Introduction
Epstein-Barr virus (EBV) is a human herpes virus
that infects over 90% of the human population worldwide (1). EBV is suggested to be an environmental
factor associated with the development of several human
malignancies, including nasopharyngeal carcinoma (NPC), Burkitt's
lymphoma, Hodgkin's disease (HD), gastric cancer, natural
killer/T-cell lymphoma, acquired immune deficiency syndrome- and
transplantation-associated lymphoma (2,3), breast
carcinoma, and hepatocellular carcinoma (4,5). EBV, as
all other herpes viruses, may establish a latent or lytic infection
in host cells (6). Notably, studies
suggest that EBV reactivation into a lytic cycle may contribute to
the pathogenesis of malignancies (7,8). EBV
lytic infection in vivo has been identified by elevated
antibody titers against EBV lytic antigens and by increased viral
DNA load in the serum/plasma, and these observations correspond
with advanced cancer stages, poor prognosis or tumor recurrence
following therapy (9,10). Additionally, serological studies have
indicated that EBV lytic infection may occur months or years prior
to a clinical diagnosis of NPC, HD or Burkitt's lymphoma, which
suggests EBV lytic infection may be a risk factor for cancer
development (11–13).
Green tea contains (−)-epigallocatechin-3-gallate
(EGCG), which is reported to have antioxidant, antibacterial and
antitumor effects (14,15). EGCG has been indicated to modulate
multiple signaling pathways, including the phosphoinositide
3-kinase (PI3-K)/Akt and mitogen-activated protein kinase (MAPK)
signaling pathways, which may enable it to exert its cancer
chemopreventive and therapeutic effects (16,17).
EBV encoded latent membrane protein 1 (LMP1), which
is considered to have oncogenic properties, has been identified in
90% of patients with NPC (18,19).
Through its cytoplasmic C-terminal, LMP1 may trigger multiple
signal transduction cascades, including the MAPK kinase
(MEK)/extracellular signal-regulated kinase (ERK), PI3-K/Akt, c-Jun
N-terminal kinase (JNK) and signal transducer and activator of
transcription 3 signaling pathways, to alter cell growth and
survival (20,21). Furthermore, LMP1 is established as a
critical viral protein required for the EBV life cycle (13,22–25).
Our previous work indicated that EGCG may inhibit
the spontaneous reactivation of EBV, which was associated with
activation of the MAPK and PI3-K/Akt pathways (26). Furthermore, EGCG has been reported to
modulate signal pathways induced by LMP1 (16). Therefore, the present study
investigated whether EGCG can suppress EBV lytic infection by
inhibiting LMP1 expression.
Materials and methods
Cell lines and culture
B95.8, an EBV-positive marmoset B cell line, was
obtained from the American Type Culture Collection (Manassas, VA,
USA) and preserved at the Cancer Research Institute, Xiangya School
of Medicine (Central South University, Changsha, China) (27). CNE1 is an LMP1-negative
EBV-associated epithelial carcinoma cell line that has been
identified to be cross-contaminated with HeLa cells and an
additional cell line of unknown origin (28). The CNE1-LMP1 cell line, which stably
expresses LMP1, was obtained from the Cancer Research Institute of
Central South University (26,29). All
these cells were also preserved at the Cancer Research Insitute at
Xiangya School of Medicine (26,27,29,30). All
cells were cultured in Roswell Park Memorial Institute (RPMI)-1640
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% fetal bovine serum (FBS; Invitrogen; Thermo Fisher
Scientific, Inc.) and 100 U/ml penicillin/streptomycin, and were
maintained at 37°C and 99% humidity, in an atmosphere containing 5%
CO2.
Plasmids and transient
transfection
An expression vector for wild-type LMP1, pSG5-LMP1,
was derived from the B95.8 EBV strain and provided by Dr Lzumi
(Brigham and Women's Hospital, Boston, MA, USA) (30). CNE1 cells (5×105
cells/well) were transfected with different concentrations of
pSG5-LMP1 plasmid (0, 0.5 and 1 µg/well) or with control pSG5
vector (Agilent Technologies, Inc., Santa Clara, CA, USA; 1
µg/well) using Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). Lipofectamine-DNA complexes were incubated with
cells at 37°C for 4 h in RPMI 1640, then washed in PBS and
maintained in RPMI supplemented with 10% FBS and 100 U/ml
penicillin/streptomycin at 37°C under 5% CO2 and 99%
humidity for 24 h, prior to being harvested for western blot
analysis.
DNAzyme (DZ1) and transfection
B95.8, CNE1-LMP1 and CNE1 cells (5×105
cells/well) were seeded in 6-well plates at 37°C in an atmosphere
containing 5% CO2 overnight. DZI/tetra (4-methylpyridyl)
porphyrine mixtures were made at a charge ratio of 1 and with 2 µM
DZ1 oligonucleotides or control oligonucleotides (ODN). The EBV
LMP1-targeted DNAzyme DZ1 is an LMP1-targeted DNAzyme that binds
and cleaves LMP1 RNA in a highly sequence-specific manner and was
synthesized by Oligos Etc., Inc. (Wilsonville, OR, USA). Tetra
(4-methylpyridyl) porphyrine was purchased from Frontier
Scientific, Inc. (Logan, UT, USA) (31). Control oligonucleotides (Takara
Biotechnology Co., Ltd., Dalian, China) were designed by inverting
the catalytic core sequence, as described previously (31–33).
Mixtures were incubated for 15 min at room temperature to form
transfection complexes. Cells were rinsed twice with phosphate
buffered saline and then incubated with the transfection mixtures
of either DZ1 or ODN at 37°C for 4 h in an atmosphere containing 5%
CO2, which was followed by the addition of complete
medium to the wells. Cells were subsequently incubated at 37°C for
24 h in an atmosphere containing 5% CO2.
EGCG and cell treatment
EGCG was purchased from Sigma-Aldrich (Merck KGaA;
Darmstadt, Germany) and prepared in autoclaved water as a stock
solution for in vitro experiments. Prior to treatment with
different concentrations of EGCG (0, 5, 10 or 20 µM) at 37°C for 24
h, CNE1-LMP1 and B95.8 cells (2×105 cells/well) were
starved in RPMI-1640 supplemented with 0.1% FBS at 37°C in an
atmosphere containing 5% CO2 for 24 h. H2O
treatment was used as a negative control (0 µM EGCG treatment
group).
Preparation of cell lysates and
western blot analysis
Whole cell lysate preparation and western blot
analysis were performed according to published methods (26). EGCG-treated B95.8 and CNE1-LMP1
cells, or post-transfection B95.8, CNE1-LMP1 and CNE1 cells were
harvested at the indicated time, lysed in lysis buffer [10 mM
Tris-HCl, pH 8.0, 1 mM EDTA, 2% SDS, 5 mM dithiothreitol, 10 mM
phenylmethyl sulfonylfluoride, 1 mM Na3VO4, 1
mM NaF and 10% (v/v) glycerol; and a protease inhibitor cocktail
tablet (Roche Diagnostics, Basel, Switzerland)], incubated on ice
for 30 min with mixing every 10 min, and subsequently centrifuged
for 10 min at 16,800 × g and 4°C. Supernatant was collected as
whole cell lysates and the protein concentration was measured using
a BCA Assay Reagent (Pierce; Thermo Fisher Scientific, Inc.).
Protein samples (50 µg/lane) were separated by 6–12% SDS-PAGE,
transferred onto a nylon membrane. The membranes were blocked with
buffer containing 5% non-fat milk in PBS with 0.05% Tween-20 (PBST)
at room temperature for 2 h, and incubated with different primary
antibodies overnight at 4°C. The following primary antibodies were
used for immuno-detection: Mouse BZLF1 monoclonal antibody
(SC-53904; Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at
1:200 dilution; EBV nuclear antigen 1 (EBNA1) antibody (ab25653;
Abcam; Cambridge, UK) at 1:1,000 dilution; BMRF1 antibody (ab6524;
Abcam), which binds to BMRF1 (Ea-D) p52/50 of EBV, at 1:1,000
dilution; β-actin (Ac-15; Sigma-Aldrich; Merck KGaA) at 1:2,000
dilution; and LMP1 monoclonal antibody (M0897; Dako; Agilent
Technologies, Inc.), which binds to full length LM1 (62 kDa) and
truncated LMP1 (42 kDa) at 1:200 dilution. Following a second wash
with PBST, the membranes were incubated with anti-mouse (sc-2005;
Santa Cruz Biotechnology, Inc.) horseradish peroxidase-conjugated
secondary antibody for 1 h at room temperature, and color was
subsequently developed using an enhanced chemiluminescence
detection kit (Pierce; Thermo Fisher Scientific, Inc.). The protein
bands were visualized following exposure of the membranes to Kodak
X-ray film. Densitometric analysis of the bands was carried out
using ImageJ software 1.42q (National Institutes of Health,
Bethesda, MD, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA from EGCG-treated B95.8 and CNE1-LMP1
cells was isolated using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) and complementary DNA was synthesized according
to a previously published method (26). LMP1 expression was measured by
RT-qPCR with the following primers: LMP1, forward,
5′-ATACCTAAGACAAGTAAGCA-3′ and reverse 5′-ACACACTGCCCTGAGGATGG-3′
(34); The PCR products underwent
electrophoresis on 2.5% agarose gel. Visualization following
ethidium bromide staining at room temperature for 30 min was
performed under UV light. qPCR was performed using a Rotor-Gene
6000 thermocycler (Qiagen GmbH, Hilden, Germany) and SYBR Premix Ex
Taq II (Takara Biotechnology Co., Ltd.) and 2 µl complementary DNA
with the following primers: LMP1, forward
5′-TGACTGGACTGGAGGAGC-3′ and reverse 5′-AGCGATGAGCAGGAGGGT−3′; and
β-actin, forward 5′-TTCCAGCCTTCCTTCCTGGG-3′ and reverse
5′-TTGCGCTCAGGAGGAGCAAT-3′. The following thermocycling conditions
were used: Initial denaturation at 95°C for 1 min, followed by 40
cycles of denaturing at 95°C for 15 sec, annealing at 55°C for 20
sec and extension at 72°C for 30 sec, and a final extension at 72°C
for 10 min with subsequent cooling to 4°C. Relative mRNA abundance
was calculated by the 2−∆∆Cq method using β-actin as the
internal control (35). For each
experiment, the mRNA levels in untreated cells were used as
controls and set as 1. The mRNA expression levels were represented
relative to those in the untreated cells.
Statistical analysis
Data were analyzed using GraphPad Prism 5 (GraphPad
Software, Inc., La Jolla, CA, USA). All values were expressed as
the mean ± standard error of the mean of triplicate experiments.
Two-group comparisons were performed using Student's t-tests, and
P<0.05 was considered to indicate a statistically significant
difference.
Results
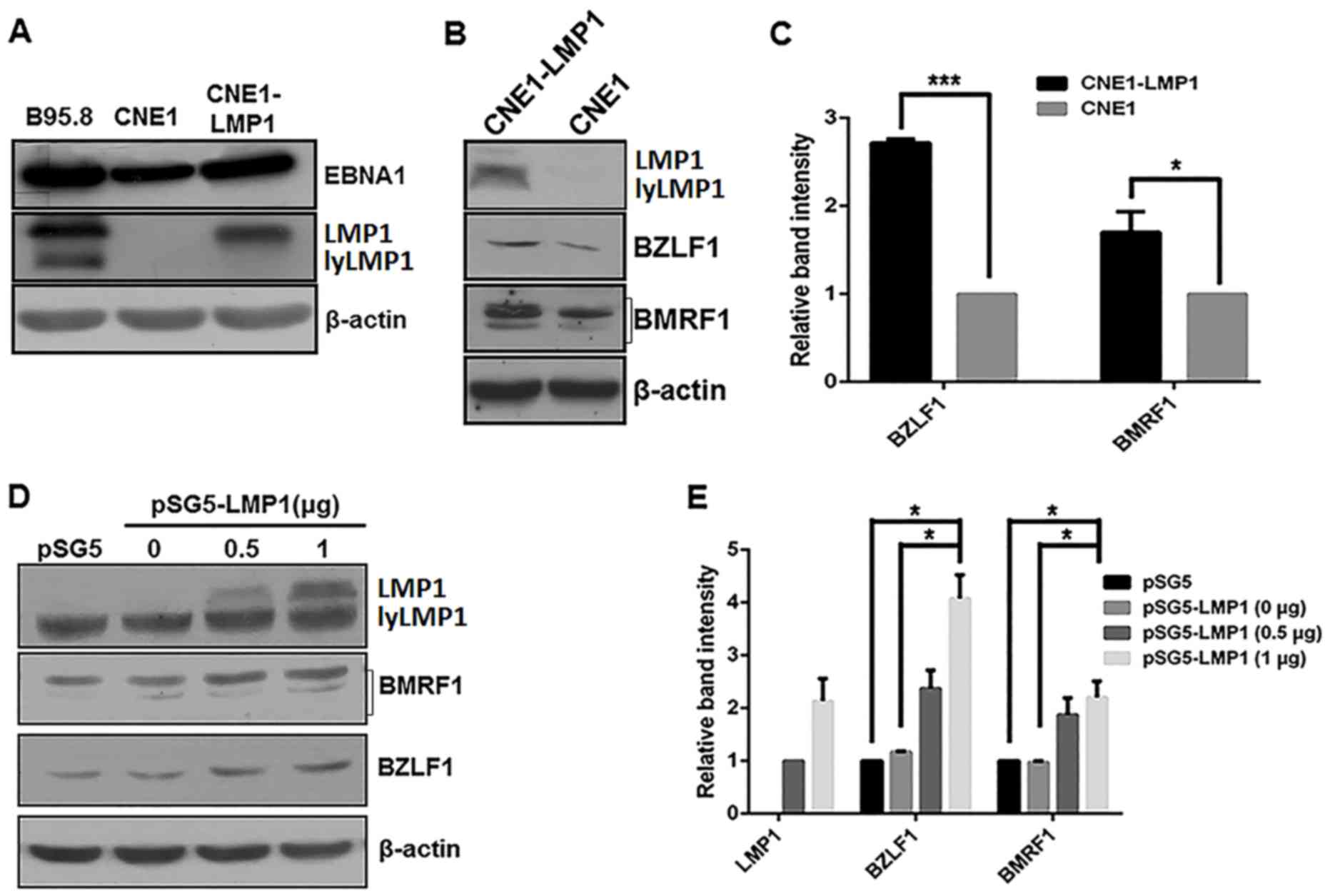
LMP1 enhances the expression levels of
EBV lytic proteins in CNE1 cells
Our previous work indicated that EBV lytic and
latent infection proteins are present in NPC tumor cells and
lymphomas (26). In the present
study, EBV latent and lytic protein expression levels were
investigated in the B95.8 and CNE1 cell lines and compared with the
constructed CNE1-LMP1 cells. B95.8 cells were considered as a
positive control as the line is permissive for viral lytic
replication (36). Western blot
analysis revealed that the EBV latent proteins, EBNA1 and LMP1
(37), were constitutively expressed
in B985.8, CNE1 and CNE1-LMP1 cell lines, which indicated that
these cell lines were EBV-positive (Fig.
1A). Additionally, the constructed CNE1-LMP1 cell line that
constitutively expressed LMP1 exhibited markedly increased
expression levels of the EBV lytic proteins BZLF1 and BMRF1
(37,38) when compared with the CNE1 control
cells (Fig. 1B). As depicted in
Fig. 1C, the differences in the
protein levels of BZLF1 and BMRF1 between the CNE1-LMP1 and CNE1
cells were determined to be statistically significant (P<0.001
and P<0.05, respectively).
To further evaluate the expression of the EBV lytic
proteins, CNE1 cells were transiently transfected with the
LMP1-expressing pSG5-LMP1 plasmid. As indicated in Fig. 1D and E, pSG5-LMP1 in the CNE1 cells
increased the protein levels of BZLF1 and BMRF1 protein in an
apparent dose-dependent manner. Furthermore, compared with the CNE1
cells treated with pSG5 or 0 µg pSG5-LMP1, the CNE1 cells treated
with 1 µg pSG5-LMP1 exhibited significantly increased protein
levels of BZLF1 and BMFR1 (P<0.05; Fig. 1E).
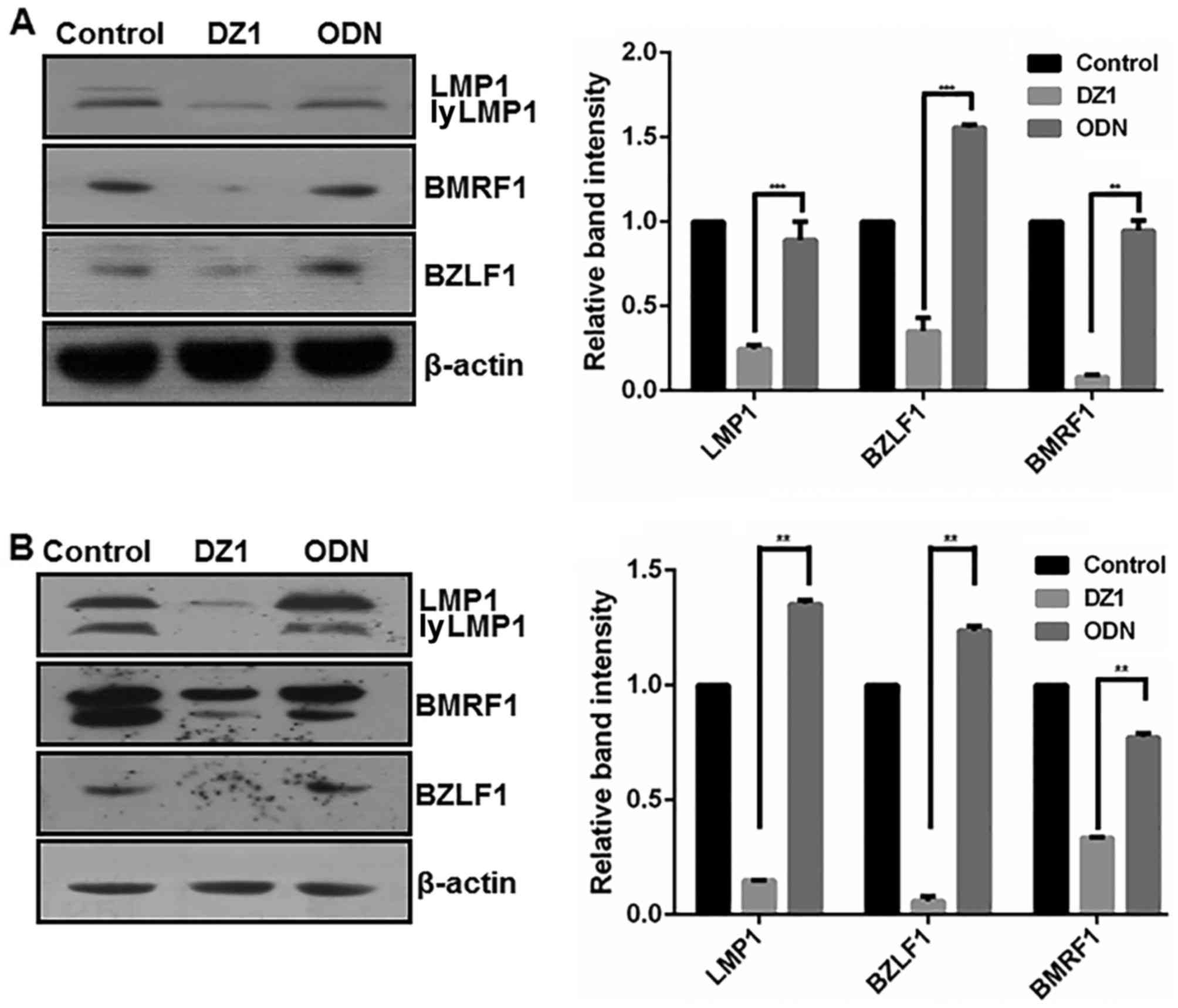
Inhibition of LMP1 expression
decreases the expression of EBV lytic proteins in LMP1-positive
cells
To clarify the potential regulatory effect of LMP1
on EBV lytic protein expression, DZ1 was used to downregulate LMP1
in B95.8 and CNE1-LMP1 cells (Fig.
2). As expected, LMP1 protein expression was significantly
downregulated in the CNE1-LMP1 and B95.8 cells following DZ1
treatment when compared with the respective ODN controls
(P<0.001 and P<0.01, respectively). More notably, following
DZ1 treatment, the CNE1-LMP1 and B95.8 cells exhibited
significantly reduced protein levels of BZLF1 (P<0.001 and
P<0.01, respectively) and BMRF1 (both P<0.01). ODN had no
significant effect on the protein levels of LMP1 or EBV lytic
proteins when compared with the untreated control cells.
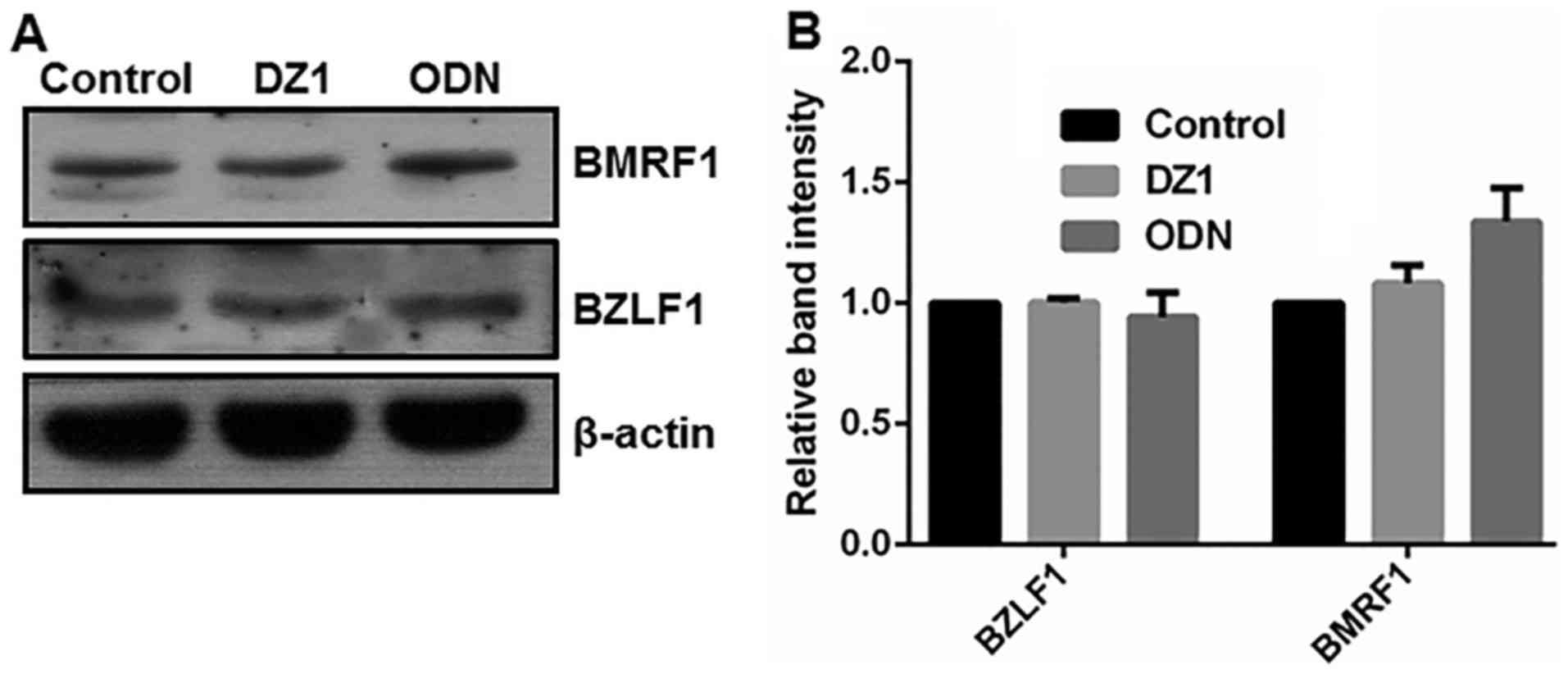
Effect of LMP-1-specific DZ1 on the
expression of EBV lytic proteins in LMP1-negative cells
To ascertain the specific cytotoxicity of DZ1,
LMP1-negative CNE1 cells were treated with 2 µM LMP1-targeting DZ1.
As depicted in Fig. 3, in
LMP1-negative CNE1 cells, DZ1 had no significant effect on the
expression of the EBV lytic proteins BZLF1 and BMRF1. Thus, DZ1 was
indicated to specifically inhibit LMP1 expression and consequently
EBV lytic protein expression in the CNE1-LMP1 cells. These findings
also verified that LMP1 had a positive regulatory effect on the
expression of the EBV lytic proteins BZLF1 and BMRF1.
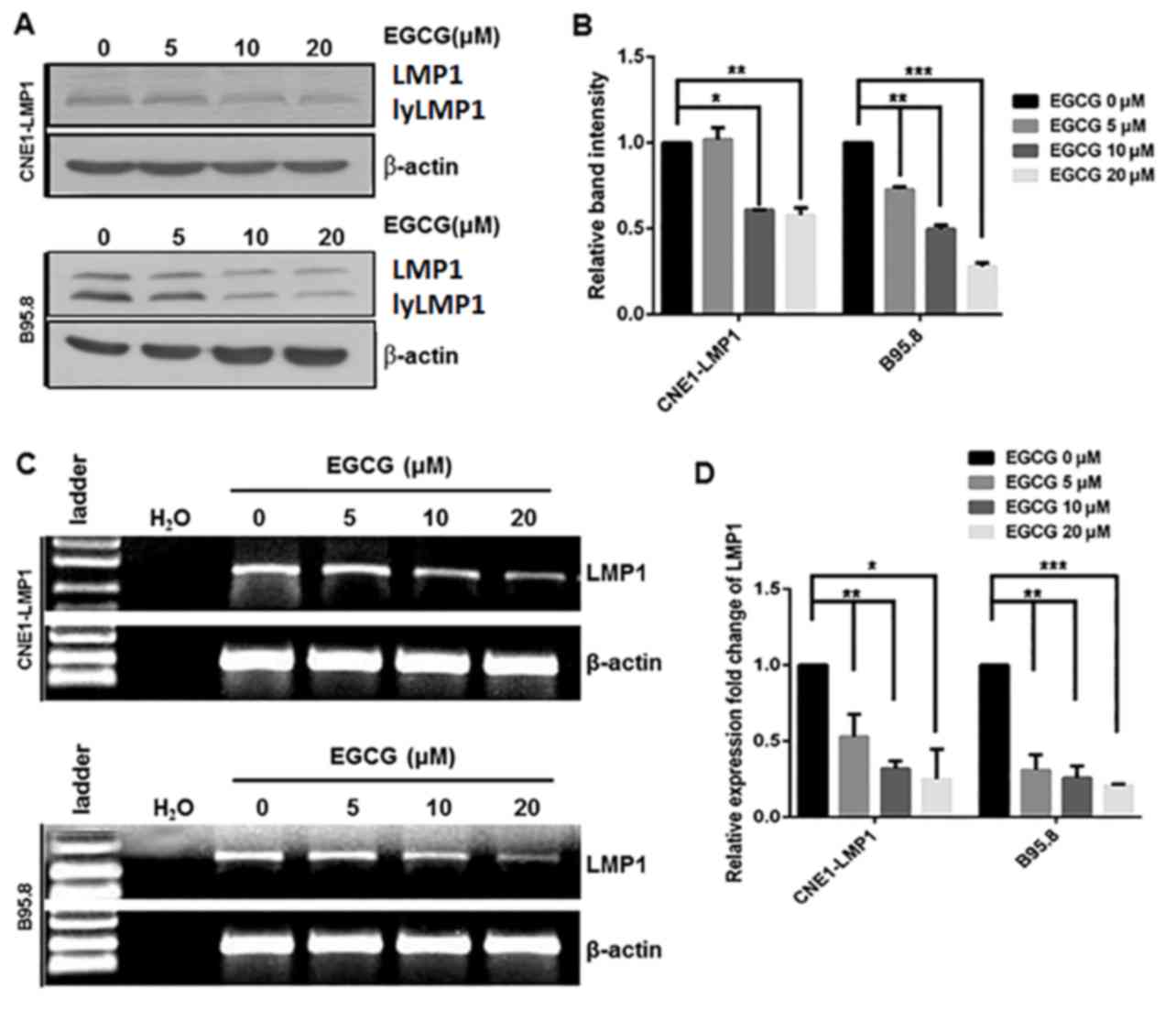
Inhibitory effect of EGCG on the
expression of LMP1
Our previous work indicated that EGCG exerted
inhibitory effects on the viability of CNE1-LMP1 and B95.8 cells
(IC50 20 µM), and that this was dose- and time-dependent
(26). Thus, EGCG (0–20 µM) was used
in the current experiments. To determine whether EGCG affected the
protein expression of LMP1, LMP1-positive CNE1-LMP1 and B95.8 cells
were treated with EGCG (0, 5, 10 and 20 µM), after which the cells
were harvested and the levels of LMP1 expression were measured by
western blot analysis. As depicted in Fig. 4A, the protein levels of LMP1 in
CNE1-LMP1 and B95.8 cells treated with EGCG were markedly reduced,
mostly notably following treatment with 20 µM EGCG, when compared
with those in the untreated CNE1-LMP1 and B95.8 cells. Subsequent
quantification of the results indicated that these differences in
the levels of LMP1 were statistically significant; in the CNE1-LMP1
cells, the protein levels of LMP1 were deemed to be significantly
decreased following treatment with 10 (P<0.05) and 20 µM
(P<0.01) EGCG, while for the B95.8 cells, the protein levels of
LMP1 were significantly decreased following treatment with 5
(P<0.01), 10 (P<0.01) and 20 µM (P<0.001) EGCG (Fig. 4B).
Decreased protein expression may be due to
downregulated mRNA; therefore, the mRNA levels of LMP1 in the
CNE1-LMP1 and B95.8 cells were determined. The results indicated
that EGCG treatment markedly downregulated LMP1 mRNA in the
CNE1-LMP1 and B95.8 cells (Fig. 4C).
Quantification of these results indicated that EGCG treatment (5–20
µM) significantly downregulated LMP1 mRNA in the CNE1-LMP1 and
B95.8 cells (P<0.05) in an apparent dose-dependent manner,
(Fig. 4D). These findings suggested
that EGCG decreased viral LMP1 expression in the EBV-associated
epithelial carcinoma cell line CNE1-LMP1 and EBV-positive B95.8
cell line at both the protein and mRNA level.
Discussion
The life cycle of EBV includes latent and lytic
stages. In the majority of asymptomatic carriers, the lytic cycle
of EBV in the host cells is periodically reactivated (7). Previous studies have focused on the
effects of EBV latent infection (2,20). Other
studies have demonstrated that the lytic cycle of EBV was able to
increase B-cell transformation efficiency at cell culture levels
and the development of B-cell lymphoma in a humanized mouse model
(39–41). Our previous data demonstrated that
EBV lytic infection proteins are present, not only in CNE1-LMP1 and
lymphoma cell lines, but also in patient biopsies (26). Similarly, the present study
identified markers of the EBV lytic cycle in EBV-associated
epithelial carcinoma and lymphoma cell lines.
LMP1 is an EBV-encoded 62-kDa integral membrane
oncogenic protein containing 386 amino acid residues that is
primarily composed of a short intracellular N-terminus, six
hydrophobic transmembrane domains and an intracellular C-terminus
that includes the three functional domains CTAR1, CTAR2 and CTAR3
(42). LMP1 has been be detected in
90% of patients with NPC (18,19).
Through its cytoplasmic C-terminus, LMP1 has been reported to
trigger multiple signal transduction cascades associated with the
EBV lytic cycle, including MEK/ERK, PI3-K/Akt, nuclear factor-κB
and JNK signaling pathways (20,21). In
addition, the LMP1 gene of several EBV strains also contains a late
lytic cycle promoter, EDL1A, which drives the expression of an
amino-terminally truncated form of LMP1, lytic LMP1 (lyLMP1).
lyLMP1, has 258 amino acid residues ~42-kD, expressed in the lytic
phase (43). Modulation of
LMP1-activated signaling pathways was the first identified
biological activity associated with lyLMP1, and this activity may
contribute to the progression of EBV's lytic cycle (43,44). The
present findings also suggested that LMP1 and lyLM1 were expressed
in B95-8 and CNE1-LMP1 cells and that there was spontaneous
reactivation of EBV in the cell lines.
A number of studies have demonstrated that LMP1
serves an important role in the EBV lytic cycle (24,45,46).
While LMP1 may be expressed in some states of EBV latency,
significant induction of full-length LMP1 is frequently observed
during virus reactivation into the lytic cycle (13,47,48).
Notably, when EBV reactivation is induced by various stimuli,
including cross-linking of surface immunoglobulin, virus
superinfection and treatment with phorbol ester, 5-azacytidine,
butyrate or histone deacetylase inhibitors, the expression of
full-length LMP1 may be significantly increased (13,22–25).
Furthermore, transfection of cells with an exogenous Rta plasmid
has been demonstrated to induce the expression of LMP1 in a variety
of epithelial cell lines such as NPC NA, EBV-infected HeLa, 293,
P3HR1 and Akata cells (24). The
close correlation between the inducible increased expression of
LMP1 and the EBV replication cycle indicates that LMP1 expression,
as a lytic cycle gene, is under the control of the lytic cycle
program (24). In a previous study,
lack of LMP1 expression severely impaired virus release into
culture supernatants, which resulted in poor infection efficiency.
These results have suggested that LMP1 serves an important role in
EBV particle release from cells during the lytic cycle and in the
infection of new host cells (46).
Furthermore, a different study identified that low expression of
LMP1 suppressed the activity of the EBV latent replication origin
oriP, and that the LMP1 binding site for tumor necrosis factor
receptor-associated factor was essential for this suppressive
effect (49).
In the present study, the protein expression levels
of the EBV lytic proteins BZLF1 and BMRF1 in the LMP1-positive
CNE1-LMP1 cells were significantly elevated when compared with the
LMP1-negative CNE1 cells. Through an induction strategy with an
LMP1 expression plasmid to induce LMP1 expression and a blockade
strategy with DZ1 to inhibit LMP1 expression, it was indicated that
LMP1 promoted the expression of the EBV lytic proteins BZLF1 and
BMRF1. To ascertain the specific cytotoxicity of DZ1, LMP1-negative
CNE1 cells were used as an experimental model, in which it was
demonstrated that DZ1 had no significant effect on the expression
of BZLF1 and BMRF1. These findings suggest a positive regulatory
effect of LMP1 on the expression of the EBV lytic proteins BZLF1
and BMRF1.
EBV lytic genes, including BZLF1 and
BMRF1, or cellular genes induced by these viral lytic
proteins, may encode paracrine factors that promote tumor growth
(39). It has been reported that
BZLF1 has various malignancy-promoting activities (50). Therefore, EBV lytic infection may be
a notable factor to consider in malignant transformation, as EBV
infection results in changes to the infected host cells or nearby
cells (45), and the biological
characteristics of these cells may be altered in a way that may
increase the degree of malignancy and promote the occurrence of
metastasis (51). Furthermore, LMP1
is an important viral protein required for the EBV lytic life cycle
(45). Therefore, selection of this
protein for augmenting virus release may be a critical evolutionary
step for EBV.
Other studies have suggested a conflicting model in
which LMP1 inhibits lytic cycle progression (52–54). For
instance, LMP1 was reported to inhibit lytic cycle induction via
the transcription factor nuclear factor-κB in an EBV-positive
Burkitt's lymphoma P3HR1-c16 cell line, which lacks LMP1 and may be
activated into a virally productive lytic cycle (52). These findings indicate that in B
cells, EBV self-limits its lytic cycle via the transcription factor
nuclear factor (NF)-κB. In addition, LMP1 inhibits lytic cycle
progress via two distinct NF-κB-independent mechanisms: One
associated with the cytosolic C-terminal activating regions and the
other with the transmembrane region of LMP1 (52). Additionally, cluster of
differentiation (CD) 40-CD40 ligand interactions and viral mimics
of activated CD40 and LMP1 suppress virus reactivation, and this
regulation of latency by CD40 and LMP1 may have important
implications for the balance between EBV and its host in normal or
immunocompromised individuals (55).
The discrepancy between these previous findings with the present
results may be due to differences in the experimental systems and
thus expression levels of LMP1 protein. Regardless, the above
findings indicate that LMP1 may serve dual roles in EBV lytic
replication.
LMP1 may create an optimum cellular environment for
efficient EBV DNA replication by promoting cell proliferation or
triggering necessary signaling pathways (2,20). The
latent form of infection allows the virus to persist for the
lifetime of the host, whereas the lytic form of infection enables
infectious virion production and transmission from cell to cell and
from host to host. Both forms of infection are essential for the
long-term success of the virus (45). It is also speculated that once EBV is
reactivated into the lytic cycle, the induced expression of LMP1 is
considered to be critical for efficient virus release and infection
of new host cells. However, high levels of LMP1 may negatively
regulate EBV lytic infection (52–54).
Therefore, EBV has developed a series of strategies to maintain
itself in host cells over the host's lifetime, and only
periodically produces infectious virons to transmit and infect new
host cells.
Previous studies on cancer chemoprevention using
EGCG have suggested that EGCG has anti-carcinogenic activity in
various organs in animal models (14,56).
Alternative studies and our previous and present studies indicate
that EGCG inhibits EBV lytic infection, though the mechanism is not
well understood (26,57–59). In
our previous study, CNE1-LMP1 and B95.8 cells with EBV spontaneous
lytic replication were used to mimic the natural state of infected
cells, and it was indicated that EGCG inhibited EBV spontaneous
lytic replication by inhibiting activation of MEK/ERK1/2 and
PI3-K/Akt signaling (26).
Considering that EGCG may modulate signaling pathways induced by
LMP1 (16), the present study
investigated the biological significance of EGCG on LMP1 expression
during the lytic cycle of viral replication, and observed that EGCG
inhibited the expression of LMP1 at the transcriptional and
translational levels. Thus, EGCG may inhibit EBV spontaneous lytic
replication by a novel mechanism involving the inhibition of LMP1
expression. Further elucidation of the molecular mechanisms
underlying EGCG activity during EBV lytic replication may
facilitate the development of therapies for EBV-positive
malignancies.
Acknowledgements
The present study was supported by the National
Nature Science Foundation of China (grant no. 81101474).
References
|
1
|
Cohen JI, Fauci AS, Varmus H and Nabel GJ:
Epstein-Barr virus: An important vaccine target for cancer
prevention. Sci Transl Med. 3:107fs72011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Young LS and Murray PG: Epstein-Barr virus
and oncogenesis: From latent genes to tumours. Oncogene.
22:5108–5121. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Young LS and Rickinson AB: Epstein-Barr
virus: 40 years on. Nat Rev Cancer. 4:757–768. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sugawara Y, Makuuchi M and Takada K:
Detection of Epstein-Barr virus DNA in hepatocellular carcinoma
tissues from hepatitis C-positive patients. Scand J Gastroenterol.
35:981–984. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kutok JL and Wang F: Spectrum of
Epstein-Barr virus-associated diseases. Annu Rev Pathol. 1:375–404.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hammerschmidt W and Sugden B: Replication
of Epstein-Barr viral DNA. Cold Spring Harb Perspect Biol.
5:a0130292013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Murata T: Regulation of Epstein-Barr virus
reactivation from latency. Microbiol Immunol. 58:307–317. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Murata T and Tsurumi T: Switching of EBV
cycles between latent and lytic states. Rev Med Virol. 24:142–153.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lei KI, Chan LY, Chan WY, Johnson PJ and
Lo YM: Quantitative analysis of circulating cell-free Epstein-Barr
virus (EBV) DNA levels in patients with EBV-associated lymphoid
malignancies. Br J Haematol. 111:239–246. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lo YM: Quantitative analysis of
Epstein-Barr virus DNA in plasma and serum: Applications to tumor
detection and monitoring. Ann N Y Acad Sci. 945:68–72. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Geser A, de Thé G, Lenoir G, Day NE and
Williams EH: Final case reporting from the Ugandan prospective
study of the relationship between EBV and Burkitt's lymphoma. Int J
Cancer. 29:397–400. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mueller N, Evans A, Harris NL, Comstock
GW, Jellum E, Magnus K, Orentreich N, Polk BF and Vogelman J:
Hodgkin's disease and Epstein-Barr virus. Altered antibody pattern
before diagnosis. N Engl J Med. 320:689–695. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Boos H, Berger R, Kuklik-Roos C, Iftner T
and Mueller-Lantzsch N: Enhancement of Epstein-Barr virus membrane
protein (LMP) expression by serum, TPA, or n-butyrate in latently
infected Raji cells. Virology. 159:161–165. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ramos S: Cancer chemoprevention and
chemotherapy: Dietary polyphenols and signalling pathways. Mol Nutr
Food Res. 52:507–526. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yang CS and Wang H: Cancer therapy
combination: Green tea and a phosphodiesterase 5 inhibitor? J Clin
Invest. 123:556–558. 2013.PubMed/NCBI
|
|
16
|
Zhao Y, Wang H, Zhao XR, Luo FJ, Tang M
and Cao Y: Epigallocatechin-3-gallate interferes with EBV-encoding
AP-1 signal transduction pathway. Zhonghua Zhong Liu Za Zhi.
26:393–397. 2004.(In Chinese). PubMed/NCBI
|
|
17
|
Kanwar J, Taskeen M, Mohammad I, Huo C,
Chan TH and Dou QP: Recent advances on tea polyphenols. Front
Biosci (Elite Ed). 4:111–131. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tsao SW, Tramoutanis G, Dawson CW, Lo AK
and Huang DP: The significance of LMP1 expression in nasopharyngeal
carcinoma. Semin Cancer Biol. 12:473–487. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dawson CW, Port RJ and Young LS: The role
of the EBV-encoded latent membrane proteins LMP1 and LMP2 in the
pathogenesis of nasopharyngeal carcinoma (NPC). Semin Cancer Biol.
22:144–153. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng H, Li LL, Hu DS, Deng XY and Cao Y:
Role of Epstein-Barr virus encoded latent membrane protein 1 in the
carcinogenesis of nasopharyngeal carcinoma. Cell Mol Immunol.
4:185–196. 2007.PubMed/NCBI
|
|
21
|
Li H, Liu S, Hu J, Luo X, Li N, M Bode A
and Cao Y: Epstein-Barr virus lytic reactivation regulation and its
pathogenic role in carcinogenesis. Int J Biol Sci. 12:1309–1318.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chang Y, Tung CH, Huang YT, Lu J, Chen JY
and Tsai CH: Requirement for cell-to-cell contact in Epstein-Barr
virus infection of nasopharyngeal carcinoma cells and
keratinocytes. J Virol. 73:8857–8866. 1999.PubMed/NCBI
|
|
23
|
Contreras-Salazar B, Ehlin-Henriksson B,
Klein G and Masucci MG: Up regulation of the Epstein-Barr virus
(EBV)-encoded membrane protein LMP in the Burkitt's lymphoma line
Daudi after exposure to n-butyrate and after EBV superinfection. J
Virol. 64:5441–5447. 1990.PubMed/NCBI
|
|
24
|
Chang Y, Lee HH, Chang SS, Hsu TY, Wang
PW, Chang YS, Takada K and Tsai CH: Induction of Epstein-Barr virus
latent membrane protein 1 by a lytic transactivator Rta. J Virol.
78:13028–13036. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nishikawa J, Kis LL, Liu A, Zhang X,
Takahara M, Bandobashi K, Kiss C, Nagy N, Okita K, Klein G and
Klein E: Upregulation of LMP1 expression by histone deacetylase
inhibitors in an EBV carrying NPC cell line. Virus Genes.
28:121–128. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu S, Li H, Chen L, Yang L, Li L, Tao Y,
Li W, Li Z, Liu H, Tang M, et al: (−)-Epigallocatechin-3-gallate
inhibition of Epstein-Barr virus spontaneous lytic infection
involves ERK1/2 and PI3-K/Akt signaling in EBV-positive cells.
Carcinogenesis. 34:627–637. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu SF, Wang H, Li ZJ, Deng XY, Xiang H,
Tao YG, Li W, Tang M and Cao Y: Aspirin induces lytic cytotoxicity
in Epstein-Barr virus-positive cells. Eur J Pharmacol. 589:8–13.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Strong MJ, Baddoo M, Nanbo A, Xu M,
Puetter A and Lin Z: Comprehensive high-throughput RNA sequencing
analysis reveals contamination of multiple nasopharyngeal carcinoma
cell lines with HeLa cell genomes. J Virol. 88:10696–10704. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ma X, Yang L, Xiao L, Tang M, Liu L, Li Z,
Deng M, Sun L and Cao Y: Down-regulation of EBV-LMP1
radio-sensitizes nasal pharyngeal carcinoma cells via NF-κB
regulated ATM expression. PLoS One. 6:e246472011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xiao L, Hu ZY, Dong X, Tan Z, Li W, Tang
M, Chen L, Yang L, Tao Y, Jiang Y, et al: Targeting Epstein-Barr
virus oncoprotein LMP1-mediated glycolysis sensitizes
nasopharyngeal carcinoma to radiation therapy. Oncogene.
33:4568–4578. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Benimetskaya L, Takle GB, Vilenchik M,
Lebedeva I, Miller P and Stein CA: Cationic porphyrins: Novel
delivery vehicles for antisense oligodeoxynucleotides. Nucleic
Acids Res. 26:5310–5317. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lu ZX, Ye M, Yan GR, Li Q, Tang M, Lee LM,
Sun LQ and Cao Y: Effect of EBV LMP1 targeted DNAzymes on cell
proliferation and apoptosis. Cancer Gene Ther. 12:647–654. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cao Y, Yang L, Jiang W, Wang X, Liao W,
Tan G, Liao Y, Qiu Y, Feng D, Tang F, et al: Therapeutic evaluation
of Epstein-Barr virus-encoded latent membrane protein-1 targeted
DNAzyme for treating of nasopharyngeal carcinomas. Mol Ther.
22:371–377. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mattia E, Chichiarelli S, Hickish T, Gaeta
A, Mancini C, Cunningham D and van Renswoude J: Inhibition of in
vitro proliferation of Epstein Barr Virus infected B cells by an
antisense oligodeoxynucleotide targeted against EBV latent membrane
protein LMP1. Oncogene. 15:489–493. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Miller G and Lipman M: Release of
infectious Epstein-Barr virus by transformed marmoset leukocytes.
Proc Natl Acad Sci USA. 70:pp. 190–194. 1973; View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Tsurumi T, Fujita M and Kudoh A: Latent
and lytic Epstein-Barr virus replication strategies. Rev Med Virol.
15:3–15. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kenney SC and Mertz JE: Regulation of the
latent-lytic switch in Epstein-Barr virus. Semin Cancer Biol.
26:60–68. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Katsumura KR, Maruo S and Takada K: EBV
lytic infection enhances transformation of B-lymphocytes infected
with EBV in the presence of T-lymphocytes. J Med Virol. 84:504–510.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kalla M, Schmeinck A, Bergbauer M, Pich D
and Hammerschmidt W: AP-1 homolog BZLF1 of Epstein-Barr virus has
two essential functions dependent on the epigenetic state of the
viral genome. Proc Natl Acad Sci USA. 107:pp. 850–855. 2010;
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ma SD, Hegde S, Young KH, Sullivan R,
Rajesh D, Zhou Y, Jankowska-Gan E, Burlingham WJ, Sun X, Gulley ML,
et al: A new model of Epstein-Barr virus infection reveals an
important role for early lytic viral protein expression in the
development of lymphomas. J Virol. 85:165–177. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li HP and Chang YS: Epstein-Barr virus
latent membrane protein 1: Structure and functions. J Biomed Sci.
10:490–504. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Vazirabadi G, Geiger TR, Coffin WF III and
Martin JM: Epstein-Barr virus latent membrane protein-1 (LMP-1) and
lytic LMP-1 localization in plasma membrane-derived extracellular
vesicles and intracellular virions. J Gen Virol. 84:1997–2008.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pandya J and Walling DM: Oncogenic
activity of Epstein-Barr virus latent membrane protein 1 (LMP-1) is
down-regulated by lytic LMP-1. J Virol. 80:8038–8046. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nawandar DM, Ohashi M, Djavadian R, Barlow
E, Makielski K, Ali A, Lee D, Lambert PF, Johannsen E and Kenney
SC: Differentiation-dependent LMP1 expression is required for
efficient lytic Epstein-Barr virus reactivation in epithelial
cells. J Virol. 91:pii: e02438–16. 2017. View Article : Google Scholar
|
|
46
|
Ahsan N, Kanda T, Nagashima K and Takada
K: Epstein-Barr virus transforming protein LMP1 plays a critical
role in virus production. J Virol. 79:4415–4424. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang D, Liebowitz D and Kieff E: The
truncated form of the Epstein-Barr virus latent-infection membrane
protein expressed in virus replication does not transform rodent
fibroblasts. J Virol. 62:2337–2346. 1988.PubMed/NCBI
|
|
48
|
Rowe M, Evans HS, Young LS, Hennessy K,
Kieff E and Rickinson AB: Monoclonal antibodies to the latent
membrane protein of Epstein-Barr virus reveal heterogeneity of the
protein and inducible expression in virus-transformed cells. J Gen
Virol. 68:1575–1586. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Shirakata M, Imadome KI, Okazaki K and
Hirai K: Activation of TRAF5 and TRAF6 signal cascades negatively
regulates the latent replication origin of Epstein-Barr virus
through p38 mitogen-activated protein kinase. J Virol.
75:5059–5068. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen C, Li D and Guo N: Regulation of
cellular and viral protein expression by the Epstein-Barr virus
transcriptional regulator Zta: Implications for therapy of EBV
associated tumors. Cancer Biol Ther. 8:987–995. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tsao SW, Tsang CM, To KF and Lo KW: The
role of Epstein-Barr virus in epithelial malignancies. J Pathol.
235:323–333. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Prince S, Keating S, Fielding C, Brennan
P, Floettmann E and Rowe M: Latent membrane protein 1 inhibits
Epstein-Barr virus lytic cycle induction and progress via different
mechanisms. J Virol. 77:5000–5007. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bentz GL, Moss CR II, Whitehurst CB, Moody
CA and Pagano JS: LMP1-induced sumoylation influences the
maintenance of Epstein-Barr virus latency through KAP1. J Virol.
89:7465–7477. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Lai KY, Chou YC, Lin JH, Liu Y, Lin KM,
Doong SL, Chen MR, Yeh TH, Lin SJ and Tsai CH: Maintenance of
Epstein-Barr virus latent status by a novel mechanism, latent
membrane protein 1-induced interleukin-32, via the protein kinase
Cδ pathway. J Virol. 89:5968–5980. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Adler B, Schaadt E, Kempkes B,
Zimber-Strobl U, Baier B and Bornkamm GW: Control of Epstein-Barr
virus reactivation by activated CD40 and viral latent membrane
protein 1. Proc Natl Acad Sci USA. 99:pp. 437–442. 2002; View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kim JW, Amin AR and Shin DM:
Chemoprevention of head and neck cancer with green tea polyphenols.
Cancer Prev Res (Phila). 3:900–909. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Choi KC, Jung MG, Lee YH, Yoon JC, Kwon
SH, Kang HB, Kim MJ, Cha JH, Kim YJ, Jun WJ, et al:
Epigallocatechin-3-gallate, a histone acetyltransferase inhibitor,
inhibits EBV-induced B lymphocyte transformation via suppression of
RelA acetylation. Cancer Res. 69:583–592. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Taniguchi S, Imayoshi Y, Kobayashi E,
Takamatsu Y, Ito H, Hatano T, Sakagami H, Tokuda H, Nishino H,
Sugita D, et al: Production of bioactive triterpenes by Eriobotrya
japonica calli. Phytochemistry. 59:315–323. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Chang LK, Wei TT, Chiu YF, Tung CP, Chuang
JY, Hung SK, Li C and Liu ST: Inhibition of Epstein-Barr virus
lytic cycle by (−)-epigallocatechin gallate. Biochem Biophys Res
Commun. 301:1062–1068. 2003. View Article : Google Scholar : PubMed/NCBI
|