Introduction
Endothelial progenitor cells (EPCs) are a cell
population participating in vessel formation in physiological and
pathological processes. EPCs may be categorized as endothelial
outgrowth cells (EOCs) or endothelial colony forming cells (ECFCs)
(1). ECFCs do not express cell
surface markers found on monocytes and macrophages whereas EOCs do
(2). ECFCs proliferate in a
relatively stable and rapid manner while EOCs have limited growth
potential in vitro. Thus, ECFCs have the greater research
value for scientific research and clinical applications. EPCs have
been detected, characterized and isolated from bone marrow,
peripheral blood, human umbilical cord (UC) and UC blood (3–5). We have
previously collected ECFCs from human UC (unpublished data).
Mesenchymal stem cells (MSCs) are multipotent cells
that can be induced to differentiate to adipocytes, osteocytes,
chondrocytes and stromal cells under certain conditions; they are
traditionally obtained from sources such as bone marrow, human UC
and UC blood (6–8). They have wide applications in tissue
regeneration and cellular therapy.
The accurate identification, characterization and
isolation of EPCs and MSCs is crucial to their use. The
disadvantages of invasive isolation, limited cell numbers, and
ethical constraints when obtaining the two types of cells from
human bone marrow, organs and foetuses has increased the use of
birth-associated tissues as a source of EPCs and MSCs and for the
evaluation of stem cells, and the UC has been the most popular
source (9). The UC is a connection
between the mother and fetus. It is currently a focus of
considerable attention, not only because of the important role it
plays during pregnancy, but also because of the many kinds of stem
cells it contains (10). Usually,
the UC has two arteries and a vein enveloped in loose Wharton's
jelly (10). Since hematopoietic
stem cells were first successfully harvested from UC blood, they
have been successfully used for the treatment of hematopoietic
diseases. Stem cell populations have also been found in other parts
of the UC, including the endothelium, umbilical blood vessel
adventitia and Wharton's jelly, which is composed of stromal cells
and extracellular matrix (11).
Numerous researchers have succeeded in isolating stem cells from UC
(11,12).
In our previous studies, we successfully isolated
MSCs from whole human UC (13) and
ECFCs from the UC vein (unpublished data). In the present study, a
simple and convenient strategy to isolate two major cell types from
a single UC at the same time was developed. The two cell types were
isolated on the basis of the different sites they occupy in the UC
and the selection of suitable media for culturing. Following the
isolation of cobble-like cells from the UC vein, those cells were
identified as EPCs with a high proliferative potential. From the
Wharton's jelly, typical fibroblast-like cells were tested as
potential MSC candidates. Their characteristics were consistent
with those of MSCs (14,15). Given the extra-embryonic nature of
the two cell types, they will be of great benefit in the field of
tissue engineering and may be valuable for possible future cellular
therapeutic applications.
Materials and methods
Isolation and culture of UC-EPCs and
MSCs
Two types of stem cells in human UC were isolated on
the basis of the different sites they occupy in the UC and the
choice of suitable media for culturing them. This follows on from a
previous study, in which we successfully applied a single enzyme
approach to isolate MSCs from human UC (13), and observed that the majority of the
MSCs were obtained from the Wharton's jelly, while EPCs were
obtained from the surroundings of the umbilical cord vein. This
study was conducted in accordance with the Declaration of Helsinki,
and with approval from the Ethics Committee of the Affiliated
Hospital of Jining Medical University (Jining, China). Written
informed consent was obtained from the participants. Four
replicates were performed.
Human UCs were obtained from cesarean section births
following full-term pregnancy, and were then kept at 4°C in DF12
(Invitrogen; Thermo Fisher Scientific, Waltham, MA, USA) and
treated within 24 h. The UCs were obtained from the General
Hospital of Chinese People's Liberation Army (Beijing, China). The
mothers of all newborns provided written informed consent. At the
beginning of the experiment, samples were flushed with
phosphate-buffered saline (PBS, pH 7.0) containing 2% gentamicin
(Thermo Fisher Scientific, Inc.) twice. The umbilical vein cavity
was also flushed with PBS to remove the residual blood. Following
the ligation of one end of the cord with a surgery line, 5–10 ml
0.1% collagenase type II (Gibco; Thermo Fisher Scientific, Inc.)
was injected into the umbilical vein cavity. With dual-port
ligation, the cord was placed in PBS for 1 h at 37°C. The digested
umbilical vein was then fully washed and the digested cells were
collected by centrifugation at 724 × g for 10 min. The resuspended
cells were cultured in fibronectin-coated T75 culture
flasks (Corning Incorporated, Corning, NY, USA) containing 12 ml
complete EGM-2 medium supplemented with 10% fetal bovine serum
(FBS; Lonza, Basel, Switzerland) at a density of 2×104
cells/cm2. The cells were incubated in a humidified
incubator at 37°C under 5% CO2. Approximately 6 days
later, non-adherent cells were removed and the adherent cells were
cultured; these were considered to be EPCs.
After the digested cells had been obtained, the UC
vessels were cleared off, and the remainder of the cord was incised
into cubes 1–2 cm3 in size. The cubes were incubated in
0.1% collagenase type II for 1 h at 37°C. The digested cubes were
collected. After passing them through a 100-µm nylon strainer, the
filtered cells were centrifuged at 322 × g for 10 min. The
resuspended cells were seeded in T75 flasks containing
12 ml DF12 medium with 10% FBS, supplemented with 100 U
penicillin/streptomycin (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) at a density of 2×104 cells/cm2. All
flasks were incubated at 37°C with 5% CO2. After 3 days,
the adherent cell population was cultured. This population was
considered to comprise MSCs. Four replicates were performed.
EPCs or MSCs were passaged at a ratio of 1:3 at
subconfluence using 0.05% trypsin/EDTA (Thermo Fisher Scientific,
Inc.). For the following experiments, all cells were used at
passage 3.
Proliferative potential of UC-EPCs and
MSCs
Firstly, colony-forming assays of the two types of
cells were conducted. The colony-forming assays were performed as
previously described (13). The
numbers of colonies that contained >50 cells were counted.
Subsequently, a growth kinetics assay was conducted. Adherent cells
were harvested with 0.25% trypsin/EDTA digestion when they had
grown to 80% confluence. Mononuclear EPCs and MSCs were separately
seeded to 24-well plates at 2×104 cells/cm2
in 0.5 ml EGM-2 medium (EPCs) or DF-12 medium (MSCs), which was
replaced every 3 days. The procedure was performed as previously
described (13). Four replicates
were performed.
Finally, cell cycle analysis was performed. The two
types of cells, which were in the logarithmic phase, were fixed
with precooled 75% ethanol at 4°C for 1 h after trypsinization.
Cells were incubated with 50 µg/ml of propidium iodide (PI) for 5
min at 4°C protected from light and then analyzed using CellQuest
software (version 5.1) and a FACSCalibur system (both BD
Biosciences, Franklin Lakes, NJ, USA). Four replicates were
performed.
Reverse transcription-polymerase chain
reaction (RT-PCR) analysis of UC-EPCs and MSCs
Total RNA was extracted using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. cDNA synthesis was performed using the MLV
RT kit (Invitrogen; Thermo Fisher Scientific, Inc.) for 50 min at
37°C. In order to evaluate the expression of human von Willebrand
factor (vWF), Fms-related tyrosine kinase 1 (Flt-1), CD31, vascular
endothelial (VE)-cadherin and kinase insert domain receptor (KDR)
in the EPCs, primers (details shown in Table I) were used as previously described
(16–19). To detect the expression of fatty acid
binding protein 4 (FABP4), peroxisome proliferator-activated
receptor γ (PPAR-γ), alkaline phosphatase (ALP) and osteocalcin in
UC-MSCs, primers (details shown in Table II) were used as previously described
(13). PCR was performed using a
Mini Cycler (MJ Research; Bio-Rad Laboratories, Inc., Hercules, CA,
USA). β-actin was used as a reference. The PCR conditions were 5
min at 95°C, then 45 cycles of 95°C for 30 sec, 60–65°C for 30 sec
and 72°C for 30 sec, then a final extension for 10 min at 72°C. The
expression of genes was detected using an Applied Biosystems 7500
Real-time PCR system (Thermo Fisher Scientific, Inc.) and SYBR
Green Master mix (Beijing TransGen Biotech Co., Ltd., Beijing,
China). The amplified samples were run on a 1% agarose gel with
ethidium bromide and photographed. Four replicates were
performed.
 | Table I.Primers for human endothelial
progenitor cells. |
Table I.
Primers for human endothelial
progenitor cells.
| Gene | Forward primer (5′ to
3′) | Reverse primer (5′ to
3′) |
|---|
| CD31 |
GCTGTTGGTGGAAGGAGTGC |
GAAGTTGGCTGGAGGTGCTC |
| KDR |
CAACAAAGTCGGGAGAGGAG |
ATGACGATGGACAAGTAGCC |
| Flt-1 |
AGCAAGTGGGAGTTTGC |
AGGTCCCGATGAATGC |
| VE-Cadherin |
AAGACATCAATGACAACTTCC |
CCTCCACAGTCAGGTTATACC |
| vWF |
GAGGCTGAGTTTGAAGTGC |
CTGCTCAGCTCATCCAC |
 | Table II.Primers for human mesenchymal stem
cells. |
Table II.
Primers for human mesenchymal stem
cells.
| Gene | Forward primer (5′
to 3′) | Reverse primer (5′
to 3′) |
|---|
| FABP4 |
GTCACAGCACCCTCCT |
AGCCCACTCCTACTTCTT |
| PPAR-γ |
AAGCCAACACTAAACCACA |
GAAATGCTGGAGAAGTCAA |
| ALP |
CCTGGGAGACAAAGCAATAA |
TCCTGGGTAGCTGGGACTA |
| Osteocalcin |
GAGGGCAGCGAGGTAGTGAAG |
CCTGAAAGCCGATGTGGTC |
Flow cytometric analysis of UC-EPCs
and MSCs
Cells were harvested and washed prior to suspension
in PBS for flow cytometry. EPCs (1×105 cells/well) were
respectively labeled with eight different antibodies, namely,
fluorescence isothiocyanate (FITC) conjugated mouse anti-human CD90
(cat. no. 555595) and HLA-DR (cat. no. 555560), phycoerythrin (PE)
conjugated mouse anti-human CD31 (cat. no. 560983), CD34 (cat. no.
555822), CD73 (cat. no. 550257), CD105 (cat. no. 560839) and
vascular endothelial growth factor receptor (VEGRF) 2 (cat. no.
560872), and peridinin chlorophyll protein complex (PerCP)
conjugated mouse anti-human CD4 5 (cat. no. 564106). MSCs
(1×105 cells/well) were separately stained using the
aforementioned antibodies with the exception of PE-conjugated mouse
anti-human CD31 and VEGRF-2. Mixtures were placed in the dark for
15 min at room temperature. All of the monoclonal antibodies were
purchased from BD Pharmingen (San Diego, CA, USA). Cells were
analyzed by flow cytometry as described above. Four replicates were
performed.
Fluorescent-labeled acetylated
low-density protein (Ac-LDL) uptake and lectin binding assay of
UC-EPCs
EPCs were placed in 24-well plates at a density of
1×105 cells/cm2 in 1 ml DF-12 medium with 10
µg/ml 1,1′-dioctadecyl-3,3,3′, 3′-tetra-methylindocarbocyanine
perchlorate-labeled Ac-LDL (DiL-Ac-LDL; Molecular Probes; Thermo
Fisher Scientific, Inc.) at 37°C for 24 h. Cells were then fixed
with 4% paraformaldehyde for 20 min and incubated in PBS containing
10 µg/m plant lectin from Ulex europaeus conjugated with FITC
(FITC-UEA-1; Sigma-Aldrich; Merck KGaA) at room temperature for 1
h. Cells were observed under an inverted microscope (Olympus IX73;
Olympus Corporation, Tokyo, Japan). Four replicates were
performed.
Matrigel assay of UC-EPCs
Matrigel basement membrane matrix (BD Biosciences)
was used according to the manufacturer's recommended protocol to
evaluate the tube formation capability of the EPCs in vitro.
A 300-µl quantity of Matrigel basement membrane matrix was added to
each well of a precooled 24-well plate and incubated at 37°C for 30
min. Monolayer cells (2×104) suspended in 300 µl DF-12
medium were added to the Matrigel basement membrane matrix. After
24 h of normal culture, cells were assessed using an inverted
microscope. Four replicates were performed.
Differentiation of UC-MSCs
Adipogenic and osteogenic differentiation of the
MSCs was carried out as previously described (15). To induce adipocyte differentiation,
adipogenic medium consisting of Dulbecco's modified Eagle's medium
(DMEM; Invitrogen; Thermo Fisher Scientific, Inc.) supplemented
with 10% FBS, 100 µg/ml 1-methyl-3-isobutylxanthine (Sigma-Aldrich;
Merck KGaA), 50 µg/ml ascorbic acid (Sigma-Aldrich; Merck KGaA) and
10−6 M dexamethasone (Sigma-Aldrich; Merck KGaA) was
used. After 21 days, the cells were fixed in 10% formalin (Merck
KGaA) for 30 min, athen stained with fresh 0.6% Oil Red O solution
(Sigma-Aldrich; Merck KGaA). DMEM supplemented with 10% FBS,
7.0×10−5 M β-glycerophosphate (Sigma-Aldrich; Merck
KGaA), 2.0×10−6 M ascorbic acid, and 10−8 M
dexamethasone was used to induce osteogenic differentiation. After
14 days, the cells were fixed in 10% formalin (Merck KGaA) for 30
min, then stained with fresh 2% Alizarin Red (Sigma-Aldrich; Merck
KGaA). Four replicates were performed.
Results
Isolation and culture of UC-EPCs and
MSCs
Two types of stem cells were isolated from human UCs
using a convenient protocol in the present study. After being
cultured for 3–7 days, a few cells from the umbilical vein started
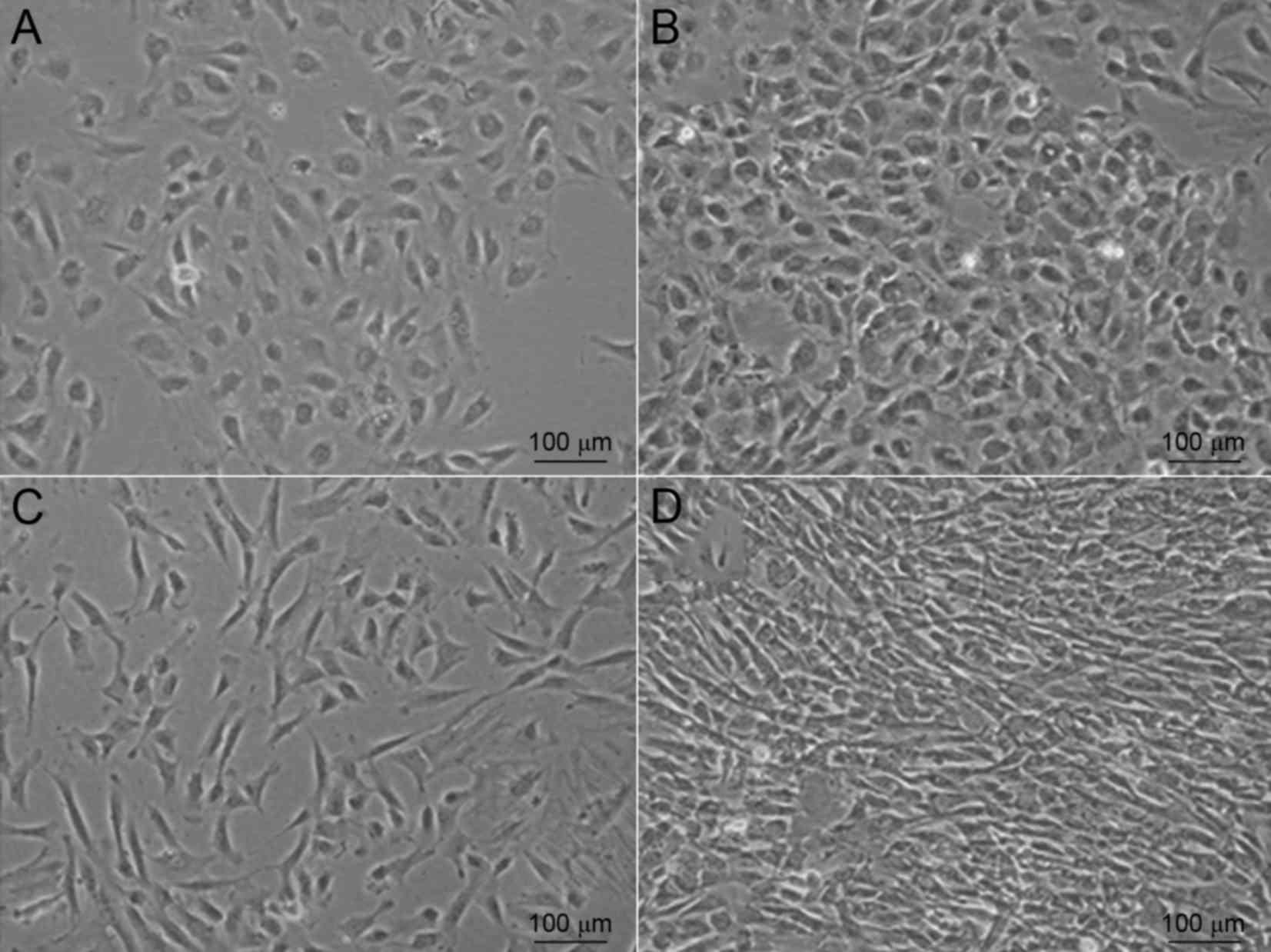
to adhere to the well (Fig. 1A).
These appeared to have a typical cobblestone appearance when
observed under a microscope. After 7–10 days, the cells became
confluent and exhibited colony growth (Fig. 1B). When these cobblestone-like cells
had reached >80% confluence in the T75 flask,
2.5×106 cells (n=15) were obtained. By contrast, primary
cells from Wharton's jelly required a shorter time to adhere to the
well. Adherent cells were observed within 24–48 h (Fig. 1C). After 2–3 weeks, the cell
morphology changed to become more homogeneous, as the
fibroblast-like cells gradually increased while the polygonal cells
decreased (Fig. 1D).
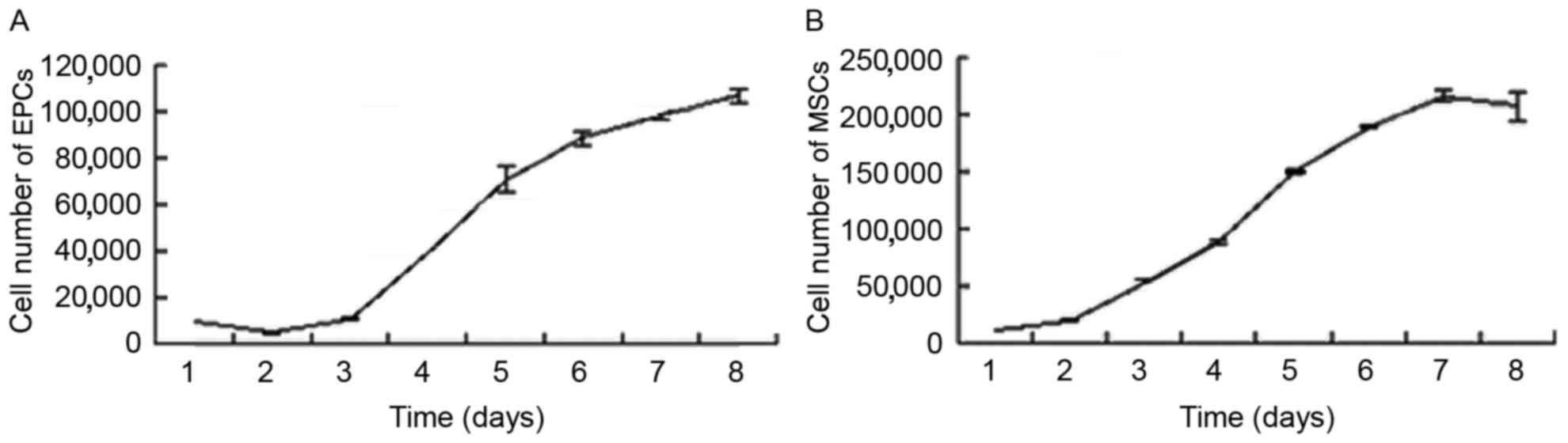
To quantitatively evaluate the proliferative
potential of the cells, three indicators were examined: Growth
kinetics, cell cycle and colony-forming ability. The growth curves
for the EPCs and MSCs were consistent with the basic law of cell
growth. All cells maintained a normal growth state. The EPC
doubling time was calculated as 43.55±5.22 h (Fig. 2A) in the logarithmic growth phase,
while the doubling time of MSCs was calculated as 24.03±0.27 h
(Fig. 2B). The DNA content of the
MSCs and EPCs was analyzed to evaluate the cellular cycle using PI.
Flow cytometric analysis demonstrated that the majority of the EPCs
and MSCs were in the G0/G1 phase, accounting for 75.58 and 85.35%
of the whole cell population, respectively. The results of cell
cycle analysis for EPCs and MSCs were consistent with their
stemness potencies. Moreover, the capacity to form colonies is one
of the characteristics of stem cells. In the colony-forming
experiment, EPC colony counts were ~36 per 1,000 single cells
seeded on the cell culture dishes, whereas MSC colony counts were
~55 per 1,000 single cells. Thus, EPCs and MSCs both underwent
relatively stable and rapid proliferation in vitro; however,
the proliferation capacity of MSCs was greater than that of
EPCs.
Flow cytometric analysis of UC-EPCs
and MSCs
Results of the flow cytometric analysis for EPCs
showed that cells were strongly positive for CD31, CD73, CD105 and
VEGFR-2, and weakly expressed the hematopoietic lineage CD34 (which
decreased during passaging), but they were negative for CD45, CD90
and HLA-DR (Fig. 3A). When cell
surface expression antigens of MSCs were analyzed, flow cytometry
showed that these cells were positive for CD73, CD90 and CD105, and
negative for CD34, CD45 and HLA-DR (Fig.
3B).
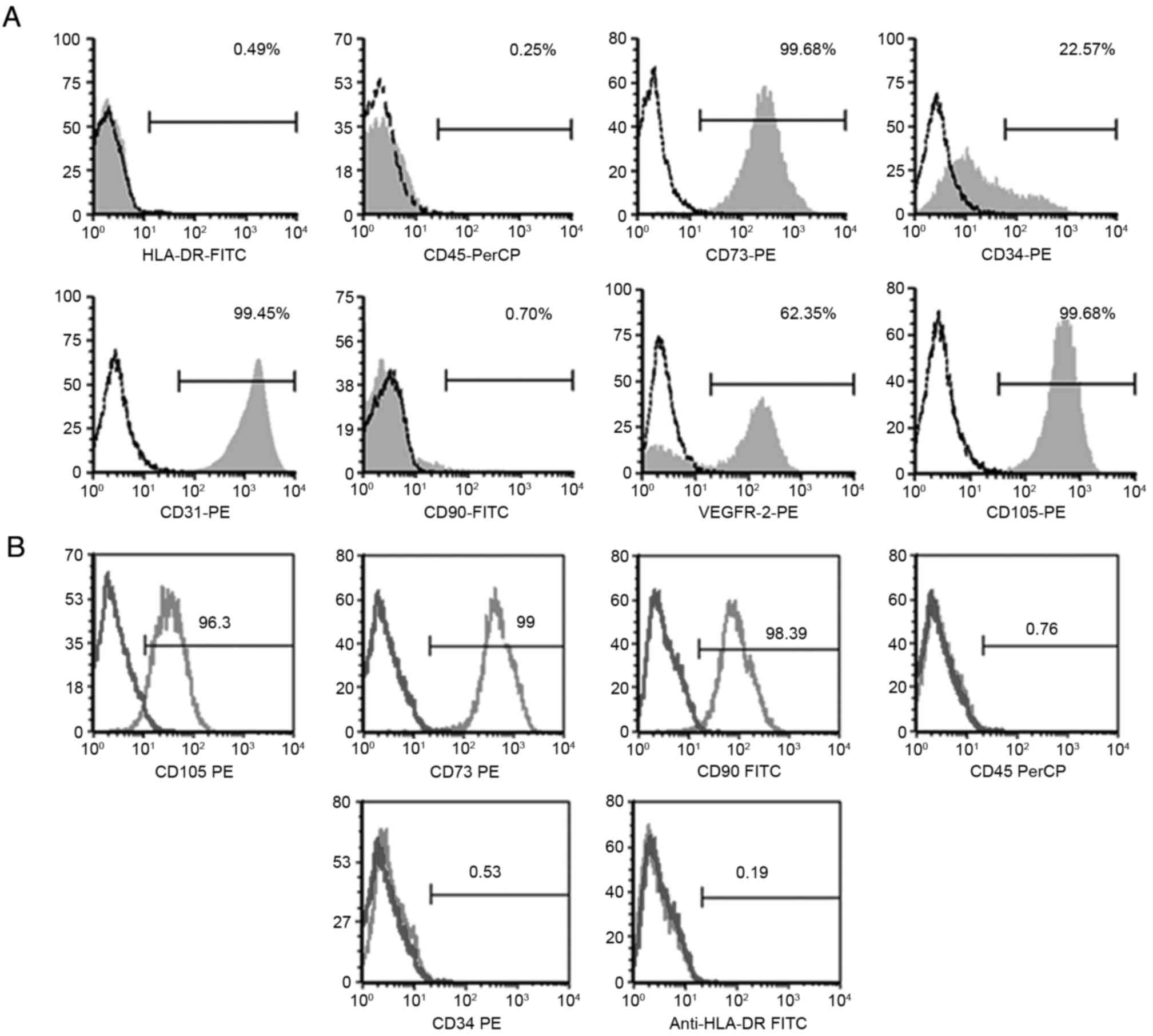 | Figure 3.Human umbilical cord cell phenotypes
as determined by flow cytometric analysis. Phenotypes of (A) EPCs
and (B) MSCs. The EPCs were positive for CD31, CD34, CD73, VEGFR-2
and CD105 and negative for CD45, CD90 and HLA-DR. The MSCs were
positive for CD73, CD90, CD105, and negative for CD34, CD45 and
HLA-DR. EPC, endothelial progenitor cell; MSC, mesenchymal stem
cell; VEGFR-2, vascular endothelial growth factor receptor 2. |
Identification of UC-EPCs
Total RNA of the UC-EPCs was obtained for RT-PCR
analysis. The cells were characterized using RT-PCR to detect the
expression of cell markers (Fig. 4).
The endothelial progenitor cell/endothelial cell RNAs vWF, Flt-1,
CD31, VE-cadherin and KDR were all expressed by the UC-EPCs. The
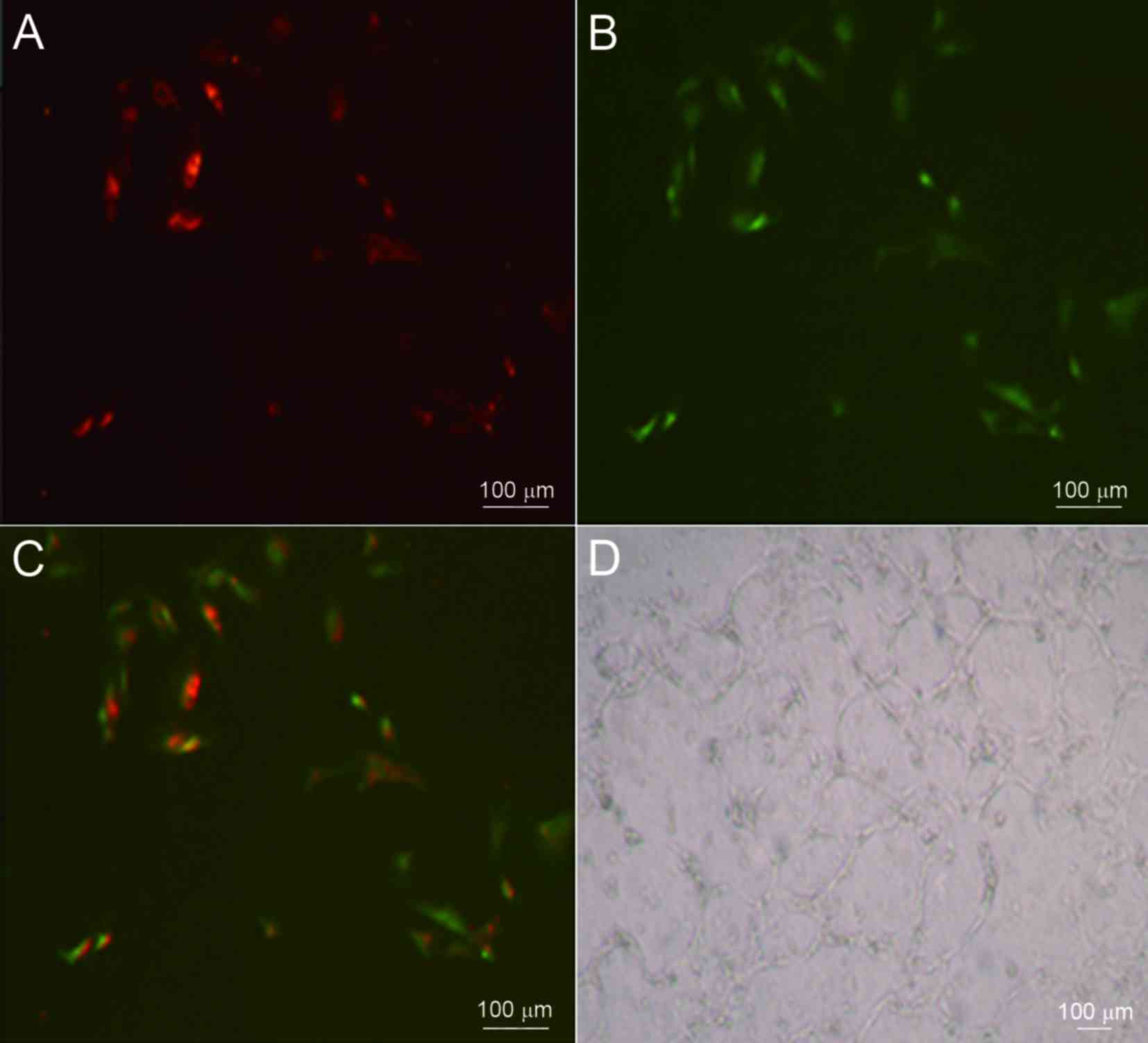
expression of GAPDH was used as a reference (Fig. 4). These adherent cells were capable
of taking up DiL-ac-LDL and binding to FITC-UEA-l (Fig. 5A-C). When these EPCs were seeded on a
Matrigel basement membrane matrix in order to investigate whether
they had true endothelial cell potential, the formation of vascular
tube-like structures was observed after 24 h (Fig. 5D).
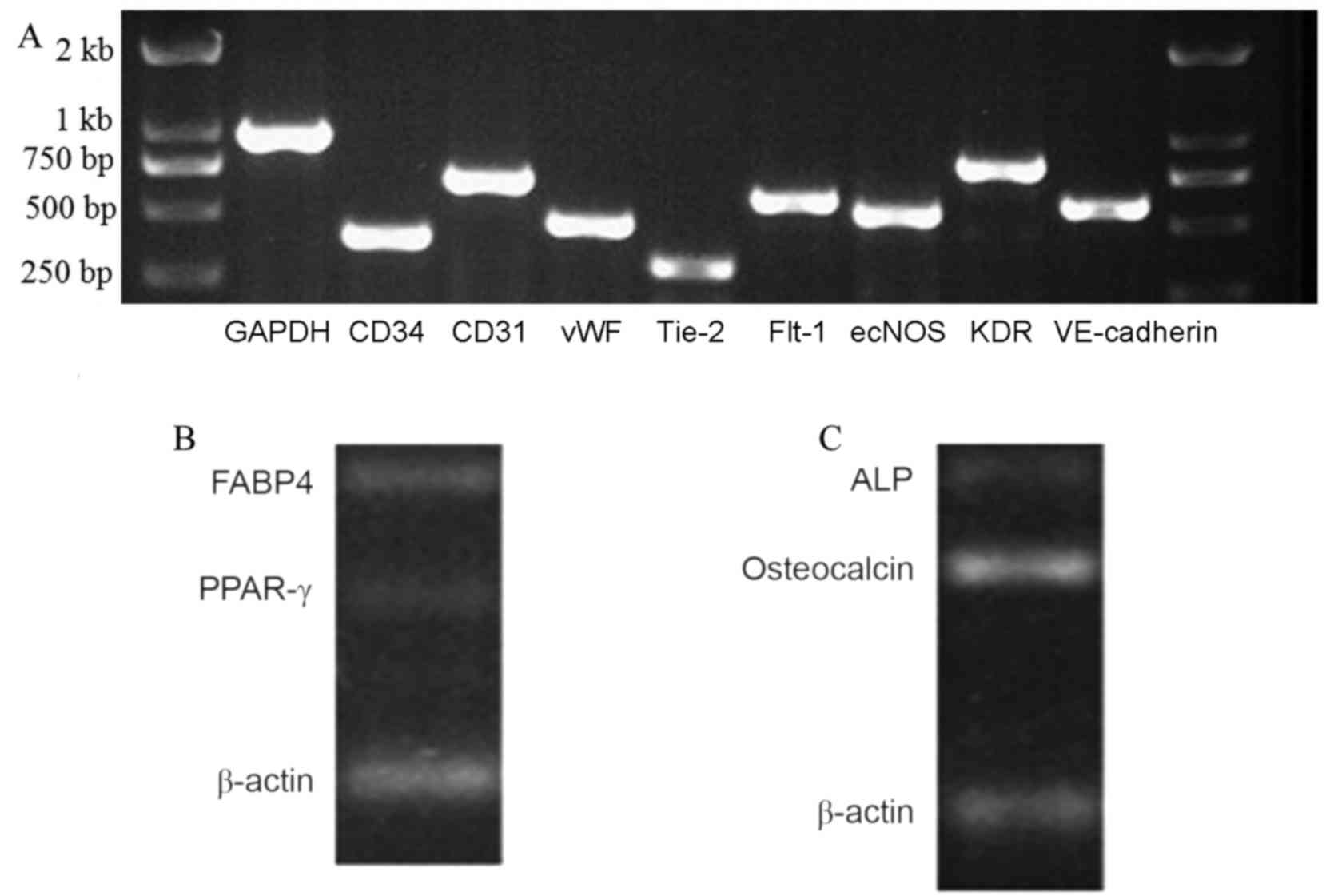 | Figure 4.Reverse transcription polymerase chain
reaction analysis of EPCs and differentiated MSCs from human
umbilical cord. (A) EPC/endothelial cell RNAs vWF, Flt-1, CD31,
VE-cadherin and KDR were expressed by the UC-EPCs. The expression
of GAPDH was used as a reference. (B) These cells expressed FABP4
and PPAR-γ RNA. (C) Osteogenic medium was used to induce osteogenic
differentiation. These cells expressed ALP and osteocalcin RNA.
EPC, endothelial progenitor cell; MSC, mesenchymal stem cell; vWF,
von Willebrand factor; Flt-1, Fms-related tyrosine kinase 1; KDR,
kinase insert domain receptor; VE, vascular endothelial; ecNOS,
endothelial constitutive nitric oxide synthase; GAPDH,
glyceraldehyde-3-phosphate dehydrogenase. |
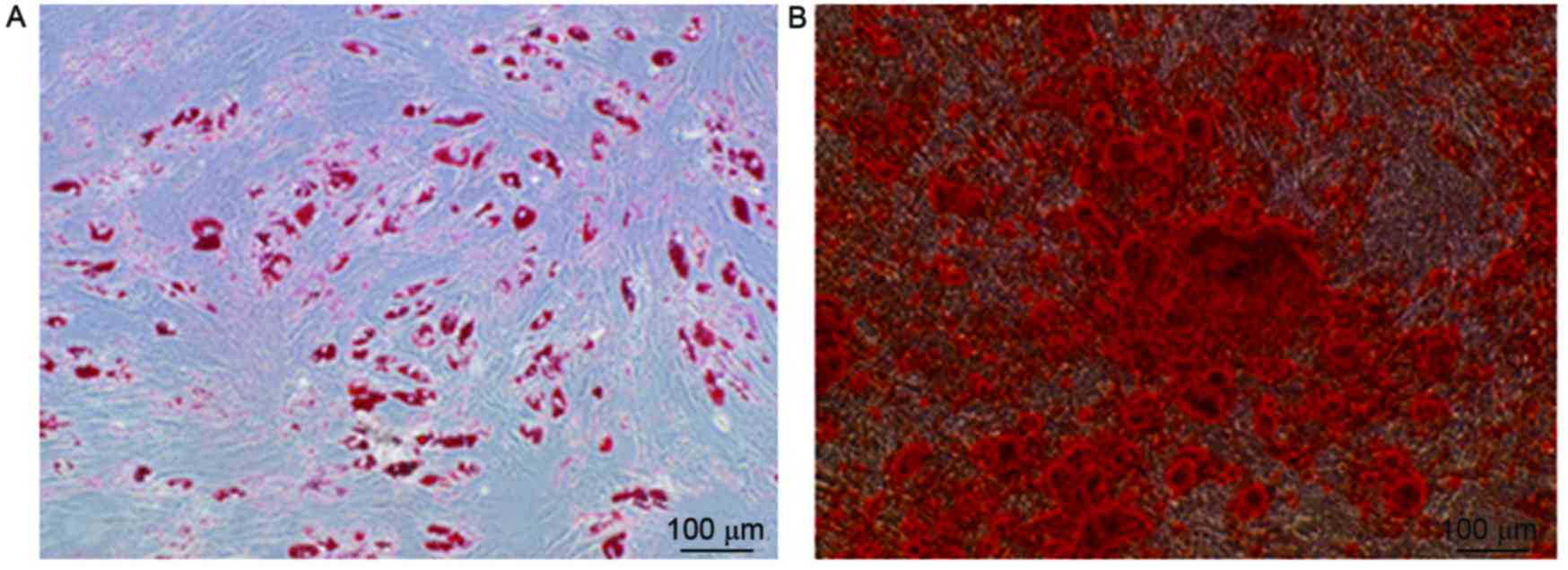
Differentiation of UC-MSCs
An adipogenic medium was used to induce adipocyte
differentiation. After 5 days, the cells had begun to store lipid
drops in the cytoplasm, which was considered a sign of adipogenic
differentiation. After 21 days, when the cells were stained with
Oil Red O solution, red staining of the cells was observed, which
confirmed that cells had changed into adipose cells (Fig. 6A). In addition, these cells expressed
FABP4 and PPAR-γ RNA, which is consistent with the adipocyte
phenotype (Fig. 4B) (20,21).
An osteogenic medium was used to induce osteogenic
differentiation. The cells underwent a series of changes on the
culture plates: Deposits formed on day 3 and gradually increased in
size until day 21. It was confirmed that cells had changed into
osteoblasts because they appeared red when treated with 2% Alizarin
Red S (Fig. 6B) and were shown to
express ALP and osteocalcin RNA (Fig.
4C).
Discussion
Recent studies have identified the human UC as a
novel source of multipotent stem cells (22–24).
There is a high demand for MSCs and EPCs, which are applied in the
treatment of patients with hematologic disorders, malignancies,
inherited immunodeficiency diseases, and even metabolic diseases
(3,5,6,10–12).
Although there have been some studies concerning the simultaneous
collection of mesenchymal stem cells, hematopoietic cells or
endothelial cells from the UC or the associated blood, the
achievements have been limited (25,26). The
present study describes a strategy to isolate MSCs and EPCs from a
single UC according to their different locations within the
cord.
EPCs have been isolated and expanded ex vivo
from UC blood or Wharton's jelly in previous research (20,21).
However, where the majority of EPCs reside in the UC is unknown. We
considered that they may be distributed around the UC vein. EPCs
were successfully isolated from the UC vein in the present study.
The isolated EPCs were demonstrated to have the properties of
progenitor cells in proliferative potential assays, including
growth curve, cell cycle and colony-forming assays. The cells had a
cobble-like morphology, and expressed Flt-1, KDR, VE-Cadherin, CD31
and vWF RNAs. They were strongly positive for CD31, CD73, CD105 and
VEGFR-2, but were negative for CD45, HLA-DR and the mesenchymal
marker CD90. In addition to absorbing DiI-ac-LDL and FITC-UEA-l,
they were able to form vascular tube-like structures on
Matrigel.
In the present study, the MSCs taken from the UC
Wharton's jelly had a spindle-shaped morphology, resembling that of
MSCs isolated from bone marrow. Flow cytometric analysis showed
that the percentage of cells expressing CD73, CD90, CD105, CD34,
CD45 HLA-DR was consistent with previously reported data (27,28).
These MSCs were able to differentiate into adipocytes that
accumulated lipid vacuoles and osteoblasts that were stained with
Alizarin Red S in vitro. They expressed
adipocyte/osteoblast-specific RNAs that were consistent with
previous reports for bone marrow MSCs (29).
In conclusion, the present study describes an
economical and commercially viable option for the harvesting of
EPCs and MSCs for use in stem cell research and cell replacement
therapy.
Acknowledgements
This study was supported by the 863 Program of the
Ministry of Science & Technology of the People's Republic of
China (grant no. 2011AA020114), the Military Clinical High-Tech Key
Program (grant no. 2010gxjs100), Clinical Feature and Application
Research of Capital (grant no. Z111107058811107), Science &
Technology Development Projects of Shandong Province (grant no.
2012YD18066), Shandong Province Commission for Population and
Family Planning (grant no. 201309), the Jining Science and
Technology Bureau (grant no. 2012jnnk03) and Youth Foundation of
Jining Medical University (grant no. JYQ14KJ30).
References
|
1
|
Timmermans F, Plum J, Yöder MC, Ingram DA,
Vandekerckhove B and Case J: Endothelial progenitor cells: Identity
defined? J Cell Mol Med. 13:87–102. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kang SD, Carlon TA, Jantzen AE, Lin FH,
Ley MM, Allen JD, Stabler TV, Haley NR, Truskey GA and Achneck HE:
Isolation of functional human endothelial cells from small volumes
of umbilical cord blood. Ann Biomed Eng. 41:2181–2192. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kalka C, Masuda H, Takahashi T, Kalka-Moll
WM, Silver M, Kearney M, Li T, Isner JM and Asahara T:
Transplantation of ex vivo expanded endothelial progenitor cells
for therapeutic neovascularization. Proc Natl Acad Sci USA. 97:pp.
3422–3427. 2000; View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fan CL, Li Y, Gao PJ, Liu JJ, Zhang XJ and
Zhu DL: Differentiation of endothelial progenitor cells from human
umbilical cord blood CD 34+ cells in vitro. Acta
Pharmacol Sin. 24:212–218. 2003.PubMed/NCBI
|
|
5
|
Rafii S and Lyden D: Therapeutic stem and
progenitor cell transplantation for organ vascularization and
regeneration. Nat Med. 9:702–712. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pittenger MF, Mackay AM, Beck SC, Jaiswal
RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S and
Marshak DR: Multilineage potential of adult human mesenchymal stem
cells. Science. 284:143–147. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Toma C, Pittenger MF, Cahill KS, Byrne BJ
and Kessler PD: Human mesenchymal stem cells differentiate to a
cardiomyocyte phenotype in the adult murine heart. Circulation.
105:93–98. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Secco M, Zucconi E, Vieira NM, Fogaça LL,
Cerqueira A, Carvalho MD, Jazedje T, Okamoto OK, Muotri AR and Zatz
M: Multipotent stem cells from umbilical cord: Cord is richer than
blood! Stem Cells. 26:1–150. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bongso A, Fong CY and Gauthaman K: Taking
stem cells to the clinic: Major challenges. J Cell Biochem.
105:1352–1360. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hayward CJ, Fradette J, Galbraith T, Rémy
M, Guignard R, Gauvin R, Germain L and Auger FA: Harvesting the
potential of the human umbilical cord: Isolation and
characterisation of four cell types for tissue engineering
applications. Cells Tissues Organs. 197:37–54. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang SH, Lin SJ, Chen YH, Lin FY, Shih JC,
Wu CC, Wu HL and Chen YL: Late outgrowth endothelial cells derived
from Wharton jelly in human umbilical cord reduce neointimal
formation after vascular injury: Involvement of pigment
epithelium-derived factor. Arterioscler Thromb Vasc Biol.
29:816–822. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chao KC, Chao KF, Fu YS and Liu SH:
Islet-like clusters derived from mesenchymal stem cells in
Wharton's Jelly of the human umbilical cord for transplantation to
control type 1 diabetes. PLoS One. 3:e14512008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang H, Zhang B, Tao Y, Cheng M, Hu J, Xu
M and Chen H: Isolation and characterization of mesenchymal stem
cells from whole human umbilical cord applying a single enzyme
approach. Cell Biochem Funct. 30:643–649. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang HS, Hung SC, Peng ST, Huang CC, Wei
HM, Guo YJ, Fu YS, Lai MC and Chen CC: Mesenchymal stem cells in
the Wharton's jelly of the human umbilical cord. Stem Cells.
22:1330–1337. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sarugaser R, Lickorish D, Baksh D,
Hosseini MM and Davies JE: Human umbilical cord perivascular
(HUCPV) cells: A source of mesenchymal progenitors. Stem Cells.
23:220–229. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang J, Kimura T, Asada R, Harada S,
Yokota S, Kawamoto Y, Fujimura Y, Tsuji T, Ikehara S and Sonoda Y:
SCID-repopulating cell activity of human cord blood-derived
CD34-cells assured by intra-bone marrow injection. Blood.
101:2924–2931. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yahata T, Ando K, Sato T, Miyatake H,
Nakamura Y, Muguruma Y, Kato S and Hotta T: A highly sensitive
strategy for SCID-repopulating cell assay by direct injection of
primitive human hematopoietic cells into NOD/SCID mice bone marrow.
Blood. 101:2905–2913. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hur J, Yoon CH, Kim HS, Choi JH, Kang HJ,
Hwang KK, Oh BH, Lee MM and Park YB: Characterization of two types
of endothelial progenitor cells and their different contributions
to neovasculogenesis. Arterioscler Thromb Vasc Biol. 24:288–293.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Murga M, Yao L and Tosato G: Derivation of
endothelial cells from CD34-umbilical cord blood. Stem Cells.
22:385–395. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Moon SH, Kim SM, Park SJ, Kim H, Bae D,
Choi YS and Chung HM: Development of a xeno-free autologous culture
system for endothelial progenitor cells derived from human
umbilical cord blood. PLoS One. 8:e752242013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Huang ZW, Liu N, Li D, Zhang HY, Wang Y,
Liu Y, Zhang LL and Ju XL: Angiopoietin-1 modified human umbilical
cord mesenchymal stem cell therapy for endotoxin-induced acute lung
injury in rats. Yonsei Med J. 58:206–216. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee MJ, Yoon TG, Kang M, Kim HJ and Kang
KS: Effect of subcutaneous treatment with human umbilical cord
blood-derived multipotent stem cells on peripheral neuropathic pain
in rats. Korean J Physiol Pharmacol. 21:153–160. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kumbhar SG and Pawar SH: Synthesis and
characterization of chitosan-alginate scaffolds for seeding human
umbilical cord derived mesenchymal stem cells. Biomed Mater Eng.
27:561–575. 2016.PubMed/NCBI
|
|
24
|
Marupanthorn K, Tantrawatpan C, Kheolamai
P, Tantikanlayaporn D and Manochantr S: Bone morphogenetic
protein-2 enhances the osteogenic differentiation capacity of
mesenchymal stromal cells derived from human bone marrow and
umbilical cord. Int J Mol Med. 39:654–662. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yin C, Liang Y, Zhang J, Ruan G, Li Z,
Pang R and Pan X: Umbilical cord-derived mesenchymal stem cells
relieve hindlimb ischemia through enhancing Angiogenesis in tree
shrews. Stem Cells Int. 2016:97420342016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zayed SA, Gaafar TM, Samy RM, Sabry D,
Nasr AS and Maksoud FA: Production of endothelial progenitor cells
obtained from human Wharton's jelly using different culture
conditions. Biotech Histochem. 91:532–539. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Meng X, Xue M, Xu P, Hu F, Sun B and Xiao
Z: MicroRNA profiling analysis revealed different cellular
senescence mechanisms in human mesenchymal stem cells derived from
different origin. Genomics. 109:147–157. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lo Iacono M, Anzalone R, La Rocca G,
Baiamonte E, Maggio A and Acuto S: Wharton's jelly mesenchymal
stromal cells as a feeder layer for the ex vivo expansion of
hematopoietic stem and progenitor cells: A review. Stem Cell Rev.
13:35–49. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Fellows CR, Matta C, Zakany R, Khan IM and
Mobasheri A: Adipose, bone marrow and synovial joint-derived
mesenchymal stem cells for cartilage repair. Front Genet.
7:2132016. View Article : Google Scholar : PubMed/NCBI
|