Introduction
Allergic rhinitis currently affects 20–40% of the
global population and is increasing in prevalence (1). For patients with allergic rhinitis,
quality of life is reduced by fatigue, cognitive impairment and
many other symptoms associated with the condition (2). Asthma is an airway disease associated
with airway hyperresponsiveness, which causes wheezing,
breathlessness and chest tightening (3). The incidence of asthma is markedly
higher in developed countries, and >300 million individuals are
estimated to have asthma worldwide (4,5). Both
allergic rhinitis and asthma are chronic, inflammatory and
reversible allergic airway diseases, which share similar
pathophysiology features, including eosinophil infiltration, goblet
cell hyperplasia and sub-epithelial fibrosis (6). Moreover, the probability of coexistence
between allergic rhinitis and asthma in patients is high, and ~78%
of patients with asthma also exhibit nasal inflammation (7,8).
Allergic rhinitis and asthma (ARA) are substantial medical and
financial burdens to healthcare systems worldwide. The pathogenesis
of ARA is not well understood, and current research if focusing on
more effective treatment methods.
Transforming growth factor-β (TGF-β), so named for
its ability to induce fibroblast proliferation, is involved in cell
differentiation, developmental biology and immunological processes
(9). The TGF-β superfamily consists
of >30 distinct molecules, including TGF-β isoforms, bone
morphogenetic proteins, activins, growth factors, inhibins and
anti-mullerian hormone (10).
Activated TGF-β complexes may bind to corresponding TGF-β
receptors, which stimulates the receptors to induce signaling
cascades that regulate the expression of different target genes
involved in cell differentiation, growth and immune responses
(11,12). TGF-β1 is an isoform of
TGF-β, and is synthesized as a 390-amino-acid protein precursor
that is proteolytically processed to produce a mature peptide
containing 112 amino acids. TGF-β1 is considered to
serve a key role in regulating the immune system. In particular, it
has been documented that TGF-β1 exerts suppressive
effects on the activities of many types of immune cells (13–16). For
instance, TGF-β1 may inhibit the proliferation of
macrophages and monocytes and suppress their production of reaction
oxygen and nitrogen intermediates (14). Furthermore, TGF-β1 has
been demonstrated to affect the differentiation of B cells and
regulate the secretion of antibodies (15). In the regulation of helper T cells
(Th), previous studies observed that TGF-β1 suppressed
the action of Th1 cells and inhibited the release of interferon-γ
(IFN-γ) and tumor necrosis factor-α (TNF-α) (16,17).
In recent studies into the pathogenesis of Th1/Th2
immune response imbalances, it was indicated that allergic rhinitis
and asthma may be caused by a hypernomic immune reaction of Th2
cells mediated by various inflammatory cytokines (6,18).
Therefore, restoration of the Th1/Th2 immune balance may be a
potential therapeutic strategy. In the present study,
TGF-β1 neutralizing antibody was used to treat a mouse
model of ARA, and was compared to treatments with dexamethasone
(DXM), montelukast (MK) and budesonide (BUD). The effects of
TGF-β1 neutralizing antibody on inflammatory symptoms,
secretion of interleukin (IL)-4 and IFN-γ, regulation of regulatory
T (Treg) cells and activation of Smad2/3 signaling pathways were
also evaluated.
Materials and methods
Animals and ARA modeling
The animal experiments were approved by the
Institutional Animal Care and Use Committee of Zhejiang University
(Hangzhou, China). A total of 60 female Balb/c mice (4–6 weeks old,
weighing 18–20 g) were purchased from the Shanghai SLAC Laboratory
Animal Co., Ltd. (Shanghai, China). Mice were maintained under a
constant ambient temperature (22–24°C) and relative humidity (55%)
with a normal 12 h light/dark cycle and free access to food and
water. A mouse model of ARA was established as described previously
with minor modification (19).
Briefly, as a sensitization treatment, mice were injected with 200
µl phosphate-buffered saline (PBS) containing 40 µg ovalbumin (OVA;
0.2 mg/ml; S7951; Sigma-Aldrich, Merck KGaA, Darmstadt, Germany)
and 2 mg aluminum hydroxide every other day for 13 days (on days 1,
3, 5, 7, 9, 11 and 13). A rest phase was applied between days 14
and 20. From days 21 to 27, the mice were challenged daily with 5%
OVA through an ultrasonic nebulizer (WH-2000; Yuehua Medical
Instrument Factory Co., Ltd., Guangdong, China) for 30 min,
followed by intranasal instillation of 20 µl OVA (40 mg/ml). Mice
in the normal control group were treated with PBS alone instead of
OVA. Mice were divided into medication groups and were treated
according to the experimental design below. All mice were
sacrificed by cervical dislocation 24 h after the final
treatments.
Experimental design
Mice were divided into the following six groups
(n=10), according to a previously described method (20): i) Normal group (treated with PBS
alone); ii) ARA group (ARA without any treatment); iii)
Anti-TGF-β1 group (ARA + TGF-β1 neutralizing
antibody), iv) DXM group (ARA + DXM); v) MK group (ARA + MK); and
vi) BUD group (ARA + BUD). Mice in the anti-TGF-β1 and
DXM groups were treated with a daily intraperitoneal injection of
TGF-β1 neutralizing antibody (10 mg/kg; ab25121; Abcam,
Cambridge, UK) and DXM (0.5 mg/kg; D1756; Sigma-Aldrich; Merck
KGaA), respectively, at 30 min prior to OVA challenge for 7 days
starting on day 21. The same treatment protocol was performed on
mice in the MK and BUD groups, but with intragastric administration
of MK (5 mg/kg; Lunan Beite Pharmaceutical Co. Ltd., Jinan, China)
and nebulization of BUD (1 ml/2 ml; AstraZeneca, Cambridge, UK),
respectively.
Histological assay
On day 28, 5 mice in each group were sacrificed, and
lung and nasal mucosa tissues were collected. Sections of nasal
mucosa were isolated at a distance of 5 mm posterior to the nasal
vestibule. All tissues were fixed with 10% formalin at room
temperature overnight and embedded in paraffin. Lung and nasal
mucosa sections were prepared at a thickness of 3 µm. Lung sections
were stained with hematoxylin and eosin to assess the infiltration
of eosinophils. Periodic acid-Schiff (PAS) staining and Masson's
trichrome (MT) staining were performed on nasal mucosa sections to
visualize goblet cell hyperplasia and collagen deposition,
respectively, in the nasal mucosa. The numbers of eosinophils were
evaluated under a light microscope at ×200 magnification.
PAS-stained goblet cells and MT-stained areas were analyzed under a
microscope at ×400 magnification using ImageJ software.
Immunohistochemistry (IHC)
To assess the levels of TGF-β1 protein in
nasal and lung tissues, IHC was performed as described previously
(21). Briefly, nasal mucosa and
lung sections were incubated with mouse monoclonal
anti-TGF-β1 antibody (1:20; rabbit monoclonal antibody;
ab170874; Abcam) at 4°C overnight, followed by incubation with
horseradish peroxidase conjugated goat anti-rabbit secondary
antibody (1:200; A0208; Beyotime Institute of Biotechnology,
Haimen, China) at 37°C for 2 h. Sections were then developed with
diaminobenzidine (DAB) in accordance with instructions of a
commercial DAB Horseradish Peroxidase Color Development kit (P0202;
Beyotime Institute of Biotechnology). Positive stained cells were
counted under a light microscope at ×200 magnification.
Enzyme-linked immunosorbent assay
(ELISA)
At 24 h after the final OVA challenge, the
peripheral blood, nasal lavage fluid (NALF) and bronchoalveolar
lavage fluid (BALF) were harvested from the remaining five mice of
each group. After ligating the upper level of the trachea, the
nasal cavities were gently rinsed with 1 ml cold PBS through a
22-gauge catheter inserted into the nasopharynx. The pulmonary
alveoli were rinsed with cold PBS via a 20-gauge needle inserted
through the main bronchus. NALF and BALF were collected from the
nose and trachea, respectively. Peripheral blood, NALF and BALF
were then centrifuged at 400 × g at 4°C for 10 min, and the
supernatant was applied to ELISA plates coated with antibodies
against IL-4 or IFN-γ. The concentrations of IL-4 and IFN-γ were
measured according to the instructions of IL-4 (cat. no. BMS613TWO)
and IFN-γ (cat. no. BMS606TWO) ELISA kits (Thermo Fisher
Scientific, Inc., Waltham, MA, USA).
Flow cytometry analysis
To identify forkhead box P3 (Foxp3)+ Treg
cells and TGF-β1+ T cells, flow cytometry
analysis was performed using a flow cytometer (Accuri C6, BD
Biosciences, Franklin Lakes, NJ, USA), according to a previously
described method (22). Peripheral
blood mononuclear cells (PBMCs; 105 cells per sample)
were obtained from the isolated peripheral blood samples using
lymphocyte separation medium (Cedarlane Laboratories, Burlington,
Canada) in accordance with the manufacturer's instructions. PBMCs
were blocked with 5% BSA-PBS solution (Beyotime Institute of
Biotechnology) for 45 min at 4°C and then surface-stained with
anti-cluster of differentiation (CD)4-fluorescein isothiocyanate
(FITC; 1:20; 11-0043-82, eBioscience; Thermo Fisher Scientific,
Inc.), anti-CD25-allophycocyanin (APC, 1:20; 17-0251-82;
eBioscience; Thermo Fisher Scientific, Inc.) and anti-CD3-FITC
(1:20; ab34275; Abcam) at 4°C for 1 h. After washing with ice-cold
PBS twice, cells were fixed with 10% formalin at 4°C for 30 min and
then permeabilized with 0.5% Triton X-100 in PBS at 4°C for 30 min.
The cells were then stained intracellularly with
anti-Foxp3-phycoerythrin (PE; 1:20; 12-5773-82, eBioscience; Thermo
Fisher Scientific, Inc.) and anti-TGF-β1 (1:20;
MAB240-100; R&D Systems, Inc., Minneapolis, MN, USA) at 4°C for
1 h. Cells were incubated with Alexa Flour 555 conjugated donkey
anti-mouse secondary antibody (1:400; A0460; Beyotime Institute of
Biotechnology) to detect intracellular staining. All the antibodies
were diluted in PBS. Data were analyzed using FlowJo software
Version 7.6 (Tree Star, Inc., Ashland, OR). A minimum of 10,000
events was collected in each analysis.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the isolated nasal
mucosa tissues using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.). Reverse transcription was carried out
using a cDNA Synthesis kit (Fermentas; Thermo Fisher Scientific,
Inc.) with incubation for 60 min at 42°C. qPCR was conducted with a
SYBR Green PCR Master Mix (Fermentas; Thermo Fisher Scientific,
Inc.) in an ABI-7300 Real-Time PCR system (Applied Biosystems;
Thermo Fisher Scientific, Inc.). All procedures were conducted in
accordance with the manufacturer's instructions. GAPDH was used as
an internal control. The primer sequences used were as follows: For
TGF-β1 (accession number, NM_001312868.1), forward,
5′-CGAGAGGCAGAGATTTATCAG-3′ and reverse, 5′-ATGTGAAGATGGGCAAGAC-3′;
and for GAPDH (accession number, NM_008084.2), forward,
5′-ATCACTGCCACCCAGAAG-3′ and reverse, 5′-TCCACGACGGACACATTG-3′.
Thermocycling conditions were as follows: 95°C for 10 min, followed
by 40 cycles of 95°C for 15 sec and 60°C for 45 sec. for 40 cycles.
Data were analyzed with the ABI Prism 7500 SDS software (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The experiments were
repeated three times in triplicate. TGF-β1 mRNA
expression was calculated relative to the expression of GAPDH.
Western blot analysis
The nasal mucosa tissue samples from 5 mice in each
group were homogenized, and the tissue homogenate was disrupted in
radioimmunoprecipitation assay lysis buffer (Beyotime Institute of
Biotechnology) at 4°C for 30 min. Following centrifugation at
13,000 × g for 30 min at 4°C, the supernatant was collected and
protein concentration was determined by using a BCA protein assay
kit (Thermo Fisher Scientific, Inc.). Following normalization for
protein concentration, samples (30 µg protein per lane) were
separated by 10% SDS-PAGE. The nitrocellulose membranes (Merck
KGaA) of electrotransferred samples were block with 5% skim milk at
room temperature for 30 min and probed with primary antibodies,
then incubated with horseradish peroxidase (HRP)-conjugated
secondary antibodies at room temperature for 1 h. Immunolabeling
was detected with an enhanced chemiluminescence substrate (Thermo
Fisher Scientific, Inc.). GAPDH was used as an internal control.
The antibodies used were as follows: Anti-Smad2/3 (1:1,000; cat.
no. 8685), anti-phospho-Smad2/3 (1:1,000; cat. no. 8828; both from
Cell Signaling Technology, Inc., Danvers, MA, USA),
anti-TGF-β1 (ab170874; 1:100; Abcam), anti-GAPDH
(1:1,500; cat. no. 5174; Cell Signaling Technology, Inc.) and
HRP-labeled secondary antibodies (1:1,000; A0208; Beyotime
Institute of Biotechnology). Experiments were repeated three times.
The protein expression was quantified using ImageJ software
(version 1.6; National Institutes of Health, Bethesda, MD,
USA).
Statistical analysis
Five independent experiments were performed for each
assay, and the results were analyzed using SPSS 13.0 software
(SPSS, Inc., Chicago, IL, USA). Data were expressed as the mean ±
standard deviation and analyzed with a one-way analysis of variance
followed by a Sidak's t-test for multiple comparisons. P<0.05
was considered to indicate a statically significant difference.
Results
TGF-β1 neutralizing
antibody attenuates upper and lower airway inflammation in ARA
The effect of TGF-β1 neutralizing
antibody on inflammatory symptoms in the lungs and nasal mucosa
were investigated by histological analysis (Fig. 1A). Following sensitization and
challenges with OVA, mice with ARA exhibited a significant
infiltration of eosinophils in the bronchi (P<0.001 vs. normal
group), and significant goblet cell hyperplasia and collagen
deposition in the nasal mucosa (P<0.01 vs. normal group;
Fig. 1B-D). Groups administered with
different drug treatments (TGF-β1 neutralizing antibody,
DXM, MK and BUD) prior to OVA challenges exhibited significant
decreases in eosinophil infiltration in the lower airways
(P<0.01), and in goblet cell hyperplasia and collagen deposition
in the upper airways (P<0.05). Moreover, treatment with
TGF-β1 neutralizing antibody exerted a significantly
greater inhibitory effect on eosinophil infiltration relative to
the DXM and BUD groups (P<0.05), and on collagen deposition
relative to the MK and BUD groups (P<0.05; Fig. 1B-D). These results indicated that
TGF-β1 neutralizing antibody reduced inflammation of the
upper and lower airways in ARA mice.
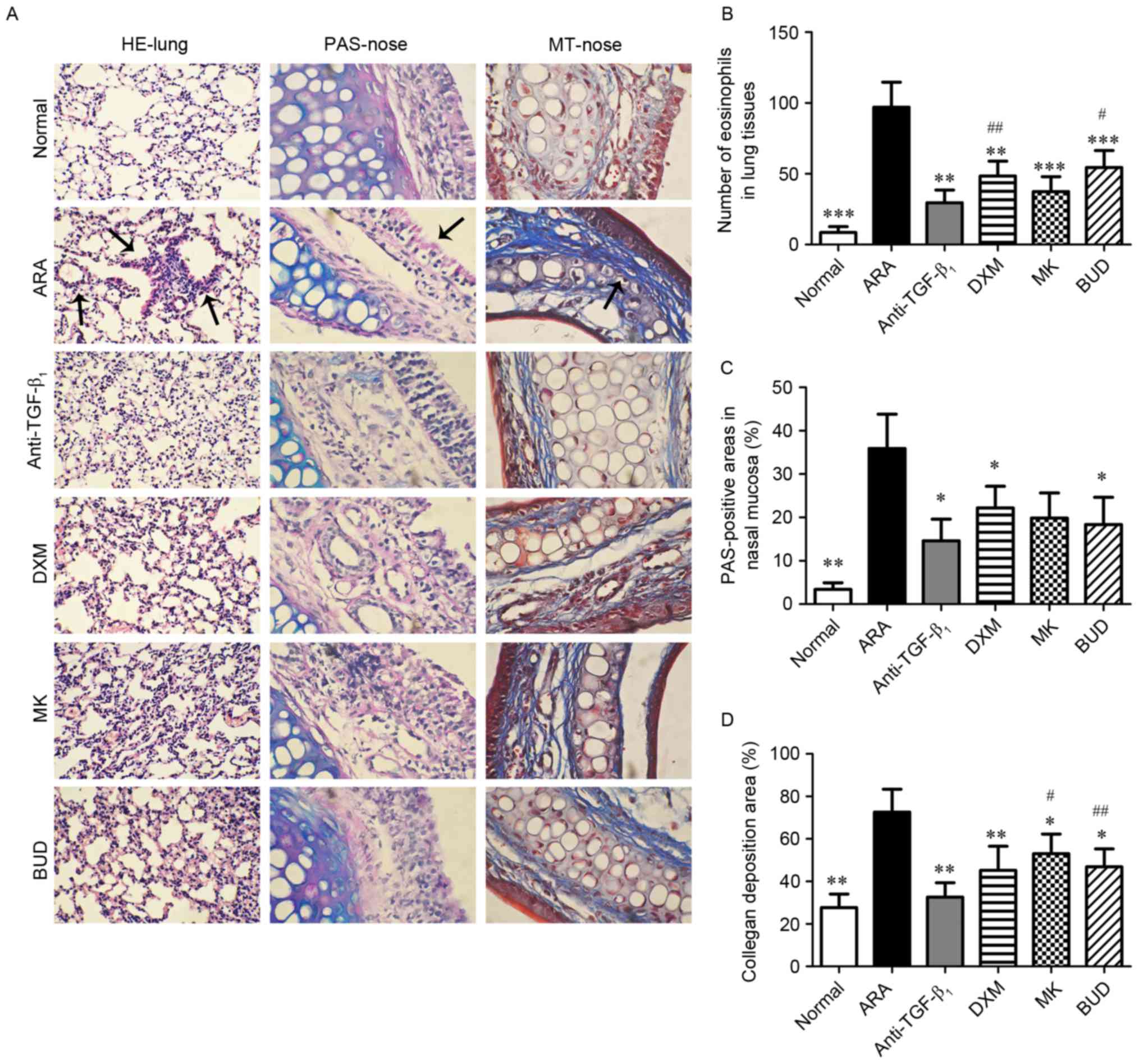 | Figure 1.Effect of drug treatments on the
symptoms of ovalbumin-induced ARA in the lung and nasal mucosa. (A)
Representative photos of H&E-stained lung tissue sections
(magnification, ×200), and PAS- and MT-stained nasal mucosa
sections (magnification, ×400) from the six experimental groups.
Stained eosinophils (H&E), goblet cells (PAS) and collagen (MT)
are indicated by the arrows. (B) Analysis of eosinophilia
infiltration in each group. (C) Analysis of goblet cell hyperplasia
in each group. (D) Analysis of positive-stained collagen areas in
each group. Data are presented as the mean ± standard deviation.
*P<0.05, **P<0.01 and ***P<0.001 vs. ARA;
#P<0.05 and ##P<0.01 vs.
anti-TGF-β1 group. ARA, allergic rhinitis and asthma;
anti-TGF-β1, tumor growth factor-β1
neutralizing antibody; DXM, dexamethasone; MK, montelukast; BUD,
budesonide; H&E, hematoxylin and eosin; PAS, periodic
acid-Schiff; MT, Masson's trichrome. |
TGF-β1 neutralizing
antibody alters levels of IL-4 and INF-γ in the NALF, BALF and
peripheral blood
The levels of cytokines secreted by Th cells in the
NALF, BALF and peripheral blood. As depicted in Fig. 2A, mice with ARA exhibited
significantly increased levels of IL-4 in the NALF, BALF and
peripheral blood (P<0.001 vs. normal group), while IL-4 levels
were significantly decreased in ARA mice post-medication
(P<0.05). The degree of IL-4 inhibition did not differ
significantly among the TGF-β1 neutralizing antibody,
DXM and BUD groups. TGF-β1 neutralizing antibody exerted
a greater inhibitory effect on IL-4 levels in NALF (P<0.001),
but not in BALF or peripheral blood. By contrast, significantly
reduced levels of IFN-γ were identified in the NALF, BALF and
peripheral blood of the ARA group (P<0.001 vs. normal group),
and the medications significantly increased IFN-γ in ARA mice
(P<0.05). Moreover, TGF-β1 neutralizing antibody
exerted a significantly greater stimulatory effect on IFN-γ levels
when compared with the other drug treatments in all three samples
(P<0.05). As IL-4 is characteristic of Th2-mediated allergic
inflammation and IFN-γ is a primary secretory product of Th1
(23), these results indicated that
TGF-β1 neutralizing antibody suppressed the action of
Th2 cells while elevating the action of Th1 cells in the nasal
mucosa, bronchia and peripheral blood.
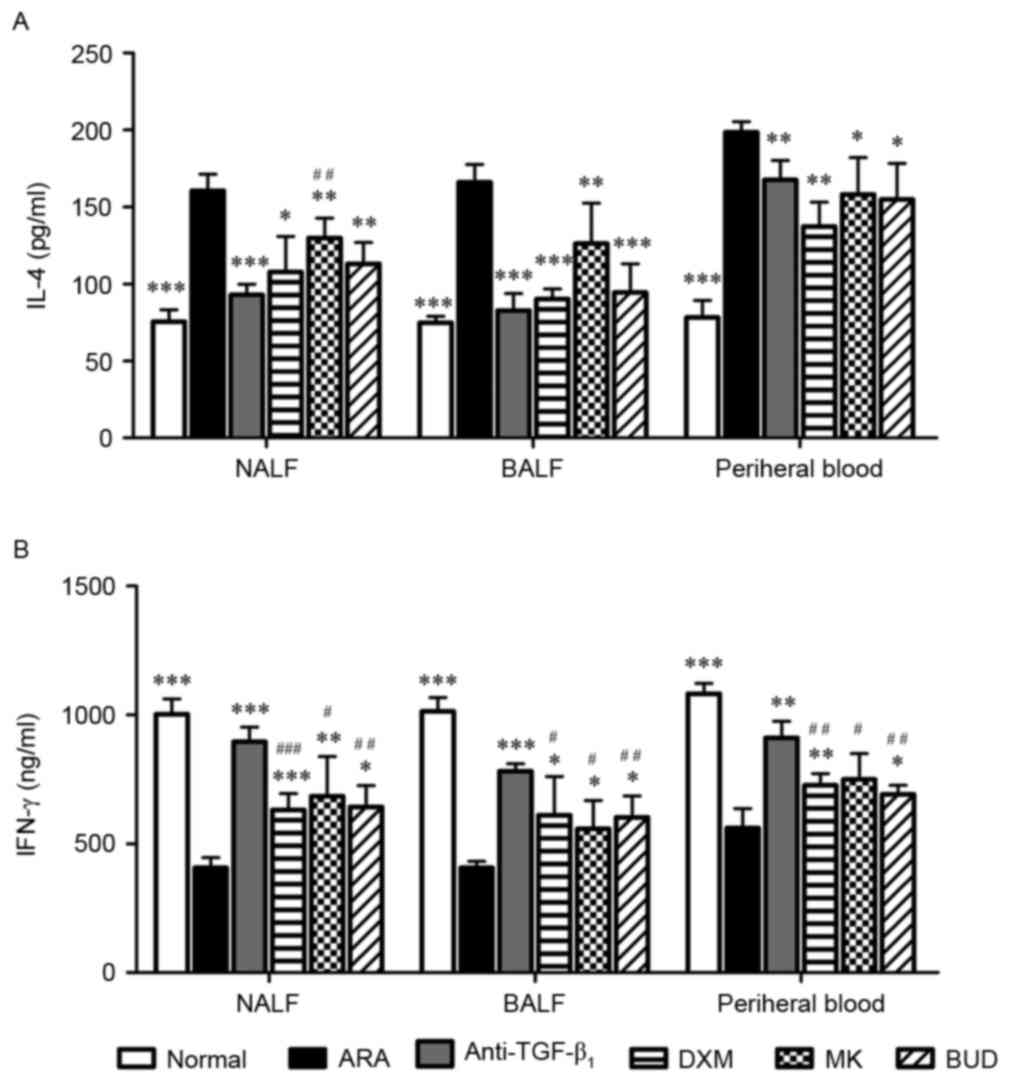 | Figure 2.Effect of drug treatments on cytokine
secretion by Th1 and Th2 cells in NALF, BALF and peripheral blood.
Assessment of (A) IL-4 and (B) IFN-γ levels in NALF, BALF and
peripheral blood in ARA mice following treatments with
anti-TGF-β1, DXM, MK and BUD. Data are presented as the
mean ± standard deviation. *P<0.05, **P<0.01 and
***P<0.001 vs. ARA; #P<0.05,
##P<0.01 and ### P<0.001 vs.
anti-TGF-β1 group. Th, helper T cells; NALF, nasal
lavage fluid; BALF, bronchoalveolar lavage fluid; IL, interleukin;
IFN, interferon; ARA, allergic rhinitis and asthma;
anti-TGF-β1, tumor growth factor-β1
neutralizing antibody; DXM, dexamethasone; MK, montelukast; BUD,
budesonide. |
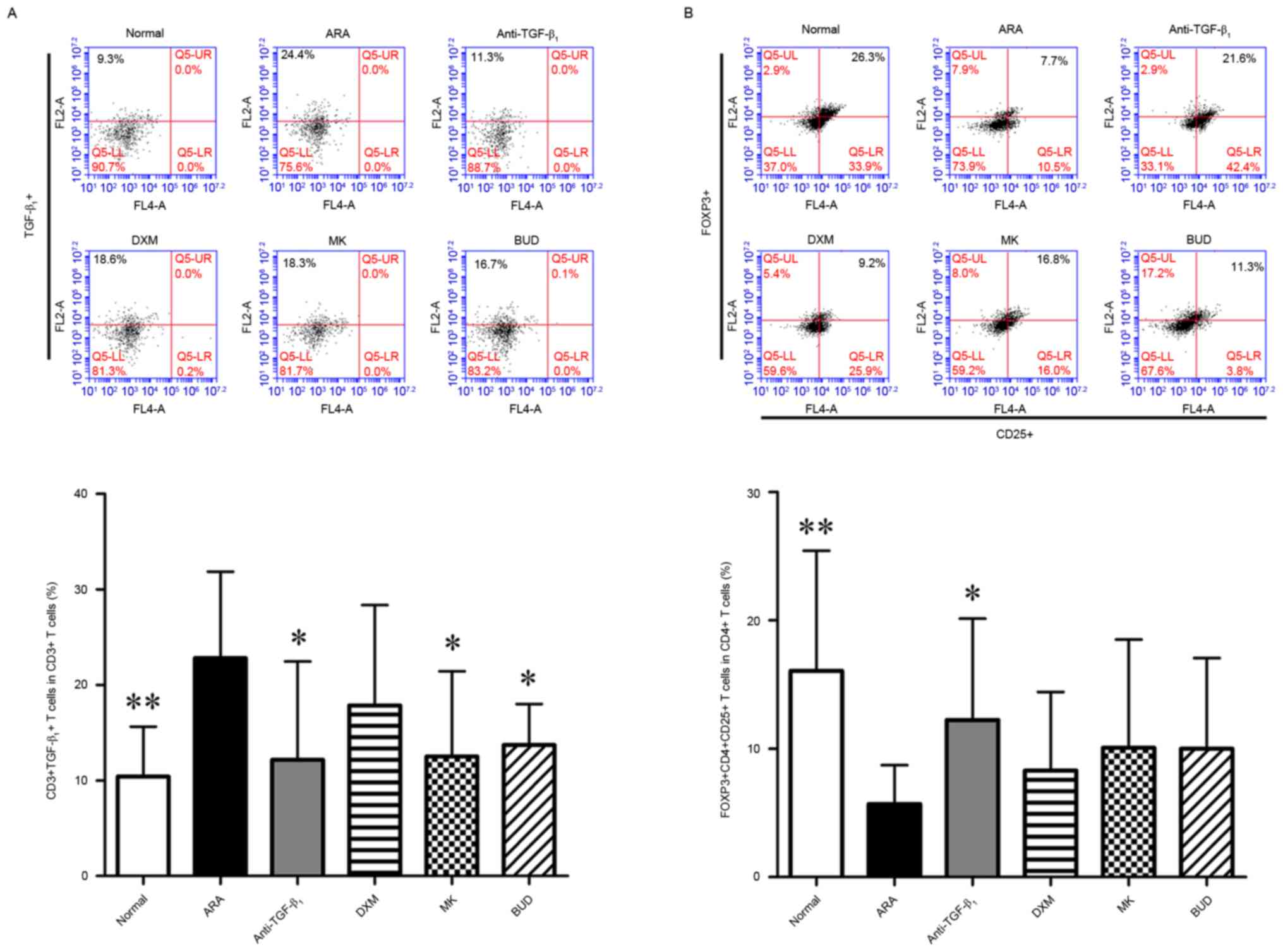
TGF-β1 neutralizing
antibody modulates Treg cell expansion in the PBMCs of ARA
mice
As TGF-β1 neutralizing antibody altered
the levels of Th1- and Th2-related cytokines, the effect of
TGF-β1 neutralizing antibody on Treg cells in ARA were
evaluated using flow cytometry. Firstly, the expression of
TGF-β1 in immunological cells was assessed. No
significant difference in the frequency of
TGF-β1+ PBMCs was observed among all six
groups (data not shown). By contrast, the frequency of
TGF-β1+ cells in the cluster of
differentiation (CD)3+ subpopulation, and
CD3+ is a marker of T cells (24) of the ARA group was significantly
elevated compared with the normal group (P<0.01; Fig. 3A). Furthermore, treatment with
TGF-β1 neutralizing antibody, MK and BUD significantly
decreased the frequency of TGF-β1+ cells in
the CD3+ subpopulation when compared with the ARA group
(P<0.05; Fig. 3A). Next, the
modulatory effects of ARA and drug treatments on Treg cells, which
were defined by the expression of CD4, CD25 and Foxp3 (25), were investigated. As depicted in
Fig. 3B, the numbers of Treg cells
were significantly decreased in the ARA group when compared with
the normal group (P<0.01). In turn, treatment with
TGF-β1 neutralizing antibody significantly increased the
frequency of CD4+ CD25+ Foxp3+
cells in the CD4+ subpopulation compared with the ARA
group (P<0.05). The numbers of Treg cells did not differ
significantly between the ARA group and remaining treatment groups.
These data suggested that TGF-β1 neutralizing antibody
inhibited the expression of TGF-β1 in T cells and
prevented Treg cell deficiency induced by ARA.
TGF-β1 neutralizing
antibody reduces the level of TGF-β1 in nasal and lung
tissues
To further analyze the function of TGF-β1
neutralizing antibody in ARA, levels of TGF-β1 in nasal
and lung tissues were assessed using IHC and RT-qPCR. As depicted
in Fig. 4A-C, levels of
TGF-β1 were significantly higher in the nasal and lung
tissues of the ARA group compared with the normal group
(P<0.001). In turn, the medications induced significant
decreases in the TGF-β1-positive areas in the nasal
mucosa and lung of ARA mice (P<0.05). Similar results of western
blotting and RT-qPCR demonstrated that the protein and mRNA
expressions of TGF-β1 were significantly reduced in the
nasal mucosa of ARA mice by the medications (P<0.01),
particularly by TGF-β1 neutralizing antibody treatment
(P<0.001; Fig. 4D and E). These
data suggested that TGF-β1 levels were significantly
higher in ARA mice, and TGF-β1 neutralizing antibody
inhibited the expression of TGF-β1 in the nasal and lung
tissues.
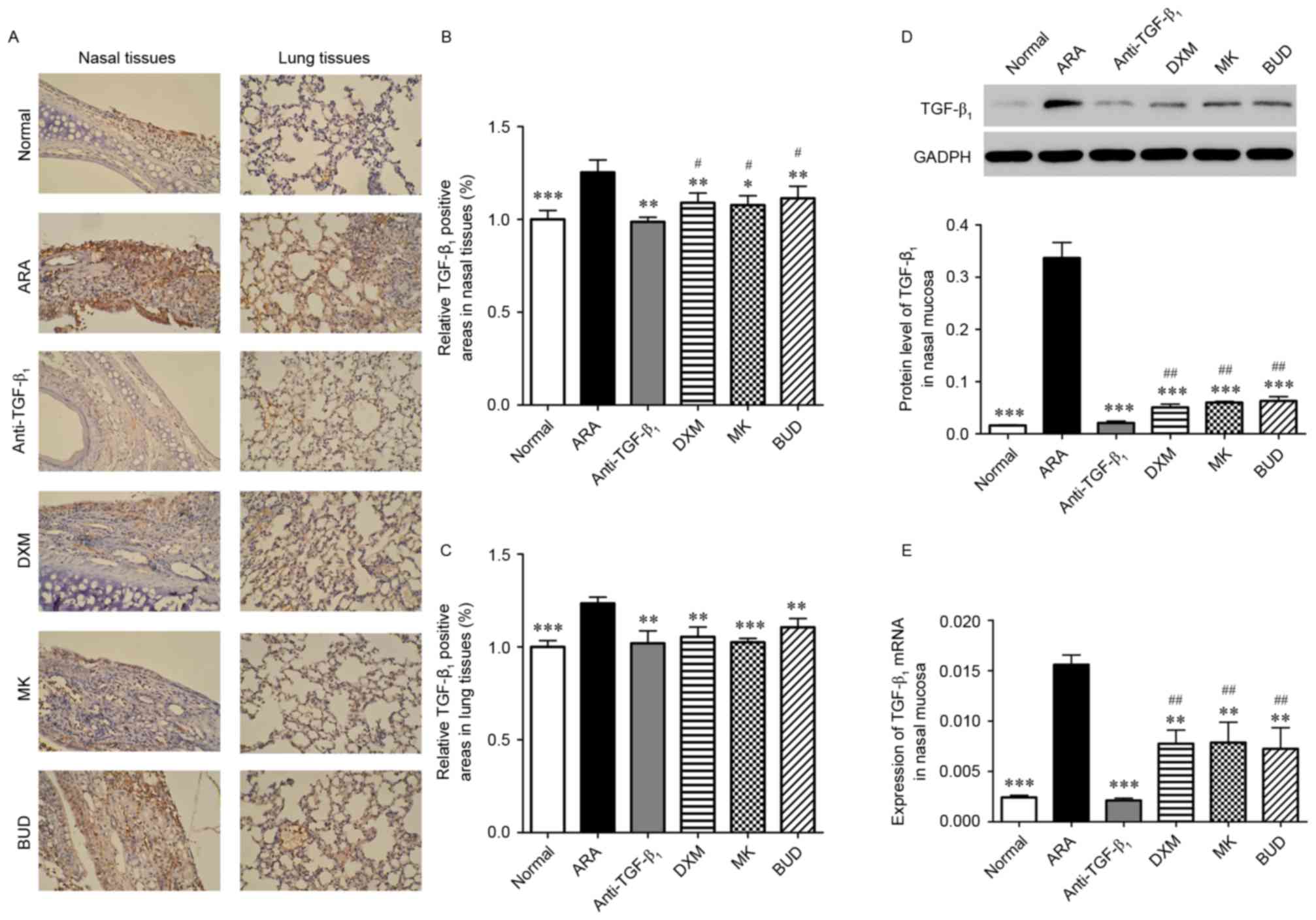 | Figure 4.Expression of TGF-β1 in
the lungs and nasal mucosa following treatment with
TGF-β1 neutralizing antibody, DXM, MK and BUD. (A-C)
TGF-β1 expression in the lungs and nasal mucosa analyzed
by immunohistochemistry (brown DAB staining). Magnification, ×200.
(D) Expression of TGF-β1 protein in the nasal mucosa
measured by western blot analysis. (E) Expression of
TGF-β1 mRNA in the nasal mucosa measured by reverse
transcription-quantitative polymerase chain reaction. Data are
presented as the mean ± standard deviation. *P<0.05, **P<0.01
and ***P<0.001 vs. ARA; #P<0.05 and
##P<0.01 vs. anti-TGF-β1 group.
TGF-β1, tumor growth factor-β1; ARA, allergic
rhinitis and asthma; anti-TGF-β1, TGF-β1
neutralizing antibody; DXM, dexamethasone; MK, montelukast; BUD,
budesonide. |
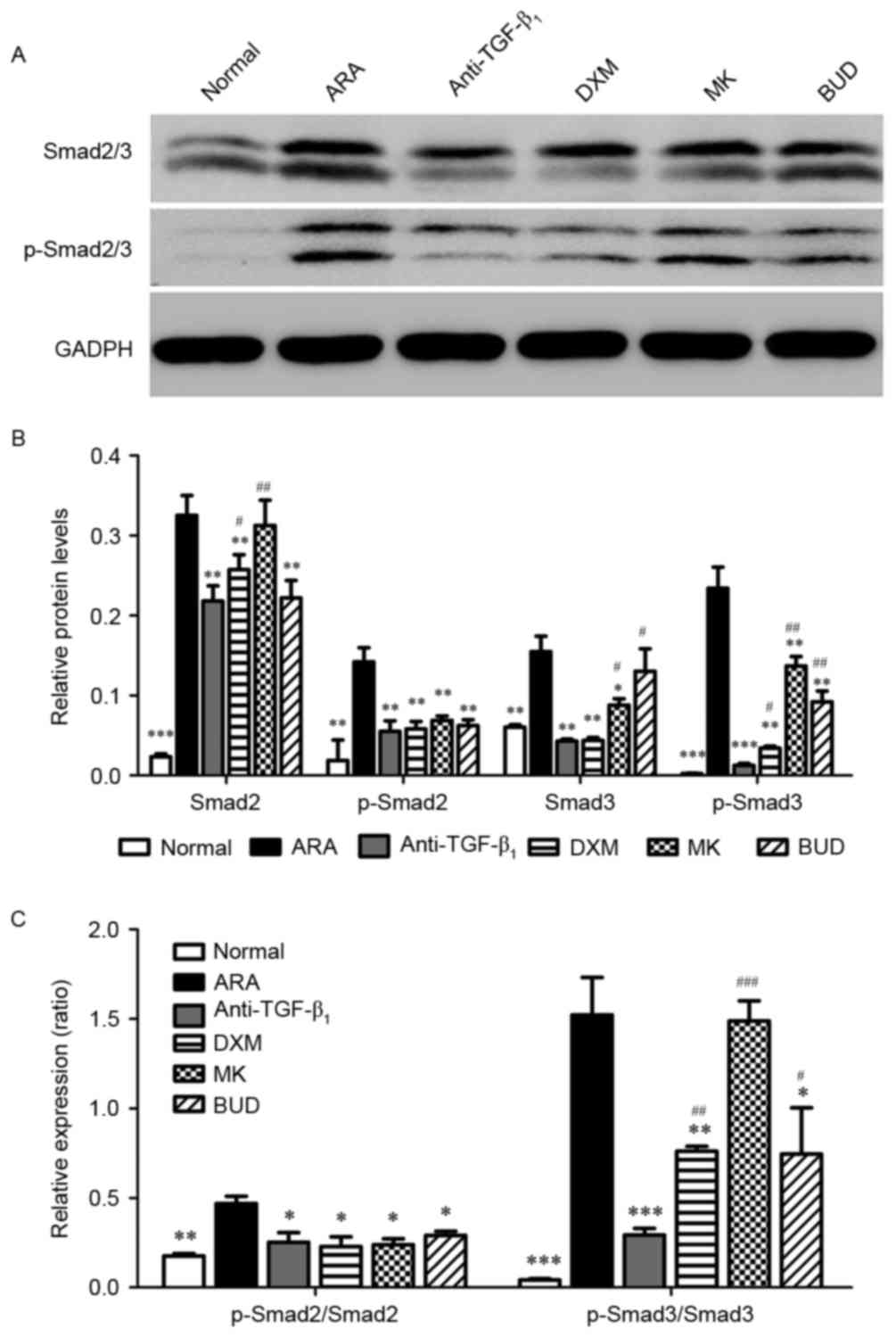
TGF-β1 neutralizing
antibody inhibits the activation of the Smad2/3 signaling
pathway
Finally, the Smad2/3 signaling pathway was
investigated using western blot analysis. The protein expression of
Smad2/3 and the phosphorylation of Smad2/3 were significantly
higher in the ARA group compared with the normal group (P<0.01).
It was observed that the protein expression of Smad2/3 and the
phosphorylation of Smad2/3 were significantly inhibited by the
medications compared with the ARA group (P<0.05; Fig. 5). Furthermore, TGF-β1
neutralizing antibody had a significantly greater inhibitory effect
on the phosphorylation of Smad3 (P<0.05) compared with the other
drug treatments and a greater inhibitory effect on Smad2 expression
than DXM and MK (P<0.05).
Discussion
TGF-β1 is as a multifunctional cytokine
involved in many biological processes. TGF-β1 is
typically considered to be an anti-inflammatory cytokine that
exerts inhibitory effects on T cells, macrophages and dendritic
cells (13). It may also lead to the
differentiation of Treg cells (16,26).
However, facilitated expression of TGF-β1 has also been
detected in the airways of some chronic respiratory diseases,
including allergic rhinitis and asthma (27,28). It
has been suggested that TGF-β1 may function in several
pro-inflammatory processes, and may serve complex roles in the
pathophysiology of ARA (13–16). The complex effects of
TGF-β1 in allergic diseases may provide novel clinical
targets. In the present study, TGF-β1 neutralizing
antibody as was used to treat allergic inflammation in upper and
lower airways of mice with ARA induced by OVA. Treatments with
three other medicines, namely DXM, MK and BUD, served as positive
control groups. Firstly, the effects of the medications on the
pathological symptoms of ARA were investigated. It was observed
that TGF-β1 neutralizing antibody exerted significant
therapeutic effects in ARA, as demonstrated by significant
inhibition of eosinophil infiltration, goblet cell hyperplasia and
collagen deposition in the upper and lower airways.
Cytokine secretion was subsequently assessed in the
NALF, BALF and peripheral blood. It was observed that treatment
with TGF-β1 neutralizing antibody significantly
decreased levels of IL-4. Moreover, relative to DXM, MK and BUD,
TGF-β1 neutralizing antibody reversed the ARA-induced
decrease in IFN-γ to a significantly greater extent. Results of
flow cytometry also suggested that TGF-β1 neutralizing
antibody inhibited the expression of TGF-β1 in T cells,
and significantly restored the ratio of Treg cells in
CD4+ T cells. Imbalance in the Th1/Th2 immune response
is considered to be involved in the pathogenesis of allergic airway
diseases (29). Secretions of Th1
cells, containing IL-2, IFN-γ and TNF, serve roles in cell-mediated
immunity and delayed-type hypersensitivity occurrence (30). However, cytokines released by Th2 may
also contribute to the initiation and maintenance of inflammation
in allergic immune responses (31,32). For
instance, IL-4 has been demonstrated to regulate the isotype
switching of IgE (33). Mutual
antagonism between Th1 and Th2 cells means that restoration of the
Th1/Th2 balance is necessary for effective ARA therapy. More
recently, Treg cell immunodeficiency has observed in allergic
diseases (34). Treg cells may
regulate the Th1/Th2 immune balance by inhibiting the function of
Th2 cells (35,36). The data in the present study
indicated that TGF-β1 neutralizing antibody restored the
Th1/Th2 immune balance and alleviated the deficiency in Treg cells
induced by ARA.
To elucidate the functional mechanism of
TGF-β1 neutralizing antibody, the expression of
TGF-β1 was measured in lung and nasal tissues, and
Smad2/3 signaling pathway activation was assessed in the nasal
mucosa by western blot analysis. Despite the anti-inflammatory
functions of TGF-β1 (16), results have suggested that the
expression of TGF-β1 is increased in ARA. Previous
studies have also demonstrated that TGF-β1 was involved
in the promotion of eosinophil infiltration and goblet cell
hyperplasia (37,38). Moreover, TGF-β1 may serve
a role in the differentiation of effector Th17 cells, which are
related to the pathogenesis of several inflammatory diseases
(39). The Smad2/3 signaling
pathways are associated with many biological processes. Rosendahl
et al (40) documented that
the TGF-β/Smad2 pathway may be activated during allergic airway
inflammation. Furthermore, the activation of Smad2/3 signaling
pathways promote IL-6 release, as a critical process in the
induction of subepithelia fibrosis and airway remodeling in asthma
(41). Activated Smad2/3 pathways
also serve roles in collagen deposition and airway remodeling
(40,42). Moreover, Antoni et al
(43) suggested that a lack of Smad3
may induce an upregulation in Foxp3 mRNA and increased numbers of
Foxp3+ cells during contact hypersensitivity responses.
In the present study, treatment with TGF-β1 neutralizing
antibody significantly downregulated TGF-β1 in the lung
and nasal tissues, and all medications with the exception of MK
inhibited the protein expression of Smad2/3 and phosphorylation of
the Smad2/3 pathway. Notably, TGF-β1 neutralizing
antibody exerted a significantly greater inhibitory effect on the
Smad3 signaling pathway when compared with the other three
medicines. These results indicated that the therapeutic effects of
TGF-β1 in ARA were based on the downregulation of TGF-β1
and inhibition of Smad2/3 signaling in the airways.
In conclusion, the present study used
TGF-β1 neutralizing antibody as a novel therapeutic
approach for the treatment of ARA. It was demonstrated that
TGF-β1 neutralizing antibody inhibited allergic
symptoms, restored the Th1/Th2 immune balance and ameliorated Treg
cell deficiencies. The functional mechanism of TGF-β1
neutralizing antibody may involve the repression of
TGF-β1 expression and Smad2/3 signaling in airway
tissues. The results of the present study suggest that the clinical
implications of TGF-β1 neutralizing antibody in ARA
therapy although the side effects have not been characterized.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 81441030), the
Research Fund for the Doctoral Program of Higher Education of China
(grant no. J20131460) and the Research Fund for the Health
Commission of Zhejiang Province, China (grant no.
JSW2013-A020).
References
|
1
|
Bousquet J: Allergic rhinitis and its
impact on asthma (ARIA). Clin Exp Allergy Rev. 3:43–45. 2003.
View Article : Google Scholar
|
|
2
|
Peters-Golden M, Gleason MM and Togias A:
Cysteinyl leukotrienes: Multi-functional mediators in allergic
rhinitis. Clin Exp Allergy. 36:689–703. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Beasley R, Keil U, von Mutius E, Pearce N,
Ait-Khaled N, Anabwani G, Anderson HR, Asher MI, Bjorkstein B, Burr
ML, et al: Worldwide variation in prevalence of symptoms of asthma,
allergic rhinoconjunctivitis and atopic eczema: ISAAC. Lancet.
351:1225–1232. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Eder W, Ege MJ and von Mutius E: The
asthma epidemic. N Engl J Med. 355:2226–2235. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gerez IF, Lee BW, van Bever HP and Shek
LP: Allergies in Asia: Differences in prevalence and management
compared with western populations. Expert Rev Clin Immunol.
6:279–289. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Galli SJ, Tsai M and Piliponsky AM: The
development of allergic inflammation. Nature. 454:445–454. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Koh YY and Kim CK: The development of
asthma in patients with allergic rhinitis. Curr Opin Allergy Clin
Immunol. 3:159–164. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bousquet J, Schünemann HJ, Samolinski B,
Demoly P, Baena-Cagnani CE, Bachert C, Bonini S, Boulet LP,
Bousquet PJ, Brozek JL, et al: Allergic Rhinitis and its Impact on
Asthma (ARIA): Achievements in 10 years and future needs. J Allergy
Clin Immunol. 130:1049–1062. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Massagué J: TGF-β signaling in development
and disease. FEBS Lett. 586:18332012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Massagué J: TGF-beta signal transduction.
Annu Rev Biochem. 67:753–791. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Camoretti-Mercado B and Solway J:
Transforming growth factor-beta1 and disorders of the lung. Cell
Biochem Biophys. 43:131–148. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Halwani R, Al-Muhsen S, Al-Jahdali H and
Hamid Q: Role of transforming growth factor-β in airway remodeling
in asthma. Am J Respir Cell Mol Biol. 44:127–133. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Li MO, Wan YY, Sanjabi S, Robertson AK and
Flavell RA: Transforming growth factor-beta regulation of immune
responses. Annu Rev Immunol. 24:99–146. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bellocq A, Azoulay E, Marullo S, Flahault
A, Fouqueray B, Philippe C, Cadranel J and Baud L: Reactive oxygen
and nitrogen intermediates increase transforming growth
factor-beta1 release from human epithelial alveolar cells through
two different mechanisms. Am J Respir Cell Mol Biol. 21:128–136.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lebman DA and Edmiston JS: The role of
TGF-beta in growth, differentiation, and maturation of B
lymphocytes. Microbes Infect. 1:1297–1304. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Letterio JJ and Roberts AB: Regulation of
immune responses by TGF-beta. Annu Rev Immunol. 16:137–161. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lúdvíksson BR, Seegers D, Resnick AS and
Strober W: The effect of TGF-beta1 on immune responses of naïve
versus memory CD4+ Th1/Th2 T cells. Eur J Immunol. 30:2101–2111.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Umetsu DT and DeKruyff RH: TH1 and TH2
CD4+ cells in human allergic diseases. J Allergy Clin Immunol.
100:1–6. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sun YQ, Deng MX, He J, Zeng QX, Wen W,
Wong DS, Tse HF, Xu G, Lian Q, Shi J and Fu QL: Human pluripotent
stem cell-derived mesenchymal stem cells prevent allergic airway
inflammation in mice. Stem Cells. 30:2692–2699. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhou K, Liu L and Shi S: Qu Feng Xuan Bi
Formula attenuates anaphylactic rhinitis-asthma symptoms via
reducing EOS count and regulating T cell function in rat ARA
models. J Ethnopharmacol. 152:568–574. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang XF, Zhu J, Geng WY, Zhao SJ, Jiang
CW, Cai SR, Cheng M, Zhou CY and Liu ZB: Electroacupuncture at
Feishu (BL13) and Zusanli (ST36) down-regulates the expression of
orexins and their receptors in rats with chronic obstructive
pulmonary disease. J Integr Med. 12:417–424. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fu QL, Chow YY, Sun SJ, Zeng QX, Li HB,
Shi JB, Sun YQ, Wen W, Tse HF, Lian Q and Xu G: Mesenchymal stem
cells derived from human induced pluripotent stem cells modulate
T-cell phenotypes in allergic rhinitis. Allergy. 67:1215–1222.
2012. View Article : Google Scholar PubMed/NCBI
|
|
23
|
Bonecchi R, Bianchi G, Bordignon PP,
D'Ambrosio D, Lang R, Borsatti A, Sozzani S, Allavena P, Gray PA,
Mantovani A and Sinigaglia F: Differential expression of chemokine
receptors and chemotactic responsiveness of type 1 T helper cells
(Th1s) and Th2s. J Exp Med. 187:129–134. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Strojnik T, Kavalar R, Barone TA and
Plunkett RJ: Experimental model and immunohistochemical comparison
of U87 human glioblastoma cell xenografts on the chicken
chorioallantoic membrane and in rat brains. Anticancer Res.
30:4851–4860. 2010.PubMed/NCBI
|
|
25
|
Buckner JH: Mechanisms of impaired
regulation by CD4(+)CD25(+)FOXP3(+) regulatory T cells in human
autoimmune diseases. Nat Rev Immunol. 10:849–859. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Geissmann F, Revy P, Regnault A,
Lepelletier Y, Dy M, Brousse N, Amigorena S, Hermine O and Durandy
A: TGF-beta 1 prevents the noncognate maturation of human dendritic
Langerhans cells. J Immunol. 162:4567–4575. 1999.PubMed/NCBI
|
|
27
|
Batra V, Musani AI, Hastie AT, Khurana S,
Carpenter KA, Zangrilli JG and Peters SP: Bronchoalveolar lavage
fluid concentrations of transforming growth factor (TGF)-beta1,
TGF-beta2, interleukin (IL)-4 and IL-13 after segmental allergen
challenge and their effects on alpha-smooth muscle actin and
collagen III synthesis by primary human lung fibroblasts. Clin Exp
Allergy. 34:437–444. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vignola AM, Chanez P, Chiappara G,
Merendino A, Pace E, Rizzo A, la Rocca AM, Bellia V, Bonsignore G
and Bousquet J: Transforming growth factor-beta expression in
mucosal biopsies in asthma and chronic bronchitis. Am J Respir Crit
Care Med. 156:591–599. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Baraniuk JN: Mechanisms of allergic
rhinitis. Curr Allergy Asthma Rep. 1:207–217. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sallusto F, Lanzavecchia A and Mackay CR:
Chemokines and chemokine receptors in T-cell priming and
Th1/Th2-mediated responses. Immunol Today. 19:568–574. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kapsenberg ML, Jansen HM, Bos JD and
Wierenga EA: Role of type 1 and type 2 T helper cells in allergic
diseases. Curr Opin Immunol. 4:788–793. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Simon HU and Blaser K: Inhibition of
programmed eosinophil death: A key pathogenic event for
eosinophilia? Immunol Today. 16:53–55. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Carballido JM, Carballido-Perrig N,
Oberli-Schrämmli A, Heusser CH and Blaser K: Regulation of IgE and
IgG4 responses by allergen specific T-cell clones to bee venom
phospholipase A2 in vitro. J Allergy Clin Immunol. 93:758–767.
1994. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chatila TA: Role of regulatory T cells in
human diseases. J Allergy Clin Immunol. 116:949–959; quiz 960.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Saito S, Nakashima A, Shima T and Ito M:
Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J
Reprod Immunol. 63:601–610. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sakaguchi S, Yamaguchi T, Nomura T and Ono
M: Regulatory T cells and immune tolerance. Cell. 133:775–787.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Makinde T, Murphy RF and Agrawal DK: The
regulatory role of TGF-beta in airway remodeling in asthma. Immunol
Cell Biol. 85:348–356. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Ouyang Y, Miyata M, Hatsushika K, Ohnuma
Y, Katoh R, Ogawa H, Okumura K, Masuyama K and Nakao A: TGF-beta
signaling may play a role in the development of goblet cell
hyperplasia in a mouse model of allergic rhinitis. Allergol Int.
59:313–319. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
He D, Wu L, Kim HK, Li H, Elmets CA and Xu
H: CD8+ IL-17-producing T cells are important in effector functions
for the elicitation of contact hypersensitivity responses. J
Immunol. 177:6852–6858. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Rosendahl A, Checchin D, Fehniger TE, ten
Dijke P, Heldin CH and Sideras P: Activation of the
TGF-beta/activin-Smad2 pathway during allergic airway inflammation.
Am J Respir Cell Mol Biol. 25:60–68. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ge Q, Moir LM, Black JL, Oliver BG and
Burgess JK: TGFβ1 induces IL-6 and inhibits IL-8 release in human
bronchial epithelial cells: The role of Smad2/3. J Cell Physiol.
225:846–854. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cho JY, Doshi A, Rosenthal P, Beppu A,
Miller M, Aceves S and Broide D: Smad3-deficient mice have reduced
esophageal fibrosis and angiogenesis in a model of egg-induced
eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 59:10–16.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Antoni M, Fyhrquist-Vanni N, Wolff H,
Alenius H and Lauerma A: Transforming growth factor-beta/Smad3
signalling regulates inflammatory responses in a murine model of
contact hypersensitivity. Br J Dermatol. 159:546–554.
2008.PubMed/NCBI
|