Introduction
Gestational diabetes mellitus (GDM) refers to the
varying degree of abnormal glucose metabolism observed during
pregnancy (1). GDM does not include
diabetes existed before pregnancy. GDM can lead to macrosomia,
oligohydramnios, premature birth and other complications. GDM can
also cause maternal postpartum metabolic syndrome, offspring
cognitive decline, abnormal glucose metabolism and other
far-reaching effects. Although the pathogenesis of GDM is still
unclear, genetic and environmental factors have significant effect
on the development of this disease. It has been proved that insulin
resistance, which can be aggregated by immune-induced chronic
inflammation, is the main mechanism of GDM (2). A variety of inflammatory factors were
also found to be involved in the occurrence and development of GDM
(3). Vascular endothelial cells can
produce a variety of inflammatory factors to participate in
inflammatory defense response, such as intercellular adhesion
molecule-1 (ICAM-1) and adiponectin (LPS) (4). ICAM-1 is believed to be an important
indicator of endothelial dysfunction (5). Therefore, in this study, expression of
ICAM-1 in umbilical artery and vein of both GDM patients and normal
pregnant women was detected to explore whether GDM can induce
changes in the function of umbilical cord vascular endothelium.
Patients and methods
Patients
In the GDM group were GDM patients selected in the
First Hospital of Lanzhou University and the Second Affiliated
Hospital of Xi'an Jiaotong University from January 2016 to December
2016. At the same time, 106 normal pregnant women were also
selected to serve as control group. The study was approved by the
Ethics Committee of our institute and all pregnant women or their
families signed informed consent. Exclusion criteria: Pregnant
women with liver and kidney dysfunctions or other complications
were excluded. General information of the groups of pregnant women
(age, gestational age, gravida, parity, BMI, systolic blood
pressure and diastolic blood pressure) are given in Table I. In GDM group, one case received
insulin treatment and other patients were subjected to diet control
and exercise therapy. GDM was diagnosed according to the
recommended guidelines of the diagnosis of GDM in China established
in 2014: Patients were subjected to 75 g oral glucose tolerance
test (OGTT). Participants were subjected to normal diet for three
days, followed by fasting for 8 h before test. Then participants
were asked to orally intake 300 ml liquid containing 75 g glucose.
Blood glucose level in venous blood was measured before and 1 and 2
h after the oral intake of glucose. Normal values of OGTT 0 h, OGTT
1 h and OGTT 2 h were 5.1, 10.0 and 8.5 mmol/l, respectively (1
mmol/l≈18 mg/dl). GDM was diagnosed if and higher value was
detected.
 | Table I.Comparison of general information
between two groups (mean ± SD). |
Table I.
Comparison of general information
between two groups (mean ± SD).
| General
information | GDM group
(n=103) | Control group
(n=106) | t-value | P-value |
|---|
| Age (years) |
29.07±4.45 |
28.21±5.78 | 1.729 | 0.168 |
| Gravida (times) |
2.12±0.58 |
2.20±0.46 | 1.834 | 0.152 |
| Parity (times) |
1.72±0.89 |
1.66±1.01 | 1.912 | 0.089 |
| Gestational age
(weeks) |
39.25±0.58 |
39.03±0.72 | 1.867 | 0.115 |
| BMI
(kg/m2) |
26.11±4.35 |
25.74±3.93 | 1.645 | 0.186 |
| Systolic blood
pressure (mmHg) |
117.79±10.38 |
115.01±11.57 | 2.126 | 0.073 |
| Diastolic blood
pressure (mmHg) |
72.79±9.38 |
71.36±8.64 | 1.981 | 0.094 |
Methods
Fasting peripheral venous blood (2 ml) was extracted
before birth to measure the levels of glycosylated hemoglobin
(HbA1c). Umbilical vessels were collected during labor stage for
blood gas analysis to record pH, pO2 and
pCO2.
After birth, umbilical cord tissue (1 ml) was
collected at the position 5 cm away from placenta and washed with
saline. Expression of ICAM-1 in umbilical cord blood vessels of two
groups was detected by immunohistochemistry. Specific steps:
Fixation; dehydration; transparency; oozing wax; embedding;
slicing; dewaxing; hydration; antigen retrieval; blocking;
incubation with primary and secondary antibody; hematoxylin and
eosin (H&E) staining; dehydration; transparency; sealing; data
analysis. All sections were analyzed by a senior pathologist.
Antibodies were purchased from Santa Cruz Biotechnology (Santa
Cruz, CA, USA) and DAB kit was purchased from Vector Laboratories
(Burlingame, CA, USA).
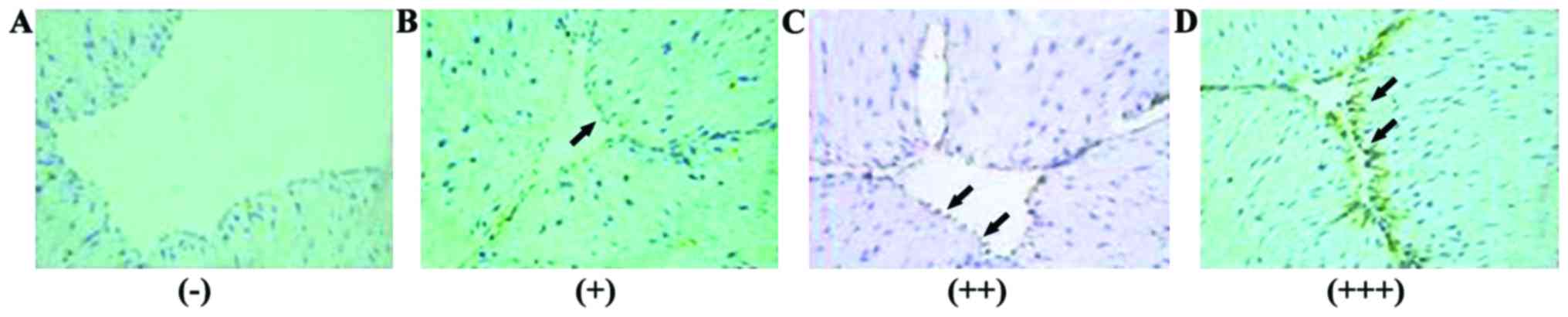
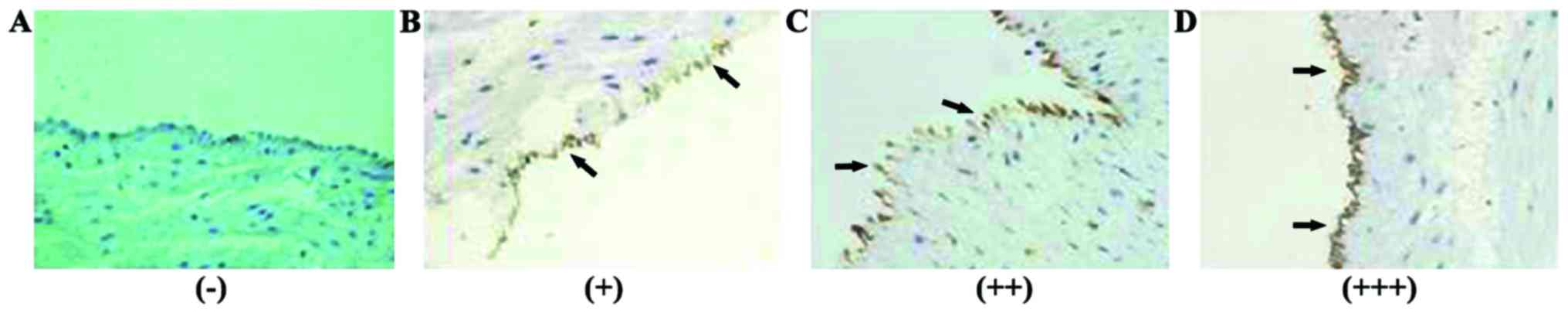
Interpretation criteria
Positive signals were yellow to brown particles in
the membrane of umbilical vascular endothelial cells. Five high
power visual fields were selected to record the degree of staining
and the percentage of positive cells. The average degree of
staining of each section was multiplied by the average percentage
of positive cells to get the final score of ICAM-1 expression.
Negative (−), 0 points; weak positive (+), 1–2 points; moderate
positive (++), 3–5 points; strong positive (+++), 6–9 points
(Table II).
 | Table II.Degree of staining and the percentage
of positive cells. |
Table II.
Degree of staining and the percentage
of positive cells.
| Cell staining | 0 points | 1 point | 2 points | 3 points |
|---|
| Degree of
staining | No color | Yellow | Yellowish-brown | Chocolate brown |
| Positive cells
(%) | ≤5 | 6–20 | 21–50 | ≥51 |
Statistical analysis
Data were analyzed by SPSS 18.0 software (SPSS Inc.,
Chicago, IL, USA). Data of the normal distribution were recorded by
mean ± standard deviation (SD). Comparison of measurement data
between two groups were performed by independent sample t-test.
Non-normal distribution data were tested by non-parametric
Mann-Whitney U test. Expression of ICAM-1 in umbilical blood
vessels was analyzed by chi-square test. P<0.05 was considered
to indicate a statistically significant difference.
Results
Comparison of laboratory indicators
between two groups
Fasting blood glucose of GDM group and control group
were 4.56±0.73 and 4.47±0.65 mmol/l, respectively. HbA1c levels of
GDM group and control group were 5.87±0.51 and 5.64±0.49%,
respectively. Umbilical cord arterial pH values of GDM group and
control group were 7.24±0.01 and 7.28±0.01, respectively.
pO2 of GDM group and control group were 2.41±0.19 and
2.40±0.20 Kpa, respectively. pCO2 of GDM group and
control group were 8.43±0.22 and 7.57±0.20 Kpa, respectively. No
significant differences in laboratory indicators were found between
the groups (p>0.05) (Table
III).
 | Table III.Comparison of laboratory indicators
between two groups (mean ± SD). |
Table III.
Comparison of laboratory indicators
between two groups (mean ± SD).
| Laboratory
indicators | GDM group
(n=103) | Control group
(n=106) | t-value | P-value |
|---|
| Fasting blood glucose
(mmol/l) |
4.56±0.73 |
4.47±0.65 | 2.013 | 0.101 |
| HbA1c (%) |
5.87±0.51 |
5.64±0.49 | 2.512 | 0.061 |
| Umbilical cord
arterial pH |
7.24±0.01 |
7.28±0.01 | 2.912 | 0.052 |
| pO2
(Kpa) |
2.41±0.19 |
2.40±0.20 | 1.867 | 0.115 |
| pCO2
(Kpa) |
8.43±0.22 |
7.57±0.20 | 2.645 | 0.056 |
ICAM-1 expression in GDM group and
control group
ICAM-1 expression was detected in umbilical cord
blood vessels of both groups (Figs.
1 and 2).
Moderately positive (++) and strongly positive (+++)
signals in umbilical artery endothelial cells of GAM group account
for 33.98 and 33.01% of all the cases, respectively. Moderately
positive (++) signals in umbilical artery endothelial cells of
control group accounted for 33.96% of all the cases. Moderately
ICAM-1 positive (++) signals in umbilical vein endothelial cells
accounted for 38.83 and 40.57% of all the cases in GDM group and
control group, respectively. No significant differences in the
expression of ICAM-1 in umbilical artery and umbilical vein were
found between the two groups (p>0.05) (Table IV).
 | Table IV.ICAM-1 expression in GDM group and
control group (mean ± SD). |
Table IV.
ICAM-1 expression in GDM group and
control group (mean ± SD).
| ICAM-1
expression | GDM group
(n=103) | Control group
(n=106) | χ2
value | P-value |
|---|
| Umbilical artery |
| − | 8 (7.77%) | 9 (8.49%) | 1.834 | 0.089 |
| + | 26 (25.24%) | 27 (25.47%) | 0.712 | 0.152 |
| ++ | 35 (33.98%) | 36 (33.96%) | 1.267 | 0.115 |
|
+++ | 34 (33.01%) | 34 (32.08%) | 0.645 | 0.186 |
| Umbilical vein |
| − | 11 (10.68%) | 13 (12.26%) | 2.034 | 0.070 |
| + | 21 (20.39%) | 22 (20.75%) | 2.013 | 0.101 |
| ++ | 40 (38.83%) | 43 (40.57%) | 1.981 | 0.094 |
|
+++ | 31 (30.10%) | 28 (26.42%) | 2.512 | 0.061 |
Discussion
The incidence of GDM is different in different races
and regions (6,7). Incidence of GDM is approximately 2–6%
in Europe (8) and 7% in United
States (9). In addition, incidence
of GDM showed an increasing trend (10,11),
seriously affecting the health of mothers and children. Studies
have shown that, compared with women without a history of GDM, the
risk of type 2 diabetes (T2DM) within 5 to 20 years after delivery
was increased by 6 times to 17–63% in women with a history of GDM
(12–14). GDM is also called ‘early T2DM’ due to
the high risk of T2DM caused by GDM (15–17). The
pathogenesis of GDM is still unclear. Incidence of GDM is higher in
Chinese than in blacks and whites (18). Vascular endothelial dysfunction is an
important initial stage of atherosclerosis (AS) (19). Increased blood glucose caused by GDM
is leading risk factor of AS and cardiovascular diseases. In view
of the high mortality of cardiovascular diseases, early prevention
and treatment of GDM is always needed. Studies have shown that
vascular endothelial dysfunction occurs at an early stage of AS
(20). Vascular endothelium can not
only play a role as a physical barrier, but also can maintain the
integrity and stability of blood vessels. Vascular endothelial
cells can release diastolic and vasoconstrictor substances through
endocrine function and paracrine synthesis to regulate and protect
vascular structure and functional integrity. Damaged vascular
endothelium cannot perform the normal functions of anticoagulation,
anti-platelet, anti-fibrinolysis, vasomotor and secretion and
abnormal secretion of cytokines can change endothelial
permeability, promote platelet aggregation, increase endothelial
structural damage, which in turn promote the formation of AS
(21).
Studies have shown that vascular endothelial
dysfunction in patients with diabetes is expected to become a new
target for prevention (22).
Vascular endothelial dysfunction in patients with diabetes is
caused by various factors including cytokines. Up to now, the
function of human vascular endothelium can only be evaluated
indirectly (21,23) and ICAM-1 is a good evaluation
indicator. ICAM-1, also known as CD54, belongs to the adhesion
molecule immunoglobulin superfamily. ICAM-1 single-stranded
transmembrane glycoprotein of 76–114 kDa and is composed of
extracellular region, transmembrane region and cytoplasmic region
(24). ICAM-1 is rarely expressed in
vascular endothelial cells under physiological conditions, so white
blood cells cannot adhere to endothelial cells. The damaged
vascular endothelium caused by external pathogenic factors (such as
hyperglycemia and oxidative stress) can activate endothelial cells
to secrete excessive ICAM-1, LPS and other adhesion molecules,
inflammatory factors and chemokines, so as to accelerate the
migration of white blood cells to the damaged region (25). Thus, studies on GDB have attracted
increasing attention.
Using asymmetric dimethylarginine (ADMA) as a
biochemical indicators of endothelial dysfunction and 44 pregnant
women with GDB and 69 normal pregnant women (32–39 years old) as
subjects, Akturk et al (26)
found that levels of blood glucose, HbA1c and ADMA in GDB group
were significantly higher than those in control group, indicating
that endothelial cells in GDM patients were activated and the
function was impaired. In contrast, with 32 GDM patients and 28
normal pregnant women with HbA1c lower than 6% as subjects, Kurt
et al (27) found that there
were no significant differences in the expression of ICAM-1 in
umbilical cord tissue and placental tissue between the two groups.
Although GDM can cause macrosomia, dystocia, eclampsia and many
other adverse effects, transient and mild increases in blood
glucose GDM patients do not seem to cause endothelial dysfunction
and vascular dysfunction. Vastagh et al (28) and others (29,30)
reported that there was no significant difference in AS between GDM
patients with normal pregnant women with similar background (age,
gestational age and BMI). In this study, no significant difference
in expression level of ICAM-1 in umbilical artery and umbilical
vein endothelial cells was found between two groups, indicating the
good blood glucose control in GDM patients. So, umbilical cord
endothelial cell damage does not seem to exist in GDM patients with
good blood glucose control. The possible reasons are: i) GDM is
caused by the increased insulin resistance after pregnancy, blood
glucose is only increased slightly after GDM; ii) the modified
cutoff score in the newly established guidelines for GDM diagnosis
allows more pregnant women to receive early intervention
management; iii) with the popularity of medical health education
and the improvement of civic health awareness diet control and
exercise therapy have been accepted by more and more people. This
study is still limited by the small sample size. Future studies
with greater sample sizes are needed to further confirm the
conclusion of this study.
References
|
1
|
American Diabetes Association, . Standards
of medical care in diabetes − 2010. Diabetes Care. 33 Suppl
1:S11–S61. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kim C: Gestational diabetes mellitus in
korean women: Similarities and differences from other racial/ethnic
groups. Diabetes Metab J. 38:1–12. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ko GT, Tam WH, Chan JC and Rogers M:
Prevalence of gestational diabetes mellitus in Hong Kong based on
the 1998 WHO criteria. Diabet Med. 19:802002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Krauss T, Emons G, Kuhn W and Augustin HG:
Predictive value of routine circulating soluble endothelial cell
adhesion molecule measurements during pregnancy. Clin Chem.
48:1418–1425. 2002.PubMed/NCBI
|
|
5
|
Bo S, Valpreda S, Menato G, Bardelli C,
Botto C, Gambino R, Rabbia C, Durazzo M, Cassader M, Massobrio M,
et al: Should we consider gestational diabetes a vascular risk
factor? Atherosclerosis. 194:e72–e79. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ferrara A, Kahn HS, Quesenberry CP, Riley
C and Hedderson MM: An increase in the incidence of gestational
diabetes mellitus: Northern California, 1991–2000. Obstet Gynecol.
103:526–533. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Getahun D, Nath C, Ananth CV, Chavez MR
and Smulian JC: Gestational diabetes in the United States: Temporal
trends 1989 through 2004. Am J Obstet Gynecol. 198:525.e1–525.e5.
2008. View Article : Google Scholar
|
|
8
|
Kaaja RJ and Greer IA: Manifestations of
chronic disease during pregnancy. JAMA. 294:2751–2757. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
American Diabetes Association, .
Gestational diabetes mellitus. Diabetes Care. 27 Suppl 1:S88–S90.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Feig DS, Zinman B, Wang X and Hux JE: Risk
of development of diabetes mellitus after diagnosis of gestational
diabetes. CMAJ. 179:229–234. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Feng J, Li HY, Wang XL, Huo Y, Liu SX and
Li L: Nicotinamide phosphoribosyltransferase enhances beta cell
expansion during pregnancy. Eur Rev Med Pharmacol Sci.
20:4965–4971. 2016.PubMed/NCBI
|
|
12
|
Bellamy L, Casas JP, Hingorani AD and
Williams D: Type 2 diabetes mellitus after gestational diabetes: A
systematic review and meta-analysis. Lancet. 373:1773–1779. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Coustan DR, Carpenter MW, O'Sullivan PS
and Carr SR: Gestational diabetes: Predictors of subsequent
disordered glucose metabolism. Am J Obstet Gynecol. 168:1139–1144.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kjos SL, Peters RK, Xiang A, Henry OA,
Montoro M and Buchanan TA: Predicting future diabetes in Latino
women with gestational diabetes. Utility of early postpartum
glucose tolerance testing. Diabetes. 44:586–591. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bloomgarden ZT: Inflammation and insulin
resistance. Diabetes Care. 26:1922–1926. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Carr DB, Utzschneider KM, Hull RL, Tong J,
Wallace TM, Kodama K, Shofer JB, Heckbert SR, Boyko EJ, Fujimoto
WY, et al: Gestational diabetes mellitus increases the risk of
cardiovascular disease in women with a family history of type 2
diabetes. Diabetes Care. 29:2078–2083. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seshiah V, Balaji V, Balaji MS,
Paneerselvam A, Arthi T, Thamizharasi M and Datta M: Gestational
diabetes mellitus manifests in all trimesters of pregnancy.
Diabetes Res Clin Pract. 77:482–484. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yue DK, Molyneaux LM, Ross GP, Constantino
MI, Child AG and Turtle JR: Why does ethnicity affect prevalence of
gestational diabetes? The underwater volcano theory. Diabet Med.
13:748–752. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Quiñones MJ, Nicholas SB and Lyon CJ:
Insulin resistance and the endothelium. Curr Diab Rep. 5:246–253.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Iudakova TN, Girsh AO, Maksimishin SV and
Mal'Kov OA: Association of cardiovascular system and endothelial
dysfunction indicators in patients with hemorrhagic shock.
Anesteziol Reanimatol. 6:11–14. 2013.(In Russian).
|
|
21
|
Hartge MM, Kintscher U and Unger T:
Endothelial dysfunction and its role in diabetic vascular disease.
Endocrinol Metab Clin North Am. 35:551–560. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Caballero AE: Endothelial dysfunction,
inflammation, and insulin resistance: A focus on subjects at risk
for type 2 diabetes. Curr Diab Rep. 4:237–246. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Matsumoto T, Kobayashi T and Kamata K:
Alterations in EDHF-type relaxation and phosphodiesterase activity
in mesenteric arteries from diabetic rats. Am J Physiol Heart Circ
Physiol. 285:H283–H291. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang Y, Jiang LL, Maimaitirexiati XM,
Zhang Y and Wu L: Irbesartan attenuates TNF-α-induced ICAM-1,
VCAM-1, and E-selectin expression through suppression of NF-κB
pathway in HUVECs. Eur Rev Med Pharmacol Sci. 19:3295–3302.
2015.PubMed/NCBI
|
|
25
|
Imhof BA and Dunon D: Leukocyte migration
and adhesion. Adv Immunol. 58:345–416. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Akturk M, Altinova A, Mert I, Dincel A,
Sargin A, Buyukkagnici U, Arslan M and Danisman N: Asymmetric
dimethylarginine concentrations are elevated in women with
gestational diabetes. Endocrine. 38:134–141. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kurt M, Zulfikaroglu E, Ucankus NL,
Omeroglu S and Ozcan U: Expression of intercellular adhesion
molecule-1 in umbilical and placental vascular tissue of
gestational diabetic and normal pregnancies. Arch Gynecol Obstet.
281:71–76. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vastagh I, Horváth T, Garamvölgyi Z, Rosta
K, Folyovich A, Rigó J, Kollai M, Bereczki D and Somogyi A:
Preserved structural and functional characteristics of common
carotid artery in properly treated normoglycemic women with
gestational diabetes mellitus. Acta Physiol Hung. 98:294–304. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bulzico DA, Zajdenverg L, Cabizuca CA, de
Oliveira JE and Salles GF: Assessment of arterial stiffness in
women with gestational diabetes. Diabet Med. 29:227–231. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Salmi AA, Zaki NM, Zakaria R, Nor Aliza AG
and Rasool AH: Arterial stiffness, inflammatory and pro-atherogenic
markers in gestational diabetes mellitus. Vasa. 41:96–104. 2012.
View Article : Google Scholar : PubMed/NCBI
|