P<0.05).
Compared with the CPB group, cleaved caspase-3 significantly
decreased in the VEGF group (P<0.05), whereas Bcl-2, VEGF, p-Akt
and p-eNOS increased significantly (P<0.05; Fig. 6).
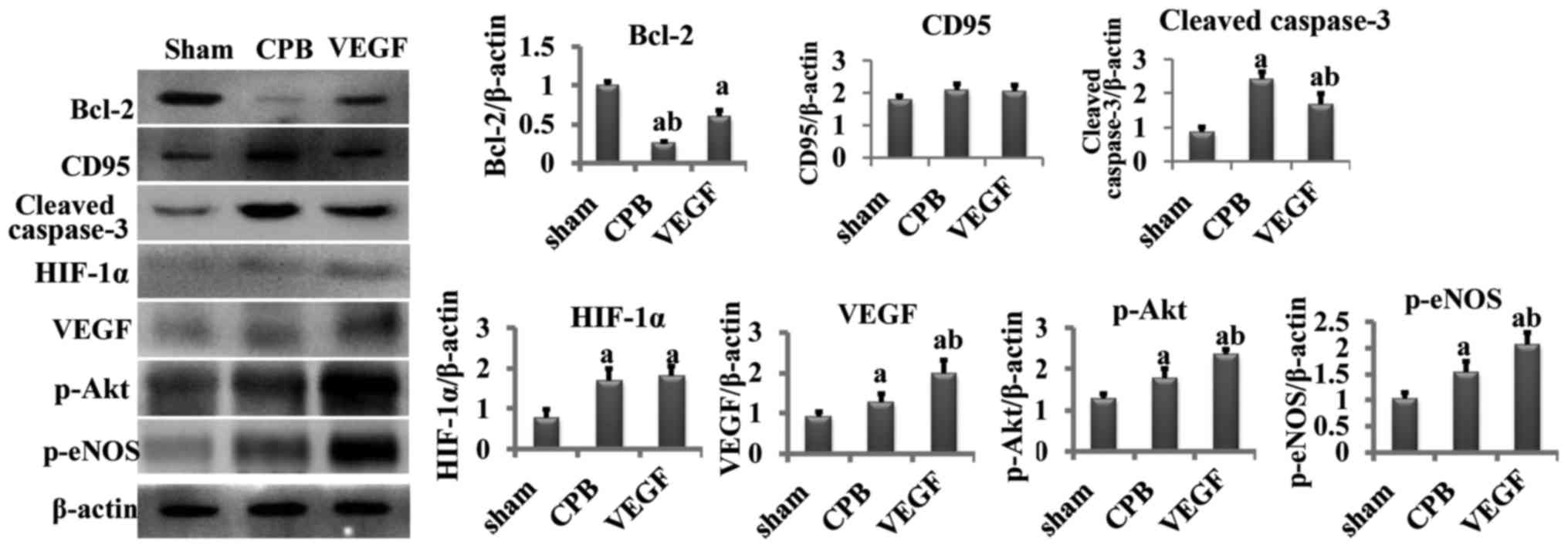 | Figure 6.Concentration of Bcl-2, CD95, cleaved
caspase-3, HIF-1α, VEGF, p-Akt, p-eNOS. aP<0.05 vs.
sham, bP<0.05 vs. CPB. Bcl-2, B-cell lymphoma 2; CD,
cluster of differentiation; VEGF, vascular endothelial growth
factor; p-eNOS, phosphorylated endothelial nitric oxide synthase;
CPB, cardiopulmonary bypass. |
Discussion
Our previous studies have demonstrated that
reduction in renal microcirculatory perfusion was associated with
AKI even within normal range of blood pressure (4,5), but we
have no renal ultrasound data from patients during CPB to evaluate
how renal microcirculatory perfusion effect on kidney for patients'
safety consideration and surgeon's feeling. Kumar and Suneja
(1) described that CPB-associated
postoperative kidney injury was associated with renal
microcirculatory and resorted to diagnostic technique and early
diagnosis qualifying severity. CEU, from which decrease of the ASR
and DPI and increase of AUC and TTP mean reduction of
microperfusion, is able to quantify changes in renal
microcirculation with advantages of real-time dynamic imaging,
convenient, fast, no radiation, and no renal toxicity (11).
We found there were significant decreases in the ASR
and DPI, increases in AUC and TTP at the beginning of CPB, at least
from 1 h after initiation of CPB, and throughout the whole
procedure. It was suggestive of decreased renal microcirculation
perfusion, and similar to our previous studies (4,5), and
confirmed by Andersson et al (12)and Pathi et al (13). By contrast, same tendency was found
after VEGF administration, but there were much better outcomes,
including ASR increased, AUC and TTP decreased significantly
throughout the whole procedure in comparison with CPB group. Our
data at least confirmed the existence of improved renal
microcirculation after VEGF administration.
Since the target of the present study was to reduce
AKI, the evaluation of a greater number of animals that develop AKI
would be beneficial. However, our ischemic model was built via a
CPB procedure for large animals, which was different from previous
ischemic or metabolic kidney injury models created by clamping the
organ arteries of small animals (14,15).
Clinical on-pump cardiac surgery was maximally stimulated in this
study, so the incidence of AKI was not 100% in beagle models. AKI
occurred in 2 beagles in the CPB group (33.3%), which was close to
previous reports (20–30%) (1,3). One
risk factor of AKI is ischemia reperfusion, so it is reasonable to
think of increase of AKI incidence rate in the re-warming phase
post-surgery, even if 2 h may be too short a time-frame to see
significant effects in this study.
There were no instances of hypotension, severe
anemia, and acid-base balance and water electrolyte abnormalities
observed in the beagles during CPB, however, BUN and SCr increased
significantly after CPB. We also found more degeneration and
necrosis from H&E staining slices and significantly increased
apoptosis from TUNEL staining slices in comparison to sham group.
Meanwhile, we observed that the changes were in consistence with
CEU data, which was similar to the studies of Kumar and Suneja
(1) and Iliescu et al
(16) who concluded that damages of
kidney endothelial microvascular system played an important role in
the process of acute renal failure. Interestingly, BUN, SCr,
degeneration and necrosis, and apoptosis were significantly
improved after VEGF administration including less edema in renal
tubules, reduction in renal tubular injury score, and decreased
apoptosis. Thinking of renal microcirculation changes, it made us
to recognize that VEGF may have protective effect via improvement
of the renal microcirculation, which was not used as an angiogenic
factor but some others.
This finding was similar to several recent studies
which highlighted the important ability of VEGF in stabilization of
the capillary structure (17–20). It
has even been predicted that VEGF could be a novel potential
therapeutic approach in ischemic renal disease (21).
In further experiments on the relationship between
CPB and renal microcirculation, we found that the expression of
hypoxia-inducible factor-1α (HIF-1α), which was upregulated in
anoxic conditions (22,23), increased after CPB under widely
accepted normal blood pressure. It intuitively drove us to consider
it a sign of renal ischemia. Meanwhile, the expression of VEGF was
also elevated, the transcription and expression of which was
induced by HIF-1α. VEGF mRNA expression was 4 h late after the
increase of HIF-1α (24,25). The increase of VEGF protein level may
due to the release of synthesized VEGF. However, the increased
concentration of VEGF in the CPB group was not enough to exert a
positive effect on the renal capillaries, so we administered
exogenous VEGF to beagles. At last, we obtained a significantly
improved renal microcirculation and elevated expression of p-Akt
and p-eNOS. P-Akt, which is activated by VEGF, could activate eNOS
and increases the ratio of p-eNOS to total eNOS. P-eNOS induces
nitric oxide (NO) (26), and
increases the cGMP concentration that leads to vasodilatation by
the activation of soluble guanylate cyclase, and improves
microcirculation (2,3). Besides the effect of vasodilatation, NO
can be beneficial to inflammatory conditions via inhibiting the
accumulation of neutrophils and leukocyte adhesion (27–29). We
found inflammation, such as IL-6 and TNF-α, were lower after VEGF
administration, which may be associated with anti-inflammatory
effect of NO.
CPB-associated decrease of renal microperfusion,
combined with ischemia reperfusion after CPB, systemic inflammatory
response, reduced postoperative cardiac output and use of vascular
active drug, may contribute to renal microcirculatory injury
(30). Renal microcirculation injury
includes two stages: functional injury and structural injury
(11,31). Function injury is at early stage, due
to endothelial damage, inflammatory cytokines release, and decrease
of expression of endothelial NO (31,32).
Elevated BUN and SCr quickly after CPB might be associated with
functional renal injury (6,33), which could be reversed by effective
improvement of the microcirculation.
Tiny tweaks may lead to big changes to patients. We
hope this study may provide important insights to support the use
of CEU during CPB in clinical on-pump cardiac surgery, and initiate
insights in renal microcirculation regimen for kidney
protection.
In conclusion, CPB-associated decrease of renal
microcirculation perfusion may predispose to AKI. VEGF may produce
protection against AKI through minimizing reduction in renal
microperfusion.
Acknowledgements
This study was financially supported by nos.
2012FZ0121 and 81300110.
References
|
1
|
Kumar AB and Suneja M: Cardiopulmonary
bypass-associated acute kidney injury. Anesthesiology. 114:964–970.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Uchino S, Bellomo R, Goldsmith D, Bates S
and Ronco S: An assessment of the RIFLE criteria for acute renal
failure in hospitalized patients. Crit Care Med. 34:1913–1917.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang Y, Lang XB, Zhang P, Lv R, Wang YF
and Chen JH: Remote ischemic preconditioning for prevention of
acute kidney injury: A meta-analysis of randomized controlled
trials. Am J Kidney Dis. 64:574–583. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang L, Zhang JY, Zhong XF, Zhu D and Liu
B: Exploration of renal microcirculation perfusion during
cardiopulmonary bypass with contrast-enhanced ultrasound. Sichuan
Da Xue Xue Bao Yi Xue Ban. 46:846–850. 2015.(In Chinese).
PubMed/NCBI
|
|
5
|
Zhong XF, Zhu D, Lu Q, Liu B and Peng YL:
Monitoring renal microcirculation perfusion alteration with
contrast-enhanced ultrasound during cardiopulmonary bypass. J
Sichuan Univ Med Sci Edi. 44:646–650. 2013.
|
|
6
|
Goldberg R and Dennen P: Long-term
outcomes of acute kidney injury. Adv Chronic Kidney Dis.
15:297–307. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Leonard EC, Friedrich JL and Basile DP:
VEGF-121 preserves renal microvessel structure and ameliorates
secondary renal disease following acute kidney injury. Am J Physiol
Renal Physiol. 295:F1648–F1657. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Masuda Y, Shimizu A, Mori T, Ishiwata T,
Kitamura H, Ohashi R, Ishizaki M, Asano G, Sugisaki Y and Yamanaka
N: Vascular endothelial growth factor enhances glomerular capillary
repair and accelerates resolution of experimentally induced
glomerulonephritis. Am J Pathol. 159:599–608. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fadel FI, Abdel Rahman AM, Mohamed MF,
Habib SA, Ibrahim MH, Sleem ZS, Bazaraa HM and Soliman MM: Plasma
neutrophil gelatinase-associated lipocalin as an early biomarker
for prediction of acute kidney injury after cardio-pulmonary bypass
in pediatric cardiac surgery. Arch Med Sci. 8:250–255. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Badylak SF, Kern KB, Tacker WA, Ewy GA,
Janas W and Carter A: The comparative pathology of open chest vs.
mechanical closed chest cardiopulmonary resuscitation in dogs.
Resuscitation. 13:249–264. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gessner R and Dayton PA: Advances in
molecular imaging with ultrasound. Mol Imaging. 9:117–127.
2010.PubMed/NCBI
|
|
12
|
Andersson LG, Bratteby LE, Ekroth R,
Hallhagen S, Joachimsson PO, van der Linden J and Wesslén O: Renal
function during cardiopulmonary bypass: Influence of pump flow and
systemic blood pressure. Eur J Cardiothorac Surg. 8:597–602. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pathi VL, Morrison J, MacPhaden A, Martin
W, McQuiston AM and Wheatley DJ: Alterations in renal
microcirculation during cardiopulmonary bypass. Ann Thorac Surg.
65:993–998. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chade AR and Kelsen S: Renal microvascular
disease determines the responses to revascularization in
experimental renovascular disease. Circ Cardiovasc Interv.
3:376–383. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chade AR, Krier JD, Textor SC, Lerman A
and Lerman LO: Endothelin-a receptor blockade improves renal
microvascular architecture and function in experimental
hypercholesterolemia. J Am Soc Nephrol. 17:3394–3403. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Iliescu R, Fernandez SR, Kelsen S, Maric C
and Chade AR: Role of renal microcirculation in experimental
renovascular disease. Nephrol Dial Transplant. 25:1079–1087. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chade AR and Kelsen S: Reversal of renal
dysfunction by targeted administration of VEGF into the stenotic
kidney: A novel potential therapeutic approach. Am J Physiol Renal
Physiol. 302:F1342–F1350. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Amadio M, Govoni S and Pascale A:
Targeting VEGF in eye neovascularization: What's new?: A
comprehensive review on current therapies and oligonucleotide-based
interventions under development. Pharmacol Res. 103:253–269. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Foster RR, Armstrong L, Baker S, Wong DW,
Wylie EC, Ramnath R, Jenkins R, Singh A, Steadman R, Welsh GI, et
al: Glycosaminoglycan regulation by VEGFA and VEGFC of the
glomerular microvascular endothelial cell glycocalyx in vitro. Am J
Pathol. 183:604–616. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Iruela-Arispe L, Gordon K, Hugo C,
Duijvestijn AM, Claffey KP, Reilly M, Couser WG, Alpers CE and
Johnson RJ: Participation of glomerular endothelial cells in the
capillary repair of glomerulonephritis. Am J Pathol. 147:1715–1727.
1995.PubMed/NCBI
|
|
21
|
Logue OC, McGowan JW, George EM and
Bidwell GL III: Therapeutic angiogenesis by vascular endothelial
growth factor supplementation for treatment of renal disease. Curr
Opin Nephrol Hypertens. 25:404–409. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Larrivee B and Karsan A: Signaling
pathways induced by vascular endothelial growth factor (Review).
Int J Mol Med. 5:447–456. 2000.PubMed/NCBI
|
|
23
|
Hicklin DJ and Ellis LM: Role of the
vascular endothelial growth factor pathway in tumor growth and
angiogenesis. J Clin Oncol. 23:1011–1027. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Lee YM, Jeong CH, Koo SY, Son MJ, Song HS,
Bae SK, Raleigh JA, Chung HY, Yoo M and Kim KW: Determination of
hypoxic region by hypoxia marker in developing mouse embryos in
vivo: A possible signal for vessel development. Dev Dyn.
220:175–186. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Vadlapatla RK, Vadlapudi AD and Mitra AK:
Hypoxia-inducible factor-1 (HIF-1): A potential target for
intervention in ocular neovascular diseases. Curr Drug Targets.
14:919–935. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Blanes MG, Oubaha M, Rautureau Y and
Gratton JP: Phosphorylation of tyrosine 801 of vascular endothelial
growth factor receptor-2 is necessary for Akt-dependent endothelial
nitric-oxide synthase activation and nitric oxide release from
endothelial cells. J Biol Chem. 282:10660–10699. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shu X, Keller TC IV, Begandt D, Butcher
JT, Biwer L, Keller AS, Columbus L and Isakson BE: Endothelial
nitric oxide synthase in the microcirculation. Cell Mol Life Sci.
72:4561–4575. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kubes P, Suzuki M and Granger DN: Nitric
oxide: An endogenous modulator of leukocyte adhesion. Proc Natl
Acad Sci USA. 88:pp. 4651–5545. 1991; View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Qin M, Landriscina A, Rosen JM, Wei G, Kao
S, Olcott W, Agak GW, Paz KB, Bonventre J, Clendaniel A, et al:
Nitric oxide-releasing nanoparticles prevent propionibacterium
acnes-induced inflammation by both clearing the organism and
inhibiting microbial stimulation of the innate immune response. J
Invest Dermatol. 135:2723–2731. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Basile DP: Challenges of targeting
vascular stability in acute kidney injury. Kidney Int. 74:257–258.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Garwood S: Cardiac surgery-associated
acute renal injury: New paradigms and innovative therapies. J
Cardiothorac Vasc Anesth. 24:990–1001. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chade AR, Rodriguez-Porcel M, Grande JP,
Krier JD, Lerman A, Romero JC, Napoli C and Lerman LO: Distinct
renal injury in early atherosclerosis and renovascular disease.
Circulation. 106:1165–1171. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Badal SS and Danesh FR: New insights into
molecular mechanisms of diabetic kidney disease. Am J Kidney Dis.
63 2 Suppl 2:S63–S83. 2014. View Article : Google Scholar : PubMed/NCBI
|