Introduction
The main function of salivary glands (SGs) is to
secret saliva, which is essential for the maintenance of oral
health. Saliva is required for lubrication, digestion, perception
of taste, protection against noxious materials and microorganisms
and for the maintenance of immune homeostasis (1). Reduction in the secretion of saliva
inevitably causes dry mouth and a series of accompanying
complications, including secondary rampant caries, chewing and
swallowing disorders, loss of the sense of taste and oral mucous
inflammation, which may significantly depress the quality of life
of affected individuals (2).
Approximately 500,000 novel cases of head and neck
cancer develop worldwide annually. Amongst them, the vast majority
of advanced patients require radiotherapy, either alone or in
combination with other treatments, such as chemotherapy, as a
primary or adjuvant mode of treatment following surgery. This
inevitably causes damage to the SGs. Approximately 40% of the
patients who receive radiotherapy develop salivary hypofunction or
xerostomia (3), and suffer from
severe pain and other complications, leading to the termination of
cancer treatment in certain cases. Current treatment strategies are
limited to conservative care based on sialagogues or salivary
substitutes (4). However, to date,
no satisfactory treatment is available to address this fundamental
issue.
A growing number of therapeutic strategies have been
devised to recover or rehabilitate damaged SGs, including gene
therapy (5,6), construction of tissue-engineered
artificial SGs (7–10), re-implantation of autologous SG cells
(11,12) and stem cell therapy (13,14). The
study of duct-ligated SGs has confirmed the presence of
stem/progenitor cells in SGs, which can differentiate into
functional hepatocytes and β-cells (15). Moreover, intraglandular
transplantation of a small amount of SG stem cells
(c-kit+ cells) has resulted in the long-term recovery of
irradiation-induced damaged SG morphology and function (11). Besides, bone marrow-derived stem
cells (BMSCs) and their soluble intracellular contents (termed as
‘BM soup’) have also been used for the treatment of
irradiation-induced SG hypofunction (14,16).
Adipose tissue-derived stem cells (ADSCs) are another type of
pluripotent adult stem cells that can differentiate into bone,
cartilage, fat and nerve cells. Furthermore, they can easily be
obtained with minimal invasion and are readily available, as the
density of mesenchymal stem cells (MSCs) is much higher in adipose
tissues than in bone marrow (17).
Therefore, ADSCs have been used in tissue engineering and
regenerative medicine with a proven ability to prevent
irradiation-induced SG damage (13,18).
However, to the best of our knowledge, there have been no studies
on treatments for permanent SG damage induced by radiotherapy.
Platelet-rich fibrin (PRF), a second-generation
platelet-rich concentrate, contains a variety of growth factors
that are slowly and continuously released (19). During the centrifugation process of
PRF production, the supernatant, referred to as acellular or
platelet-poor plasma (PPP), is also known to promote cell
proliferation (19,20). A study by Liu et al (21) has corroborated that PRF can improve
the survival rate of transplanted adipose tissue and increase its
angiogenic properties. The study also reported that PRF can release
growth factors for at least 2 weeks in vitro, thereby
suggesting the presence of a variety of growth factors in the PRF
extract (PRFe) obtained from PRF immersed in PPP (21).
The purpose of the present study was to determine
whether the administration of ADSCs combined with PRFe is more
effective than ADSCs alone for the treatment of radiation-induced
SG damage. At 3 months post irradiation, ADSCs, PRFe or ADSCs
combined with PRFe were transplanted into the submandibular glands
of C3H mice with permanent SG damage induced by radiotherapy.
Functional and morphological changes in the SGs were assessed using
transmission electron microscopy (TEM) and immunofluorescence in
order to determine the salivary flow rate (SFR), histopathological
changes and microvessel density at 3 months post
transplantation.
Materials and methods
Ethics statement
The protocols of the present study were reviewed and
approved by the Institutional Animal Care and Use Committee at the
Fourth Military Medical University (Xi'an, China). Animals were
cared for according to established institutional guidelines and all
efforts were made to minimize suffering. Surgeries were performed
under anesthesia with xylazine (10 mg/kg; Meridian Life Science,
Inc., Memphis, TN, USA) premedication and an intraperitoneal
injection of ketamine (110 mg/kg; Meridian Life Science, Inc.).
Irradiation-induced SG damage in C3H
mice
A total of 80 8–12-week old female C3H mice (weight,
28–34 g) were purchased from Vital River Laboratory Animal
Technology Co., Ltd. (Beijing, China). The mice were maintained in
a specific pathogen-free, microisolated environment (temperature,
18–29°C; humidity, 50–80%) with a 12 h light/dark cycle at the
Laboratory Animal Center of the School of Stomatology (the Fourth
Military Medical University, Xi'an, China) and were provided with a
standard pellet diet along with free access to sterilized
water.
The animals were anesthetized by intraperitoneal
injection of 50 mg/kg sodium pentobarbital (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) and irradiated with a single dose of 18
Gy at a focus-to-skin distance of 100 cm, using a 4 MV X-ray from a
linear accelerator (Mevatron MD; Siemens Medical Laboratories,
Inc., Munich, Germany). The mice were locally irradiated in the
head and neck region, including the SGs, while the body was
protected by a 12 mm-thick lead block. This radiation dose is known
to induce sufficient damage without compromising the general health
of the animal and results in permanent, irreversible salivary
dysfunction within 6 months of exposure (22).
Preparation of ADSCs
Mouse ADSCs were isolated and expanded according to
methods described previously (21),
with minor modifications. In brief, the inguinal fat pads were
obtained from 20 8-week-old, male C3H mice under general anesthesia
by intraperitoneal injection of 50 mg/kg sodium pentobarbital under
sterile conditions, following which mice were sacrificed with an
overdose of 3% isoflurane gas (Beijing Guochengruitai Science &
Technology Co., Ltd, Beijing, China). Following the removal of the
thin fascia and blood vessels covering the adipose tissue, the pads
were minced into small fragments of ~1 mm3 and
enzymatically dissociated with 0.2% collagenase type I
(Sigma-Aldrich; Merck KGaA) on a shaking table with continuous
agitation for 40 min at 37°C to separate the stromal cell fraction
from adipocytes. The stromal cell fraction was then filtered
through a 100-µm cell strainer (BD Biosciences, San Jose, CA, USA)
and centrifuged at 1,200 × g for 5 min at 26°C. The cell pellet was
resuspended into Dulbecco's modified Eagle's medium-F12 (DMEM-F12;
Gibco, Thermo Fisher Scientific, Inc., Waltham, MA, USA) containing
10% fetal bovine serum (FBS; Gibco, Thermo Fisher Scientific,
Inc.), 0.272 g/l L-glutamine (Sigma-Aldrich; Merck KGaA), and 2%
antibiotics (200 mg/ml penicillin and 200 mg/ml streptomycin;
Gibco, Thermo Fisher Scientific, Inc.). Next, the cell suspension
was plated in 100-mm cell culture dishes and standard medium was
added to reach a volume of 10 ml. The cells were incubated at 37°C
in an atmosphere containing 5% CO2 with 100% humidity.
After 3 days, the medium and all floating cells were removed and
fresh medium was added to the remaining adherent cells, which were
considered as the ADSCs. The medium was replaced every 3 days until
the cells reached confluence, following which they were subcultured
at a ratio of 1:3. ADSCs from the third passage were used in the
present study. In order to identify the characteristics of the
cultured cells, flow cytometry (FACSCalibur; BD Biosciences) as
well as tests to examine the pluripotent plasticity of stem cells,
including osteogenic and adipogenic differentiation, were
performed. Briefly, cells were labeled and incubated with mouse
monoclonal antibodies against CD29 (cat. no. ab95623; 1:200), CD31
(cat. no. ab24590; 1:200), and CD34 (cat. no. ab187282; 1:200) (all
from Abcam, Cambridge, MA, USA) at 4°C for 30 min, with an
isotype-identical antibody (IgG) as a control (ab37355; 1:200;
Abcam), and were subsequently incubated with fluorescein
isothiocyanate-labeled goat anti-mouse IgG antibody in the dark at
4°C for 30 min, PBS washed, and analyzed on a BD FACSCalibur. For
osteogenic differentiation, ADSCs were cultured at 37°C in
osteogenic media for three weeks, and osteogenesis was subsequently
assessed using Alizarin red staining. For adipogenic
differentiation, ADSCs were cultured at 37°C in adipogenic media
for two weeks, and adipogenesis was assessed by Oil-Red-O staining
for the presence of lipid vacuoles using light microscopy (DX51;
Olympus Corporation, Tokyo, Japan). Furthermore, in order to meet
the requirements for transplantation of huge numbers of ADSCs, a
3-D dynamic culture system based on the use of a rotating cell
culture system bioreactor (RCCS; Synthecon Inc., Houston, TX, USA)
was used to culture and expand stem cells according to a previously
described method (23,24), with minor modifications. In brief,
cytodex-3 microcarrier beads (GE Healthcare, Chalfont, UK) were
soaked in 0.1 M PBS (Sigma-Aldrich; Merck KGaA) overnight and then
washed three times in PBS, followed by autoclaving at 115°C for 30
min. Subsequently, the beads were washed twice using serum-free
DMEM-F12, placed in DMEM-F12 containing 10% FBS complete media and
stored at 4°C. The ADSCs were inoculated at a density of
1×105/ml with cytodex-3 (5 mg/ml), loaded into the
rotating cell culture system and rotated in an incubator containing
5% CO2 at 37°C. For the control group, stem cells were
seeded in a 24-well culture plate at a density of
1×105/ml. The stem cells were labeled using the
fluorescent reactive dye DiI (Molecular Probes; Thermo Fisher
Scientific, Inc.) to display adhesion and growth of the cells on
the cytodex-3 beads, and the microstructure was observed by
scanning electron microscopy (SEM; S-4800, Hitachi, Tokyo,
Japan).
Preparation of PRFe from New Zealand
rabbits
A total of 6 3-month-old male New Zealand rabbits
(mean weight, 2.5 kg) were obtained from the animal holding unit of
the Fourth Military Medical University. The rabbits were housed at
18–29°C with 50–80% humidity and a 12 h light/dark cycle at the
Laboratory Animal Center of the School of Stomatology. Rabbits were
provided with a standard pellet diet with free access to sterilized
water. The preparation of PRF was performed as described previously
(21), with minor modifications. In
brief, 10 ml of blood was collected from each rabbit in 10-ml dried
microcentrifuge tubes without anticoagulant and immediately
centrifuged for 10 min at 1,200 × g at 26°C. The centrifuged
product consisted of three layers; the PRF clot was located in the
middle of the tube, just between the red corpuscles at the bottom
and PPP on top. The acellular plasma was collected in a 4-ml
microcentrifuge tube for further use. The PRF clot was harvested
with tweezers and, using soft compression for 10 sec, gently
pressed onto a membrane, between two sterile pieces of gauze, in
order to keep the membrane wet. The obtained PRF membranes were cut
into small fragments in sterile dishes and then immersed into
microcentrifuge tubes containing acellular plasma. Finally, in
order to release the growth factors, the tubes were placed on a
shaking table with continuous agitation at 37°C for 1 week. The
supernatant, which was the PRFe, was harvested for further
transplantation.
Transplantation
The irradiation-induced, SG-damaged C3H mice (n=40)
were randomly divided into the following groups consisting of 10
mice each: IR+ADSCs+PRFe treatment group (received ADSCs in
combination with PRFe at 12 weeks post irradiation); IR+ADSC
treatment group (received ADSCs alone at 12 weeks post
irradiation); IR+PRFe treatment group (received PRFe alone at 12
weeks post irradiation); and IR+PBS negative control group (an
irradiated/untreated group that was injected with 100 µl PBS). A
fifth, non-irradiated group (normal, positive control) comprising
10 mice, was also included in the study. Mice were anesthetized via
intraperitoneal injection of 50 mg/kg sodium pentobarbital, and
subsequently ADSCs, alone or in combination with PRFe, and PRFe
alone were injected through the capsule of both submandibular
glands. Two injections of 50 µl each per gland were given, which
contained 2×105 ADSCs. In the IR+ADSCs group,
2×105 cells were suspended in 100 µl PBS, whereas in the
IR+ADSCs+PRF group, 2×105 cells were suspended in 100 µl
PRFe supernatant. Only 100 µl PRFe was injected into the mice of
the IR+PRFe group. This infusion was repeated weekly for three
consecutive weeks.
Furthermore, in order to confirm the successful
injection of ADSCs in the submandibular gland, the stem cells were
labeled using the fluorescent reactive dye DiI. Four weeks after
intraglandular injection, the mice were sacrificed by cervical
dislocation and the submandibular glands were harvested and snap
frozen in liquid nitrogen. Frozen SG tissue sections were analyzed
by fluorescence microscopy for detection of DiI-positive stem cells
in the SGs (25).
Morphological and functional
evaluation
Salivary flow rate
Salivary secretory function was determined by
measuring the SFR at 12 weeks after transplantation. Saliva was
collected from the floor of the mouth using a micropipette for a
period of 5 min after stimulation with an intraperitoneal injection
of pilocarpine (2 mg/kg; Toronto Research Chemicals, Inc., North
York, ON, Canada). The collected saliva was placed in a pre-weighed
1.5-ml microcentrifuge tube and the SFR (µl/min) was calculated by
dividing the weight (mg) of saliva collected by the duration of
collection (min) (saliva was assumed to have a specific gravity of
1 mg/ml).
Measurement of gland and body
weight
At 12 weeks after transplantation, the gland and
body weights of the mice were measured, followed by saliva
collection and, finally, sacrifice by cervical dislocation. The
submandibular glands were harvested and the surrounding fat and
connective tissues were removed. The weight of the harvested glands
was individually determined prior to fixation in 10% neutral
formalin buffer and embedding in paraffin.
Histological and immunohistochemical
evaluation of changes in the structure and function of acinar
cells
Following deparaffinization and rehydration, the
tissue sections were analyzed by hematoxylin and eosin (H&E)
staining. The quantification of acinar cells was performed by
counting the number of cells using light microscopy (DX51; Olympus
Corporation) at ×200 magnification. The assessments were performed
in three randomly selected sections by two independent
observers.
Moreover, Periodic acid-Schiff (PAS; Sigma-Aldrich;
Merck KGaA) staining was also performed to evaluate the changes.
The ratio of the surface area occupied by PAS-positive cells to the
total measured area was quantified in five random fields under ×400
magnification using a light microscope. The assessments were
performed in three randomly selected sections by two independent
observers using the Image J software (National Institutes of
Health, Bethesda, MD, USA).
The function of acinar cells was studied by
measuring α-amylase (AMY) production (Abcam) using
immunohistochemistry in SG tissues as previously described
(22,26). The percentage of surface area
occupied by AMY-containing acinar cells was determined by
densitometry using light microscopy at ×400 magnification. Three
sections were prepared for each gland and at least three fields per
section were examined by two blinded investigators using Metamorph
software (Version 7.6.4; Molecular Devices Corp., Sunnyvale, CA,
USA).
CD31 staining for microvessel density
analysis
Microvessel density analysis was performed using
Weidner's method (27), with minor
modifications. Following de-paraffinization and rehydration, tissue
sections were analyzed by immunohistochemical staining for CD31. As
previously described (Abcam) (26).
At ×100 magnification, a region of dense, regenerated blood vessels
was selected (hot spot), and cells with brown (positive) staining
were then identified within this ‘hot spot’ at ×200 magnification.
Each positively stained cell or cell cluster was regarded as a
newly formed blood vessel, while the positively stained red blood
cells in the vessel cava were excluded. If the size of a vessel
cavum was larger than that of eight blood cells, it was not
considered as a newly formed blood vessel and was excluded. Data
obtained from five ‘hot spots’, examined at ×200 magnification
using the Image J software (National Institutes of Health), were
statistically analyzed (21).
TEM
The specimens were fixed in 2.5% glutaraldehyde
solution for 12 h and post-fixed in 1% osmium tetroxide for 2 h
(Sigma-Aldrich; Merck KGaA). Following dehydration and embedding in
epoxy resin, the specimens were cut into semi-thin (2 µm),
longitudinal sections and examined with a TEM (JEM-1230; Jeol,
Tokyo, Japan).
Detection of apoptosis
Apoptotic cells in the submandibular glands were
visualized using the Apoptag Plus Fluorescein in situ
Apoptosis Detection kit (Millipore, Bedford, MA, USA), which uses
terminal deoxynucleotidyl transferase dUTP nick end labeling
(TUNEL) to detect DNA cleavage and chromatin condensation.
Following deparaffinization and rehydration, the slides were
incubated with the TUNEL reaction mixture containing TdT enzyme for
1 h at 37°C, and then with anti-digoxigenin fluorescein for 30 min
at room temperature. Nuclei were visualized using DAPI. Two blinded
examiners independently counted the number of apoptotic cells in
three random fields per tissue section at a magnification of ×400.
At least three random tissue sections per gland were mounted on
each slide.
Detection of cell proliferation
The Zymed proliferating cell nuclear antigen (PCNA)
staining kit (Invitrogen; Thermo Fisher Scientific, Inc.) was used
to detect cell proliferation. After deparaffinization, rehydration,
antigen retrieval and peroxidase blocking, the slides were
processed using the avidin biotin complex method for PCNA staining.
Two independent observers counted the absolute number of
PCNA-positive cells under a light microscope (magnification, ×400)
in five random fields per section. Three randomly selected sections
per specimen were subjected to analysis.
Statistical analysis
Statistical analysis was performed using the Graph
Pad Prism 5 package (GraphPad Software Inc., La Jolla, CA, USA).
The Mann-Whitney U-test was used to determine differences between
two groups, and analysis of variance was used to determine
differences within the groups, followed by Tukey's honestly
significant difference tests. P<0.05 was considered to indicate
a statistically significant difference.
Results
Characteristics of ADSCs and PRF
The mesenchymal stem cells used in the present study
were highly purified and had CD29-positive as well as CD31- and
CD34-negative immunophenotypes. Multiple differentiation capacities
towards osteogenic and adipogenic lineages were also confirmed
in vitro (Fig. 1A-C). The
doubling time (2.89±0.11 days) of cells cultured in the
three-dimensional (3-D) dynamic system was significantly shorter
than that of cells cultured under conventional 2-D conditions
(3.63±0.26 days; P<0.05). Fluorescence microscopy and SEM
revealed good adhesion and growth of stem cells on the beads
(Fig. 1D). In addition, the
dispersed beads began to aggregate at day 3 (Fig. 1E-a), which gradually increased at day
7 (Fig. 1E-b) and day 14 (Fig. 1E-c). After 3 weeks of culture,
following the addition of microcarriers, the cells grew into
visible cell clusters of 6–8 mm in size (Fig. 1E-d).
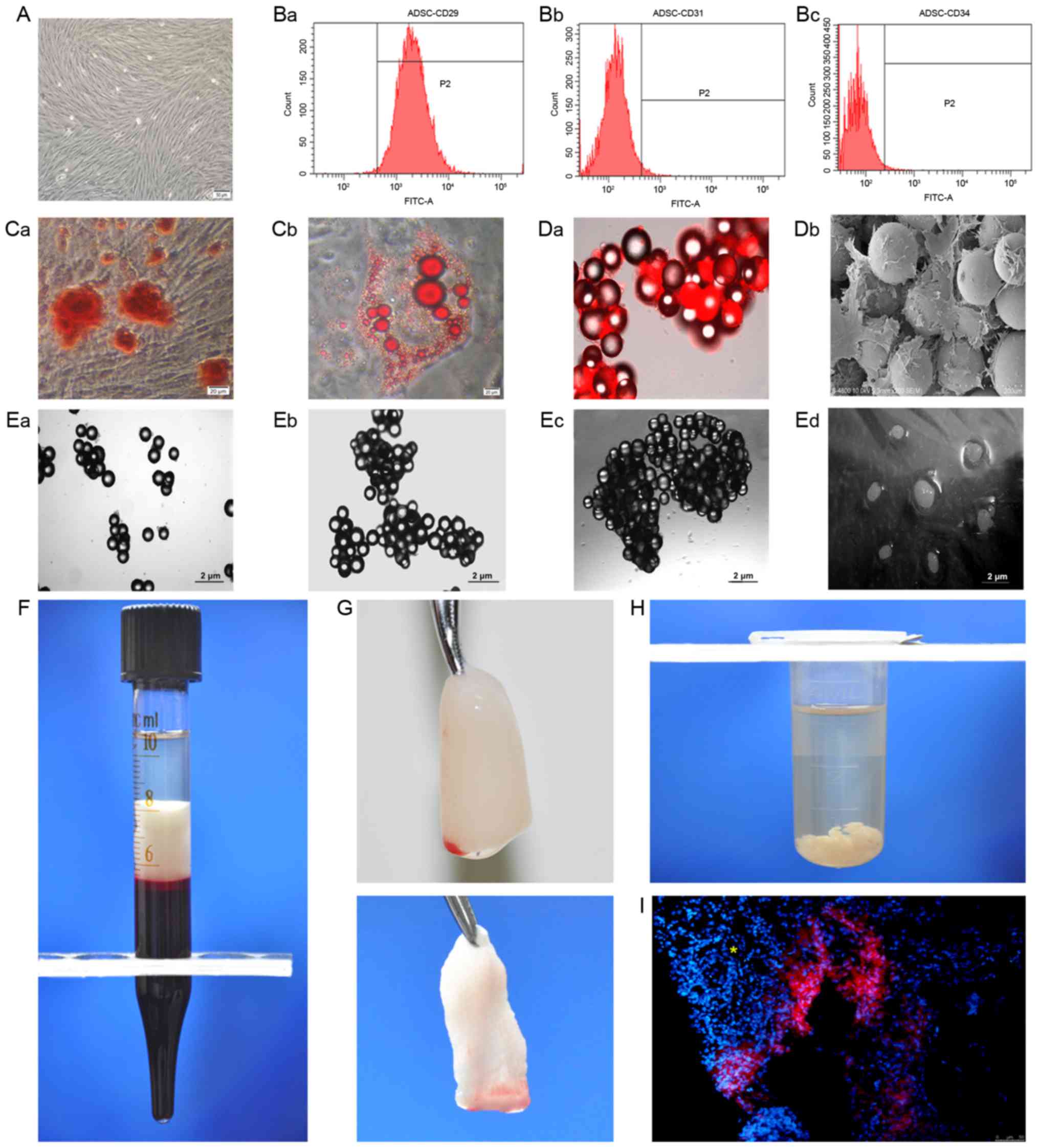 | Figure 1.Preparation of ADSCs and PRFe. (A)
Phase contrast micrograph of ADSCs at passage 2 (scale bar, 50 µm).
(B) The cells were positive for (a) the mesenchymal stem cell
marker CD29, but negative for the hematopoietic markers (b) CD31
(c) and CD34. (C) Pluripotent differentiation potentials towards
(a) osteogenic and (b) adipogenic lineages were also confirmed
in vitro (scale bar, 20 µm). (D-a) Fluorescence microscopy
and (b) scanning electron microscopy (scale bar, 200 µm) revealed
good adhesion and growth of stem cells on the microcarrier beads.
(E-a) The dispersed beads began to aggregate on day 3, with gradual
increased in aggregation on (b) day 7 and (c) day 14 of culture.
(d) The stem cell/bead mixture finally grew into visible cell
clusters of 6–8 mm in size at 3 weeks after culture. (F) A fibrin
clot is shown in the middle layer of a tube, between the red blood
cells at the bottom and the acellular plasma on top. (G) The PRF
clot was harvested (top panel) and the PRF membranes were obtained
(bottom panel). (H) Shredded PRF membranes were immersed in
microcentrifuge tubes containing acellular plasma in order to
prepare the PRFe. (I) In the IR+ADSCs treatment group, DiI-positive
stem cells were dispersed in the salivary glands of experimental
mice. The conduit structure is indicated by a yellow asterisk.
FITC, fluorescein isothiocyanate; ADSC, adipose-derived mesenchymal
stem cell; PRFe, platelet-rich fibrin extract. |
The blood samples were collected without
anticoagulants into 10-ml tubes and immediately centrifuged at
1,200 × g for 10 min. The formation of fibrin clots was visible in
the middle layer of each tube, between the red blood cells at the
bottom and the acellular plasma at the top (Fig. 1F). The PRF clot located in the middle
layer was harvested with tweezers and gently pressed onto a
membrane between two sterile pieces of gauze, using soft
compression for 10 sec in order to keep the membrane wet, and the
PRF membranes obtained were then prepared as described previously
(Fig. 1G) (21); subsequently, the membranes were cut
into fragments of a few millimeters in size and inserted into
microcentrifuge tubes containing acellular plasma (Fig. 1H). Frozen sections revealed
DiI-positive stem cells dispersed in the SGs of experimental mice
in the IR+ADSC group (Fig. 1I),
indicating the effectiveness of the intraglandular injections.
ADSCs+PRFe injection reverses
irradiation-induced reduction in body weight and saliva
production
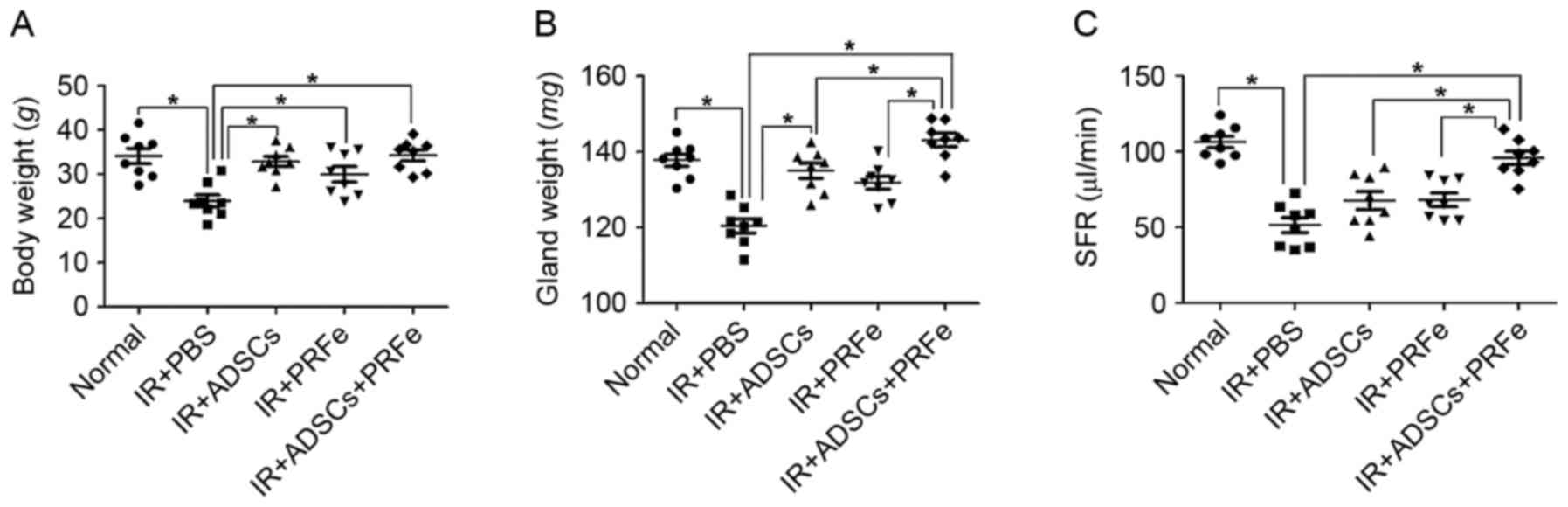
Macromorphological findings at 12 weeks post
transplantation demonstrated a significant reduction in body weight
in the irradiated untreated mice compared with that of the
non-irradiated normal mice (23.95±3.91 vs. 34.13±4.85 g, P<0.05,
Fig. 2A). In the mice injected with
ADSCs, PRFe, and ADSCs combined with PRFe, the body weight was
significantly increased to 32.86±3.23, 29.98±3.73 and 34.26±3.46 g,
respectively, compared to that in the irradiation group
(P<0.05). Moreover, the SG weight at 12 weeks post
transplantation was significantly increased in the ADSCs combined
with PRFe group when compared to that in the irradiated untreated
group (P<0.05, Fig. 2B).
To determine whether the administration of ADSCs,
PRFe, or ADSCs+PRFe improved the salivary secretory function, the
SFR was measured at 12 weeks post transplantation. The irradiated
group showed a significantly reduced ability to produce saliva
(51.51±6.97 µl/min) when compared to the normal group (106.34±10.57
µl/min, P<0.05, Fig. 2C). In the
treatment groups, the post stimulation SFR values were all
significantly increased (P<0.05 vs. irradiation group), with
those in the ADSCs+PRFe group (98.5±10.95 µl/min) being
significantly higher than those in the ADSC (76.2±14.06 µl/min,
P<0.05) and PRFe (65.3±9.47 µl/min) groups (P<0.05).
ADSCs+PRFe injection reverses
irradiation-induced changes in the micromorphology and function of
SGs
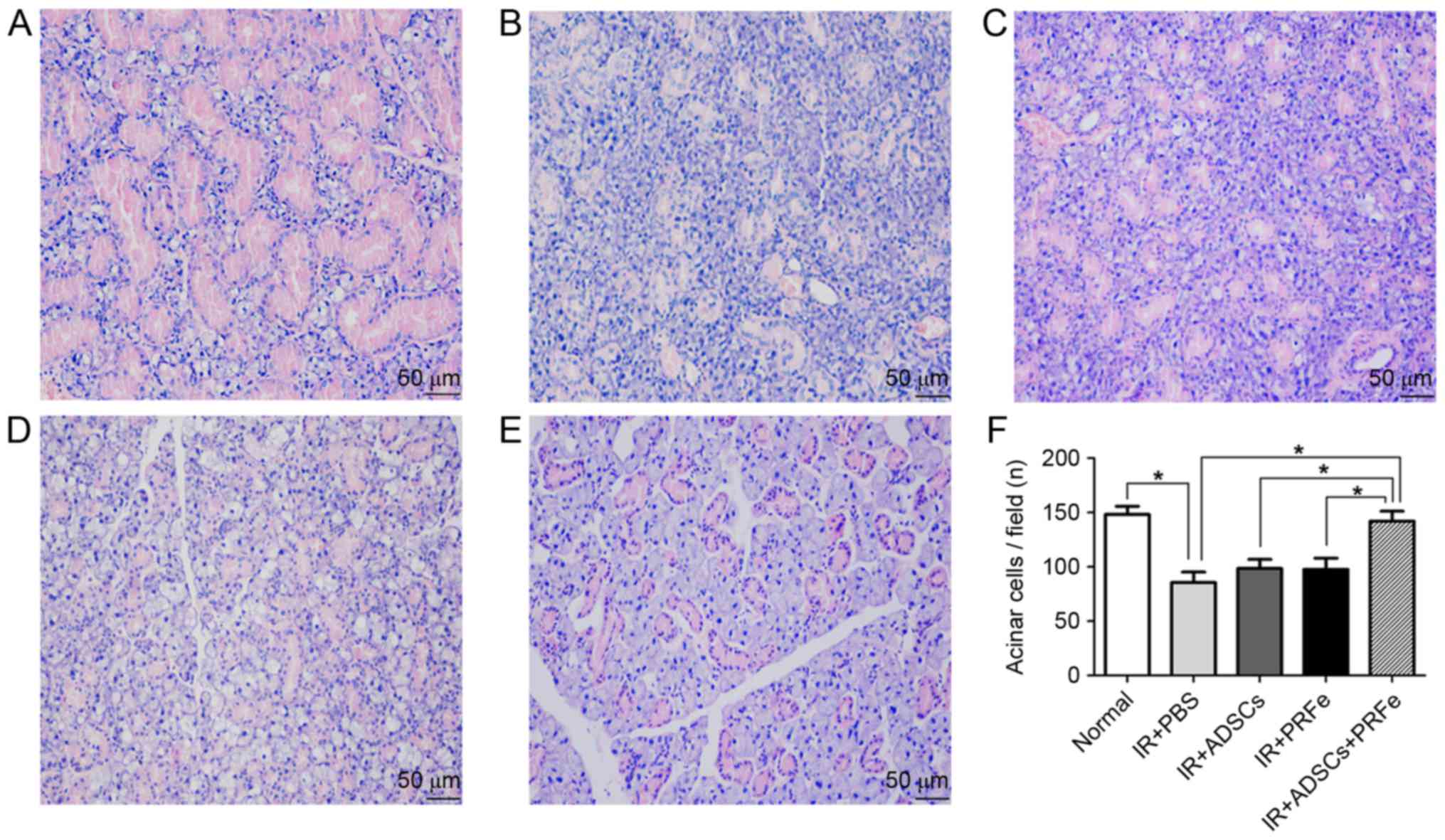
Microscopic morphological changes were visualized by
H&E staining. It was revealed that at 12 weeks post
transplantation, SGs in the combined treatment group presented with
more preserved structures and greater numbers of acini than the
irradiated and untreated SGs, while monotreatment did not markedly
affect irradiation-induced structural changes (Fig. 3A-E). Quantification analysis revealed
a significant reduction in the number of acinar cells in the
irradiated group (85.5±9.75, when compared to the normal group
(148.3.5±13.5, P<0.05), while the number was significantly
increased in the ADSCs+PRFe group (142.3±15.6, P<0.05 vs.
irradiated group) (Fig. 3F).
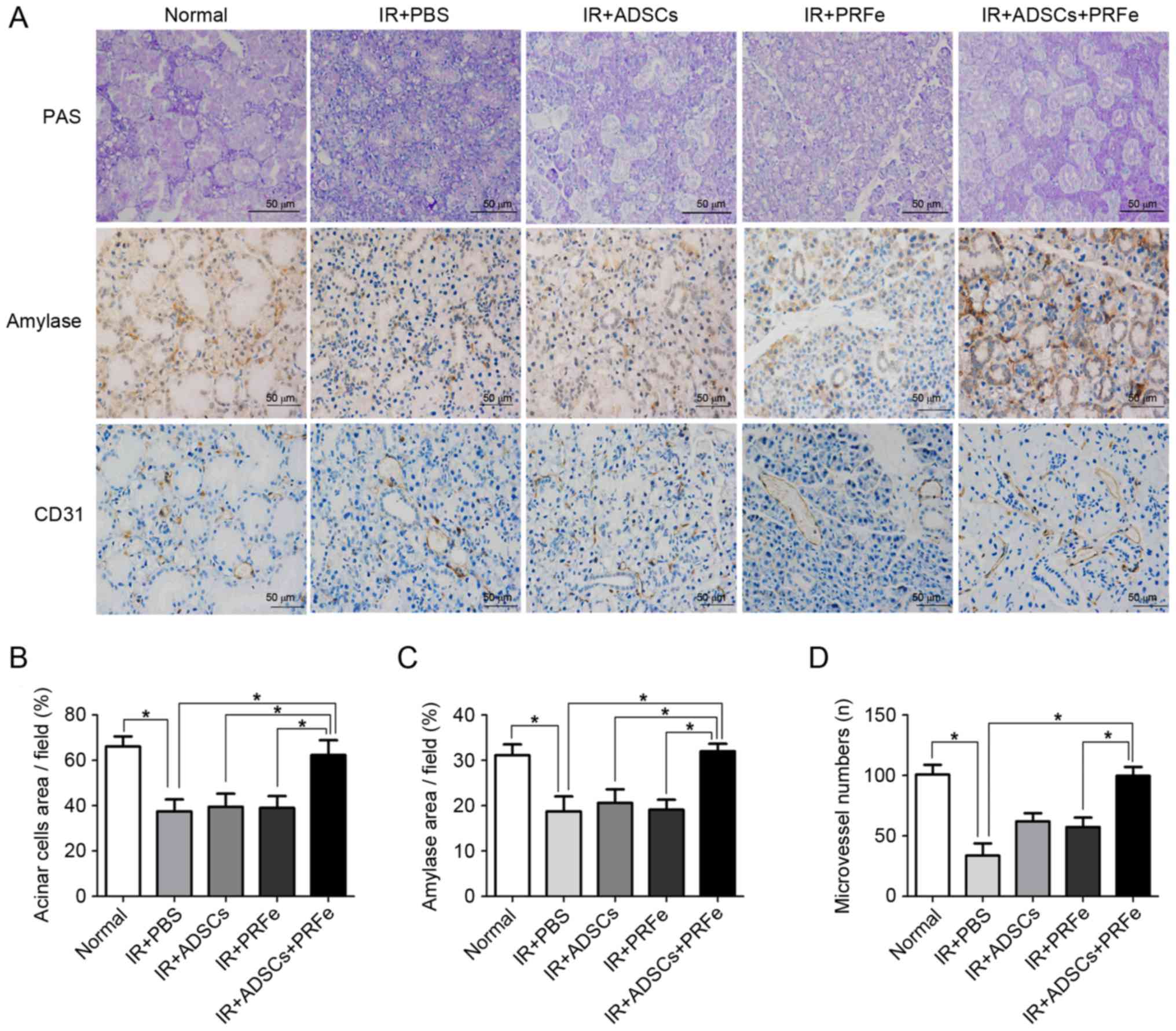
The results of the PAS staining were similar to
those observed by H&E staining; the number of PAS-positive
acinar cells was significantly reduced in the irradiated group
compared to the normal group. The ADSCs+PRFe-treated glands
presented with a higher number of mucopolysaccharide-containing
acinar cells than the irradiated glands (P<0.05, Fig. 4A and B), whereas the number of
PAS-positive acinar cells was not significantly affected by
treatment with ADSCs or PRFe alone (P>0.05, Fig. 4B).
ADSCs, PRFe, and ADSCs+PRFe were found to protect
several cell populations in the irradiated and treated groups.
Immunohistochemistry was used to measure α-amylase (AMY) production
in the SGs in order to assess acinar function, revealing
significantly lower AMY levels in the irradiated SGs compared with
those in the normal group (P<0.05, Fig. 4A and C). In addition, the SGs in the
ADSCs+PRFe treatment group showed significantly higher levels of
AMY production when compared with that in the irradiated group
(P<0.05, Fig. 4C). However,
treatment with ADSC or PRFe alone did not significantly affect AMY
levels compared with those in the irradiated group.
Microvessel density analysis was performed by CD31
immunostaining at 4 weeks post transplantation. The number of
microvessels was found to be higher in the ADSCs+PRFe-treated mice
when compared with that in the irradiation group (P<0.05,
Fig. 4D), which had the lowest
microvessel density. Moreover, a significant difference in
microvessel density was observed between the PRFe and the
ADSCs+PRFe groups (P<0.05, Fig.
4D). The difference between the irradiation group and the ADSCs
group was also significant (P<0.05, Fig. 4D).
In the normal group, TEM revealed a healthy cell
membrane with various cytoplasmic organelles, including a large
number of mitochondria and endoplasmic reticula surrounding the
cell nucleui, along with the presence of the characteristic zymogen
granules (Fig. 5A). By contrast, the
irradiation group displayed cell disintegration, karyopyknosis and
cell organelles that were evidently damaged, particularly the
mitochondria, which were swollen with liquefaction, degeneration,
and vacuolization (Fig. 5B). Of
note, the cellular ultramicrostructure was found to be intact,
except for a certain amount of mitochondrial swelling and
liquefaction along with a few areas of dilatation and degeneration
of the endoplasmic reticulum in the mice transplanted with ADSCs
(Fig. 5C). Similar findings were
observed in the PRFe group (Fig.
5D). However, no significant ultramicrostructural damage was
observed in the IR+ADSCs+PRFe group. The nuclei were regularly
shaped and clearly visible, a large number of mitochondria and
endoplasmic reticula were present in the cytoplasm, cell organelles
were intact and a large number of secretory zymogen granules were
also clearly visible (Fig. 5E).
Furthermore, normal, healthy, small blood vessels were also
observed in the IR+ADSCs+PRFe group (Fig. 5E and F).
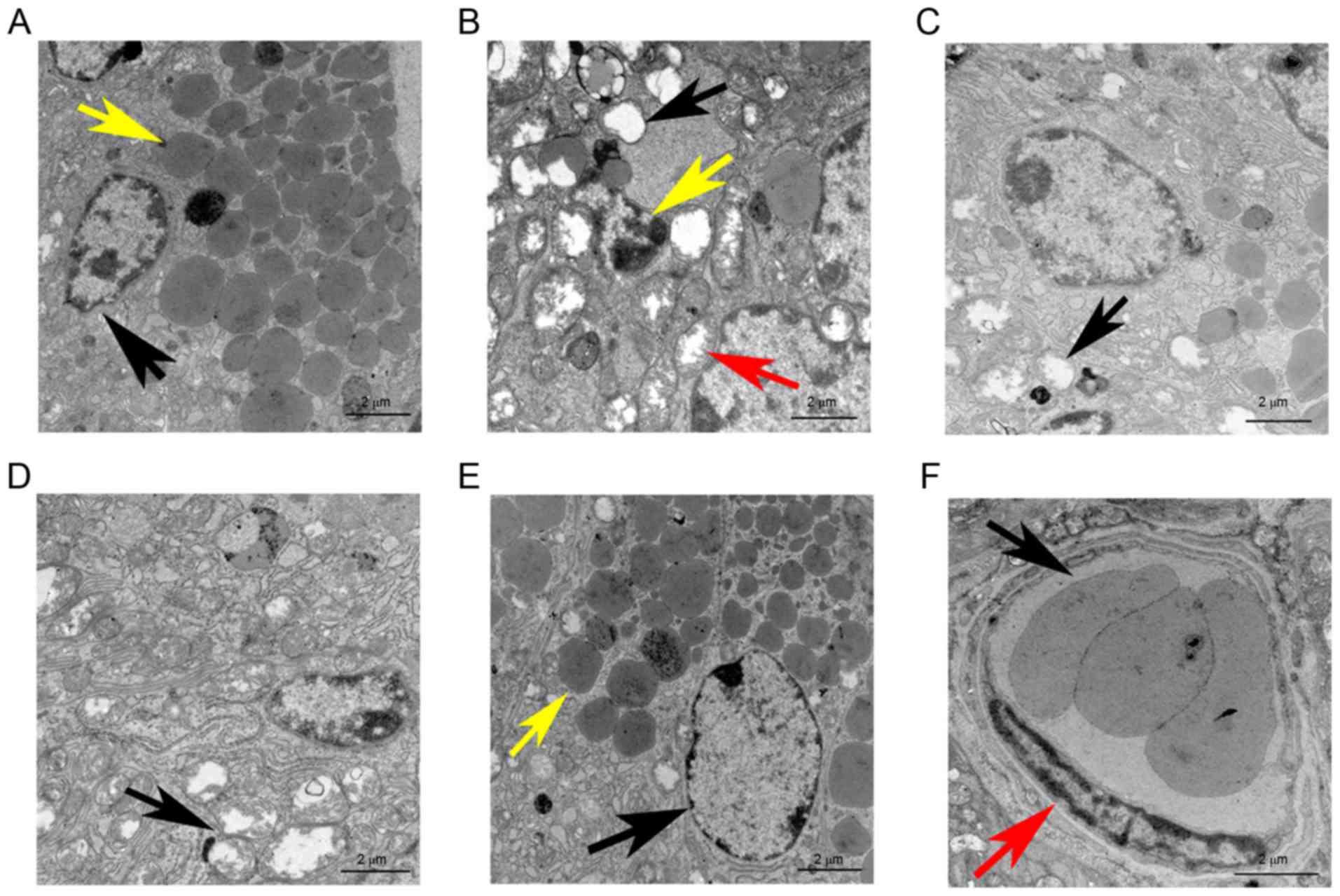 | Figure 5.TEM images of salivary glands at 12
weeks after transplantation (scale bar, 2 µm). (A) TEM revealed
well-maintained cell membranes in the normal group; the cell nuclei
were surrounded by a large number of mitochondria and endoplasmic
reticula (black arrow) and characteristic zymogen granules (yellow
arrow) were present. (B) In the IR+PBS group, cell disintegration,
karyopyknosis (yellow arrow) and damage to cell organelles,
including swelling, liquefaction degeneration and vacuolization of
the mitochondria (red and black arrows), were evident. (C) After
ADSC transplantation therapy, the cell ultramicrostructure was kept
intact, except for a certain amount of mitochondrial swelling and
liquefaction as well as a few dilated and degenerated endoplasmic
reticula (black arrow). (D) In the PRFe group, widespread
intracytoplasmic vacuolization and destruction of the mitochondrial
crest (black arrow) were seen. (E) No significant cell
ultramicrostructural damage was observed in the IR+ADSCs+PRFe
group. The cell nuclei were regular and clearly visible (black
arrow), large numbers of mitochondria and endoplasmic reticula were
visible in the cytoplasm, cell organelles were substantially intact
and immature zymogen granules were clearly visible (yellow arrow).
(F) Normal, healthy, small blood vessels were also observed in the
IR+ADSCs+PRFe group; normal endothelial cells (red arrow) and
erythrocytes (black arrow) are also indicated. PBS,
phosphate-buffered saline; IR, irradiation; ADSC, adipose-derived
mesenchymal stem cell; PRFe, platelet-rich fibrin extract; TEM,
transmission electron microscopy. |
Protective effect of ADSCs or ADSCs
combined with PRFe against irradiation-induced salivary tissue
damage
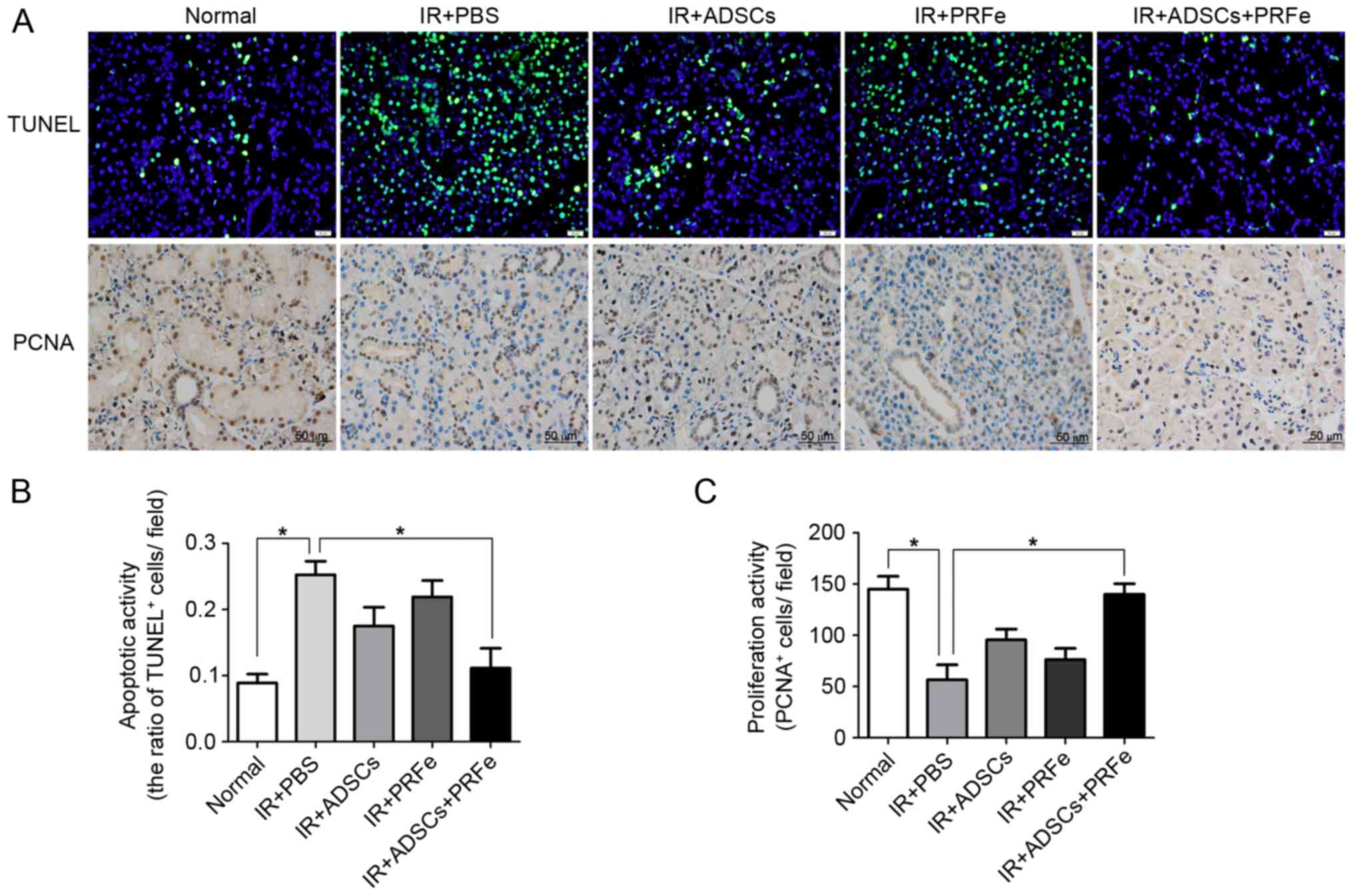
The present study further explored the mechanisms
responsible for the morphological and functional improvements in
the ADSC-, PRFe- and ADSCs+PRFe-treated irradiated SGs. Cell
apoptosis and proliferation were analyzed by TUNEL and PCNA
antibody staining, respectively. Few TUNEL-positive apoptotic cells
were observed in the normal SGs, whereas significantly higher
numbers were seen in the irradiated SGs (P<0.05, Fig. 6A and B). Administration of ADSCs
combined with PRFe significantly reduced the number of
TUNEL-positive apoptotic SG cells when compared with that in the
irradiated group (P<0.05, Fig. 6A and
B). However, no significant differences between the irradiated
group and the ADSC or PRFe monotreatment groups were noted
(P>0.05, Fig. 6B). PCNA staining
also revealed increased proliferation in the ADSCs+PRFe group when
compared with that in the irradiated group (P<0.05, Fig. 6A and C), while no significant effect
was seen in the monotreatment groups. These results suggested that
systemic transplantation of ADSCs combined with PRFe protects SGs
against radiation-induced damage via inhibition of cell apoptosis
and induction of cell proliferation.
Discussion
The present study showed that the intraglandular
transplantation of highly homogenous ADSCs combined with
heterologous PRFe was able to improve or restore the acinoductal
integrity and secretory function of irradiation-induced SGs.
Furthermore, the results from the present study indicated that
implantation of ADSCs and PRFe can i) recover saliva production and
ii) inhibit apoptosis, restore cell proliferation and regenerate
the tissue.
Secretory dysfunction of SGs is inevitable,
particularly after irradiation for the treatment of head and neck
cancer; therefore, SG regeneration post irradiation is of utmost
importance (13). Numerous studies
have been performed to address this issue, including those
involving the construction of tissue-engineered artificial SGs
(7–10), isolation and culture of adult
SG-derived stem/progenitor cells (28), reimplantation of autologous SG cells
(11,12) and stem cell therapy (13,14). In
the present study, a mouse model of irradiation-induced SG was
generated according to previously described methods (22). Three months after transplantation
with ADSCs combined with PRFe, the SFRs returned to near normal
values in the irradiated mice, while those in the irradiated group
were significantly lower. Histological examination including
H&E staining, and TEM verified that the SG tissues from mice
subjected to irradiation were significantly damaged when compared
to those in normal mice. However, the secretory functions and
tissue morphologies of the SGs in the IR+ADSCs+PRFe group were
restored and recovered 3 months post transplantation, indicating
that the combination of ADSCs and PRFe may be used for the
treatment of irradiation-damaged SGs in the clinic.
ADSCs have been widely used in numerous instances
where cell therapies (via local or systemic administration) were
indicated, including rheumatic disease and thyroiditis (29,30). In
addition, the therapeutic properties of ADSCs for the treatment of
ischemic skeletal muscle and myocardial infarction have also been
reported (31,32). The mechanisms by which ADSCs enable
the restoration of damaged tissues include cell fusion,
vasculogenesis, cell transdifferentiation and paracrine activities
(18). A study by Kojima et
al (13) confirmed that
irradiation induces inflammation and apoptosis of acinar cells in
SGs, leading to a decrease in blood flow and subsequent
hyposalivation. ADSCs not only possess the ability to secrete
angiogenic growth factors, such as vascular endothelial growth
factor (VEGF) and hepatocyte growth factor, but can also
differentiate into endothelial cells (13). These findings suggested that ADSCs
stimulate tissue repair via paracrine activities and
vasculogenesis. The results of the present study, demonstrating the
role of ADSCs in treating SGs with irradiation-induced damage, are
in accordance with those reported previously (13,18).
However, the aforementioned studies used injected cells at a
concentration of 1×106 through the tail vein, whereas in
the present study, mice were subjected to intra-SG injection of
2×105 ADSCs, as the administration of 1×106
ADSCs intraglandularly had proven fatal for the mice in preliminary
experiments. In addition, in a previous study, PRF was made from 10
ml blood so that ~2 ml of acellular plasma were obtained by blood
centrifugation (21). Therefore,
ADSCs were resuspended in 2 ml PRFe containing growth factors
derived from PRF and acellular plasma.
ADSCs have been shown to protect SGs from
irradiation-induced damage if transplanted immediately after
irradiation (13). However, in the
present study, a model of radiation-induced SG damage was developed
that was transplanted with ADSCs combined with PRFe 3 months later.
Of note, TEM, immunofluorescence, microvessel density measurements
and the TUNEL assay indicated that the combination of ADSCs and
PRFe provided an effective treatment outcome for the fixed damaged
SGs, whereas ADSCs or PRFe alone only mildly improved the tissue
morphology and functional activity. This may be attributed to the
fact that the SGs in the irradiated mice had suffered severe,
irreparable damage, which could not be restored by ADSCs or PRFe
alone. The body weights of mice in the IR+PRFe group were
increased, but the gland weight and SFR did not. These results may
be due to the protective efficacy of growth factors derived from
PRFe on mice. PRFe alleviated the irradiation-induced damage in the
mice, but failed to restore SG structure and function. However, the
administration of ADSCs combined with PRFe successfully restored
the severely damaged SGs by providing it with large quantities of
stem cells as well as growth factors, including such as
platelet-derived growth factor, transforming growth factor-beta 1,
insulin-like growth factor-1, VEGF, endothelial cell growth factor
and fibroblast growth factor-2 (19,21). As
the efficacy of ADSCs alone was similarly limited, it is indicated
that growth factors are important for regeneration, based on the
finding that ADSCs and PRFe appeared to have synergistic effects. A
previous study showed that MSCs could adopt a salivary epithelial
phenotype by mesenchymal-to-epithelial transition induced by
crosstalk between cells and the microenvironment (33). Therefore, with the addition of PRFe,
it is more likely that the vasculogenic and paracrine effects of
ADSCs, rather than their transdifferentiation potential, are
responsible for the functional restoration of the SGs via
neovascularization, as well as by increasing acinar cell survival
and decreasing apoptosis. To the best of our knowledge, the present
study was the first to demonstrate the functional restoration of
permanent irradiation-induced SG damage by intraglandular
transplantation of ADSCs combined with PRFe. However, further
studies are warranted to expound the process by which the
combination of ADSCs and PRFe mediates SG regeneration.
Research efforts to provide a safe and reliable
method for MSC infusion has led to the development of systemic cell
administration for the treatment of various types of disease
(34). Moreover, Kojima et al
(13) have demonstrated that
transplantation of allogenic ADSCs is nontoxic and safe. In the
present study, ADSCs were cultured and expanded using the 3-D cell
culture method based on the rotating cell culture bioreactor, which
is an acceptable III-D dynamic culture system for cell growth and
differentiation, as it aids in increasing the diffusion of
nutrients and oxygen, thereby promoting cell viability and
proliferation (35). In addition, in
the present study, xenogenic PRFe was successfully transplanted
into the SGs of mice without any signs of toxicity at doses of
2×105 cells. PRFe provided growth factors in combination
with the ADSCs to remedy the irradiation-induced SG damage and also
to restore the SGs' secretory function. However, while xenogenic
models are beneficial, their outcomes are controversial. PRFe
contains a variety of growth factors and has no cellular components
due to the centrifugation and filtration steps during its
preparation; therefore, it cannot cause any immunological
rejection. In the present study, xenogenicity did not hinder the
effect of ADSCs and PRFe on the SG cells. However, in the present
study, the immune evasion strategies of the xenogenic PRFe were
presumed to be associated with the immune-privileged properties of
stem cells, such as the modulation of immune cell function,
hypoimmunogenicity and the creation of a suppressive
microenvironment (36).
Several issues require addressing prior to using
ADSCs in combination with PRFe for the functional restoration of
SGs with fixed, irradiation-induced damage. First, a preclinical
animal model suitable for evaluating the therapeutic potential of
human stem cells combined with PRFe transplantation should be
established. Second, the ideal number of stem cells and the amount
of PRFe transplanted must be determined. Third, appropriate routes
of transplantation, including intravenous injection, intraglandular
transplantation by percutaneous injection and retroductal
administration via an excretory duct should be confirmed. Finally,
the basic mechanism of SG regeneration mediated by ADSCs combined
with PRFe should be elucidated. The method described in the present
study may be useful for preclinical research purposes and can be
used to protect as well as to restore the structure and secretory
function of SGs after irradiation-induced damage.
In conclusion, the present study was the first to
use a 3-D dynamic cell culture method to obtain a large number of
ADSCs in order to meet the required number of cells for
transplantation. Allogenic ADSCs combined with xenogenic PRFe,
directly transplanted into SGs with permanent, irradiation-induced
damage, were able to ameliorate salivary hypofunction. Furthermore,
the combination of ADSCs and PRFe offered an effective treatment
outcome for irradiation-induced SG damage when compared to ADSCs or
PRFe alone. Thus, the combination of ADSCs and PRFe should be
regarded as novel mode of cell-based therapy for the restoration of
irradiation-induced SG damage and hypofunction.
Acknowledgements
The present study was funded by the National Natural
Science Foundation of China (no. 31170942).
References
|
1
|
Pedersen AM, Bardow A, Jensen SB and
Nauntofte B: Saliva and gastrointestinal functions of taste,
mastication, swallowing and digestion. Oral Dis. 8:117–129. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vissink A, Burlage FR, Spijkervet FK,
Jansma J and Coppes RP: Prevention and treatment of the
consequences of head and neck radiotherapy. Crit Rev Oral Biol Med.
14:213–225. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jensen SB, Pedersen AM, Vissink A,
Andersen E, Brown CG, Davies AN, Dutilh J, Fulton JS, Jankovic L,
Lopes NN, et al: A systematic review of salivary gland hypofunction
and xerostomia induced by cancer therapies: Prevalence, severity
and impact on quality of life. Support Care Cancer. 18:1039–1060.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chambers MS, Garden AS, Kies MS and Martin
JW: Radiation-induced xerostomia in patients with head and neck
cancer: Pathogenesis, impact on quality of life, and management.
Head Neck. 26:796–807. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zheng C, Cotrim AP, Rowzee A, Swaim W,
Sowers A, Mitchell JB and Baum BJ: Prevention of radiation-induced
salivary hypofunction following hKGF gene delivery to murine
submandibular glands. Clin Cancer Res. 17:2842–2851. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Arany S, Benoit DS, Dewhurst S and Ovitt
CE: Nanoparticle-mediated gene silencing confers radioprotection to
salivary glands in vivo. Mol Ther. 21:1182–1194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang TL and Young TH: The enhancement of
submandibular gland branch formation on chitosan membranes.
Biomaterials. 29:2501–2508. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Soscia DA, Sequeira SJ, Schramm RA,
Jayarathanam K, Cantara SI, Larsen M and Castracane J: Salivary
gland cell differentiation and organization on micropatterned PLGA
nanofiber craters. Biomaterials. 34:6773–6784. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Joraku A, Sullivan CA, Yoo J and Atala A:
In-vitro reconstitution of three-dimensional human salivary gland
tissue structures. Differentiation. 75:318–324. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jean-Gilles R, Soscia D, Sequeira S, Melfi
M, Gadre A, Castracane J and Larsen M: Novel modeling approach to
generate a polymeric nanofiber scaffold for salivary gland cells. J
Nanotechnol Eng Med. 1:310082010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nanduri LS, Lombaert IM, van der Zwaag M,
Faber H, Brunsting JF, van Os RP and Coppes RP: Salisphere derived
c-Kit+ cell transplantation restores tissue homeostasis in
irradiated salivary gland. Radiother Oncol. 108:458–463. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lombaert IM, Brunsting JF, Wierenga PK,
Faber H, Stokman MA, Kok T, Visser WH, Kampinga HH, de Haan G and
Coppes RP: Rescue of salivary gland function after stem cell
transplantation in irradiated glands. PLoS One. 3:e20632008.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kojima T, Kanemaru S, Hirano S, Tateya I,
Ohno S, Nakamura T and Ito J: Regeneration of radiation damaged
salivary glands with adipose-derived stromal cells. Laryngoscope.
121:1864–1869. 2011.PubMed/NCBI
|
|
14
|
Lim JY, Yi T, Choi JS, Jang YH, Lee S, Kim
HJ, Song SU and Kim YM: Intraglandular transplantation of bone
marrow-derived clonal mesenchymal stem cells for amelioration of
post-irradiation salivary gland damage. Oral Oncol. 49:136–143.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Matsumoto S, Okumura K, Ogata A, Hisatomi
Y, Sato A, Hattori K, Matsumoto M, Kaji Y, Takahashi M, Yamamoto T,
et al: Isolation of tissue progenitor cells from duct-ligated
salivary glands of swine. Cloning Stem Cells. 9:176–190. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tran SD, Liu Y, Xia D, Maria OM, Khalili
S, Wang RW, Quan VH, Hu S and Seuntjens J: Paracrine effects of
bone marrow soup restore organ function, regeneration, and repair
in salivary glands damaged by irradiation. PLoS One. 8:e616322013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gomillion CT and Burg KJ: Stem cells and
adipose tissue engineering. Biomaterials. 27:6052–6063. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lim JY, Ra JC, Shin IS, Jang YH, An HY,
Choi JS, Kim WC and Kim YM: Systemic transplantation of human
adipose tissue-derived mesenchymal stem cells for the regeneration
of irradiation-induced salivary gland damage. PLoS One.
8:e711672013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Dohan DM, Choukroun J, Diss A, Dohan SL,
Dohan AJ, Mouhyi J and Gogly B: Platelet-rich fibrin (PRF): A
second-generation platelet concentrate. Part I: Technological
concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol
Endod. 101:e37–e44. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sunitha Raja V and Munirathnam Naidu E:
Platelet-rich fibrin: Evolution of a second-generation platelet
concentrate. Indian J Dent Res. 19:42–46. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu B, Tan XY, Liu YP, Xu XF, Li L, Xu HY,
An R and Chen FM: The adjuvant use of stromal vascular fraction and
platelet-rich fibrin for autologous adipose tissue transplantation.
Tissue Eng Part C Methods. 19:1–14. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sumita Y, Liu Y, Khalili S, Maria OM, Xia
D, Key S, Cotrim AP, Mezey E and Tran SD: Bone marrow-derived cells
rescue salivary gland function in mice with head and neck
irradiation. Int J Biochem Cell Biol. 43:80–87. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zwezdaryk KJ, Warner JA, Machado HL,
Morris CA and Hönerzu Bentrup K: Rotating cell culture systems for
human cell culture: Human trophoblast cells as a model. J Vis Exp.
pii:33672012.
|
|
24
|
Li P, Zhang Y, Wang YM, Duan CM, Hao T, Wu
BL and Wang CY: RCCS enhances EOE cell proliferation and their
differentiation into ameloblasts. Mol Biol Rep. 39:309–317. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Picken MM: Options for amyloid typing in
renal pathology: The advantages of frozen section
immunofluorescence and a summary of general recommendations
regarding immunohistochemistry methodsAmyloid and Related
Disorders. Current Clinical Pathology. Picken M, Herrera G and
Dogan A: Humana Press; New York, NY: pp. 283–293. 2015, View Article : Google Scholar
|
|
26
|
Li Z, Wang Y, Xing HY, Wang Z, Hu H, An R,
Xu H, Liu Y and Liu B: Protective efficacy of intravenous
transplantation of adipose-derived stem cells for the prevention of
radiation-induced salivary gland damage. Arch Oral Biol.
60:1488–1496. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Weidner N, Semple JP, Welch WR and Folkman
J: Tumor angiogenesis and metastasis-correlation in invasive breast
carcinoma. N Engl J Med. 324:1–8. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Baek H, Noh YH, Lee JH, Yeon SI, Jeong J
and Kwon H: Autonomous isolation, long-term culture and
differentiation potential of adult salivary gland-derived
stem/progenitor cells. J Tissue Eng Regen Med. 8:717–727. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhou B, Yuan J, Zhou Y, Ghawji M Jr, Deng
YP, Lee AJ, Lee AJ, Nair U, Kang AH, Brand DD and Yoo TJ:
Administering human adipose-derived mesenchymal stem cells to
prevent and treat experimental arthritis. Clin Immunol.
141:328–337. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Choi EW, Shin IS, Lee HW, Park SY, Park
JH, Nam MH, Kim JS, Woo SK, Yoon EJ, Kang SK, et al:
Transplantation of CTLA4Ig gene-transduced adipose tissue-derived
mesenchymal stem cells reduces inflammatory immune response and
improves Th1/Th2 balance in experimental autoimmune thyroiditis. J
Gene Med. 13:3–16. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang DZ, Gai LY, Liu HW, Jin QH, Huang JH
and Zhu XY: Transplantation of autologous adipose-derived stem
cells ameliorates cardiac function in rabbits with myocardial
infarction. Chin Med J (Engl). 120:300–307. 2007.PubMed/NCBI
|
|
32
|
Shevchenko EK, Makarevich PI, Tsokolaeva
ZI, Boldyreva MA, Sysoeva VY, Tkachuk VA and Parfyonova YV:
Transplantation of modified human adipose derived stromal cells
expressing VEGF165 results in more efficient angiogenic response in
ischemic skeletal muscle. J Transl Med. 11:1382013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Maria OM and Tran SD: Human mesenchymal
stem cells cultured with salivary gland biopsies adopt an
epithelial phenotype. Stem Cells Dev. 20:959–967. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kang SK, Shin IS, Ko MS, Jo JY and Ra JC:
Journey of mesenchymal stem cells for homing: Strategies to enhance
efficacy and safety of stem cell therapy. Stem Cells Int.
2012:3429682012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Lei X, Deng Z, Zhang H, Zhao H, Zhou J,
Liu S, Chen Q, Ning L, Cao Y, Wang X, et al: Rotary suspension
culture enhances mesendoderm differentiation of embryonic stem
cells through modulation of Wnt/β-catenin pathway. Stem Cell Rev.
10:526–538. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ryan JM, Barry FP, Murphy JM and Mahon BP:
Mesenchymal stem cells avoid allogeneic rejection. J Inflamm
(Lond). 2:82005. View Article : Google Scholar : PubMed/NCBI
|