Introduction
Nonalcoholic fatty liver disease is a type of common
chronic liver disease, with a gradually increasing number of cases
in China. This disease threatens people's physical and mental
health (1). The pathogenesis of
nonalcoholic fatty liver disease remains unclear, and most studies
suggest that (2–4) this disease may be closely related to
many factors such as abnormal lipid metabolism and an inflammatory
response. Nonalcoholic steatohepatitis is an important stage during
the development and progression of nonalcoholic fatty liver
disease. In recent years, many studies have demonstrated that the
imbalance of intestinal flora in the body leads to obesity, which
is closely related to nonalcoholic steatohepatitis (5). Interleukin-10 (IL-10) and IL-17 are
relatively common pro-inflammatory factors. Currently, there are
few studies on the correlation between changes in intestinal flora
and the inflammatory factors IL-10 and IL-17 in patients with
nonalcoholic steatohepatitis. Here we analyzed the correlation
between changes in intestinal flora and IL-10 and IL-17 in order to
reveal the pathogenesis of nonalcoholic fatty liver disease.
Patients and methods
General data
Ninety patients that were diagnosed with
nonalcoholic steatohepatitis and treated at Xuzhou Infectious
Disease Hospital from February 2016 to February 2017 were selected
as the patient group. Their diagnosis conformed to the diagnostic
criteria in Diagnosis and Treatment Guidance on Nonalcoholic Fatty
Liver Disease (6). Patients with
viral or autoimmune liver disease, pregnant or lactating patients,
and patients with prior use of medicine that would affect the
intestinal flora in the body in the prior 15 days were eliminated.
In the patient group, there were 50 males and 40 females aged 25–66
years with an average age of 45.7±1.5 years. In the control group,
there were 80 healthy people without intestinal, hepatic and
biliary diseases who were undergoing physical examination in the
hospital during the same time period and were selected as the
control group, including 38 males and 32 females aged 24–65 years
with an average age of 45.2±1.3 years. All the patients and/or
guardians signed the informed consent. The study was approved by
the Ethics Committee of Xuzhou Infectious Disease Hospital.
Methods
Determination of intestinal flora
A total of 1.5 g of fresh excreta was collected from
all subjects, and the log value of the colony forming unit in wet
weight per gram of excreta (log CFU/g) was used for the viable
count of intestinal beneficial bacteria, such as
Bifidobacterium and Lactobacillus, and pathogenic
bacteria, such as Enterobacter and Enterococcus. The
ratio of Bifidobacterium to Enterobacter (B/E value)
was calculated to indicate the index of intestinal colonization
resistance, thus evaluating the changes in intestinal flora of
subjects.
Determination of relative expression
levels of IL-10 mRNA and IL-17 mRNA in peripheral blood mononuclear
cells
A total of 5 ml of fasting venous blood was
collected from the subjects, and the changes in relative expression
levels of IL-10 mRNA and IL-17 mRNA in the peripheral blood
mononuclear cells were detected via reverse
transcription-polymerase chain reaction (RT-PCR).
Determination of serum IL-10 and IL-17
levels
The serum IL-10 and IL-17 levels in subjects were
detected via ELISA.
Statistical analysis
The SPSS 20.0 (IBM Corp., New York, NY, USA)
statistical software was used for analysis. Measurement data were
presented as mean ± standard deviation, and the Chi-square test was
used for measurement data. Paired samples t-test was used for
enumeration data, and the Pearson's correlation analysis was used
for the correlation analysis. P<0.05 is considered to indicate a
statistically significant difference.
Results
Comparisons of intestinal flora amount
and B/E value between the two groups
The number of beneficial bacteria, such as
Bifidobacterium and Lactobacillus, in patient group
was significantly lower than in the control group (P<0.05). The
number of pathogenic bacteria, such as Enterobacteriaceae and
Enterococcus, in the patient group was significantly higher
than that in the control group (P<0.05), and the B/E value in
the patient group was significantly lower than in the control group
(P<0.05) (Table I).
 | Table I.Comparison of intestinal flora amount
and B/E value between the two groups (log CFU/g). |
Table I.
Comparison of intestinal flora amount
and B/E value between the two groups (log CFU/g).
| Groups | n |
Bifidobacterium |
Lactobacillus |
Enterobacteriaceae |
Enterococcus | B/E value |
|---|
| Patient | 90 | 8.56±0.34 | 9.07±0.02 | 9.88±0.42 | 8.02±0.25 | 0.87±0.03 |
| Control | 80 | 9.68±0.55 | 9.84±0.36 | 8.63±0.17 | 7.35±0.14 | 1.04±0.05 |
| t-test |
| 9.561 | 10.942 | 7.338 | 8.796 | 9.005 |
| P-value |
| <0.05 | <0.05 | <0.05 | <0.05 | <0.05 |
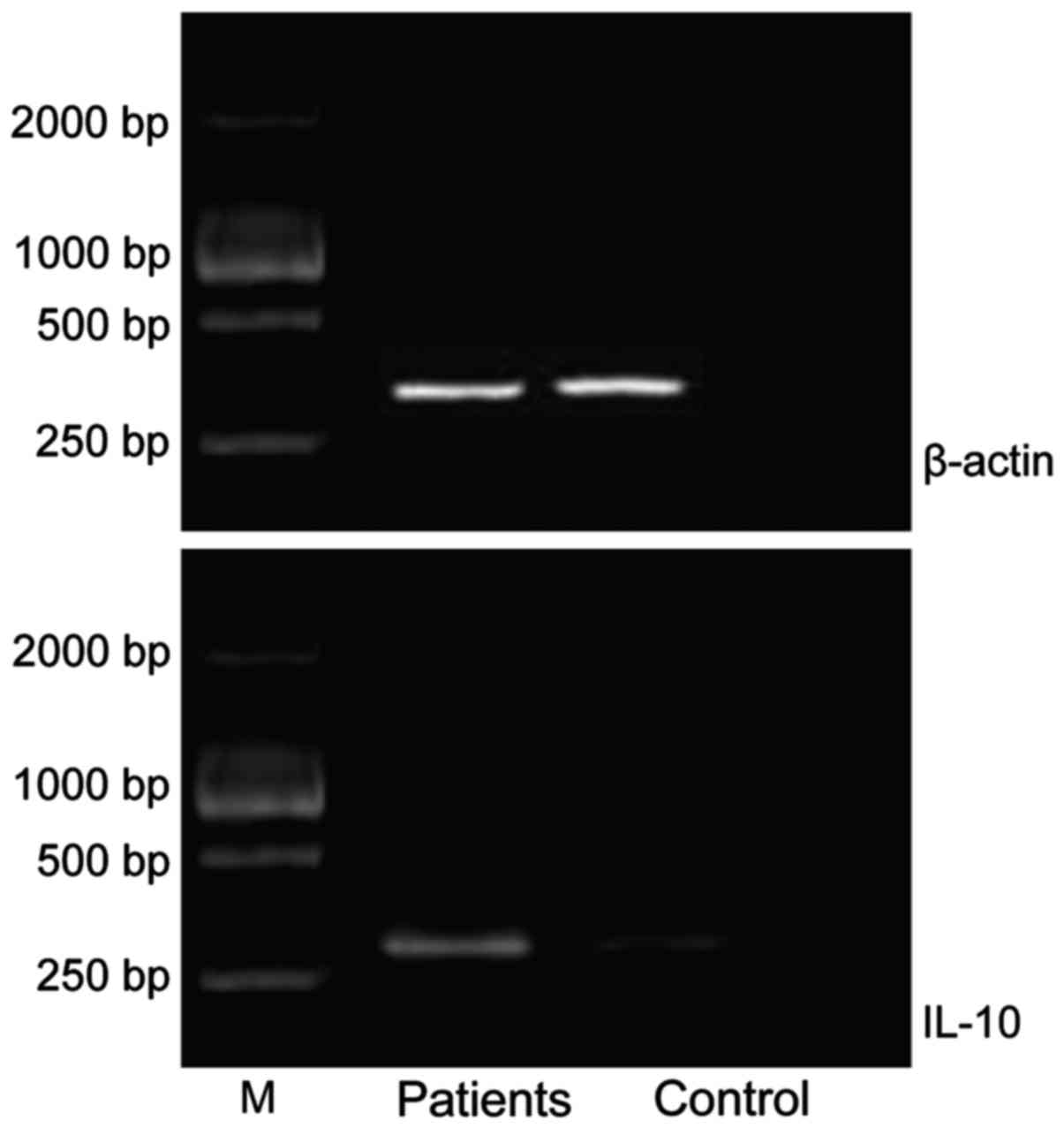
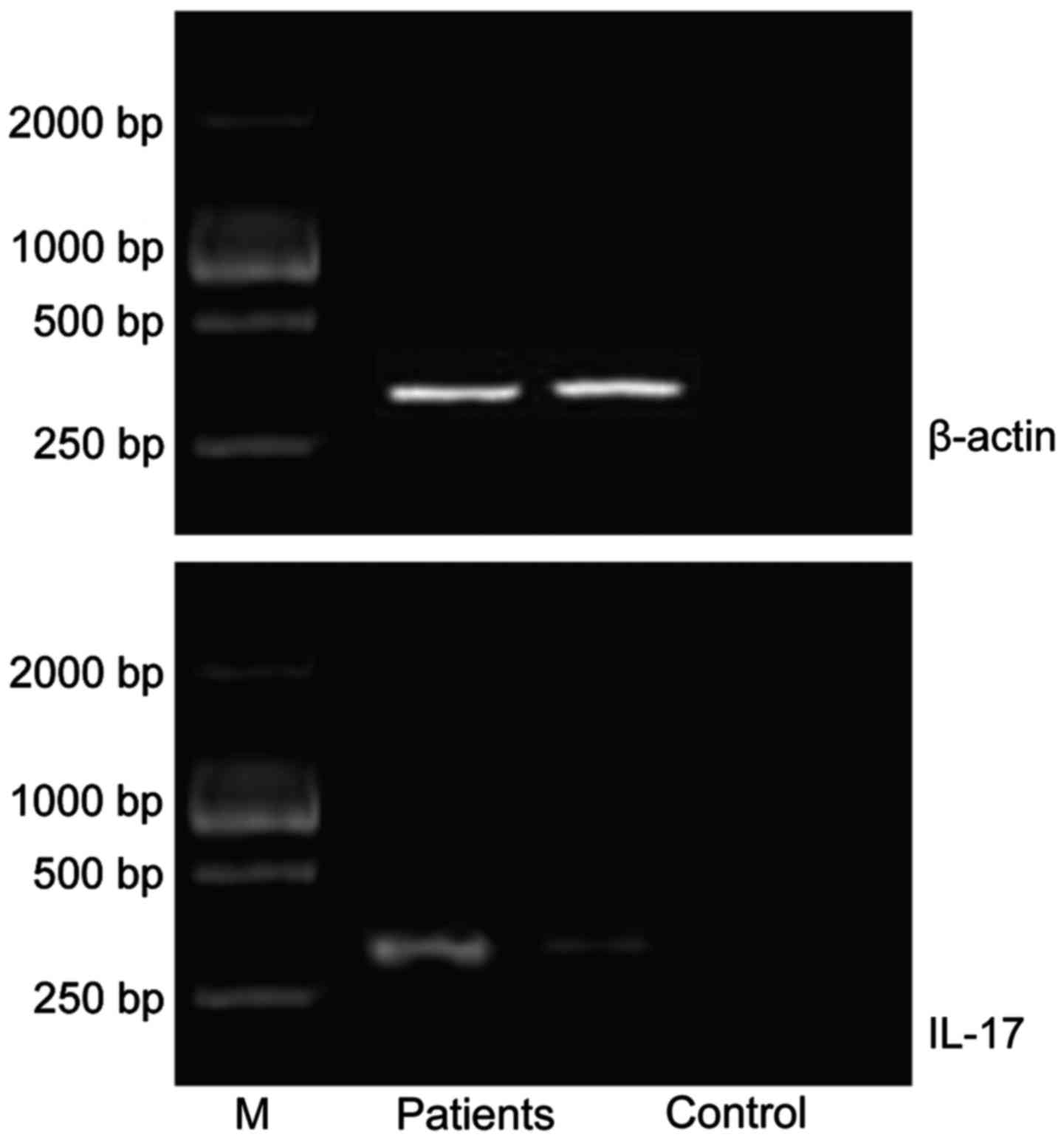
Comparisons of relative expression
levels of IL-10 mRNA and IL-17 mRNA between the two groups
The relative expression levels of IL-10 mRNA and
IL-17 mRNA in the patient groups were significantly higher than
those in control group (P<0.05) (Table II; Figs.
1 and 2).
 | Table II.Comparisons of relative expression
levels of IL-10 mRNA and IL-17 mRNA between the two groups (mean ±
standard deviation). |
Table II.
Comparisons of relative expression
levels of IL-10 mRNA and IL-17 mRNA between the two groups (mean ±
standard deviation).
| Groups | n | IL-10 mRNA | IL-17 mRNA |
|---|
| Patient | 90 | 0.67±0.06 | 0.62±0.04 |
| Control | 80 | 0.18±0.03 | 0.16±0.02 |
| t-test |
| 9.116 | 9.832 |
| P-value |
| <0.05 | <0.05 |
Comparison of serum IL-10 and IL-17
levels between the two groups
The serum IL-10 levels in the patients group were
1.17±0.15 pg/ml, which was significantly higher than that in
control group at 0.32±0.04 pg/ml (P<0.05). The serum IL-17 level
in the patient group was 0.96±0.11 pg/ml, which was significantly
higher than that in the control group (0.28±0.01 pg/ml) (P<0.05)
(Table III).
 | Table III.Comparison of serum IL-10 and IL-17
levels between the two groups (mean ± standard deviation,
pg/ml). |
Table III.
Comparison of serum IL-10 and IL-17
levels between the two groups (mean ± standard deviation,
pg/ml).
| Groups | n | IL-10 | IL-17 |
|---|
| Patient | 90 | 1.17±0.15 | 0.96±0.11 |
| Control | 80 | 0.32±0.04 | 0.28±0.01 |
| t-test |
| 8.042 | 9.735 |
| P-value |
| <0.05 | <0.05 |
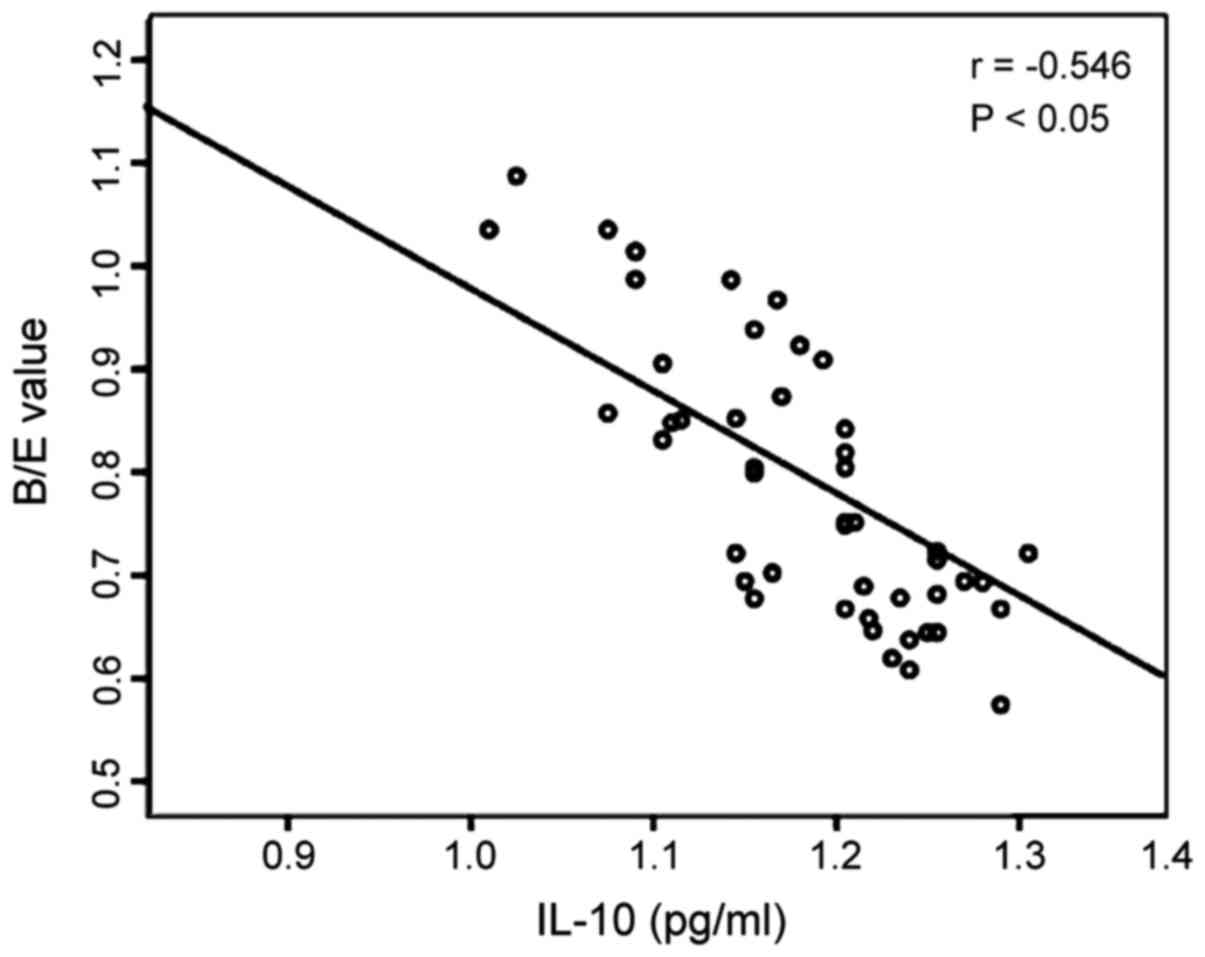
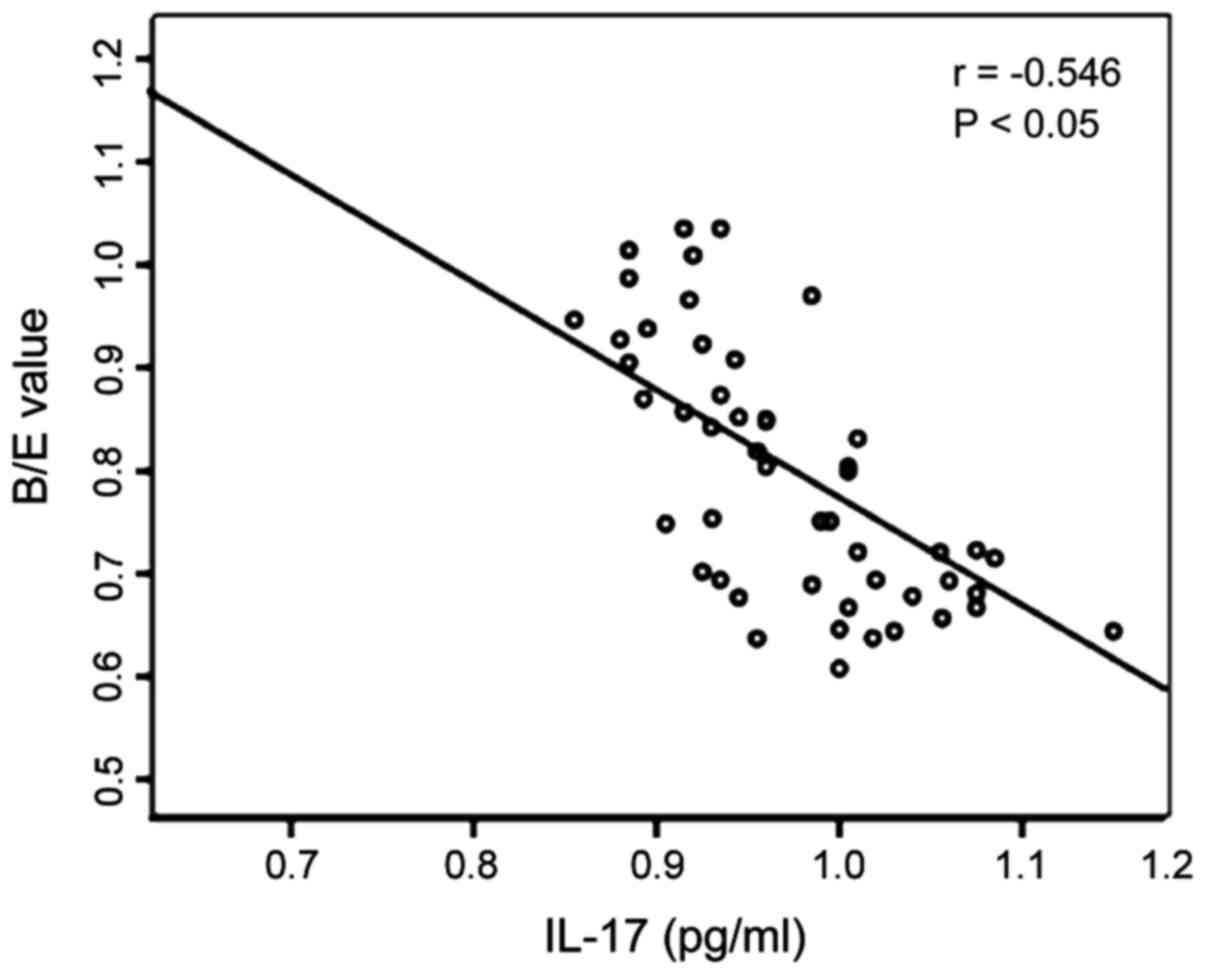
Analysis of correlation between
changes in intestinal flora and serum IL-10 and IL-17 in the
patient group
The Pearson's correlation analysis shows that the
B/E value representing the changes in intestinal flora in the
patient group were negatively correlated to serum IL-10 (r=−0.546,
P<0.05), and also negatively correlated with serum IL-17
(r=−0.535, P<0.05) (Figs. 3 and
4).
Discussion
Intestinal flora in the human body comprise a
complex micro-ecosystem composed of probiotics and pathogens. In
this micro-ecosystem, the beneficial bacteria and pathogenic
bacteria combine in accordance to a certain proportion, and they
are extremely important in digestion and absorption within the
human body via mutual regulation (7–9).
Research shows that (10) the
development and progression of liver diseases are closely related
to the intestinal microbiology. It has also been reported that
(11,12) intestinal probiotics can reduce
oxidative stress and inflammatory injury in the liver, which plays
a very important roles in the prevention of nonalcoholic fatty
liver diseases. The B/E value can generally be used to assess the
changes in intestinal flora. This study shows that the number of
beneficial bacteria, such as Bifidobacterium and
Lactobacillus, in the patient group was significantly lower
than in the control group (P<0.05). On the other hand, the
number of pathogenic bacteria, such as Enterobacteriaceae and
Enterococcus, in the patient group was significantly higher
than that in control group (P<0.05). The B/E value in the
patient group was significantly lower than that in control group
(P<0.05), which suggests that the imbalance of intestinal flora
exists in nonalcoholic steatohepatitis, and leads to the injury of
intestinal colonization resistance.
Studies have suggested that (5,13) the
pathogenesis of nonalcoholic fatty liver disease may be associated
with oxidative stress, inflammatory response and other factors.
Oxidative stress generally promotes the inflammatory response and
releases a variety of inflammatory factors, such as IL-10 and IL-17
(14–17). This study demonstrates that the
relative expression levels of IL-10 and IL-17 mRNA in the patient
group were significantly higher than those in the control group
(P<0.05). The serum IL-10 levels in patient group were 1.17±0.15
pg/ml, which was significantly higher than that in the control
group at 0.32±0.04 pg/ml (P<0.05). Furthermore, the serum IL-17
levels in the patient group was 0.96±0.11 pg/ml, which was
significantly higher than that in the control group (0.28±0.01
pg/ml) (P<0.05), which suggests that the increased IL-10 and
IL-17 levels are closely related to the development and progression
of nonalcoholic steatohepatitis and can reflect the severity of
nonalcoholic steatohepatitis.
Due to the close correlation between the changes in
intestinal flora, inflammatory factors and the incidence of
nonalcoholic steatohepatitis, the B/E value was used to reflect the
changes in the intestinal flora, and the correlation analysis was
performed for IL-10 and IL-17 in order to provide a related basis
for the pathogenesis of nonalcoholic steatohepatitis. The results
of this study show that the B/E value in the patient group was
negatively correlated with serum IL-10 (r=−0.546, P<0.05), and
also negatively correlated with serum IL-17 (r=−0.535, P<0.05),
which suggests that the imbalance of intestinal flora is related to
the damage extent of intestinal colonization resistance and changes
in the levels of inflammatory factors, IL-10 and IL-17. It is
speculated that the hepatic pathological changes, the imbalance of
intestinal flora and the release of inflammatory factors may occur
in nonalcoholic steatohepatitis, thus promoting liver injury in the
human body (3,18).
In conclusion, IL-10 and IL-17 are highly expressed
in the peripheral blood of patients with nonalcoholic
steatohepatitis compared to healthy people. The changes in
intestinal flora in patients with nonalcoholic steatohepatitis are
closely related to the changes in serum IL-10 and IL-17 levels, and
they interact and actively participate in the development and
progression of nonalcoholic steatohepatitis.
References
|
1
|
Zhu L, Baker SS, Gill C, Liu W, Alkhouri
R, Baker RD and Gill SR: Characterization of gut microbiomes in
nonalcoholic steatohepatitis (NASH) patients: A connection between
endogenous alcohol and NASH. Hepatology. 57:601–609. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ilan Y: Leaky gut and the liver: A role
for bacterial translocation in nonalcoholic steatohepatitis. World
J Gastroenterol. 18:2609–2618. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wu WC, Zhao W and Li S: Small intestinal
bacteria overgrowth decreases small intestinal motility in the NASH
rats. World J Gastroenterol. 14:313–317. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsujimoto T, Kawaratani H, Kitazawa T,
Uemura M and Fukui H: Innate immune reactivity of the ileum-liver
axis in nonalcoholic steatohepatitis. Dig Dis Sci. 57:1144–1151.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Safadi R, Konikoff FM, Mahamid M,
Zelber-Sagi S, Halpern M, Gilat T, Oren R, Safadi R, Konikoff FM,
Hershkovitz A, et al FLORA Group, : The fatty acid-bile acid
conjugate Aramchol reduces liver fat content in patients with
nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol.
12:2085–91.e1. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Santos LF, Hernández G, Puerta AV, Beltrán
O, Botero RC and Mejía G: Non alcoholic fatty liver disease: The
new millennium pandemia. Rev Col Gastroenterol. 25:380–398.
2010.
|
|
7
|
Schartum-Hansen H, Pedersen ER, Svingen
GF, Ueland PM, Seifert R, Ebbing M, Strand E, Bleie Ø and Nygård O:
Plasma choline, smoking, and long-term prognosis in patients with
stable angina pectoris. Eur J Prev Cardiol. 22:606–614. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Søndergaard B, Olsson J, Ohlson K,
Svensson U, Bytzer P and Ekesbo R: Effects of probiotic fermented
milk on symptoms and intestinal flora in patients with irritable
bowel syndrome: A randomized, placebo-controlled trial. Scand J
Gastroenterol. 46:663–672. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Armand-Lefèvre L, Angebault C, Barbier F,
Hamelet E, Defrance G, Ruppé E, Bronchard R, Lepeule R, Lucet JC,
El Mniai A, et al: Emergence of imipenem-resistant gram-negative
bacilli in intestinal flora of intensive care patients. Antimicrob
Agents Chemother. 57:1488–1495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Steensels D, Slabbaert K, De Wever L,
Vermeersch P, Van Poppel H and Verhaegen J:
Fluoroquinolone-resistant E. coli in intestinal flora of patients
undergoing transrectal ultrasound-guided prostate biopsy - should
we reassess our practices for antibiotic prophylaxis? Clin
Microbiol Infect. 18:575–581. 2012.PubMed/NCBI
|
|
11
|
Hotten P, Marotta F, Naito Y, Minelli E,
Helmy A, Lighthouse J, Fuji H and Fesce E: Effects of probiotics,
lactitol and rifaximin on intestinal flora and fecal excretion of
organic acids in cirrhotic patients. Chin J Dig Dis. 4:13–18. 2003.
View Article : Google Scholar
|
|
12
|
Xu RY, Wan YP, Fang QY, Lu W and Cai W:
Supplementation with probiotics modifies gut flora and attenuates
liver fat accumulation in rat nonalcoholic fatty liver disease
model. J Clin Biochem Nutr. 50:72–77. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Omura Y, Kitamoto M, Hyogo H, Yamanoue T,
Tada Y, Boku N, Nishisaka T, Miyauchi M, Takata T and Chayama K:
Morbidly obese patient with non-alcoholic steatohepatitis-related
cirrhosis who died from sepsis caused by dental infection of
Porphyromonas gingivalis: A case report. Hepatol Res. 46:E210–E215.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Habior A: Nonalcoholic fatty liver disease
and obesity. Nutr Clin Pract. 22:1–10. 2013.(In Polish).
|
|
15
|
El-Bassiouni NE, El Messery LO, Zayed RA,
Metwally OB, Zahran MY, Mahmoud OM, Ibrahim RA and El Bassiouny AE:
Tissue factor expression on blood monocytes in patients with
hepatitis C virus-induced chronic liver disease. Comp Clin Pathol.
23:1159–1166. 2014. View Article : Google Scholar
|
|
16
|
Tarantino G: Nutrition: A promising route
for prevention and management of obesity-related nonalcoholic fatty
liver disease. Horm Mol Biol Clin Investig. 20:39–41.
2014.PubMed/NCBI
|
|
17
|
Mcavoy NC, Ferguson JW, Campbell IW and
Hayes PC: Review: Non-alcoholic fatty liver disease: natural
history, pathogenesis and treatment. Br J Diabetes Vasc Dis.
6:251–260. 2006. View Article : Google Scholar
|
|
18
|
Kwak DS, Jun DW, Seo JG, Chung WS, Park
SE, Lee KN, Khalid-Saeed W, Lee HL, Lee OY, Yoon BC, et al:
Short-term probiotic therapy alleviates small intestinal bacterial
overgrowth, but does not improve intestinal permeability in chronic
liver disease. Eur J Gastroenterol Hepatol. 26:1353–1359.
2014.PubMed/NCBI
|